Abstract
Hypertension is particularly prevalent in patients aged ⩾65 years, those with a body mass index ⩾30 kg m−2, Blacks and those with type II diabetes. Here we report a prespecified secondary analysis of the efficacy of amlodipine (10 mg day−1), olmesartan medoxomil (40 mg day−1), a combination of the two and placebo in these subgroups. Patients were randomized to treatment for 8 weeks. The primary efficacy endpoint was the change from baseline in mean seated diastolic blood pressure (DBP). Secondary efficacy endpoints included the change from baseline in mean seated systolic BP (SBP), proportions of patients achieving BP goal (<140/90 mm Hg or <130/80 mm Hg in patients with diabetes), and the number and percentage of patients achieving a range of BP targets. Safety and tolerability of amlodipine 5 and 10 mg, olmesartan medoxomil 10, 20 and 40 mg, and all possible combinations of the two were also assessed. For each prespecified subgroup, all active treatments resulted in significant BP reductions from baseline (P<0.05). The antihypertensive effect of the combination of amlodipine+olmesartan medoxomil was generally greater than the constituent amlodipine or olmesartan medoxomil monotherapies, regardless of subgroup. In general, more patients receiving combination therapy achieved BP goal than those treated with monotherapies. The safety and tolerability of combinations were similar to monotherapies across the subgroups. These results suggest that the combination of amlodipine+olmesartan medoxomil provides a safe and effective option for the treatment of hypertension in challenging patient populations.
Keywords: angiotensin receptor blocker, calcium channel blocker, elderly, obesity, race, type II diabetes
Introduction
It is well established that effective blood pressure (BP) control reduces the risk of cardiovascular disease and stroke in patients with hypertension.1, 2 For every 20 mm Hg decrease in systolic BP (SBP), there are 30 and 40% reductions in ischaemic heart disease and stroke mortality, respectively.3
However, only a small proportion of patients achieve BP goal (<140/90 mm Hg or <130/80 mm Hg in patients with diabetes) with antihypertensive monotherapy.1, 2 The Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) and the European Societies of Hypertension and Cardiology guidelines recognize that in the majority of patients, combination therapy will be required.1, 2
The prevalence of hypertension (BP ⩾140/90 mm Hg or ⩾130/80 mm Hg in patients with diabetes) is approximately 33% in the general population and even higher in certain patient populations.4, 5 In the elderly, 63.6% of women and 73.9% of men have hypertension.4, 5 Hypertension prevalence is also higher in Blacks (41.4%), patients with diabetes (76.8%) and those who are obese (body mass index (BMI) ⩾30 kg m−2; 40.8%).5, 6, 7, 8 In the case of elderly or patients with diabetes, this is due, in part, to arterial stiffness as a consequence of the pathobiology of ageing or diabetes.9, 10 Causes of the increased prevalence of hypertension in Blacks have not been fully elucidated, though it appears that physiological factors may have a part.11 In addition, Black children have significantly higher BP than age-matched Caucasian children of <10 years of age, and Blacks with hypertension generally present with more severe disease.4, 5 In obese patients, the renin–angiotensin system may be dysregulated contributing to the development of hypertension.12
Treating hypertension in patients with diabetes is challenging because of a stringent BP goal (<130/80 mm Hg) that has been shown to reduce the progression of diabetic nephropathy onto end-stage renal disease and other complications of diabetes.1, 2, 13 Treatment guidelines from scientific committees recommend the use of two or more antihypertensive agents for control of BP in Blacks or patients with diabetes, and generally recommend the use of two or more antihypertensive agents if BP is ⩾20/10 mm Hg above goal in any patient.1, 2, 13
Combinations of angiotensin receptor blockers (ARBs) and calcium channel blockers (CCBs) are recognized by the European Society of Hypertension and the European Society of Cardiology as effective and well-tolerated therapeutic options.2 It has been shown that combination therapy with an ARB or an angiotensin-converting enzyme inhibitor with a CCB may minimize the adverse effects of the CCB, such as peripheral oedema.14, 15 ARBs also provide protection against renal and cardiac end-organ failure,16 which is of particular importance in patients with diabetes and hypertension. A recent long-term clinical outcomes study, ACCOMPLISH (Avoiding Cardiovascular Events in Combination Therapy in Patients Living With Systolic Hypertension), has shown that the combination of renin–angiotensin system blockade (angiotensin-converting enzyme inhibitor—benazepril) with a CCB (amlodipine) was more effective in reducing cardiovascular complications than the combination of renin–angiotensin system blockade (benazepril) with a diuretic (hydrochlorothiazide).17, 18
The results of the COACH (Combination of Olmesartan Medoxomil and Amlodipine Besylate in Controlling High Blood Pressure) study, a clinical trial that assessed the efficacy and safety of amlodipine besylate (dihydropyridine CCB) in combination with olmesartan medoxomil (ARB) in patients with mild-to-severe hypertension, have been published elsewhere.19 Here we report a prespecified subgroup analysis of the COACH study in patients with diabetes, Blacks, elderly (⩾65 years) patients and those who are overweight/obese with a BMI⩾30 kg m−2.
Materials and methods
Study population
This was an 8-week multicenter, randomized, double-blind, factorial design study conducted at 172 sites in the United States of America. Inclusion criteria have been described in detail elsewhere.19 Briefly, patients who were aged ⩾18 years with both a mean seated diastolic BP (SeDBP) of 95–120 mm Hg from 1 week before randomization and at the randomization visit with a mean SeDBP difference of ⩽10 mm Hg from the two separate visits were randomized to treatment. Subgroup analyses were carried out for each of the following variables: diabetes status (yes/no), age (<65 years and ⩾65 years) and baseline BMI (⩾30 kg m−2, <30 kg m−2). A further subgroup analysis was made on the basis of race (Black/non-Black).
