Abstract
Omics approaches to the study of complex biological systems with potential applications to molecular medicine are attracting great interest in clinical as well as in basic biological research. Genomics, transcriptomics and proteomics are characterized by the lack of an a priori definition of scope, and this gives sufficient leeway for investigators (a) to discern all at once a globally altered pattern of gene/protein expression and (b) to examine the complex interactions that regulate entire biological processes. Two popular platforms in “omics” are DNA microarrays, which measure messenger RNA transcript levels, and proteomic analyses, which identify and quantify proteins. Because of their intrinsic strengths and weaknesses, no single approach can fully unravel the complexities of fundamental biological events. However, an appropriate combination of different tools could lead to integrative analyses that would furnish new insights not accessible through one-dimensional datasets. In this review, we will outline some of the challenges associated with integrative analyses relating to the changes in metabolic pathways that occur in complex pathophysiological conditions (viz. ageing and altered thyroid state) in relevant metabolically active tissues. In addition, we discuss several new applications of proteomic analysis to the investigation of mitochondrial activity.
1. Introduction
Genomic and proteomic data analyses have proven to be essential for an understanding of the underlying factors involved in human disease and for the discovery of diagnostic biomarkers, as well as for the provision of further insights into the metabolic effects mediated by signaling molecules.
All classes of biological compounds, from genes through mRNA to proteins and metabolites, can be analyzed by the respective “omic” approaches, namely, genomics, transcriptomics, proteomics, or metabonomics. Such an “omic” approach leads to a broader view of the complex biological system, including the pathology of diseases. Indeed, while the data obtained from genomics may explain the disposition of diseases (i.e., increased risk of acquiring a certain disease), several other mechanisms that are not gene mediated may be involved in the onset of disease. Moreover, a single gene can be processed to result in several different mRNAs or proteins, which directly determine different cellular functions. Variations in metabolite fluxes, which may be taken as the downstream result of changes in gene expression and protein translation, may be expected to be amplified relative to changes in the transcriptome and proteome. However, time-dependent measurements and determinations of metabolite content at a single time-point can be misleading as these fluxes vary quickly. Therefore, while genomics/transcriptomics enables assessments of all potential information, proteomics enables us to assess the programs that are actually executed, and metabolomics will mostly display the results of such executions.
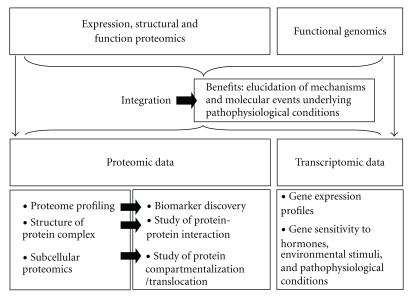
In the postgenomic era, functional analysis of genes and their products constitutes a novel and powerful approach since the expression levels of multiple genes and proteins can thereby be analyzed simultaneously, in both health and disease (Figure 1). Among the techniques used in functional genomics, both DNA microarrays [1–3] and classical and ongoing proteomic approaches (finalized to protein separation and identification) [4–6] hold great promise for the study of complex biological systems and have applications in molecular medicine. These technologies allow high-throughput analysis as they are complementary to each other, and they may lead to a better understanding of the regulatory events involved in physiological, and disease, processes. Proteins are excellent targets in disease diagnostics, prognostics, and therapeutics. Consequently, proteomic approaches (such as two-dimensional gel electrophoresis (2D-E), two-dimensional liquid chromatography (2-DL), and mass spectrometry (MS)), which allow the simultaneous measurement and comparison of the expression levels of hundreds of proteins, represent powerful tools for (a) the discovery of novel hormone/drug targets and biomarkers and (b) studies of cellular metabolism and protein expressions [7, 8]. Increasingly, proteomic techniques are being adopted—in particular, to avoid the limitations inherent in the more classical approaches—to solve analytical problems and obtain a more comprehensive identification and characterization of molecular events associated with pathophysiological conditions (Figure 1).
Figure 1.
Categories and potential applications of proteomics and benefits of integration of proteomics and transcriptomics in the study of complex biological systems.
In this paper, we will discuss a variety of mainly recent transcriptomic- and proteomic-based studies that have provided a comprehensive mechanistic insight into two very complex biological phenomena, namely, age-associated muscle sarcopenia and thyroid-hormone signaling. Moreover, as mitochondria are severely affected during ageing and it is generally believed that dysfunctions of mitochondria also cause ageing and muscle sarcopenia, we will also discuss proteomic analysis of the alterations in rat skeletal muscle mitochondria caused by ageing.
2. Ageing Sarcopenia
Ageing, one of the most complex biological phenomena, is a multifaceted process in which several physiological changes occur at both the tissue and the whole-organism level. Indeed, the age-associated decline in the healthy functioning of multiple organs/systems leads to an increased incidence of, and mortality from, diseases such as type II diabetes mellitus, neurodegenerative diseases, cancer, and cardiovascular disease [9].
One of the major engagements of gerontology is the understanding of the complex mechanisms involved in ageing at the molecular, cellular, and organ levels that would facilitate our understanding of age-related diseases. Research in this area has accelerated with the application of high-throughput technologies such as microarrays. To judge from such studies, several metabolic pathways are affected during ageing, and the picture becomes even more complex when we realize that most of them are interconnected.
Sarcopenia, the age-related decline in skeletal muscle mass and strength, is a major complication in the elderly [10, 11]. Since skeletal muscle represents the most abundant tissue in the body, fiber degeneration has severe consequences for posture, movement, the overall integration of metabolism, and heat homeostasis [12]. Although various metabolic and functional defects in ageing muscle have been described over the last decade, senescence-related muscle wasting is not well understood at the molecular and cellular levels. Consequently, no effective treatment has yet been developed to counteract age-related fiber degeneration [13].
Over the last decades, an attractive approach to the understanding of the molecular mechanisms involved in sarcopenia has been to screen all genetic pathways at one time, by the use of full-genome oligonucleotide chips, as well as the entire protein complement, by the use of using proteomic tools. These approaches, when applied together to the multifactorial muscle-wasting pathology observed in aged fibers, have allowed the identification of a variety of molecular and cellular changes. These include increased oxidative stress, mitochondrial abnormalities, disturbed microcirculation, hormonal imbalance, incomplete ion homeostasis, denervation, and impaired excitation-contraction coupling, as well as a decreased regenerative potential (see, Sections 2.1 and 2.2). In addition, altered posttranslational modifications, such as tyrosine nitration, glycosylation, and phosphorylation, were recently described as occurring in an age-related manner in numerous skeletal muscle proteins (see, Section 2.3).
2.1. Transcriptomic Analysis Pertaining to Ageing Skeletal Muscle
Knowledge of differential mRNA expressions (i.e., the transcriptome) constitutes the first essential level of information when studying integrated cell functions and cell-specific gene-expression profiles. Since the development of DNA microarray technology, it has been possible to survey thousands of genes in parallel, thereby obtaining information regarding transcriptional changes on a global scale. Such an approach has been used to study the transcriptional alterations induced by ageing both in rodent models and in humans. Ageing-related transcriptomic studies have been performed both on home-spotted microarrays containing about 4000–6000 transcripts [14–16] and on commercial Affymetrix microarrays with from 12000 [17, 18] to about 54000 [19–26] transcripts on each array.
Concerning studies on humans, the design commonly used involved a cross-sectional comparison of young and elderly healthy individuals, with about eight individuals maximum per group, or an analysis of individuals across an age-range. In these studies, several pathways were highlighted by genes that were differentially expressed between young (19–29 years) and elderly (65–85 years) individuals [14], including genes involved in energy metabolism, the cell cycle, signal transduction, and DNA repair [19–22].
Biological pathways found to be changed with age in human skeletal muscle are listed in Table 1 and schematized in Figure 2. They include genes involved in the mitochondrial electron transport chain, cell cycle, and extracellular matrix. Zahn et al. [21], by comparing the transcriptional profile of ageing in muscle with previous transcriptional profiles of ageing in the kidney [22] and brain [17], found a common signature for ageing in these diverse human tissues. This common ageing signature consists of six genetic pathways; four display increased expression (genes in the extracellular matrix, genes involved in cell growth, genes encoding factors involved in complement activation, and genes encoding components of the cytosolic ribosome) and two display decreased expression in the aged muscle. These results indicate that those pathways, but not necessarily individual genes, are common elements in the age-related expression changes among different tissues [21]. This may imply that in addition to tissue-specific effects, a common ageing signature may be found in any tissue that reflects the age of the whole organism. This could have major implications for human epidemiological studies, for which frequently only blood is available.
Table 1.
Summary of the models used and of the major findings obtained by applying microarray technologies to the study of ageing skeletal muscle.
| Authors | Experimental model | Number of analyzed genes | Identified affected pathways |
|---|---|---|---|
| Mouse | |||
| Lee et al., 1999 [25] | Studied tissue: aged skeletal muscle. | 6347 | Stress response, energy metabolism. |
| Rat | |||
| Sreekumar et al., 2002 [28] | 12-months-old Sprague-Dawley rats. Studied tissue: gastrocnemius muscle. | 800 | Energy metabolism, signal transduction, stress response, glucose/lipid metabolism, and structural/contractile function. |
| Altun et al., 2007 [29] | 4- and 30-months-old rats.Studied tissue: gastrocnemius muscle. | 6240 | Redox homeostasis, iron load, regulation of contractile proteins, glycolysis, and oxidative phosphorylation. |
| Lombardi et al., 2009 [26] | 3- and 24-months-old rats.Studied tissue: gastrocnemius muscle. | 1176 | Energy metabolism, mitochondrial pathways, myofibrillar filaments, and detoxification. |
| Human | |||
| Welle et al., 2003 [23] | 21–27 yr of age and 67–75 yr of age.Studied tissue: vastus lateralis muscle. | 44 000 | Cell cycle/cell growth, inflammation, signal transduction, protein metabolism, transcription, stress response/DNA repair, energy metabolism, and hormonal. |
| Welle et al., 2004 [19] | 20–29 yr of age and 65–71 yr of age, women.Studied tissue: vastus lateralis muscle. | 1000 | Stress response/DNA repair, energy metabolism. |
| Zahn et al., 2006 [21] | 16 and 89 yr of age. Studied tissue: skeletal muscle. | 54 675 | Electron transport chain, cell cycle/cell growth, extracellular matrix, chloride transport, complement activation, ribosomes. |
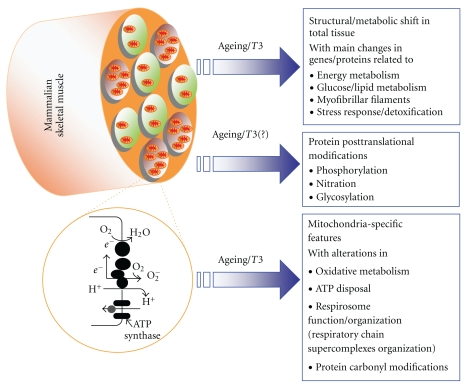
Figure 2.
Integrated overview of the main ageing/T3-induced transcriptomic and proteomic alterations occurring in mammalian skeletal muscle. Schematic representation of the common events and mechanisms underlying the response of skeletal muscle to either ageing or T3 according to data obtained from cDNA microarray/proteomic-based studies in various mammalian models of ageing and thyroid state (mouse, rat, and human) (for details, see text and Tables 1, 2, 3, and 4).
Transcriptomic studies have been performed in laboratory animals using commercially available microarrays. As in humans, the main design used for measuring changes related to chronological age is a comparison between young and old individuals. These studies are usually performed on inbred strains, and so the variation between individual animals is smaller than among human individuals. The range of tissues studied includes liver, heart, skeletal muscle, aorta, and brain. Across all species, and in most experimental designs there is an influence of gender on ageing features and gene expressions [24]. Biological pathways found to be changed with age in rodent (mouse and rat) skeletal muscle are listed in Table 1.
