Abstract
Purpose
The purpose of this manuscript is to investigate the presence of nucleoside/nucleotide efflux transporter in cornea and to evaluate the role in ocular drug efflux.
Methods
RT-PCR, immunoprecipitation followed by Western blot analysis and immunostaining were employed to establish molecular presence of multidrug resistance associated protein 5 (MRP5) on cornea. Corneal efflux by MRP5 was studied with bis(POM)-PMEA and acyclovir using rabbit and human corneal epithelial cells along with MRP5 over expressing cells (MDCKII-MRP5). Ex vivo studies using excised rabbit cornea and in vivo ocular microdialysis in male New Zealand white rabbits were used to further evaluate the role of MRP5 in conferring ocular drug resistance.
Results
RT-PCR confirms the expression of MRP5 in both rabbit and human corneal epithelial cells along with MDCKII-MRP5 cells. Immunoprecipitation followed by Western blot analysis using a rat (M511–54) monoclonal antibody that reacts with human epitope confirms the expression of MRP5 protein in human corneal epithelial cells and MDCKII-MRP5 cells. Immunostaining performed on human cornea indicates the localization of this efflux pump on both epithelium and endothelium. Efflux studies reveal that depletion of ATP decreased PMEA efflux significantly. MRP5 inhibitors also diminished PMEA and acyclovir efflux. However, depletion of glutathione did not alter efflux. MDR1 and MRP2 did not contribute to PMEA efflux. However, MRP2 is involved in acyclovir efflux while MDR1 do not participate in this process. TLC/autoradiography suggested the conversion of bis(POM)-PMEA to PMEA in rabbit and human corneal epithelial cells. Two well known antiglaucoma drugs, bimatoprost and latanoprost were rapidly effluxed by MRP5. Ex vivo study on intact rabbit corneas demonstrated accumulation of PMEA in cornea in the presence of ATP-depleting medium. In vivo ocular pharmacokinetics also revealed a significant increase in maximum aqueous humor concentration (Cmax) and area under the aqueous humor time curve (AUC) of acyclovir in the presence of MK-571, a specific MRP inhibitor.
Conclusions
Taken together immunolocalization on human cornea, in vitro efflux in human, rabbit corneal and MRP5 over expressing cells, ex vivo and in vivo studies in intact rabbit cornea suggest that MRP5 on cornea can significantly lower the permeability of antiviral and glaucoma drugs. These findings may be valuable in developing formulation strategies to optimize ocular bioavailability of topically administered ocular agents.
Introduction
Cornea is considered a major barrier for topical ocular drug delivery. Lipoidal nature of corneal epithelium and tight junctions of epithelial cells are primarily responsible for poor permeation.1,2 However, Pgp an efflux pump primarily responsible for emergence of drug resistance to a wide spectrum of drugs was reported to be expressed in rabbit1 and human2 corneal epithelial cells. Also, various Pgp inhibitors caused significant alteration in pharmacokinetics leading to enhanced anterior chamber bioavailability.3 Both Pgp and multidrug resistance associated proteins (MRP) belong to ATP-binding cassette (ABC) transporter family of efflux pumps. Recently, expression of organic anion transporter efflux pumps MRP1 and MRP2 were identified for the first time in rabbit and human corneal epithelial cells from our laboratory. MRP2 was indeed shown to confer significant resistance to drug disposition across cornea into the anterior chamber.4,5 Substrate specificity for the MRPs appears to vary. Tissue distribution also varies, however the exact function of the proteins in the cornea remains to be established.6 MRP4 and MRP5, two prominent members of MRP family are known to efflux the nucleotides. MRP5 appears to be localized on both the apical side of brain microvascular endothelial cells and on the basolateral side of gut and liver leading to drug resistance.7,8 Efflux of PMEA by MRP5 in the microglia has been demonstrated.9 MRP5-mediated drug resistance is currently being investigated.6,10