Patients with a history of cardiovascular disease, SeDBP>120 mm Hg, uncontrolled diabetes (glycosylated haemoglobin (HbA1c) >9.0%), history of drug or alcohol abuse, those who smoked >1 pack of cigarettes per day, and those with any medical condition judged by investigators to possibly jeopardize the evaluation of efficacy and safety of therapy were excluded from the study. Patients were also excluded for whom participation in the study constituted a significant risk.
The study was conducted in accordance with the institutional review board committee regulations and the Declaration of Helsinki. Written informed consent was obtained from each patient at screening.
Study design
Patients who are currently not taking any antihypertensive medications (for example, naïve) or those who have completed a washout of their current antihypertensive medications and who have met BP enrollment criteria were randomized to 1 of the 12 treatment regimens: placebo, amlodipine monotherapy (5 or 10 mg day−1), olmesartan medoxomil monotherapy (10, 20 or 40 mg day−1) or combination therapy with amlodipine+olmesartan medoxomil, including all possible dose combinations of the monotherapy groups.19 Randomization included a stratification based on age (<65 years and ⩾65 years) and diabetes status. Efficacy data reported here comprise the highest US Food and Drug Administration-approved dosage of amlodipine (10 mg day−1) combined with olmesartan medoxomil (40 mg day−1), the constituent monotherapies and placebo. Efficacy data are not reported for patients receiving olmesartan medoxomil 10 mg day−1, 20 mg day−1, amlodipine 5 mg day−1, amlodipine+olmesartan medoxomil 5+10 mg day−1, 5+20 mg day−1, 5+40 mg day−1, 10+10 mg day−1 and 10+20 mg day−1 but are available in Supplementary Tables 1 and 2.
Patients were instructed to take their medication at the same time each day (±2 h). Clinic visits were scheduled to allow a trough BP measurement 24 h after the normal dosing time, and patients were refrained from taking their daily medication until all the measurements were completed.
Therapeutic efficacy and safety were evaluated at weeks 2, 4, 6 and 8. Vital signs, including BP and heart rate, were obtained at all scheduled visits. BP was assessed at all participating sites using an automated BP-monitoring device (Omron Model HEM-705CP). After a 5-min rest period, three separate seated BP (SeBP) measurements were taken at 1 min apart, and the mean of the three readings was recorded.
For the efficacy evaluation, the intent-to-treat population was the primary-analysis population and included all the patients who took at least one dose of double-blind study medication and had one post-baseline BP measurement. Patients who took at least one dose of randomized, double-blind study medication were included in the safety population.
Efficacy variables
The primary efficacy variable was the change from baseline in mean SeDBP at week 8, using the last observation carried forward (LOCF) for patients who did not complete the study protocol (week 8/LOCF). The secondary efficacy variable was the change from baseline in mean SeSBP at week 8/LOCF. Other efficacy variables assessed included the following: change from baseline in mean SeDBP and mean SeSBP at weeks 2, 4, 6 and 8 without LOCF; proportion of patients achieving BP goal (combined term of <140/90 mm Hg or <130/80 mm Hg for patients with diabetes) at weeks 2, 4, 6, 8 and 8/LOCF; proportion of patients achieving BP targets at week 8/LOCF (<140/90 mm Hg and <120/80 mm Hg); and comparison of reductions in mean SeSBP and mean SeDBP between combination therapy and its constituent monotherapy components. These efficacy assessments were also applied to prespecified subgroups including age (<65 years, ⩾65 years), race (Black and non-Black), diabetes status and baseline BMI (<30 kg m−2 and ⩾30 kg m−2).
Safety variables
Adverse events (AEs) and serious AEs were recorded at all visits. This study used the definition of a serious AE provided by the US Food and Drug Administration. The occurrence and severity of peripheral oedema were proactively assessed at all scheduled clinic visits, and when oedema was present, the severity was rated on a 5-point scale based on the following categories: no oedema; mild pitting, slight indentation; moderate pitting or indentation; deep pitting, indentation remains; and leg remains swollen. Investigators were encouraged to report increases in the severity of oedema as AEs. Safety data are presented for all monotherapies, drug combinations and placebo.
Statistical analysis
Summary statistics were determined for baseline, endpoint, and the mean change in SeDBP and SeSBP at week 8/LOCF for each level of the subgroup variables. One-sided P-values for testing the significance of combination therapy versus each monotherapy component were derived from an analysis of covariance model that used treatment group, subgroup and treatment-by-subgroup interaction as fixed effects and study baseline BP as a covariate. As P-values were one-sided for this comparison, differences between combination therapies and monotherapy components were significant if P<0.025. Though the analyses were prespecified, the study was only powered to show statistical differences between combination therapy and its constituent monotherapy components in the overall study cohort and was not powered to show statistical significance within the treatment regimens in each of the prespecified subgroups.
All SeSBP and SeDBP reductions are reported here as least-squares means. Least-squares means, corresponding standard errors, and 95% two-sided confidence intervals, as well as the difference in least-squares means, corresponding standard error, and 95% two-sided confidence intervals, were also derived from this analysis of covariance model. A χ2-test was employed to determine the significance of differences between treatment groups for the number of patients reaching BP goal. One-sided P-values were obtained from individual Fisher's exact tests.