Transcriptomic analysis of gastrocnemius muscle from 5- and 30-month-old male C57BL/6 mice revealed that ageing resulted in a differential gene expression pattern [25]. Of the genes that increased in expression with age, 16% were mediators of stress responses, including heat shock-response genes, oxidative stress-inducible genes, and DNA damage inducible genes (Table 1). Genes involved in energy metabolism were downregulated with ageing, including genes associated with mitochondrial function and turnover. This suppression of metabolic activity was accompanied by a concerted decline in the expressions of genes involved in glycolysis, glycogen metabolism, and the glycerophosphate shunt (Table 1). Ageing was also characterized by the induction of genes involved in neuronal growth and large reductions in the expressions of biosynthetic enzymes.
We recently performed a transcriptomic study on gastrocnemius muscle from rats aged 3 months (young) and 24 months (old) via a DNA array [26]. Transcript levels for genes associated with cellular damage were elevated in the older muscle, while transcript levels for genes involved in energy metabolism were reduced with age. Among the biological classes of transcripts significantly decreased by ageing, there were transcription factors as well as ribosomal proteins, indicative of a lower transcription/translation activity in old than in young skeletal muscle (Table 1). In agreement with previous microarray studies on the skeletal muscles of humans and rodents [18, 25, 27], we found that ageing is accompanied by a decline in the expressions of genes associated with energy-metabolism functions [26] (Table 1). Alterations in oxidative phosphorylation were revealed by decreased expression levels of cytochrome c oxidase, ATPase subunit, and carbonic anhydrase III [26]. The capacity of mitochondria to import and oxidize fatty acids would presumably be impaired since the mRNA levels for acylCoA synthase as well as carnitine palmitoyl transferase 1 (CPT 1β) were reduced during ageing. Downregulation of the AK1 isoform of adenylate kinase [26] points toward decreased AMP production and hence decreased activity of AMP-activated protein kinase (AMPK), an inducer of glucose and fatty acid uptake and fatty acid oxidation. Gastrocnemius muscle from the old rats revealed increased expressions of various factors involved in muscle differentiation toward the “slow” phenotype (type I; oxidative fibers), including p27kip and muscle LIM protein (MLP) [26] (Table 1). As a whole, the above data support an ageing-induced shift towards moderate fat burning.
Ageing has been found to increase the mRNA levels of scavengers of free radicals such as phospholipids hydroperoxide glutathione peroxidase and the cytosolic superoxide dismutase Cu/Zn SOD (SOD1). In addition, 14-kDa ubiquitin-conjugating enzyme E2 mRNA (a component of the complex that adds ubiquitin to target proteins, making them capable of destruction by the proteasome machinery) and both proteasome subunit C5 and proteasome delta subunit precursor were downregulated in aged muscle. Since the proteasome is the major proteolytic complex responsible for the selective degradation of oxidized proteins, these data point toward a defective action of the proteasome. With regard to the lysosomal pathway of protein breakdown, cathepsin L (which acts upstream of the ubiquitin-proteasome system) was also downregulated in aged muscle [26], once again supporting a decline in proteolysis during ageing.
The above studies have been successful in elucidating some of the transcriptional changes that occur with age in muscle, as well as in other tissues, and in providing insights about age-related changes common to animals with different lifespans.
2.2. Proteomic Analysis Pertaining to Ageing Skeletal Muscle
Proteomic analysis has proved valuable in informing our understanding of the molecular mechanisms involved in the ageing process through the identification both of changes in protein levels and of various posttranslational modifications such as phosphorylation [30], nitration [31, 32], and glycosylation [33] that progress with age. In order to identify novel biomarkers of age-dependent skeletal muscle sarcopenia, mass spectrometry-based proteomics has been applied to the study of global muscle protein expression patterns. Mass spectrometric peptide fingerprinting, chemical peptide sequencing, electrophoretic mobility comparison using international 2-D gel databanks, and/or large-scale immunoblot analysis are among the most frequently utilized techniques.
Over the last years, several proteomic studies have catalogued the accessible skeletal muscle protein complement from various species and also investigated changes in protein expression levels in the sarcopenia of old age. The data obtained so far have furnished databanks that form an important prerequisite for future large-scale proteomic investigations into muscle ageing.
Table 2 lists proteomic studies on age-related changes in skeletal muscle in rodent and human models of ageing. Although the lists of individual proteins found to be affected by the ageing process differ considerably between individual proteomic surveys, the main trend of the altered proteins involved in energy metabolism, cellular signaling, the stress response, cytoskeleton, and contraction shows agreement among the various studies. Gelfi et al. [34] performed a quantitative differential analysis of muscle protein expression in elderly and young subjects using a 2-D DIGE approach. The main difference observed in the elderly group included downregulation of regulatory myosin light chains, particularly the phosphorylated isoforms, a higher proportion of myosin heavy chain isoforms 1 and 2A, and enhanced oxidative and reduced glycolytic capacities.
Table 2.
Summary of the models used and of the major findings obtained by applying proteomic approaches to the study of the ageing skeletal muscle.
| Authors | Experimental model | Proteomic analysis | Identified affected pathways and major findings |
|---|---|---|---|
| Mouse | |||
| Chang et al., 2003 [39] | 18-months-old C57B16 mice. Studied tissue: skeletal muscle. | Two-dimensional polyacrilamide gel electrophoresis. | Reproducibility of the 2-D PAGE system. |
| Rats | |||
| Cai et al., 2000 [35] | 12-, 18-, and 24-months-old rats.Studied tissue: extensor digitorum longus muscle and soleus muscle. | Two-dimensional gel electrophoresis. | Analysis of aqueous proteins of skeletal muscle during aging. |
| Cai et al., 2001 [40] | 8-, 18-, and 24-months-old rats.Studied tissue: extensor digitorum longus muscle and soleus muscle. | Two-dimensional gel electrophoresis. | Analysis of parvalbumin expression in rat skeletal muscles. |
| Kanski et al., 2003 [31] | 4- and -24 months old Fisher 344 rats and -6 and -34 months old Fisher 344/BN F1 rats.Studied tissue: skeletal muscle. | 2-D gel electrophoresis, Western blot analysis, MALDI-TOF MS and ESI-MS/MS analysis. | Age-dependent nitration in muscle energy metabolism. |
| Piec et al., 2005 [36] | 7-, 18- and 30-months-old LOU/c/jall rats.Studied tissue: gastrocnemius muscle. | Two-dimensional gel electrophoresis, MALDI-ToF MS analyses, and immunoblotting. | Myofibrillar regulatory proteins, signal transduction, cytosolic and mitochondrial energy metabolisms, oxidative stress, detoxification, and RNA metabolism. |
| Kanski et al., 2005 [32] | 34-months-old Fisher 344/Brown Norway F1 hybrid rats.Studied tissue: skeletal muscle. | 2-D gel electrophoresis, Western Blot analysis, MALDI-TOF and NSI-MS/MS analysis. | Proteomic analysis of protein nitration. |
| Dencher et al., 2006 [41] | Studied tissue: skeletal muscle. | Blue-native/colorless-native gel electrophoresis, 2D-SDS-PAGE and MALDI MS. | Cellular dysfunction, ageing, and cellular death. |
| O'Connell et al., 2007 [37] | 3- and 30-months-old rat. Studied tissue: gastrocnemius muscle | Two-dimensional gel electrophoresis, MALDI-ToF, DALT-Twelve gel electrophoretic separation system, 2-D immunoblotting. | Proteomic profiling of senescent fibres: stress response, contractile apparatus, and metabolic regulation. |
| Altun et al., 2007 [29] | 4- and 30-months old rats.Studied tissue: gastrocnemius muscle. | Two-dimensional gel electrophoresis, MALDI-ToF/ToF, MALDI-MS/MS, ESI-LC-MS/MS and Western Blot analysis. | Redox homeostasis, iron load, regulation of contractile proteins, glycolisis, and oxidative phosphorylation. |
| O'Connell et al., 2008 [33] | 3- and 30-months old rats. Studied tissue: gastrocnemius muscle | Two-dimensional gel electrophoresis, MALDI-ToF MS analysis. | Proteomic profiling of senescent fibers. |
| Gannon et al., 2008 [30] | 3- and -30-months old rats.Studied tissue: gastrocnemius muscle. | Two-dimensional gel electrophoresis, MALDI-ToF MS analysis. | Phosphoproteomic analysis of aged skeletal muscle. |
| Feng et al., 2008 [42] | 12- and 26-months-old Fischer 344 rats.Studied tissue: soleus, semimembranosus, plantaris, extensor digitorum longus, and tibialis anterior muscles. | SDS-polyacrylamide gel electrophoresis, μLC-ESI MS/MS analysis and Ingenuity Systems Analysis. | Carbonyl modifications, cellular function and maintenance, fatty acid metabolism, and citrate cycle. |
| Lombardi et al., 2009 [26] | 3- and 24-months-old rats.Studied tissue: gastrocnemius muscle. | Two-dimensional gel electrophoresis, Blue-Native PAGE, and MALDI-ToF MS analysis. | Energy metabolism, mitochondrial pathways, myofibrillar filaments, and detoxification. |
| Human | |||
| Cobon et al., 2002 [43] | 56–79 yr of age.Studied tissue: vastus lateralis muscle. | Two-dimensional polyacrilamide gel electrophoresis and MALDI-TOF MS. | Human aged skeletal muscle protein profile. |
| Gelfi et al., 2006 [34] | Elderly and young subjects.Studied tissue: vastus lateralis muscle. | Two-dimensional difference gel electrophoresis, SDS-PAGE and ESI-MS/MS. | Elderly group: downregulation of regulatory myosin light chains, (phosphorylated isoforms), higher proportion of myosin heavy chain isoforms 1 and 2A, and enhanced oxidative and reduced glycolytic capacity. |
Proteomic profiling of rodent muscle during ageing has been performed in several studies, resulting in the identification of a large cohort of sarcopenic biomarkers (for a schema, see Figure 2).
Age-dependent differential regulation in rodent muscle has been identified for several glycolytic and mitochondrial enzymes, which are important for energy metabolism. The glycolytic enzymes triosephosphate isomerase, glyoxalase I, and β-enolase were downregulated in aged muscle. Other features indicating perturbation of energy metabolism were downregulation of creatine kinase, of pyruvate kinase, and of the NADH-shuttle glycerol 3-phosphate dehydrogenase. At the mitochondrial level, key enzymes such as isocitrate dehydrogenase, cytochrome c oxidase, ATP synthase β subunit, and pyruvate dehydrogenase were all decreased in ageing muscle whereas there was an upregulation of aldehyde dehydrogenase [26, 29, 35–38].
Differential proteomic analyses have revealed that ageing is associated with perturbations of the myofibrillar network [26, 29, 35–38]. Notably, there is a downregulation of several isoforms of myosin long chain and of alpha-actin, as well as a differential expression of their major regulators. In contrast to the downregulation of myofibrillar components, old muscles display upregulation of many proteins of the intermediate filament, microtubules and microfilament cytoskeleton, among which are B-tubulin, desmin, and gelsolin. This suggests a mechanism affecting the cytoskeleton that compensates for perturbations in myofibrillar structure and so prevents extensive damage to the myofibers. Muscle ageing is also associated with the differential expression of enzymes implicated in the detoxification of cytotoxic products. The cytoplasmic Cu/Zn superoxide dismutase (SOD1) and H ferritin isoform, as well as the levels of glutathione transferase and mitochondrial aldehyde dehydrogenase, were found to be increased in older rats, while evidence of age-associated protein misfolding was provided by the upregulation of molecular chaperones (including HSP 27 and disulfide isomerase ER60) [26, 29, 35–38].
Most of the proteins identified by differential proteomics were previously unrecognized in ageing skeletal muscle. Their identification has not only provided further insight into the potential mechanisms of ageing, but may lead to the development of biomarkers of sarcopenia [26, 29, 35–38].