Nucleoside analog such as acyclovir is indicated in the treatment of severe corneal keratitis and stromal keratitis which is one of the leading causes of blindness in the United States.11 However, a very high dose of acyclovir needs to be administered orally because of poor aqueous solubility and low corneal permeability12 that renders acyclovir ineffective via the topical route.13,14 So far, no evidence of MDR mediated resistance related to topical acyclovir has been reported. MRP2, a major efflux pump identified on intact rabbit cornea was not shown to efflux the nucleotide molecules like PMEA.15 Prostaglandin analogs like bimatoprost and latanoprost are currently employed extensively in the treatment of glaucoma. Bimatoprost is available in the market as 0.03% ophthalmic solution and is manufactured under the trade name Lumigan by Allergan Inc. (Irvine, CA). Latanoprost is manufactured under the trade name Xalatan by Pfizer Inc. (New York) and is composed of 50 μg/mL of latanoprost along with 0.02% benzalkonium chloride. Both drugs are administered as eye drops. Since MRP5-mediated efflux of prostaglandin analogs (PGE1 and PGA1) was reported earlier16 it is important to investigate the role of MRP5 on the ocular permeation of drugs like bimatoprost, latanoprost along with PMEA and acyclovir. PMEA was the first drug of choice as it was proven to be a strong substrate of MRP5 and also used in the MRP5 characterization studies.9 In the present study, transport of MRP5 was examined in both rabbit and human corneal epithelial cells. We have shown that MRP5 is expressed at gene and protein level in human corneal cells and is functionally active. We have also shown the localization of MRP5 in human cornea and demonstrated the functional activity with ex vivo and in vivo studies in rabbit cornea. Thus this article provides the first evidence for the corneal expression of MRP5-generating rapid efflux of antiviral and glaucoma drugs.
Materials and Methods
3H-bis(POM)PMEA, 3H-PMEA, and 3H-acyclovir were obtained from Moravek Biochemicals and Radiochemicals (Mercury Lane Brea, CA). Bimatoprost and latanoprost were purchased from Cayman chemical (Ann Arbor, MI). Indomethacin and sodium azide were obtained from Sigma Chemical Co. (St. Louis, MO). Sulfinpyrazone was procured from Alexis Biochemical (San Diego, CA). D,L-Buthionine (S,R)-Sulfoximine (BSO) and 2-Deoxy-d-glucose were acquired from Acros Organics (Morris Plains, NJ). MK-571 was received from Biomol International, L.P. (Plymouth Meeting, PA).
Cell culture
Transfected human corneal epithelial cells (SV40-HCEC)
SV40-immortalized human corneal epithelial cell line17 was purchased from Riken cell bank (Ibaraki, Japan). Protocol for cell culture was provided by the cell bank. Mycoplasma-free SV40-HCEC were grown at 37°C, humidified 5% CO2/95% air atmosphere/95% humidity in a culture medium containing 50% of Dulbecco's modified Eagle's medium (DMEM) and 50% of Ham's nutrient mixture F-12 (from Gibco, Paisley, UK) supplemented with 15% (v/v) fetal bovine serum (FBS, from Atlanta biologics), antibiotic, gentamycin (30 μg/mL) and 0.5 % dimethyl sulfoxide (DMSO). Cells were harvested with trypsin-EDTA.
Statens Seruminstitut rabbit corneal epithelial cells (SIRC)
SIRCs were plated in a T-75 culture flask and MEM containing Sodium bicarbonate (3.8 mg/mL), HEPES (4.8 mg/mL), Lactalbumin (1.76 mg/mL), Penicillin (0.1 mg/mL), Streptomycin Sulfate (0.125 mg/mL), Fetal Bovine Serum (10%), and MEM Nonessential amino acids (10%) were used. Medium was changed twice a week, and cells were sub-cultured every 5–7 days as 70%–80% confluence is reached (subculture ratio, 1:5).
Primary culture of rabbit corneal epithelial cells (rPCECs)
Rabbit corneal epithelial cells were isolated and cultured as per the protocol established in our laboratory.2 Cells were grown using the same medium composition mentioned earlier for SIRC. The medium was changed twice a week, and the cells were subcultured every 7–10 days (subculture ratio, 1:5).