Results
A total of 4234 patients were screened, 1940 patients were randomized and 1689 completed the 8-week double-blind portion of the study.19 The mean age for the study cohort was 54.0 years, 19.8% were ⩾65 years, 54.3% were male and 24.8% were Black. The mean baseline BMI was 33.5 kg m−2, 64.7% of patients were classified as obese (BMI⩾30 kg m−2) and patients with diabetes comprised 13.5% of the total study population. The overall mean baseline SeBP was 163.8/101.6 mm Hg. The baseline demographics for each subgroup are presented in Table 1.
Table 1. Baseline patient demographics according to subgroup (intent-to-treat population).
| Age | Race | Diabetes status | BMI | |||||
|---|---|---|---|---|---|---|---|---|
| <65 years (n=1541) | ⩾65 years (n=382) | Black (n=474) | Non-Black (n=1449) | Diabetes (n=258) | No diabetes (n=1665) | <30 kg m−2 (n=658) | ⩾30 kg m−2 (n=1251) | |
| Mean age, years±s.d. | 50.2±8.5 | 69.8±3.3 | 50.8±10.8 | 55.1±11.0 | 57.0±10.7 | 53.6±11.1 | 56.4±11.1 | 52.8±10.9 |
| Aged⩾65 years, n (%) | NA | 382 (100%) | 62 (13.1%) | 320 (22.1%) | 73 (28.3%) | 309 (18.6%) | 179 (27.2%) | 199 (15.9%) |
| Males, n (%) | 836 (54.3%) | 209 (54.7%) | 191 (40.3%) | 854 (58.9%) | 151 (58.5%) | 894 (53.7%) | 385 (58.5%) | 649 (51.9%) |
| Hispanic or Latino, n (%) | 195 (12.7%) | 46 (12.0%) | 15 (3.2%) | 226 (15.6%) | 54 (20.9%) | 187 (11.2%) | 88 (13.4%) | 151 (12.1%) |
| Black, n (%) | 412 (26.7%) | 62 (16.2%) | 474 (100%) | NA | 66 (25.6%) | 408 (24.5%) | 143 (21.7%) | 328 (26.2%) |
| Diabetes, n (%) | 185 (12.0%) | 73 (19.1%) | 66 (13.9%) | 192 (13.3%) | 258 (100%) | NA | 57 (8.7%) | 199 (15.9%) |
| Previous antihyper- tensive therapy, n (%) | 962 (62.4%) | 304 (79.6%) | 324 (68.4%) | 942 (65.0%) | 191 (74.0%) | 1075 (64.6%) | 445 (67.6%) | 812 (64.9%) |
| Oedema present, n (%) | 199 (12.9%) | 64 (16.8%) | 75 (15.9%) | 188 (13.0%) | 51 (19.8%) | 212 (12.7%) | 38 (5.8%) | 224 (17.9%) |
| Mean BMI, kg m−2±s.d. | 33.9±7.2 | 31.6±6.4 | 34.6±7.9 | 33.1±6.8 | 35.2±6.8 | 33.2±7.1 | 26.7±2.6 | 37.0±6.0 |
| BMI⩾30 kg m−2, n (%) | 1052 (68.3%) | 199 (52.1%) | 328 (69.2%) | 923 (63.7%) | 199 (77.1%) | 1052 (63.2%) | NA | 1251 (100%) |
| Baseline BP | ||||||||
| Mean SeSBP, mm Hg±s.d. | 161.4±14.8 | 173.6±17.4 | 163.9±16.6 | 163.8±15.9 | 168.6±16.6 | 163.1±15.9 | 165.1±16.0 | 163.1±16.0 |
| Mean SeDBP, mm Hg±s.d. | 102.0±5.2 | 100.3±4.5 | 102.4±5.4 | 101.4±5.0 | 101.1±5.1 | 101.7±5.2 | 100.8±4.7 | 102.1±5.3 |
Abbreviations: BMI, body mass index; BP, blood pressure; NA, not applicable; s.d., standard deviation; SeDBP, seated diastolic BP; SeSBP, seated systolic BP.
Total n for each subgroup and data reported reflect all 12 treatment arms.
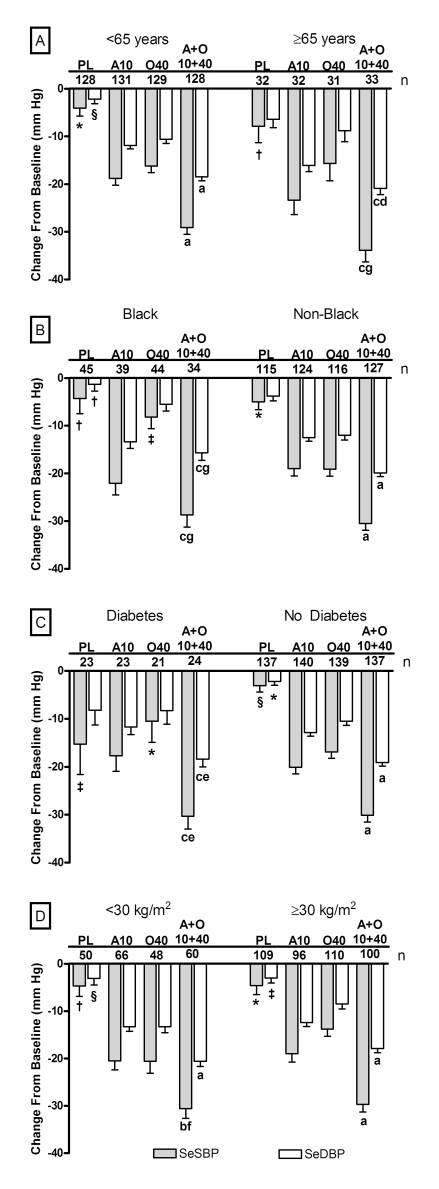
For each subgroup analysed, by week 8/LOCF, SeDBP and SeSBP were significantly reduced from baseline for all olmesartan medoxomil monotherapy (P<0.05), amlodipine monotherapy (P<0.0001) and amlodipine+olmesartan medoxomil (P<0.0001) treatment regimens. Generally, the greatest reduction from baseline occurred in patients receiving amlodipine+olmesartan medoxomil 10+40 mg day−1. These efficacy data for each subgroup and the comparisons with amlodipine 10 mg day−1, olmesartan medoxomil 40 mg day−1 and placebo are presented (Figure 1).