2.3. Proteomic Analysis Pertaining to Ageing Skeletal Muscle: Analyses of Protein Phosphorylation, Nitration, and Glycosylation
Since posttranslational modifications are key modulators of protein structure, function, signaling, and regulation, various subdisciplines of proteomics have emerged that focus on the cataloguing and functional characterization of proteins with extensively modified side chains [57]. In aged skeletal muscle, proteins undergo considerable changes in their posttranslational modifications [58]. These include, among others, phosphorylation, nitration, and glycosylation. Phosphorylation represents one of the most frequent peptide modifications [59], and abnormal phosphorylation is associated with various pathologies. A recent phosphoproteomic survey of aged muscle detected increased phosphorylation levels for myosin light chain 2, tropomyosin α, lactate dehydrogenase, desmin, actin, albumin, and aconitase [30]. In contrast, decreased phospho-specific dye binding was observed for cytochrome c oxidase, creatine kinase, and enolase. Thus, ageing-induced alterations in phosphoproteins appear to involve the contractile machinery and the cytoskeleton, as well as cytosolic and mitochondrial metabolism.
The nitration of protein tyrosine residues represents an oxidative and important posttranslational modification occurring under nitrative/oxidative stress during biological ageing. Comprehensive proteomic studies have identified an age-related increase in the nitration of numerous skeletal muscle proteins. These include enolase, aldolase, creatine kinase, tropomyosin, glyceraldehyde-3-phosphate dehydrogenase, myosin light chain, pyruvate kinase, actinin, actin, and the ryanodine receptor [31, 32]. The nitration of these essential muscle proteins may therefore be a significant causative factor in the age-related decline in muscle strength [31, 32].
Glycosylation is one of the most frequent posttranslational modifications found in proteins, and it plays a central role in cellular mechanisms in both health and disease [60]. Oligosaccharide attachment represents a common protein modification that influences the folding of the nascent peptide chain and the stability of glycoproteins, modifies enzymatic activity, controls protein-secretion events, presents critical information about the cellular targeting of a newly synthesized protein, and provides specific recognition motifs for other proteins in cell-cell interactions [61]. The identified muscle components belong mostly to the family of metabolic enzymes. They included glycolytic enzymes, such as pyruvate kinase, enolase, phosphoglycerate kinase, aldolase, glyceraldehyde-3-phosphate dehydrogenase, and phosphoglycerate-mutase, aconitase, carbonic anhydrase, and creatine kinase [33].
These data confirm that the sarcopenia of old age represents a complex neuromuscular pathology that is associated with drastic changes not only in the abundance, but also in the structure of key muscle proteins (Figure 2).
2.4. Proteomic Analysis Pertaining to Ageing Skeletal Muscle Mitochondria
Analysis of the protein profile of mitochondria, and of the changes in it that occur with age, represents a promising approach to the unraveling of the mechanisms involved in ageing. Although the role of mitochondria was long thought to be restricted to an influence on fuel metabolism, the importance of the activity of these organelles has recently been extended to the regulation of developmental/ageing processes [62]. Mitochondria have their own genome (mt-DNA) and specific mechanisms for replication, transcription, and protein synthesis. However, in terms of protein composition they are “hybrid” organelles resulting from the coordinated expression of the nuclear and their own genome. A bidirectional flow of information allows the two kinds of subcellular compartments to communicate with each other under the control of metabolic signals and several signal-transduction pathways that function across the cell. These pathways are differentially regulated by environmental and developmental signals, and under patho/physiological conditions, they allow tissues to adjust their energy production according to different energy demands possibly modulating/altering the mitochondrial phenotype. It is now beyond doubt that mitochondria are severely affected by ageing, and it is generally believed that dysfunctions of mitochondria trigger key steps in the ageing process [62].
Mitochondrial proteomes (mitoproteomes) are currently under vigorous investigation by way of both structural and comparative proteomics. In particular, we would like to emphasize the value of comparative proteomics as a tool capable of providing us with valuable information on mitochondrial physiology and on the role of these organelles in ageing muscle. First, mitochondria can be highly purified, leading to simplified 2D gels, which greatly facilitates the analysis and detection of less-abundant proteins. Second, mitochondrial proteins are generally distributed across wide ranges of both pH and molecular mass on 2D gels, leading to accurate protein resolution with only a few protein-spot overlaps. Third, most of the mitochondrial membrane-protein complexes exhibit soluble subunits that can be analyzed on 2D gels even though the hydrophobic subunits aggregate. Various detection methods are already available that allow us to monitor quantitative changes in the proteome. Of these, 2-DE-based methods appear quite promising, with isoelectric focusing (IEF), BN-SDS, and benzyldimethyl-nhexadecylammonium chloride (16-BAC)-PAGE at the forefront. However, application of IEF is restricted to proteins that are not highly hydrophobic or have no extreme isoelectric points. Indeed, by the use of classical 2D-E it is difficult to detect very acidic or very basic proteins or to distinguish small changes in the expressions of weakly expressed proteins. On the other hand, BN-SDS-PAGE deals efficiently with even hydrophobic membrane proteins, although some compromises in resolution have to be made [63]. Another advantage of the BN-PAGE system is the conservation of protein-protein interactions, enabling simultaneous elucidation of multimeric and multiprotein assemblies of soluble and membrane proteins [64]. Such a procedure might be a viable alternative to other methods, such as yeast two-hybridization.
Comparative transcriptomic and proteomic studies have been initiated to determine global changes in mitochondria from young versus aged skeletal muscle [26, 39, 41, 62, 65–68]. Native-difference gel electrophoresis (DIGE) is an approach that facilitates sensitive quantitative assessment of changes in membrane and soluble proteins. Recently, O'Connell et al. [68] analyzed the mitochondria-rich fraction from aged rat skeletal muscle by DIGE. This proteomic analysis showed a clear age-related increase in key mitochondrial proteins, such as NADH dehydrogenase, the mitochondrial inner membrane protein mitofilin, peroxiredoxin isoform PRX-III, ATPase synthase, succinate dehydrogenase, mitochondrial fission protein Fis1, succinate-coenzyme A ligase, acyl-coenzyme A dehydrogenase, porin isoform VDAC2, ubiquinol-cytochrome c reductase core I protein, and prohibitin [68].
To gain deeper insights both into ageing mechanisms and into the resulting mitoproteome alterations, mitochondria have been studied by the blue-native gel approach, both with respect to protein abundance and the supramolecular organization of OXPHOS complexes [26].
The profiles obtained for muscle crude mitochondria from young and old rats—after detergent extraction with either dodecylmaltoside or digitonin, and subsequent BN-PAGE—have been reported by us within the past years [26]. The use of dodecylmaltoside allows individual resolution of the respiratory complexes. Our densitometric analysis revealed that gastrocnemius muscle mitochondria from old rats, versus those from young rats, contained significantly lower amounts of complex I (NADH:ubiquinone oxidoreductase), V (FoF1-ATP synthase), and III (ubiquinol:cytochrome c oxidoreductase) (−35%, −40%, −25%, resp.). The same mitochondria, on the other hand, contained a significantly larger amount of complex II (succinate: ubiquinone oxidoreductase) (+25%) and an unchanged amount of complex IV (cytochrome c oxidase, COX). The use of a combination of BN-PAGE and catalytic staining allowed us to detect reduced activity of all the complexes in ageing muscle. The observed reductions in the activities of respiratory complexes I, III, and V reflected lower protein levels, but the reduction in complex II activity was associated with an increase in the amount of the same complex. To elucidate whether the ageing process also alters the functional/structural organization of the respiratory chain in terms of the assembly of supercomplexes, mitochondria were extracted using the mild detergent digitonin since this extensively retains inner mitochondrial membrane supercomplexes [69]. In both young and old mitochondria, monomeric complex I and dimeric complex III were significantly reduced versus dodecylmaltoside solubilization. However, the missing amounts were found to be assembled in two major supercomplexes, a and b, and two minor ones, c and d, all within the molecular mass range of 1500–2100 kDA. The supercomplex profile of the older rats was significantly modified, band a being less represented in the profile than the heavier supercomplexes, such as bands c and d. A significant increase was detected in the supramolecular assembly of respiratory chain complexes into respirosomes (each one being formed by complex I assembled with a dimeric complex III and a variable copy number of complex IV, represented by bands c and d). Possibly, this could be a compensatory mechanism that, in ageing muscle, is functionally directed towards substrate channeling and catalytic enhancement advantaging. Indeed, mitochondrial oxidative phosphorylation seems to be more efficient in aged than in young skeletal muscle, since old rats exhibited an increased respiratory control ratio that was attributed principally to a reduction in the reactions able to dissipate the proton motive force not associated with ATP synthesis. This could be interpreted as a compensation for the reduced level and activity of F1F0-ATP synthase.
The above data point up the ability of skeletal muscle to face the consequences of ageing in a metabolically economic way and highlight the occurrence of structural and metabolic adaptations. A comparison between these two studies [26, 68] each employing different proteomic approaches leads to the conclusion that beyond the expression/abundance changes in proteins, an insight can be obtained about the structural and functional heterogeneity in a given mitoproteome.
Another possible protein modification in skeletal muscle mitochondria, possibly contributing to its functional decline with age, is carbonylation, which can be considered an oxidative modification that may render a protein more prone to degradation. Feng et al. [42] recently identified mitochondrial proteins that were susceptible to carbonylation in a manner that was dependent on muscle type (slow- versus fast-twitch) and on age. Carbonylated mitochondrial proteins were more abundant in fast-twitch than in slow-twitch muscle. Twenty-two proteins displayed significant changes in carbonylation state with age, the majority of these increasing in their amount of carbonylation. Ingenuity pathway analysis revealed that these proteins belong to various functional classes and pathways, including cellular function and maintenance, fatty acid metabolism, and the citrate cycle. This study [42] provides a unique catalogue of promising protein targets deserving further investigation because of their potential role in the decline exhibited by ageing muscle. Since carbonylation is not repairable, this modification may, however, be of special importance in directing the affected protein to the path toward degradation.
Of note, in view of the importance of the functional mitochondrial membrane compartmentalization, together with proteomic approaches, lipidomic ones would be desirable to gain further insight into the understanding of the modification of lipids either as membrane components or energy store following aging processes.
3. Thyroid Hormones and Thyroid States
Thyroid hormones [THs; 3,5,3′,5′-tetraiodo-L-thyronine, otherwise known as thyroxine (T4), and 3,5,3′-triiodo-L-thyronine (T3)] are essential for the regulation both of energy metabolism and of development and growth in all vertebrates. In humans, the early developmental role of THs is vividly illustrated by the distinctive clinical features of cretinism, as observed in iodine-deficient areas. In adults, the primary effects of THs are manifested by alterations in metabolism. Even subclinical hyper- and hypothyroidism can have important consequences, such as atherosclerosis, obesity, and alterations in bone mineral density and heart rate [70, 71]. The effects induced by THs in the regulation of metabolism include changes in oxygen consumption and in protein, carbohydrate, lipid, and vitamin metabolism. Hyperthyroidism is associated with an increase (calorigenic effect), while hypothyroidism is associated with a decrease in metabolic rate. Of particular note, is that the number and the complexity of the clinical features of hyperthyroidism and hypothyroidism emphasize the pleiotropic effects of THs on many different pathways and target organs. Although great efforts have been made to elucidate the signaling pathways underlying the physiopathological effects of thyroid hormones, the network of factors and cellular events involved, as well as the possible role of derivatives of THs, is complicated and incompletely understood, as is the ultimate effect of THs on tissue transcriptomes and proteomes.
3.1. The Complexity of Action of Thyroid Hormones: An Overview
Most thyroid-related, direct genomic actions leading to protein changes appear to be attributable to T3. The mechanism of action that has gained general acceptance for this iodothyronine involves the binding of specific nuclear receptors (TRs) to thyroid hormone response elements (TREs) in target genes [72]. Within the nucleus, TRs dimers (hetero- or homodimers) bind to TREs and modulate gene activity by either silencing or activating transcription by recruitment of either corepressor or coactivator complexes, depending on the absence or presence of thyroid hormone [73–78]. In mammals, two genes encoding TRs have been characterized, c-erb Aα and c-erb Aβ [79–81], and these encode several proteins (α and β isoforms) with different binding properties and patterns of tissue expression. For example, c-Erb Aβ1 is expressed across a wide range of tissues, while c-ErbAβ2 is found almost exclusively in the pituitary, where it inhibits thyrotrophin (TSH) α- and β-subunit gene transcription [82] by binding to negative TREs present on these genes [83, 84]. New information on the mechanisms of action of THs have been obtained from TR gene knockout (KO) and knock-in studies [85].