Figure 1.
Mean reductions in SeSBP and SeDBP from baseline to week 8/LOCF according to subgroup and treatment regimen. (a) Age subgroups; (b) race subgroups; (c) diabetes subgroups; and (d) baseline BMI subgroups. AML, amlodipine; LOCF, last observation carried forward; PL: placebo; A10: amlodipine 10 mg; OM40: olmesartan medoxomil 40 mg; and A+0 10+40: amlodipine+olmesartan 10+40 mg. Statistics for comparisons to baseline are two-sided. n represents number of patients who received indicated treatment. P<0.0001 versus baseline for each SeBP change from baseline unless otherwise noted. *P<0.01 versus baseline; †Not significant versus baseline; ‡P<0.001 versus baseline; §P<0.05 versus baseline. Statistics for combinations versus monotherapy components used least-squares mean derived from an analysis of covariance model with treatment, subgroup and treatment-by-subgroup interaction as fixed effects and baseline BP as covariate and are one-sided. Symbol for comparison with olmesartan medoxomil monotherapy precedes symbol for comparison with AML monotherapy component. aP<0.0001 versus both monotherapy components; bP<0.01 versus olmesartan medoxomil monotherapy component; cP<0.0001 versus olmesartan medoxomil monotherapy component; dP<0.025 versus AML monotherapy component; eP<0.01 versus AML monotherapy component; fP<0.001 versus AML monotherapy component; gNot significant versus AML component. Bars represent standard errors of the mean.
Age
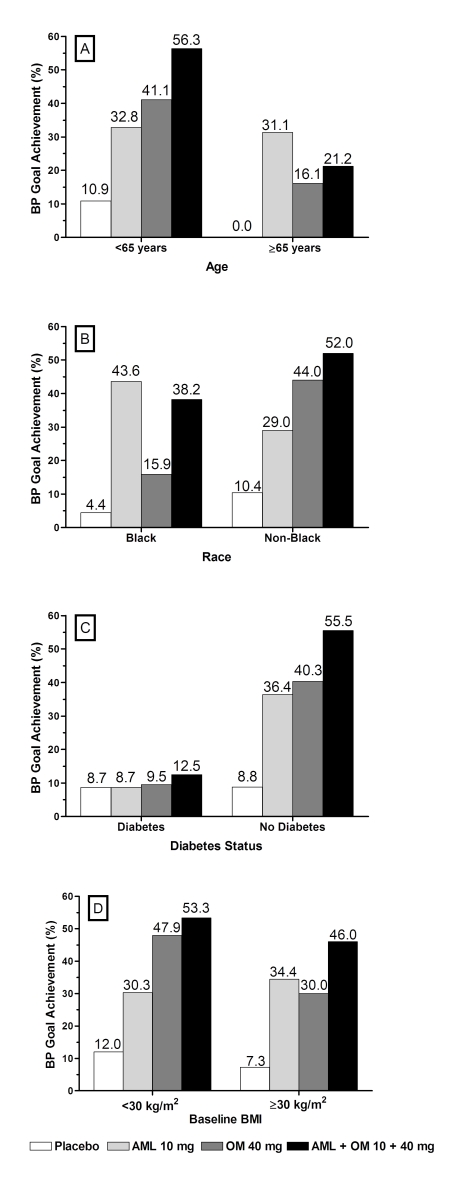
At week 8/LOCF, changes in mean SeBP were −29.1/−18.5 and −33.9/−20.9 mm Hg for patients aged <65 years and ⩾65 years, respectively, who received amlodipine+olmesartan medoxomil 10+40 mg day−1 (Figure 1). All SeBP changes were significant for combination therapy compared with the monotherapy components with the exception of the reduction in mean SeSBP in patients aged ⩾65 years versus amlodipine. The combined BP goal of <140/90 mm Hg, and <130/80 mm Hg for those with diabetes, was achieved by 56.3% of patients aged <65 years (Figure 2). In patients aged ⩾65 years, this goal was achieved by fewer patients (21.2%) who received combination therapy compared with amlodipine monotherapy (31.1%) despite the greater SeBP reductions observed. This discrepancy may be because of the lower number of patients in this subgroup. For example, 43.8% of patients aged ⩾65 years who received the amlodipine+olmesartan medoxomil 10+20 mg day−1 regimen achieved BP goal (data not shown). Achievement of the prespecified BP targets of <140/90, <130/80 and <120/80 mm Hg was also measured cumulatively through the duration of the study. These targets were achieved at the highest rates in patients who received amlodipine+olmesartan medoxomil combinations that included amlodipine 10 mg day−1, regardless of age. (Supplementary Table 2).
Figure 2.
Proportion of patients reaching BP treatment goal (<140/90 mm Hg or <130/80 mm Hg for patients with diabetes) by week 8/LOCF according to subgroup and treatment regimen. (a) Age subgroups; (b) race subgroups; (c) diabetes subgroups; and (d) baseline BMI subgroups. AML, amlodipine; BMI, body mass index; LOCF, last observation carried forward; and OM, olmesartan medoxomil.
Race
At week 8/LOCF, changes in mean SeBP were −28.7/−15.7 and −30.5/−19.9 mm Hg for Black and non-Black patients, respectively, who received amlodipine+olmesartan medoxomil 10+40 mg day−1 (Figure 1). At this dosage, the SeBP changes were significant for combination therapy compared with the monotherapy components for non-Black patients and for Black patients with olmesartan medoxomil but not with amlodipine monotherapy. In non-Black patients, BP goal was achieved by 52.0% of those who received combination therapy, a greater proportion than observed for the monotherapy groups (Figure 2). More Black patients who received amlodipine 10 mg day−1 (43.6%) achieved the BP goal compared with combination therapy comprising amlodipine+olmesartan medoxomil 10+40 mg day−1 (38.2%), although this may also be because of the lower number of patients in this subgroup. For example, 45.7% of Black patients who received the amlodipine+olmesartan medoxomil 10+20 mg day−1 regimen achieved BP goal (data not shown). Regardless of race, combination therapies enabled more patients to achieve the prespecified BP targets of <140/90, <130/80 and <120/80 mm Hg compared with monotherapy (Supplementary Table 2).
Diabetes status
In patients with and without diabetes similar changes in mean SeBP from baseline were observed. At week 8/LOCF, changes in mean SeBP were −30.3/−18.4 and −30.1/−19.1 mm Hg for patients with and without diabetes, respectively, who received amlodipine+olmesartan medoxomil 10+40 mg day−1 (Figure 1). All SeBP changes were significant for combination therapy compared with the monotherapy components. In patients without diabetes, 55.5% achieved BP goal (<140/90 mm Hg) at week 8/LOCF when treated with combination therapy (Figure 2).The more stringent BP goal of <130/80 mm Hg was achieved by 12.5% of patients with diabetes who received combination therapy. Achievement of the prespecified BP targets of <140/90, <130/80 and <120/80 mm Hg are shown in Supplementary Table 2.
Baseline BMI
In both BMI subgroups, patients who received amlodipine+olmesartan medoxomil combination therapy enabled significantly greater reductions in SeDBP and SeSBP than their component monotherapies (Figure 1). At Week 8/LOCF, changes in mean SeBP were −30.6/−20.6 and −29.7/−17.9 mm Hg for patients with BMI <30 kg m−2 and ⩾30 kg m−2, respectively, who received amlodipine+olmesartan medoxomil 10+40 mg day−1 (Figure 1). The BP goal was achieved by similar proportions of patients in each BMI subgroup. For patients treated with combination therapy, 53.3% of patients with baseline BMI <30 kg m−2 and 46.0% with baseline BMI⩾30 kg m−2 achieved BP goal (Figure 2). There was a trend for more patients with a baseline BMI <30 kg m−2 to achieve BP targets of <120/80 and <130/80 mm Hg compared with patients with a baseline BMI⩾30 kg m−2 (Supplementary Table 2).
Safety
When comparing the safety profile obtained in those patients aged ⩾65 years or <65 years, Black or non-Blacks, those with or without diabetes, or in patients with a BMI⩾30 kg m−2 or <30 kg m−2, no marked differences were observed. There was one serious AE (cerebrovascular accident) considered to be drug-related, occurring in a Black, obese female patient aged <65 years with type II diabetes in the olmesartan medoxomil 20 mg day−1 group. Safety data are reported categorically for all the 12 treatment groups who received placebo, amlodipine monotherapy, olmesartan medoxomil monotherapy and amlodipine+olmesartan medoxomil combination therapy (Table 2).
Table 2. Number and percentage of patients with TEAE of interest, by subgroup and treatment regimen (combined groups by monotherapy or combination therapy status).
| Age | Patients⩾65 years | Patients <65 years | ||||||
|---|---|---|---|---|---|---|---|---|
| Placebo (n=32) | AML monotherapy (n=64) | OM monotherapy (n=94) | AML+OM combination therapy (n=194) | Placebo (n=130) | AML monotherapy (n=260) | OM monotherapy (n=390) | AML+OM combination therapy (n=776) | |
| Lack of drug effect,a n (%) | 4 (12.5) | 0 (0.0) | 6 (6.4) | 1 (0.5) | 10 (7.7) | 6 (2.3) | 16 (4.1) | 7 (0.9) |
| Oedema,b n (%) | 5 (15.6) | 10 (15.6) | 12 (12.8) | 44 (22.7) | 15 (11.5) | 71 (27.3) | 57 (14.6) | 171 (22.0) |
| Hypotension,c n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (1.0) | 0 (0.0) | 1 (0.4) | 1 (0.3) | 5 (0.6) |