In terms of cellular effects, theories proposed so far to explain the actions of THs on metabolic rate also include mechanisms such as: uncoupling of oxidative phosphorylation, stimulation of energy expenditure by activation of Na+-K+ ATPase activity, and direct modulation by THs of transporters and enzymes located within the plasma membrane and mitochondria [86–89]. Moreover, T3-mediated nuclear gene expression leads in turn to coordinated and synergistic effects on the mitochondrial genome [90]. Actually, it has been postulated that T3's actions on this genome are achieved through both an induction of nuclear-encoded mitochondrial factors and a direct binding of T3 to specific ligand-dependent mitochondrial transcription factors [90–94]. These last are nuclear-receptor homologs and are thought to act on a number of mt-DNA response elements [95]. Indeed, T3 directly regulates the mitochondrial genes encoding ATPase subunit six [96], NADH dehydrogenase subunit three [97], and subunits of cytochrome-c-oxidase [98].
The complexity of action of T3 is broadened by the existence of nongenomic or TRE-independent actions, which have been extensively described and are now accepted [99]. Importantly, these can be either independent or dependent on the binding of T3 to TRs. They occur rapidly and are unaffected by inhibitors of transcription and protein synthesis [90, 93, 100–102]. Nongenomic actions of thyroid hormones have been described at the plasma membrane, in the cytoplasm, and within cellular organelles ([100] and references therein). These actions include modulations of Na+, K+, Ca2+, and glucose transport, activations of PKC, PKA, and ERK/MAPK, and regulation of phospholipid metabolism via activations of PLC and PLD [103], and they can be independent of the presence of nuclear TRs and mediated even by TH analogs [102]. For example, it has recently been shown that cytosolic TRβ can interact with the p85 subunit of PI3K and thereby activate the PI3K-Akt/PKB signaling cascade [99, 104]. Moreover, it has been shown that THs activate the MAPK cascade and stimulate angiogenesis via their binding to integrin α Vβ 3 [100]. Importantly, it appears now to be well established that an interplay exists between the genomic and nongenomic actions when gene expression is regulated by the TR-T3 complex and the activity of the enzyme is modulated by a nongenomic process [100].
3.2. Transcriptomic Analysis Pertaining to the Actions of Thyroid Hormones
Although the molecular actions of THs have been thoroughly studied, their pleiotropic effects are not well understood and appear to be mediated by complex changes in the expressions of numerous, but still largely unknown, target genes in various tissues. DNA microarrays have been successfully used to identify T3-target genes in mouse, rat, and human tissues, cell lines, and tumors. Actually, pioneering systematic studies in the search for T3-target genes were performed by Seelig and coworkers as long ago as 1981 [105].
Feng et al. [44] first applied cDNA-microarray technology to the study of the in vivo T3 regulation of hepatic genes in the mouse. They identified new T3-target genes, the majority of which had not previously been reported to be regulated by the hormone. Surprisingly, many of these target genes were negatively regulated. The identity of the genes indicated that multiple cellular pathways are actually affected by T3, including glycogenolysis, gluconeogenesis, lipogenesis, cell proliferation, apoptosis, the action of insulin, immunogenicity, and protein glycosylation.
Weitzel et al. [49] detected novel T3-target genes and identified two T3-mediated gene-expression patterns after the administration of T3 to hypothyroid rats. In line with the long-known observation that T3 has profound influences over mitochondrial biogenesis and metabolic balance, the authors reported that numerous genes implicated in metabolic pathways (ANT2, apolipoprotein AIV, HMG-CoA synthase, and ATP synthase β subunit) are affected by T3, as also are genes associated with a wide variety of cellular pathways (encompassing translation, protein turnover, cell structure, and apoptosis-associated proteins). These observations gave support to the idea that alongside the “classical” pathway of T3-mediated gene regulation (involving thyroid hormone-receptor binding to TREs), there appears to be an additional pathway mediated by transcription factors (such as NRF-1 and PPARγ) and coactivators (such as the PGC-1 family of coactivators).
Flores-Morales et al. [45] verified the effect of T3 on liver in mice with a targeted mutation in the TRβ gene. In accordance with the results of Weitzel [49], they reported that T3 regulates the expressions of functionally different sets of genes in temporally distinct ways. Importantly, using TRβ−/− animals they also defined a number of T3-responsive genes that are dependent on TRβ in vivo, thereby opening the way for the use of similar experimental strategies to identify the contributions made by specific transcription factors to the in vivo actions of multiple hormones and trophic factors.
Miller et al. [50] identified genes involved in glucose metabolism, biosynthesis, transcriptional regulation, protein degradation, and detoxification that were associated with T3-induced cell proliferation. Of particular significance were the findings that T3 rapidly suppresses the expressions of key regulators of the Wnt signaling pathway and that it suppresses the transcriptional downstream elements of the β-catenin-T-cell factor complex.
With the aim of defining the molecular basis of the target-tissue phenotype related to the hereditary TRβ mutations causing resistance to thyroid hormone (RTH), Miller et al. [46] showed that in T3-target tissues such as cerebellum, heart, and WAT in animal models of both RTH and hyperthyroidism, T3 acts primarily to suppress gene expression, and that TRβ has a greater modulating effect in the heart than originally thought. Moreover, their comprehensive multitissue gene-expression analysis uncovered complex multiple signaling pathways mediating the molecular actions of TRβ mutants in vivo. It also revealed some T3-independent, but mutant-dependent, genomic responses contributing to those “changes-of-function” present in TRβ mutants that are linked to the pathogenesis of RTH.
Dong et al. [48] studied the molecular mechanisms involved in the responses shown by developing mice to disruptions in maternal thyroid-hormone homeostasis. Among differentially expressed genes, Nr4a1 (nuclear receptor subfamily 4, group A, member 1), was upregulated by 3-fold in the hypothyroid juvenile mouse liver, while treatment of HepG2 cells with T3 resulted in its downregulation. A potential thyroid response element −1218 to −1188 bp upstream of the promoter region of Nr4a1 was identified and demonstrated to bind TRα and TRβ receptors.
Notably, in recent years microarray approaches have been used to characterize the effects of T3 on gene expression profiles in the postnatal developing brain as well as in the adult mouse/rat brain [106–108].
The effects of THs on gene expression profiles have been studied less intensively in human tissues than in animal above all because of the poor availability and accessibility of tissue. However, both in vitro [51, 52] and in vivo [53, 109] studies have been performed. Viguerie et al. [51], who showed that T3 regulates a large repertoire of genes in human adipocytes, provided support for the effect of T3 on catecholamine-induced lipolysis, and suggested downregulation of SREBP1c as a link between hyperthyroidism and insulin resistance. Moreover, in accordance with other array studies, the data showed that thyroid hormone can affect cellular processes such as signal transduction, apoptosis, and inflammatory responses. Moeller et al. [52] identified 91 T3-upregulated and 5 T3-downregulated genes in skin fibroblasts from normal humans. Some of these genes were not previously known to be induced by T3, namely aldo-keto reductase family 1 C1-3, collagen type VI alpha 3, member RAS oncogene family brain antigen RAB3B, platelet phosphofructokinase, hypoxia-inducible factor-1 alpha, and enolase 1 alpha. Importantly, these genes have a variety of regulatory functions in both development and metabolism.
Clèment et al. [53] studied the effects of thyroid hormone on human skeletal muscle in vivo. Their data not only helped to explain the effects of T3 on protein turnover and energy metabolism, but also revealed new putative mechanisms extending beyond the classic metabolic effects of the hormone, and importantly, added to our understanding of the permissive effects of T3 on several cellular events (such as signal-transduction cascades, intracellular transport, and tissue remodeling).
Very recently, Visser et al. [54] examined the skeletal muscle transcriptome in thyroidectomized patients being treated for differentiated thyroid carcinoma, and compared it between those who were off or on L-thyroxine replacement. They reported for the first time that in humans as in animals, a large proportion of muscle genes (∼43%) is significantly downregulated by L-thyroxine treatment. They also reported significant regulation of the primary transcripts of the noncoding RNAs miR-206 and miR-133b, which are key regulators in muscle differentiation and proliferation and may affect numerous target genes. The potential of T3 to regulate miRs may be of particular importance since this level of control would add an additional layer of complexity by which T3 may regulate cellular processes.
Collectively, these studies (summarized in Table 3) have provided a cornucopia of novel information (schematized in Figures 2 and 3) on the regulation of transcription by THs. However, the intrinsic nature of these studies means that they provide no information concerning the status of the corresponding encoded proteins, and this is particularly relevant because of the influence of thyroid hormone on protein half-life.
Table 3.
Summary of the models used and of the major findings obtained by applying microarray technologies to the study of THs effects on relevant metabolically active tissues.
| Authors | Experimental model | Treatments | Number of genes | Number of genes affected by T3 | Identified affected pathways and major findings |
|---|---|---|---|---|---|
| Mouse (in vivo and in vitro studies) | |||||
| Feng et al., 2000 [44] | Six-week-old mice. Studied tissue: liver. | Hypothyroidism induced by low-iodine diet supplemented with 0.15% PTU for 4 weeks, then hyperthyroidism induced by single i.p. injection of L-T3 or T4 100 μg/100 g body weight. | 2225 | Of 55 genes identified as target of T3, 41 were negatively regulated. | Glycogenolysis, gluconeogenesis, lipogenesis, proliferation, apoptosis, insulin signaling, immunogenity, and protein glycosylation. |
| Flores-Morales et al., 2002 [45] | 2 to 3.5-months old (WT and TRβ−/−) male mice.Studied tissue: liver. | At the onset of the experiment, all groups of animals were provided a low-iodine diet for 14 d to accustom them to the synthetic chow. Hypothyroidism was then induced by inclusion of 0.05% methimazole and 1% potassium perchlorate in the drinking water for 21 d while still on the low-iodine diet. From d 35, one group of animals was injected daily with 5 μg T3 for an additional 5-d period to induce hyperthyroidism. Another group was injected with 5 μg T3 and 5 μg T4; on d 35, the animals in this group were killed 2 hours after the T3/T4 injection. | 4000 | T3 found to regulate more than 200 genes, more than 100 of which were not previously described. 60% of these genes showed dependence on the TRβ gene for T3 regulation. | Rapid or transient effects of T3 on lipogenic genes. Long-term effects of T3 on genes for the mitochondrial respiratory chain transcription factors and protein turnover. |
| Miller et al., 2004 [46] | 8- to 10-week-old (TRβ+/+ and TRβPV/PV) male siblings (mice).Studied tissues: cerebellum, heart, and white adipose. | TR βPV mice contain a cytosine insertion in exon 10 of the TRβ1 gene at nucleotide position 1,642 of the TRβ1 cDNA that leads to a frameshift of the carboxy- terminal 14 amino acids of TRβ1. For 7 days, T3 (5 μg/mouse/day) was administered by i.p. injection. | 11500 | 163 genes responsive to T3 treatment and 187 genes differentially expressed between TRβPV/PV mice and wild-type littermates. | T3 primarily acts to repress gene expression. TRβ has a powerful modulating effect in the heart. Novel physiologic candidates for T3 action are changes in immune-gene expression and in the induction of antiproliferative genes. The relative levels of TR isoforms lead to dramatic differential effects on gene expression. |
| Ventura-Holman et al., 2007 [47] | Murine non-transfected hepatocyte cell line AML 12, expressing endogenous TRs. | RNA obtained from cells incubated for 3 hours or 24 hours +/− 10 nM T3, in the presence of 10% stripped fetal bovine serum. Cells also incubated in the presence of cycloheximide (10 ìg/ml) +/− 10 nM T3 for 3 hours to discriminate between primary and secondary responses. | 15000 | 12 genes upregulated and 5 genes downregulated in the presence of T3. | Novel T3 responsive genes were identified. Insights were obtained into the role of T3 in processes such as cholesterol metabolism, bile acid secretion, and oncogenesis. |
| Dong et al., 2007 [48] | Hypothyroid juvenile mice.Studied tissue: liver | Gene expression analyzed in livers of mice rendered hypothyroid by treating pregnant mice from gestational d 13 to postnatal d 15 with 6-propyl-2-thiouracil in drinking water. | approximately 20000 | 96 differentially expressed genes were identified. Of these, 72 genes encode proteins of known function, 15 of which had previously been identified as regulated by TH. | Metabolism, development, cell proliferation, apoptosis, and signal transduction. A potential thyroid response element −1218 to −1188 bp upstream of the promoter region of Nr4a1 was identified and demonstrated to bind TH receptor TRα and TRβ. |
| Rat (in vivo and in vitro studies) | |||||
| Weitzel et al., 2001 [49] | Adult male Wistar rats.Studied tissue: liver. | Hypothyroidism induced by i.p. injection of Na131I (250 μCi/100 g body weight) 28 days before the experiments. Hyperthyroidism provoked by i.p. injection of T3 (50 μg/100 g body weight); repeated after 24 hours. Rats killed at 0, 6, 24, and 48 hours after thyroid hormone. | 4608 | Sixty-two of the genes were reproducibly T3-responsive. | Beside the “classical” pathway of T3-mediated gene regulation by TRs binding to TREs, an additional pathway appears to be mediated by transcription factors like NRF-1 and PPARγ and coactivators (like the PGC-1 family of coactivators). |
| Miller et al., 2001 [50] | GC cells (rat pituitary cell-line expressing functional TRs). | Cells were incubated without or with T3 (100 nM) for 1, 3, 6, 12, 24, or 72 hours. At each time-point, cells were harvested for total RNA preparation. | 4400 | 358 responsive genes were identified. 88% had not previously been reported to be modulated by T3. A few genes showed biphasic expression patterns. In total, 203 genes were upregulated and the remainder were downregulated by T3. | Glucose metabolism, biosynthesis, transcriptional regulation, protein degradation, and detoxification in T3-induced cell proliferation. |
| Human (in vivo and in vitro studies) | |||||
| Viguerie and Langin, 2003 [51] | Human adipose tissue obtained from the s.c. abdominal fat depots of Caucasian women for cDNA array and RT competitive PCR experiments. | Surgical adipose tissue samples were dissected from skin and vessels, and cultured adipocytes were obtained. Cultures were treated with T3 (100 nm) or vehicle for 24 hours. Medium-free T3 concentration was measured at 1 and 24 hours after addition of T3 (using RIA kits). | 1 176 | Among the statistically significant changes in mRNA levels of more than 1.3-fold, 13 and 6 genes were positively or negatively regulated, respectively. | Signal transduction, lipid metabolism, apoptosis, and inflammatory responses. |
| Moeller et al., 2005 [52] | Skin fibroblasts of normal individuals. | Human skin was obtained by punch biopsy from three normal individuals and two patients with RTH. Fibroblasts were grown in supplemented with 10% bovine calf serum (BCS). At confluency, the medium was replaced with one containing TH-depleted BCS (TxBCS), obtained from a thyroidectomized calf. For microarrays, incubation with T3 was for 24 hours. | more than 15000 | Microarray analysis identified 148 genes induced by 1.4-fold or more and five genes repressed to 0.7 or less 24 hours after treatment with 2 × 10−9 M T3. Taking into account duplicate genes, these represented 91 up-regulated and five downregulated genes, respectively. | Aldo-keto reductase family 1 C1-3, collagen type VI α3, member RAS oncogene family brain antigen RAB3B, platelet phosphofructokinase, hypoxia-inducible factor-1α, and enolase 1α genes, previously known to be induced by TH, were identified and validated. These genes have a variety of regulatory functions in development and metabolism. |
| Clèment et al., 2002 [53] | Healthy male Caucasian volunteers (22–33 years old). The same investigations were performed on day 0 and day 14. Studied tissue: vastus lateralis muscle by percutaneous biopsies. | Participants took one tablet of 25 μg of T3 three times a day (75 μg per day) for 14 days. | 24000 | A transcriptional profile of 383 genes regulated by T3 was obtained (381 were upregulated and only two downregulated). | Novel target genes for T3 were identified. They belong to functional classes including transcriptional control, mRNA maturation, protein turnover, signal transduction, cellular trafficking, and energy metabolism. |
| Visser et al., 2009 [54] | Thyroidectomized patients treated for differentiated thyroid carcinoma (DTC) off and on L-thyroxine replacement.Studied tissue: skeletal muscle | Included were patients who had been diagnosed with DTC and had received initial therapy consisting of near-total thyroidectomy and radioiodine ablation therapy. Four weeks after L-thyroxine withdrawal and 8 wk after subsequent L-thyroxine replacement, patients were admitted to the clinical research unit. A catheter was inserted in a dorsal hand vein to collect blood samples for measurement of serum TSH, free T4 (fT4), and T3. Muscle biopsies were taken from the quadriceps muscle (vastus lateralis). | 54674 | 607 differentially expressed genes on L-thyroxine treatment were identified, of which approximately 60% were positively and approximately 40% were negatively regulated. | New genes associated with thyroid state and involved in energy and fuel metabolism were overrepresented among the up-regulated genes. L-thyroxine therapy induced a large downregulation of the primary transcripts of the noncoding microRNA pair miR-206/miR-133b. |
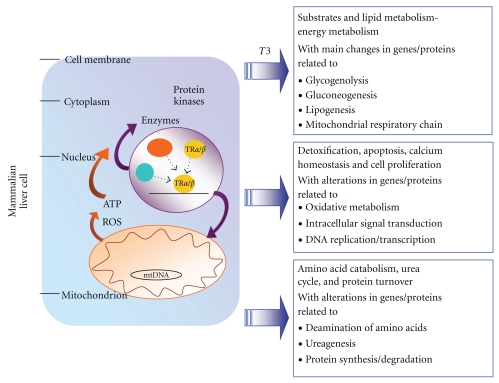
Figure 3.
Overview of the main T3-induced transcriptomic and proteomic alterations occurring in mammalian liver. Schematic representation of the alterations in gene/protein expression underlying the response of liver to T3. Schematized are the main events and mechanisms underlying the actions of T3. Summarized are data obtained in cDNA microarray/proteomic-based studies in various mammalian models (mouse, rat, and human) (for details, see text and Tables 3 and 4).
3.3. Proteomic Analysis Pertaining to the Actions of Thyroid Hormones
As stated above, overall T3 signaling can be modulated at many levels (i.e., the thyroid hormone-receptor isoforms present in the tissue, the DNA-response element in the regulated gene, the availability of receptor-binding partners, interactions with coactivators and corepressors, ligand availability, mRNA and protein stabilities, protein translocation, and metabolic interference) [72, 90–93]. Consequently, for a deeper investigation of the biological events modulated by T3 within target organs, a systematic analysis of the T3-induced changes in protein profile would appear to be appropriate.
We recently performed, on samples taken from rats in different thyroid states, high-resolution differential proteomic analysis, combining 2D-E and subsequent MALDI-ToF MS [55, 56]. These studies (summarized in Table 4) were the first application of proteomic technology to the study of the modulations that T3 exerts in vivo over tissue proteins, and they provided the first systematic identification of T3-induced changes in the protein expression profiles of rat liver and skeletal muscle. In the liver, we unambiguously identified 14 differentially expressed proteins involved in substrate and lipid metabolism, energy metabolism, detoxification of cytotoxic products, calcium homeostasis, amino acid catabolism, and the urea cycle [55]. We found that T3 treatment affected the expressions of enzymes such as mitochondrial aldehyde dehydrogenase, α-enolase, sorbitol dehydrogenase, acyl-CoA dehydrogenase, 3-ketoacyl-CoA thiolase, and 3-hydroxyanthranilate 3,4-dioxygenase. Interestingly, the first two enzymes were upregulated, while the others were downregulated.
Table 4.
Summary of the models used and of the major findings obtained by applying 2D-E and MS to the study of THs effects.
| Authors | Experimental model | Treatments | Number of protein spots analyzed | Number of identified proteins affected by T3 | Identified affected pathways and major findings |
|---|---|---|---|---|---|
| Rat | |||||
| Silvestri et al., 2006 [55] | 3-months-old male Wistar rats. Studied tissue: liver. | Hypothyroidism was induced by i.p. administration of PTU (1 mg/100 g BW) for 4 weeks together with a weekly i.p. injection of IOP (6 mg/100 g BW). T3 was chronically administered by giving seven daily i.p. injections of 15 μg T3/100 g BW to hypothyroid rats, while the control euthyroid and hypothyroid rats received saline injections. | 600 | 14 | The whole cell protein content of rat liver was analyzed following T3 administration. Identified proteins were involved in substrates and lipid metabolism, energy metabolism, detoxification of cytotoxic products, calcium homeostasis, amino acid catabolism, and the urea cycle. |
| Silvestri et al., 2007 [56] | 3-months-old male Wistar rats. Studied tissue: skeletal muscle. | Hypothyroidism and hyperthyroidism were induced as above (Silvestri et al 2006 [55]). | 220 | 20 | The whole-cell protein content of gastrocnemius muscles was analyzed. The differentially expressed proteins unambiguously identified were involved in substrates and energy metabolism, stress response, cell structure, and gene expression. |
Our data were in accordance with the reported role played by thyroid hormone in the stimulation of the rate of ethanol elimination [110], and they provided further insight into the mechanisms actuated by T3 in that pathway. T3 is known to stimulate gluconeogenesis and glucose production in the liver, thereby opposing the action of insulin on hepatic glucose production [111]. Our results extended this knowledge by showing that T3 significantly enhances the level of α-enolase, thereby participating in glycolysis and gluconeogenesis. In addition, T3 administration induced a significant increase in the hepatic ATP synthase α-chain content (in accordance with the ability of T3 to stimulate ATP synthesis) and concomitantly reduced the expression level of electron transfer flavoprotein α-subunit (α-ETF), and also that of the acyl-CoA dehydrogenases [112]. T3 treatment is associated with significant reductions in the expression levels of both peroxisomal catalase and cytoplasmic glutathione-S-transferase [55], the former being important in the protection of cells against the toxic effects of hydrogen peroxide while the latter is implicated in the cellular detoxification of a number of xenobiotics by means of their conjugation to reduced glutathione. T3 treatment of hypothyroid rats is also associated with a selective upregulation of HSP60, a molecular chaperone [113]. SMP30, also known as regucalcin, which was previously not known to be affected by T3, has now been identified as a T3 target [55]. This opens new perspectives in our understanding of the molecular pathways related to intracellular T3-dependent signaling, raising the possibility that T3 may modulate a plethora of cellular events while also acting on multifunctional proteins such as SMP30, which in turn is able to modulate the levels of second messengers such as calcium.
T3-treated rats exhibit significant reductions in the protein levels of both ornithine carbamoyltransferase and arginase 1 [55]. These data are in accordance with a previous report [114], and in line with the idea that in the hypothyroid state there are decreases in protein synthesis and turnover.
Concerning skeletal muscle, the whole-cell protein content of gastrocnemius muscle has been analyzed, and twenty differentially expressed proteins among euthyroid, hypothyroid, and hyperthyroid rats have been identified [56]. The largest group of affected proteins (50%) was involved in substrate and energy metabolism, another important group was represented by stress-induced proteins (HSPs) (21.4%), and the remainder were implicated in structural features or gene expression (transcription, translation), each of these two groups representing 14.3% of the identified proteins [56]. The thyroid state was found to induce structural shifts in gastrocnemius muscle, toward a slower phenotype in hypothyroidism and toward a faster phenotype in hyperthyroidism [56].
Among the proteins involved in substrate metabolism, three glycolytic enzymes have been identified, namely, beta-enolase, pyruvate kinase, and triosephosphate isomerase. Beta-enolase protein levels were increased following T3 treatment (hyperthyroidism), while pyruvate kinase and triosephosphate isomerase levels were decreased in hypothyroidism and elevated in hyperthyroidism [56]. This is in accordance with (a) a major T3-dependence on pyruvate kinase and triosephosphate isomerase and a generally decreased metabolic dependence on glycolysis in hypothyroidism, and (b) an increased reliance on glycolysis in hyperthyroidism [115]. Accordingly, hyperthyroidism was found to be associated with an increased expression of cytoplasmic malate dehydrogenase. Moreover, phosphatidylethanolamine-binding protein, a basic protein that shows preferential affinity in vitro for phosphatidylethanolamine, was significantly increased in both the hypo- and hyperthyroid gastrocnemius (versus the euthyroid controls), most likely reflecting a thyroid state-associated cell-remodeling [56].