| Headache, n (%) | 4 (12.5) | 1 (1.6) | 8 (8.5) | 4 (2.1) | 19 (14.6) | 20 (7.7) | 29 (7.4) | 45 (5.8) |
| Dizziness/vertigo,d n (%) | 3 (9.4) | 0 (0.0) | 6 (6.4) | 8 (4.1) | 7 (5.4) | 9 (3.5) | 19 (4.9) | 35 (4.5) |
| Discontinuations, n (%) | 5 (15.6) | 4 (6.3) | 9 (9.6) | 9 (4.6) | 14 (10.8) | 15 (5.8) | 29 (7.4) | 24 (3.1) |
| Race | Black patients | Non-Black patients | ||||||
| Placebo (n=45) | AML monotherapy (n=81) | OM monotherapy (n=112) | AML+OM combination therapy (n=243) | Placebo (n=117) | AML monotherapy (n=243) | OM monotherapy (n=372) | AML+OM combination therapy (n=727) | |
| Lack of drug effect,a n (%) | 2 (4.4) | 1 (1.2) | 8 (7.1) | 3 (1.2) | 12 (10.3) | 5 (2.1) | 14 (3.8) | 5 (0.7) |
| Oedema,b n (%) | 6 (13.3) | 13 (16.0) | 16 (14.3) | 51 (21.0) | 14 (12.0) | 68 (28.0) | 53 (14.2) | 164 (22.6) |
| Hypotension,c n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.4) | 0 (0.0) | 1 (0.4) | 1 (0.3) | 6 (0.8) |
| Headache, n (%) | 9 (20.0) | 9 (11.1) | 11 (9.8) | 21 (8.6) | 14 (12.0) | 12 (4.9) | 26 (7.0) | 28 (3.9) |
| Dizziness/vertigo,d n (%) | 2 (4.4) | 3 (3.7) | 3 (2.7) | 6 (2.5) | 8 (6.8) | 6 (2.5) | 22 (5.9) | 37 (5.1) |
| Discontinuations, n (%) | 4 (8.9) | 1 (1.2) | 10 (8.9) | 6 (2.5) | 15 (12.8) | 18 (7.4) | 28 (7.5) | 27 (3.7) |
| Diabetes status | Diabetes | No diabetes | ||||||
| Placebo (n=23) | AML monotherapy (n=45) | OM monotherapy (n=64) | AML+OM combination therapy (n=129) | Placebo (n=139) | AML monotherapy (n=279) | OM monotherapy (n=420) | AML+OM combination therapy (n=841) | |
| Lack of drug effect,a n (%) | 2 (8.7) | 1 (2.2) | 1 (1.6) | 1 (0.8) | 12 (8.6) | 5 (1.8) | 21 (5.0) | 7 (0.8) |
| Oedema,b n (%) | 4 (17.4) | 7 (15.6) | 7 (10.9) | 25 (19.4) | 16 (11.5) | 74 (26.5) | 62 (14.8) | 190 (22.6) |
| Hypotension,c n (%) | 0 (0.0) | 0 (0.0) | 1 (1.6) | 0 (0.0) | 0 (0.0) | 1 (0.4) | 0 (0.0) | 7 (0.8) |
| Headache, n (%) | 4 (17.4) | 3 (6.7) | 5 (7.8) | 5 (3.9) | 19 (13.7) | 18 (6.5) | 32 (7.6) | 44 (5.2) |
| Dizziness/vertigo,d n (%) | 2 (8.7) | 1 (2.2) | 0 (0.0) | 4 (3.1) | 8 (5.8) | 8 (2.9) | 25 (6.0) | 39 (4.6) |
| Discontinuations, n (%) | 3 (13.0) | 3 (6.7) | 3 (4.7) | 3 (2.3) | 16 (11.5) | 16 (5.7) | 35 (8.3) | 30 (3.6) |
| Baseline BMI | BMI⩾30 kg m−2 | BMI<30 kg m−2 | ||||||
| Placebo (n=109) | AML monotherapy (n=200) | OM monotherapy (n=323) | AML+OM combination therapy (n=624) | Placebo (n=52) | AML monotherapy (n=122) | OM monotherapy (n=155) | AML+OM combination therapy (n=340) | |
| Lack of drug effect,a n (%) | 10 (9.2) | 4 (2.0) | 15 (4.6) | 5 (0.8) | 4 (7.7) | 2 (1.6) | 7 (4.5) | 3 (0.9) |
| Oedema,b n (%) | 18 (16.5) | 63 (31.5) | 57 (17.6) | 155 (24.8) | 2 (3.8) | 17 (13.9) | 11 (7.1) | 59 (17.4) |
| Hypotension,c n (%) | 0 (0.0) | 1 (0.5) | 1 (0.3) | 3 (0.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (1.2) |
| Headache, n (%) | 18 (16.5) | 11 (5.5) | 22 (6.8) | 33 (5.3) | 5 (9.6) | 9 (7.4) | 15 (9.7) | 16 (4.7) |
| Dizziness/vertigo,d n (%) | 6 (5.5) | 6 (3.0) | 16 (5.0) | 22 (3.5) | 4 (7.7) | 3 (2.5) | 9 (5.8) | 21 (6.2) |
| Discontinuations, n (%) | 14 (12.8) | 13 (6.5) | 25 (7.7) | 20 (3.2) | 5 (9.6) | 6 (4.9) | 13 (8.4) | 13 (3.8) |
Abbreviations: AML, amlodipine; BMI, body mass index; BP, blood pressure; DBP, diastolic BP; OM, olmesartan medoxomil; TEAE, treatment-emergent adverse event.
Total n for each subgroup and data reported reflect all 12 treatment arms.
Lack of drug effect includes terms: BP increased, DBP increased, hypertension, BP inadequately controlled, accelerated hypertension, systolic hypertension, diastolic hypertension and drug ineffective.
Oedema includes terms: peripheral oedema, oedema, pitting oedema, generalized oedema and localized oedema.
Hypotension includes terms: hypotension and orthostatic hypotension.
Dizziness includes terms: dizziness and postural dizziness.
Age
Among patients aged ⩾65 years, 7.0% (27 out of 384) discontinued from the study because of treatment-emergent AEs (TEAEs), 4.2% (16 out of 384) of which were considered drug related. Among patients <65 years of age, 5.3% (82 out of 1556) discontinued because of TEAEs, 3.7% (58 out of 1556) of which were considered drug related. The number and proportion of specific drug-related TEAEs associated with CCB and/or ARB antihypertensive medications are summarized in Table 2. Rates of hypotension were similar in both subgroups.
Race
Twenty-one Black patients (4.4%) discontinued due to TEAEs, 13 (2.7%) of which were considered drug-related. In non-Black patients, 88 (6.0%) discontinued due to TEAEs, 61 (4.2%) of which were considered to be drug-related. The number and proportion of specific TEAEs associated with CCB and/or ARB antihypertensive medications are summarized in Table 2. The incidence of oedema was lower in non-Black patients that received combination therapy, compared with amlodipine monotherapy. However, as with the total study cohort19 oedema was lower in patients with diabetes who received amlodipine+olmesartan medoxomil 10+40mgday−1 compared with amlodipine 10mgday−1 monotherapy (data not shown).
Diabetes status
Among patients with diabetes, 12 (4.6%) discontinued because of TEAEs, 9 (3.4%) of which were considered to be drug related. Among patients without diabetes, 97 (5.8%) discontinued because of TEAEs and 65 (3.9%) were considered to be drug related. The number and proportion of specific TEAEs associated with CCB and/or ARB antihypertensive medications are summarized in Table 2. In general, patients with diabetes who received combination therapy had slightly higher incidences of oedema compared with respective monotherapies.
Baseline BMI
In patients with a baseline BMI⩾30 kg m−2, 72 (5.7%) discontinued because of TEAEs and 54 (4.3%) were considered to be drug related. In patients with a BMI <30 kg m−2, 37 and 20 (5.5 and 3.0%) discontinued because of TEAEs and drug-related TEAEs, respectively. The number and proportion of specific TEAEs associated with CCB and/or ARB antihypertensive medications are summarized in Table 2.
Discussion
Achieving target BP is a continuing challenge for the management of patients with hypertension. As such, it is acknowledged that the majority of patients, especially those with multiple risk factors, will require combination therapy with at least two antihypertensive agents to achieve optimal BP control.1, 2 Nevertheless, evidence indicates that patients are often not treated to their target BP goal.7, 20 The combination of amlodipine+olmesartan medoxomil lowers BP through different but complementary mechanisms of action. This results in additive BP-lowering effects through multiple inhibitory mechanisms, complementing the efficacy of the other antihypertensive agent and may offer wider benefits over and above BP control.14, 15
In this study, there was a consistent dose-response effect across a range of prespecified patient groups, analysed according to age, race, diabetes status and BMI. Across all subgroups, high doses of amlodipine+olmesartan medoxomil combinations produced greater reductions in BP than either of the monotherapy components, allowing a greater proportion of these perceived difficult-to-treat patients to achieve the target BP goal. For the primary endpoint, amlodipine+olmesartan medoxomil 10+40 mg day−1 produced the greatest reductions in mean SeDBP at week 8/LOCF in all subgroups. However, unlike the overall cohort, it should be noted that this study was not powered to show statistical differences between combination therapy and its component monotherapies within the subgroups.
Age did not appear to affect the magnitude of SeDBP reduction, and there were generally greater reductions in patients that received combination therapy compared with monotherapy. Numerically greater reductions in SeSBP were seen in patients ⩾65 years of age compared with those aged <65 years. However, the 10 mm Hg higher baseline SeSBP in patients aged ⩾65 years likely contributed to the lower proportions of these patients achieving the recommended BP goal of <140/90 mm Hg or <130/80 mm Hg in patients with diabetes.
Black patients tended to have higher baseline BP than the non-Black patients. When treated with olmesartan medoxomil monotherapy, Blacks had numerically lower reductions in mean SeDBP and SeSBP compared with non-Blacks. However, the addition of amlodipine in the combination treatment groups provided comparable BP reductions in Blacks and non-Blacks. Greater reductions were seen with amlodipine+olmesartan medoxomil 10+40 mg day−1, where mean BP reductions reached approximately 29/16 mm Hg in the Black subgroup and 31/20 mm Hg in non-Black patients. In both race subgroups, more patients on combination therapy achieved their BP goal compared with patients on monotherapy.
Similar overall reductions in mean SeDBP and SeSBP were observed in patients with and without diabetes. However, the lower BP goal of <130/80 mm Hg and the higher baseline BP resulted in fewer patients with diabetes achieving the BP goal, compared with patients without diabetes who had a less stringent goal (BP<140/90 mm Hg). These results support the assertion that patients with diabetes are likely to require >2 antihypertensive drugs to achieve the BP goals recommended by the American Diabetes Association.6, 13
Similar overall mean SeBP reductions from baseline were also observed in patients irrespective of baseline BMI. Generally, more patients achieved BP goal when administered combination therapy of amlodipine+olmesartan medoxomil compared with either drug as monotherapy. The incidence of oedema was greater in patients with a baseline BMI>30 kg m−2. This may have been due, in part, to obesity confounding the diagnosis of oedema.
Overall, combinations of amlodipine+olmesartan medoxomil generally produced greater BP reductions than component monotherapies regardless of age, race, diabetes status or BMI. The amlodipine+olmesartan medoxomil 10+40 mg day−1 combination produced the greatest reductions in mean SeDBP in all subgroups and in SeSBP in all subgroups except non-Blacks. In non-Blacks, the greatest reduction in mean SeSBP was produced by amlodipine+olmesartan medoxomil 10+20 mg day−1 (data not shown). In addition, safety and tolerability profiles were similar when comparing subgroups with difficult-to-treat hypertension with their counterparts with more easily treated disease as well as for the monotherapy-treated cohorts. In these subgroups, as with the total study cohort,19 oedema was higher in patients who received amlodipine 10 mg day−1 monotherapy compared with amlodipine + olmesartan medoxomil 10 + 40 mg day−1 (data not shown). A fixed-dose combination of amlodipine+olmesartan medoxomil has been approved by the US Food and Drug Administration as initial therapy for patients with hypertension unlikely to reach BP goal on monotherapy and may provide more flexibility for the successful treatment of hard-to-treat hypertensive patients.