The expression level of the ATP synthase beta subunit was increased in both hypothyroid and hyperthyroid muscle (versus euthyroid controls), with a slight decrease in hyperthyroid animals versus hypothyroid ones. Cytosolic creatine kinase, on the other hand, was decreased in hypothyroidism versus both euthyroidism and hyperthyroidism, suggesting a decreased dependence of energy metabolism on the creatine kinase shuttle in hypothyroid muscle [56].
The expression level of HSP70 was found to be significantly increased in hypothyroid muscle (versus both euthyroid and hyperthyroid muscle), paralleling the changes in MHCIb [56]. A similar expression pattern was found for HSP20 which, despite not being a heat-inducible HSP, is biologically regulated by several cellular signalling pathways. Also identified was HSP27, which has been demonstrated to play important roles in smooth muscle cells (actin polymerization, remodeling, and even cross-bridge cycling), and which can, moreover, act as a chaperone in the regulation of contractile-protein activation [116] and also combat insulin resistance [117].
Concerning cell structure, in accordance with a predominant expression of MHCIb over MHCIIb in hypothyroidism and a reversal of the ratio between the two fiber-type isoforms after T3 administration, the expression level of myosin regulatory light chain 2, typical of slow-twitch fibers, was strongly increased in hypothyroidism, with hyperthyroidism significantly reducing it (in each case, versus euthyroidism) [56].
Finally, both hypothyroidism and hyperthyroidism induced chromodomain-helicase-DNA-binding protein 1 (CHD 1), as well as eukaryotic translation initiation factor 3 subunit 10 (IF3A), two proteins that play important roles in different steps of gene expression: (1) initiation of transcription; and (2) initiation of translation [56].
4. Conclusions
In conclusion, although the biochemical and cellular mechanisms that underlie sarcopenia in ageing muscle and the effects elicited by thyroid hormones are only beginning to be elucidated, array-based transcriptomic studies, together with MS-based proteomic ones, are producing new insights into the pathophysiological mechanisms behind such complex phenomena.
As can be seen from the above discussion, the approaches used in the cited studies have allowed the identification of previously unrecognized proteins, thereby increasing our awareness of the large repertoire of proteins, and the multiple cell processes and signaling pathways that are affected by T3 and by ageing (for a schematic representation, see Figures 2 and 3). However, as the majority of the cited studies were performed in vivo, the possibility remains that certain hormones and/or other factors that are affected by such metabolic situations may have been partially responsible for the observed results.
On the basis of what has been achieved so far, the authors feel justified in championing the use of combined transcriptomic and proteomic approaches in living animals for the study of complex physiological, as well as pathophysiological, systems. Such approaches should also prove valuable for drug-design, enabling the agonist and/or antagonist properties of drugs (as well as their side effects) to be characterized on the basis of the changes they induce in protein-expression patterns.
Acknowledgments
This work was supported by Grant MIUR-COFIN 20089SRS2X and by Grant Regione Campania 2008.
References
- 1.Schena M, Shalon D, Davis RW, Brown PO. Quantitative monitoring of gene expression patterns with a complementary DNA microarray. Science. 1995;270(5235):467–470. doi: 10.1126/science.270.5235.467. [DOI] [PubMed] [Google Scholar]
- 2.Gerhold D, Rushmore T, Caskey CT. DNA chips: promising toys have become powerful tools. Trends in Biochemical Sciences. 1999;24(5):168–173. doi: 10.1016/s0968-0004(99)01382-1. [DOI] [PubMed] [Google Scholar]
- 3.Lennon GG. High-throughput gene expression analysis for drug discovery. Drug Discovery Today. 2000;5(2):59–66. doi: 10.1016/s1359-6446(99)01448-8. [DOI] [PubMed] [Google Scholar]
- 4.Celis JE, Østergaard M, Jensen NA, Gromova I, Rasmussen HH, Gromov P. Human and mouse proteomic databases: novel resources in the protein universe. FEBS Letters. 1998;430(1-2):64–72. doi: 10.1016/s0014-5793(98)00527-4. [DOI] [PubMed] [Google Scholar]
- 5.Appel RD, Hoogland C, Bairoch A, Hochstrasser DF. Constructing a 2-D database for the World Wide Web. Methods in Molecular Biology. 1999;112:411–416. doi: 10.1385/1-59259-584-7:411. [DOI] [PubMed] [Google Scholar]
- 6.Dunn MJ. Studying heart disease using the proteomic approach. Drug Discovery Today. 2000;5(2):76–84. doi: 10.1016/s1359-6446(99)01449-x. [DOI] [PubMed] [Google Scholar]
- 7.Righetti PG, Castagna A, Antonucci F, et al. Critical survey of quantitative proteomics in two-dimensional electrophoretic approaches. Journal of Chromatography A. 2004;1051(1-2):3–17. doi: 10.1016/j.chroma.2004.05.106. [DOI] [PubMed] [Google Scholar]
- 8.Vlahou A, Fountoulakis M. Proteomic approaches in the search for disease biomarkers. Journal of Chromatography B. 2005;814(1):11–19. doi: 10.1016/j.jchromb.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 9.Vandervoort AA. Aging of the human neuromuscular system. Muscle and Nerve. 2002;25(1):17–25. doi: 10.1002/mus.1215. [DOI] [PubMed] [Google Scholar]
- 10.Melton LJ, III, Khosla S, Crowson CS, O’Connor MK, O’Fallon WM, Riggs BL. Epidemiology of sarcopenia. Journal of the American Geriatrics Society. 2000;48(6):625–630. [PubMed] [Google Scholar]
- 11.Greenlund LJS, Nair KS. Sarcopenia—consequences, mechanisms, and potential therapies. Mechanisms of Ageing and Development. 2003;124(3):287–299. doi: 10.1016/s0047-6374(02)00196-3. [DOI] [PubMed] [Google Scholar]
- 12.Carmeli E, Coleman R, Reznick AZ. The biochemistry of aging muscle. Experimental Gerontology. 2002;37(4):477–489. doi: 10.1016/s0531-5565(01)00220-0. [DOI] [PubMed] [Google Scholar]
- 13.Larsson L. The age-related motor disability: underlying mechanisms in skeletal muscle at the motor unit, cellular and molecular level. Acta Physiologica Scandinavica. 1998;163(3):S27–S29. doi: 10.1046/j.1365-201x.1998.00375.x. [DOI] [PubMed] [Google Scholar]
- 14.Rao DV, Boyle GM, Parsons PG, Watson K, Jones GL. Influence of ageing, heat shock treatment and in vivo total antioxidant status on gene-expression profile and protein synthesis in human peripheral lymphocytes. Mechanisms of Ageing and Development. 2003;124(1):55–69. doi: 10.1016/s0047-6374(02)00170-7. [DOI] [PubMed] [Google Scholar]
- 15.Ly DH, Lockhart DJ, Lerner RA, Schultz PG. Mitotic misregulation and human aging. Science. 2000;287(5462):2486–2492. doi: 10.1126/science.287.5462.2486. [DOI] [PubMed] [Google Scholar]
- 16.Kyng KJ, May A, Kølvraa S, Bohr VA. Gene expression profiling in Werner syndrome closely resembles that of normal aging. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(21):12259–12264. doi: 10.1073/pnas.2130723100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu T, Pan Y, Kao S-Y, et al. Gene regulation and DNA damage in the ageing human brain. Nature. 2004;429(6994):883–891. doi: 10.1038/nature02661. [DOI] [PubMed] [Google Scholar]
- 18.Erraji-Benchekroun L, Underwood MD, Arango V, et al. Molecular aging in human prefrontal cortex is selective and continuous throughout adult life. Biological Psychiatry. 2005;57(5):549–558. doi: 10.1016/j.biopsych.2004.10.034. [DOI] [PubMed] [Google Scholar]
- 19.Welle S, Brooks AI, Delehanty JM, et al. Skeletal muscle gene expression profiles in 20-29 year old and 65-71 year old women. Experimental Gerontology. 2004;39(3):369–377. doi: 10.1016/j.exger.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 20.Csoka AB, English SB, Simkevich CP, et al. Genome-scale expression profiling of Hutchinson-Gilford progeria syndrome reveals widespread transcriptional misregulation leading to mesodermal/mesenchymal defects and accelarated atherosclerosis. Aging Cell. 2004;3(4):235–243. doi: 10.1111/j.1474-9728.2004.00105.x. [DOI] [PubMed] [Google Scholar]
- 21.Zahn JM, Sonu R, Vogel H, et al. Transcriptional profiling of aging in human muscle reveals a common aging signature. PLoS genetics. 2006;2(7, article e115) doi: 10.1371/journal.pgen.0020115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodwell GEJ, Sonu R, Zahn JM, et al. A transcriptional profile of aging in the human kidney. PLoS Biology. 2004;2(12, article e427) doi: 10.1371/journal.pbio.0020427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Welle S, Brooks AI, Delehanty JM, Needler N, Thornton CA. Gene expression profile of aging in human muscle. Physiological Genomics. 2003;14:149–159. doi: 10.1152/physiolgenomics.00049.2003. [DOI] [PubMed] [Google Scholar]
- 24.Tower J. Sex-specific regulation of aging and apoptosis. Mechanisms of Ageing and Development. 2006;127(9):705–718. doi: 10.1016/j.mad.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 25.Lee C-K, Klopp RG, Weindruch R, Prolla TA. Gene expression profile of aging and its retardation by caloric restriction. Science. 1999;285(5432):1390–1393. doi: 10.1126/science.285.5432.1390. [DOI] [PubMed] [Google Scholar]
- 26.Lombardi A, Silvestri E, Cioffi F, et al. Defining the transcriptomic and proteomic profiles of rat ageing skeletal muscle by the use of a cDNA array, 2D- and Blue native-PAGE approach. Journal of Proteomics. 2009;72(4):708–721. doi: 10.1016/j.jprot.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 27.Kayo T, Allison DB, Weindruch R, Prolla TA. Influences of aging and caloric restriction on the transcriptional profile of skeletal muscle from rhesus monkeys. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(9):5093–5098. doi: 10.1073/pnas.081061898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sreekumar R, Unnikrishnan J, Fu A, et al. Effects of caloric restriction on mitochondrial function and gene transcripts in rat muscle. American Journal of Physiology - Endocrinology and Metabolism. 2002;283(1):E38–43. doi: 10.1152/ajpendo.00387.2001. [DOI] [PubMed] [Google Scholar]
- 29.Altun M, Edström E, Spooner E, et al. Iron load and redox stress in skeletal muscle of aged rats. Muscle and Nerve. 2007;36(2):223–233. doi: 10.1002/mus.20808. [DOI] [PubMed] [Google Scholar]
- 30.Gannon J, Staunton L, O’Connell K, Doran P, Ohlendieck K. Phosphoproteomic analysis of aged skeletal muscle. International Journal of Molecular Medicine. 2008;22(1):33–42. [PubMed] [Google Scholar]
- 31.Kanski J, Alterman MA, Schöneich C. Proteomic identification of age-dependent protein nitration in rat skeletal muscle. Free Radical Biology and Medicine. 2003;35(10):1229–1239. doi: 10.1016/s0891-5849(03)00500-8. [DOI] [PubMed] [Google Scholar]