Acknowledgments
This study was sponsored by Daiichi Sankyo, Inc. We thank Jennifer M Kulak, PhD, and Christopher J Jones, PhD, of Wolters Kluwer, for providing editorial assistance in the preparation of this manuscript.
Steven G Chrysant, MD, has received grant/research support from, has served as a consultant for, and has served on the Speaker's Bureau for Daiichi Sankyo Pharma Development. James Lee, PhD; Michael Melino, PhD; Sulekha Karki, BAMS; and Reinilde Heyrman, MD, are all employees of Daiichi Sankyo Pharma Development.
Footnotes
Supplementary Information accompanies the paper on the Journal of Human Hypertension website (http://www.nature.com/jhh)
Supplementary Material
References
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, et al. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2007;25:1105–1187. doi: 10.1097/HJH.0b013e3281fc975a. [DOI] [PubMed] [Google Scholar]
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- Douglas JG, Bakris GL, Epstein M, Ferdinand KC, Ferrario C, Flack JM, et al. Management of high blood pressure in African Americans: consensus statement of the Hypertension in African Americans Working Group of the International Society on Hypertension in Blacks. Arch Intern Med. 2003;163:525–541. doi: 10.1001/archinte.163.5.525. [DOI] [PubMed] [Google Scholar]
- Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, et al. Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- Arauz-Pacheco C, Parrott MA, Raskin P. The treatment of hypertension in adult patients with diabetes. Diabetes Care. 2002;25:134–147. doi: 10.2337/diacare.25.1.134. [DOI] [PubMed] [Google Scholar]
- Ong KL, Cheung BM, Man YB, Lau CP, Lam KS. Prevalence, awareness, treatment, and control of hypertension among United States adults 1999–2004. Hypertension. 2007;49:69–75. doi: 10.1161/01.HYP.0000252676.46043.18. [DOI] [PubMed] [Google Scholar]
- Wong ND, Lopez VA, L'Italien G, Chen R, Kline SE, Franklin SS. Inadequate control of hypertension in US adults with cardiovascular disease comorbidities in 2003–2004. Arch Intern Med. 2007;167:2431–2436. doi: 10.1001/archinte.167.22.2431. [DOI] [PubMed] [Google Scholar]
- O'Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension. 2005;46:200–204. doi: 10.1161/01.HYP.0000168052.00426.65. [DOI] [PubMed] [Google Scholar]
- Pessina AC. Target organs of individuals with diabetes caught between arterial stiffness and damage to the microcirculation. J Hypertens Suppl. 2007;25 (Suppl 1:S13–S18. doi: 10.1097/01.hjh.0000271504.62325.a4. [DOI] [PubMed] [Google Scholar]
- Watson KE. Cardiovascular risk reduction among African Americans: a call to action. J Natl Med Assoc. 2008;100:18–26. doi: 10.1016/s0027-9684(15)31170-6. [DOI] [PubMed] [Google Scholar]
- Sarzani R, Salvi F, Dessi-Fulgheri P, Rappelli A. Renin-angiotensin system, natriuretic peptides, obesity, metabolic syndrome, and hypertension: an integrated view in humans. J Hypertens. 2008;26:831–843. doi: 10.1097/HJH.0b013e3282f624a0. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association Standards of medical care in diabetes—2008. Diabetes Care. 2008;31 (Suppl 1:S12–S54. doi: 10.2337/dc08-S012. [DOI] [PubMed] [Google Scholar]
- Messerli FH. Vasodilatory edema: a common side effect of antihypertensive therapy. Curr Cardiol Rep. 2002;4:479–482. doi: 10.1007/s11886-002-0110-9. [DOI] [PubMed] [Google Scholar]
- Weir MR. Incidence of pedal edema formation with dihydropyridine calcium channel blockers: issues and practical significance. J Clin Hypertens (Greenwich) 2003;5:330–335. doi: 10.1111/j.1524-6175.2003.02216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe M. Hypertension therapy: mixing, matching, and meeting targets. Adv Ther. 2004;21:107–122. doi: 10.1007/BF02850338. [DOI] [PubMed] [Google Scholar]
- Jamerson K, Weber MA, Bakris GL, Dahlof B, Pitt B, Shi V, et al. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med. 2008;359:2417–2428. doi: 10.1056/NEJMoa0806182. [DOI] [PubMed] [Google Scholar]
- Kjeldsen SE, Jamerson KA, Bakris GL, Pitt B, Dahlof B, Velazquez EJ, et al. Predictors of blood pressure response to intensified and fixed combination treatment of hypertension: the ACCOMPLISH study. Blood Press. 2008;17:7–17. doi: 10.1080/08037050801972857. [DOI] [PubMed] [Google Scholar]
- Chrysant S, Melino M, Karki S, Lee J, Heyrman R. The combination of olmesartan medoxomil and amlodipine besylate in controlling high blood pressure: COACH, a randomized, double-blind, placebo-controlled, 8-week factorial efficacy and safety study. Clin Ther. 2008;30:587–604. doi: 10.1016/j.clinthera.2008.04.002. [DOI] [PubMed] [Google Scholar]
- McInnes GT. How important is optimal blood pressure control. Clin Ther. 2004;26 (Suppl A:A3–A11. doi: 10.1016/s0149-2918(04)90140-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.