- 32.Kanski J, Hong SJ, Schöneich C. Proteomic analysis of protein nitration in aging skeletal muscle and identification of nitrotyrosine-containing sequences in vivo by nanoelectrospray ionization tandem mass spectrometry. The Journal of Biological Chemistry. 2005;280(25):24261–24266. doi: 10.1074/jbc.M501773200. [DOI] [PubMed] [Google Scholar]
- 33.O’Connell K, Doran P, Gannon J, Ohlendieck K. Lectin-based proteomic profiling of aged skeletal muscle: decreased pyruvate kinase isozyme M1 exhibits drastically increased levels of N-glycosylation. European Journal of Cell Biology. 2008;87(10):793–805. doi: 10.1016/j.ejcb.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 34.Gelfi C, Viganò A, Ripamonti M, et al. The human muscle proteome in aging. Journal of Proteome Research. 2006;5(6):1344–1353. doi: 10.1021/pr050414x. [DOI] [PubMed] [Google Scholar]
- 35.Cai D, Li M, Lee K, Lee K, Wong W, Chan K. Age-related changes of aqueous protein profiles in rat fast and slow twitch skeletal muscles. Electrophoresis. 2000;21(2):465–472. doi: 10.1002/(SICI)1522-2683(20000101)21:2<465::AID-ELPS465>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 36.Piec I, Listrat A, Alliot J, Chambon C, Taylor RG, Bechet D. Differential proteome analysis of aging in rat skeletal muscle. The FASEB Journal. 2005;19(9):1143–1145. doi: 10.1096/fj.04-3084fje. [DOI] [PubMed] [Google Scholar]
- 37.O’Connell K, Gannon J, Doran P, Ohlendieck K. Proteomic profiling reveals a severely perturbed protein expression pattern in aged skeletal muscle. International Journal of Molecular Medicine. 2007;20(2):145–153. [PubMed] [Google Scholar]
- 38.Doran P, O’Connell K, Gannon J, Kavanagh M, Ohlendieck K. Opposite pathobiochemical fate of pyruvate kinase and adenylate kinase in aged rat skeletal muscle as revealed by proteomic DIGE analysis. Proteomics. 2008;8(2):364–377. doi: 10.1002/pmic.200700475. [DOI] [PubMed] [Google Scholar]
- 39.Chang J, Van Remmen H, Cornell J, Richardson A, Ward WF. Comparative proteomics: characterization of a two-dimensional gel electrophoresis system to study the effect of aging on mitochondrial proteins. Mechanisms of Ageing and Development. 2003;124(1):33–41. doi: 10.1016/s0047-6374(02)00167-7. [DOI] [PubMed] [Google Scholar]
- 40.Cai DQ, Li M, Lee KK, et al. Parvalbumin expression is downregulated in rat fast-twitch skeletal muscles during aging. Archives of Biochemistry and Biophysics. 2001;387(2):202–208. doi: 10.1006/abbi.2001.2231. [DOI] [PubMed] [Google Scholar]
- 41.Dencher NA, Goto S, Reifschneider NH, Sugawa M, Krause F. Unraveling age-dependent variation of the mitochondrial proteome. Annals of the New York Academy of Sciences. 2006;1067(1):116–119. doi: 10.1196/annals.1354.014. [DOI] [PubMed] [Google Scholar]
- 42.Feng J, Xie H, Meany DL, Thompson LV, Arriaga EA, Griffin TJ. Quantitative proteomic profiling of muscle type-dependent and age-dependent protein carbonylation in rat skeletal muscle mitochondria. Journals of Gerontology A. 2008;63(11):1137–1152. doi: 10.1093/gerona/63.11.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cobon GS, Verrills N, Papakostopoulos P, et al. Proteomics of ageing. Biogerontology. 2002;3(1-2):133–136. doi: 10.1023/a:1015240304287. [DOI] [PubMed] [Google Scholar]
- 44.Feng X, Jiang Y, Meltzer P, Yen PM. Thyroid hormone regulation of hepatic genes in vivo detected by complementary DNA microarray. Molecular Endocrinology. 2000;14(7):947–955. doi: 10.1210/mend.14.7.0470. [DOI] [PubMed] [Google Scholar]
- 45.Flores-Morales A, Gullberg H, Fernandez L, et al. Patterns of liver gene expression governed by TRβ. Molecular Endocrinology. 2002;16(6):1257–1268. doi: 10.1210/mend.16.6.0846. [DOI] [PubMed] [Google Scholar]
- 46.Miller LD, McPhie P, Suzuki H, Kato Y, Liu ET, Cheng SY. Multi-tissue gene-expression analysis in a mouse model of thyroid hormone resistance. Genome Biology. 2004;5(5, article R31) doi: 10.1186/gb-2004-5-5-r31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ventura-Holman T, Mamoon A, Maher JF, et al. Thyroid hormone responsive genes in the murine hepatocyte cell line AML 12. Gene. 2007;396(2):332–327. doi: 10.1016/j.gene.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 48.Dong H, Yauk CL, Williams A, Lee A, Douglas GR, Wade MG. Hepatic gene expression changes in hypothyroid juvenile mice: characterization of a novel negative thyroid-responsive element. Endocrinology. 2007;148(8):3932–3940. doi: 10.1210/en.2007-0452. [DOI] [PubMed] [Google Scholar]
- 49.Weitzel JM, Radtke C, Seitz HJ. Two thyroid hormone-mediated gene expression patterns in vivo identified by cDNA expression arrays in rat. Nucleic Acids Research. 2001;29(24):5148–5155. doi: 10.1093/nar/29.24.5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller LD, Park KS, Guo QM, et al. Silencing of Wnt signaling and activation of multiple metabolic pathways in response to thyroid hormone-stimulated cell proliferation. Molecular and Cellular Biology. 2001;21(19):6626–6639. doi: 10.1128/MCB.21.19.6626-6639.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Viguerie N, Langin D. Effect of thyroid hormone on gene expression. Current Opinion in Clinical Nutrition and Metabolic Care. 2003;6(4):377–381. doi: 10.1097/01.mco.0000078998.96795.e7. [DOI] [PubMed] [Google Scholar]
- 52.Moeller LC, Dumitrescu AM, Walker RL, Meltzer PS, Refetoff S. Thyroid hormone responsive genes in cultured human fibroblasts. Journal of Clinical Endocrinology and Metabolism. 2005;90(2):936–943. doi: 10.1210/jc.2004-1768. [DOI] [PubMed] [Google Scholar]
- 53.Clément K, Viguerie N, Diehn M, et al. In vivo regulation of human skeletal muscle gene expression by thyroid hormone. Genome Research. 2002;12(2):281–291. doi: 10.1101/gr.207702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Visser WE, Heemstra KA, Swagemakers SMA, et al. Physiological thyroid hormone levels regulate numerous skeletal muscle transcripts. Journal of Clinical Endocrinology and Metabolism. 2009;94(9):3487–3496. doi: 10.1210/jc.2009-0782. [DOI] [PubMed] [Google Scholar]
- 55.Silvestri E, Moreno M, Schiavo L, et al. A proteomics approach to identify protein expression changes in rat liver following administration of 3,5,3′-triiodo-L-thyronine. Journal of Proteome Research. 2006;5(9):2317–2327. doi: 10.1021/pr060141l. [DOI] [PubMed] [Google Scholar]
- 56.Silvestri E, Burrone L, de Lange P, et al. Thyroid-state influence on protein-expression profile of rat skeletal muscle. Journal of Proteome Research. 2007;6(8):3187–3196. doi: 10.1021/pr0701299. [DOI] [PubMed] [Google Scholar]
- 57.Mann M, Jensen ON. Proteomic analysis of post-translational modifications. Nature Biotechnology. 2003;21(3):255–261. doi: 10.1038/nbt0303-255. [DOI] [PubMed] [Google Scholar]
- 58.Schöneich C. Protein modification in aging: an update. Experimental Gerontology. 2006;41(9):807–812. doi: 10.1016/j.exger.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 59.Olsen JV, Blagoev B, Gnad F, et al. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell. 2006;127(3):635–648. doi: 10.1016/j.cell.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 60.Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell. 2006;126(5):855–867. doi: 10.1016/j.cell.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 61.Freeze HH. Genetic defects in the human glycome. Nature Reviews Genetics. 2006;7(8):660–674. doi: 10.1038/nrg1894. [DOI] [PubMed] [Google Scholar]
- 62.Dencher NA, Frenzel M, Reifschneider NH, Sugawa M, Krause F. Proteome alterations in rat mitochondria caused by aging. Annals of the New York Academy of Sciences. 2007;1100:291–298. doi: 10.1196/annals.1395.030. [DOI] [PubMed] [Google Scholar]
- 63.Rexroth S, Meyer zu Tittingdorf JMW, Krause F, Dencher NA, Seelert H. Thylakoid membrane at altered metabolic state: challenging the forgotten realms of the proteome. Electrophoresis. 2003;24(16):2814–2823. doi: 10.1002/elps.200305543. [DOI] [PubMed] [Google Scholar]
- 64.Krause F. Detection and analysis of protein-protein interactions in organellar and prokaryotic proteomes by native gel electrophoresis: (Membrane) protein complexes and supercomplexes. Electrophoresis. 2006;27(13):2759–2781. doi: 10.1002/elps.200600049. [DOI] [PubMed] [Google Scholar]
- 65.Pesce V, Cormio A, Fracasso F, et al. Age-related mitochondrial genotypic and phenotypic alterations in human skeletal muscle. Free Radical Biology and Medicine. 2001;30(11):1223–1233. doi: 10.1016/s0891-5849(01)00517-2. [DOI] [PubMed] [Google Scholar]
- 66.Dani D, Dencher NA. Native-DIGE: a new look at the mitochondrial membrane proteome. Biotechnology Journal. 2008;3(6):817–822. doi: 10.1002/biot.200800030. [DOI] [PubMed] [Google Scholar]
- 67.Chang J, Cornell JE, Van Remmen H, Hakala K, Ward WF, Richardson A. Effect of aging and caloric restriction on the mitochondrial proteome. Journals of Gerontology A. 2007;62(3):223–234. doi: 10.1093/gerona/62.3.223. [DOI] [PubMed] [Google Scholar]
- 68.O'Connell K, Ohlendieck K. Proteomic DIGE analysis of the mitochondria-enriched fraction from aged rat skeletal muscle. Proteomics. 2009;9(24):5509–5524. doi: 10.1002/pmic.200900472. [DOI] [PubMed] [Google Scholar]
- 69.Schägger H, Pfeiffer K. Supercomplexes in the respiratory chains of yeast and mammalian mitochondria. The EMBO Journal. 2000;19(8):1777–1783. doi: 10.1093/emboj/19.8.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cooper DS. Subclinical hypothyroidism. The New England Journal of Medicine. 2001;345(4):260–265. doi: 10.1056/NEJM200107263450406. [DOI] [PubMed] [Google Scholar]
- 71.Knudsen N, Laurberg P, Rasmussen LB, et al. Small differences in thyroid function may be important for body mass index and the occurrence of obesity in the population. Journal of Clinical Endocrinology and Metabolism. 2005;90(7):4019–4024. doi: 10.1210/jc.2004-2225. [DOI] [PubMed] [Google Scholar]
- 72.Oetting A, Yen PM. New insights into thyroid hormone action. Best Practice and Research in Clinical Endocrinology and Metabolism. 2007;21(2):193–208. doi: 10.1016/j.beem.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 73.Robyr D, Wolffe AP, Wahli W. Nuclear hormone receptor coregulators in action: diversity for shared tasks. Molecular Endocrinology. 2000;14(3):329–347. doi: 10.1210/mend.14.3.0411. [DOI] [PubMed] [Google Scholar]
- 74.Zhang J, Lazar MA. The mechanism of action of thyroid hormones. Annual Review of Physiology. 2000;62:439–466. doi: 10.1146/annurev.physiol.62.1.439. [DOI] [PubMed] [Google Scholar]
- 75.Flamant F, Gauthier K, Samarut J. Thyroid hormones signaling is getting more complex: STORMs are coming. Molecular Endocrinology. 2007;21(2):321–333. doi: 10.1210/me.2006-0035. [DOI] [PubMed] [Google Scholar]
- 76.Busson M, Daury L, Seyer P, et al. Avian MyoD and c-Jun coordinately induce transcriptional activity of the 3,5,3′-triiodothyronine nuclear receptor c-ErbAα1 in proliferating myoblasts. Endocrinology. 2006;147(7):3408–3418. doi: 10.1210/en.2006-0101. [DOI] [PubMed] [Google Scholar]
- 77.Desbois C, Aubert D, Legrand C, Pain B, Samarut J. A novel mechanism of action for v-ErbA: abrogation of the inactivation of transcription factor AP-1 by retinoic acid and thyroid hormone receptors. Cell. 1991;67(4):731–740. doi: 10.1016/0092-8674(91)90068-a. [DOI] [PubMed] [Google Scholar]
- 78.Daury L, Busson M, Casas F, Cassar-Malek I, Wrutniak-Cabello C, Cabello G. The triiodothyronine nuclear receptor c-ErbAα1 inhibits avian MyoD transcriptional activity in myoblasts. FEBS Letters. 2001;508(2):236–240. doi: 10.1016/s0014-5793(01)03063-0. [DOI] [PubMed] [Google Scholar]
- 79.Lazar MA. Thyroid hormone receptors: multiple forms, multiple possibilities. Endocrine Reviews. 1993;14(2):184–193. doi: 10.1210/edrv-14-2-184. [DOI] [PubMed] [Google Scholar]
- 80.Brent GA. Mechanisms of disease: the molecular basis of thyroid hormone action. The New England Journal of Medicine. 1994;331(13):847–853. doi: 10.1056/NEJM199409293311306. [DOI] [PubMed] [Google Scholar]
- 81.Sap J, Munoz A, Damm K. The c-erb-A protein is a high-affinity receptor for thyroid hormone. Nature. 1986;324(6098):635–640. doi: 10.1038/324635a0. [DOI] [PubMed] [Google Scholar]
- 82.Abel ED, Moura EG, Ahima RS, et al. Dominant inhibition of thyroid hormone action selectively in the pituitary of thyroid hormone-receptor-β null mice abolishes the regulation of thyrotropin by thyroid hormone. Molecular Endocrinology. 2003;17(9):1767–1776. doi: 10.1210/me.2003-0109. [DOI] [PubMed] [Google Scholar]
- 83.Burnside J, Sarling DS, Carr FE, Chin WW. Thyroid hormone regulation of the rat glycoprotein hormone α-subunit gene promoter activity. The Journal of Biological Chemistry. 1989;264(12):6886–6891. [PubMed] [Google Scholar]
- 84.Bodenner DL, Mroczynski MA, Weintraub BD, Radovick S, Wondisford FE. A detailed functional and structural analysis of a major thyroid hormone inhibitory element in the human thyrotropin β-subunit gene. The Journal of Biological Chemistry. 1991;266(32):21666–21673. [PubMed] [Google Scholar]
- 85.Forrest D, Vennström B. Functions of thyroid hormone receptors in mice. Thyroid. 2000;10(1):41–52. doi: 10.1089/thy.2000.10.41. [DOI] [PubMed] [Google Scholar]
- 86.Lanni A, Moreno M, Lombardi A, De Lange P, Goglia F. Control of energy metabolism by iodothyronines. Journal of Endocrinological Investigation. 2001;24(11):897–913. doi: 10.1007/BF03343949. [DOI] [PubMed] [Google Scholar]
- 87.Lanni A, Moreno M, Lombardi A, Goglia F. Thyroid hormone and uncoupling proteins. FEBS Letters. 2003;543(1∗#8211;3):5–10. doi: 10.1016/s0014-5793(03)00320-x. [DOI] [PubMed] [Google Scholar]
- 88.Silvestri E, Schiavo L, Lombardi A, Goglia F. Thyroid hormones as molecular determinants of thermogenesis. Acta Physiologica Scandinavica. 2005;184(4):265–283. doi: 10.1111/j.1365-201X.2005.01463.x. [DOI] [PubMed] [Google Scholar]
- 89.Silva JE. Thermogenic mechanisms and their hormonal regulation. Physiological Reviews. 2006;86(2):435–464. doi: 10.1152/physrev.00009.2005. [DOI] [PubMed] [Google Scholar]
- 90.Goglia F, Moreno M, Lanni A. Action of thyroid hormones at the cellular level: the mitochondrial target. FEBS Letters. 1999;452(3):115–120. doi: 10.1016/s0014-5793(99)00642-0. [DOI] [PubMed] [Google Scholar]
- 91.Gaspari M, Larsson N-G, Gustafsson CM. The transcription machinery in mammalian mitochondria. Biochimica et Biophysica Acta. 2004;1659(2-3):148–152. doi: 10.1016/j.bbabio.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 92.Scarpulla RC. Nuclear control of respiratory gene expression in mammalian cells. Journal of Cellular Biochemistry. 2006;97(4):673–683. doi: 10.1002/jcb.20743. [DOI] [PubMed] [Google Scholar]
- 93.Wrutniak-Cabello C, Casas F, Cabello G. Thyroid hormone action in mitochondria. Journal of Molecular Endocrinology. 2001;26(1):67–77. doi: 10.1677/jme.0.0260067. [DOI] [PubMed] [Google Scholar]
- 94.Psarra A-MG, Solakidi S, Sekeris CE. The mitochondrion as a primary site of action of steroid and thyroid hormones: presence and action of steroid and thyroid hormone receptors in mitochondria of animal cells. Molecular and Cellular Endocrinology. 2006;246(1-2):21–33. doi: 10.1016/j.mce.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 95.Wrutniak C, Rochard P, Casas F, Fraysse A, Charrier J, Cabello G. Physiological importance of the T3 mitochondrial pathway. Annals of the New York Academy of Sciences. 1998;839:93–100. doi: 10.1111/j.1749-6632.1998.tb10738.x. [DOI] [PubMed] [Google Scholar]
- 96.Gouveia CH, Schultz JJ, Jackson DJ, Williams GR, Brent GA. Thyroid hormone gene targets in ROS 17/2.8 osteoblast like cells identified by differential display analysis. Thyroid. 2002;12(8):663–671. doi: 10.1089/105072502760258631. [DOI] [PubMed] [Google Scholar]
- 97.Iglesias T, Caubín J, Zaballos A, Bernal J, Munoz A. Identification of the mitochondrial NADH dehydrogenase subunit 3 (ND3) as a thyroid hormone regulated gene by whole genome PCR analysis. Biochemical and Biophysical Research Communications. 1995;210(3):995–1000. doi: 10.1006/bbrc.1995.1755. [DOI] [PubMed] [Google Scholar]
- 98.Wiesner RJ, Kurowski TT, Zak R. Regulation by thyroid hormone of nuclear and mitochondrial genes encoding subunits of cytochrome-c oxidase in rat liver and skeletal muscle. Molecular Endocrinology. 1992;6(9):1458–1467. doi: 10.1210/mend.6.9.1331777. [DOI] [PubMed] [Google Scholar]
- 99.Hiroi Y, Kim H-H, Ying H, et al. Rapid nongenomic actions of thyroid hormone. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(38):14104–14109. doi: 10.1073/pnas.0601600103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cheng S-Y, Leonard JL, Davis PJ. Molecular aspects of thyroid hormone actions. Endocrine Reviews. 2010;31(2):139–170. doi: 10.1210/er.2009-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hulbert AJ. Thyroid hormones and their effects: a new perspective. Biological Reviews of the Cambridge Philosophical Society. 2000;75(4):519–631. doi: 10.1017/s146479310000556x. [DOI] [PubMed] [Google Scholar]
- 102.Moreno M, de Lange P, Lombardi A, Silvestri E, Lanni A, Goglia F. Metabolic effects of thyroid hormone derivatives. Thyroid. 2008;18(2):239–253. doi: 10.1089/thy.2007.0248. [DOI] [PubMed] [Google Scholar]
- 103.Kavok NS, Krasilnikova OA, Babenko NA. Thyroxine signal transduction in liver cells involves phospholipase C and phospholipase D activation. Genomic independent action of thyroid hormone. BMC Cell Biology. 2001;2, article 5 doi: 10.1186/1471-2121-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cao X, Kambe F, Moeller LC, Refetoff S, Seo H. Thyroid hormone induces rapid activation of Akt/protein kinase B-mammalian target of rapamycin-p70S6K cascade through phosphatidylinositol 3-kinase in human fibroblasts. Molecular Endocrinology. 2005;19(1):102–112. doi: 10.1210/me.2004-0093. [DOI] [PubMed] [Google Scholar]
- 105.Seelig S, Liaw C, Towle HC, Oppenheimer JH. Thyroid hormone attenuates and augments hepatic gene expression at a pretranslational level. Proceedings of the National Academy of Sciences of the United States of America. 1981;78(8):4733–4737. doi: 10.1073/pnas.78.8.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dong H, Wade M, Williams A, Lee A, Douglas GR, Yauk C. Molecular insight into the effects of hypothyroidism on the developing cerebellum. Biochemical and Biophysical Research Communications. 2005;330(4):1182–1193. doi: 10.1016/j.bbrc.2005.03.099. [DOI] [PubMed] [Google Scholar]
- 107.Zhang H-M, Su Q, Luo M. Thyroid hormone regulates the expression of SNAP-25 during rat brain development. Molecular and Cellular Biochemistry. 2008;307(1-2):169–175. doi: 10.1007/s11010-007-9596-1. [DOI] [PubMed] [Google Scholar]
- 108.Diez D, Grijota-Martinez C, Agretti P, et al. Thyroid hormone action in the adult brain: gene expression profiling of the effects of single and multiple doses of triiodo-L-thyronine in the rat striatum. Endocrinology. 2008;149(8):3989–4000. doi: 10.1210/en.2008-0350. [DOI] [PubMed] [Google Scholar]
- 109.Visser WE, Friesema ECH, Jansen J, Visser TJ. Thyroid hormone transport by monocarboxylate transporters. Best Practice and Research in Clinical Endocrinology and Metabolism. 2007;21(2):223–236. doi: 10.1016/j.beem.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 110.Li J, Nguyen V, French BA, et al. Mechanism of the alcohol cyclic pattern: role of the hypothalamic-pituitary-thyroid axis. American Journal of Physiology. 2000;279(1):G118–G125. doi: 10.1152/ajpgi.2000.279.1.G118. [DOI] [PubMed] [Google Scholar]
- 111.Merkulova T, Keller A, Oliviero P, et al. Thyroid hormones differentially modulate enolase isozymes during rat skeletal and cardiac muscle development. American Journal of Physiology. 2000;278(2):E330–E339. doi: 10.1152/ajpendo.2000.278.2.E330. [DOI] [PubMed] [Google Scholar]
- 112.Nagao M, Parimoo B, Tanaka K. Developmental, nutritional, and hormonal regulation of tissue-specific expression of the genes encoding various acyl-CoA dehydrogenases and α-subunit of electron transfer flavoprotein in rat. The Journal of Biological Chemistry. 1993;268(32):24114–24124. [PubMed] [Google Scholar]
- 113.Hood DA, Joseph A-M. Mitochondrial assembly: protein import. Proceedings of the Nutrition Society. 2004;63(2):293–300. doi: 10.1079/PNS2004342. [DOI] [PubMed] [Google Scholar]
- 114.Sochor M, McLean P, Brown J, Greenbaum AL. Regulation of pathways of ornithine metabolism. Effects of thyroid hormone and diabetes on the activity of enzymes at the “ornithine crossroads” in rat liver. Enzyme. 1981;26(1):15–23. [PubMed] [Google Scholar]
- 115.Dimitriadis GD, Leighton B, Parry-Billings M, West D, Newsholme EA. Effect of hypothyroidism on the sensitivity of glycolysis and glycogen synthesis to insulin in the soleus muscle of the rat. Biochemical Journal. 1989;257(2):369–373. doi: 10.1042/bj2570369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Somara S, Bitar KN. Tropomyosin interacts with phosphorylated HSP27 in agonist-induced contraction of smooth muscle. American Journal of Physiology. 2004;286(6):C1290–C1301. doi: 10.1152/ajpcell.00458.2003. [DOI] [PubMed] [Google Scholar]
- 117.McCarty MF. Induction of heat shock proteins may combat insulin resistance. Medical Hypotheses. 2006;66(3):527–534. doi: 10.1016/j.mehy.2004.08.033. [DOI] [PubMed] [Google Scholar]