MDCKII-Wt, MDCKII-MDR1, MDCKII-MRP2, and MDCKII-MRP5 cells
MDCKII-Wt and MDCKII cells overexpressing human MDR1, MRP2, and MRP5 genes at protein level were obtained as a gift from Dr. Piet Borst (Netherlands Cancer Institute, Amsterdam). Cells were maintained in DMEM supplemented with 10% calf serum, 100 IU/mL penicillin, 100 μg/mL streptomycin, and 20 mmol/L HEPES, pH 7.4. Cells were plated at a density of 100,000/cm2 in 12-well tissue culture–treated plastic dishes. MDCKII cells were incubated at 37°C in humidified atmosphere of 5%CO2/95% humidity and were allowed to grow for 5–8 days.
Reverse transcriptase polymerase chain reaction (RT-PCR)
Human, rabbit corneal epithelial cells and MRP5 over expressing cells (SV40-HCEC, rPCECs, SIRC, and MDCKII-MRP5) were grown for mRNA extraction. Cells (3–5 million) from a confluent flask were taken, and 800 μL of Tri-reagent LS (Molecular Research Center, Inc., Cincinnati, OH) was added. The mixture was homogenized and transferred to eppendorf tubes. RNA was extracted by the phenol-CHCl3-isopropranolol method, purified, and dissolved in 20 μL of RNase-DNase-free water. RT-PCR was performed based on established protocol with modifications using ~1 μg of total RNA.18 MRP5 primers were designed from human MRP5 cDNA (Genbank Accession No: AB209454; http://www.ncbi.nlm.nih.gov/Genbank; made available in the public domain by the National Center for Biotechnology Information [NCBI], Bethesda, MD). The forward and reverse primers designed for MRP5 were 5′-ACCCGTTGTTGCCATCTTAG-3′ and 5′-GCTTTGACCCAGGCATACAT-3′, respectively. These primers correspond to the nucleotide position 879–1106. RT-PCR was performed with a kit (SuperScript III First-Strand Synthesis System for RT-PCR; Invitrogen, Carlsbad, CA). Conditions for reverse transcription were denaturation of the template RNA for 10 min at 70°C and reverse transcription for 60 min at 42°C. Conditions for PCR amplification were denaturation for 1 min at 94°C, annealing for 1 min at 58.5°C and extension for 1 min at 72°C for 35 cycles, and a final extension for 10 min at 72°C. RT-PCR products were analyzed by electrophoresis on 2% agarose gel. Sequencing analysis was performed by Agencourt Biosciences (Beverly, MA) using Quicklane sequencing technology.
Immunoprecipitation—Western blot
SV40-HCEC and MDCKII-MRP5 cells grown on T-75 (75 cm2 growth area) were washed twice with PBS and harvested with a cell scraper into 5 mL of PBS. Mem-PER Eukaryotic Membrane Protein Extraction Reagent Kit (Pierce Biotechnology, Inc., Rockford, IL) was used to separate membrane fraction from cytoplasmic fraction. The membrane fraction and supernatant were separated and stored at −80°C until further use. Protein content was determined with Bradford method (Bio-Rad protein estimation kit; Bio-Rad, Hercules, CA). Immunoprecipitation followed by Western blot was performed utilizing Seize Classic (G) Immunoprecipitation Kit (Pierce Biotechnology, Inc., Rockford, IL). Twenty micrograms of protein (100 μL lysate) were immunoprecipitated overnight at 4°C with the ABCAM (#M511–54) rat monoclonal antibody for MRP5 (Abcam Inc, Cambridge, MA). Protein G-sepharose beads containing antigen–antibody complexes were collected by centrifugation, washed three times with immunoprecipitation buffer, resuspended in lane marker sample buffer (0.3 M Tris–HCl, 5% SDS, 50% glycerol, lane marker tracking dye; pH-6.8) (Pierce Biotechnology, Inc., Rockford, IL) and boiled for 5 min. All immunoprecipitated samples and molecular weight protein markers were separated by SDS-PAGE (8% Tris-glycine gels for 2 h at 120 V). Protein was transferred onto nitrocellulose membranes and stored for 2 h at 250 mA on ice. Protein transfer efficiency was checked by staining the nitrocellulose membranes in 0.2% ponceau S (in 3% wt/vol trichloroacetic acid and 3% wt/vol sulfosalicylic acid) for 10 min. A portion of the blot containing MRP5 samples was incubated in freshly prepared blocking buffer (3% wt/vol BSA and 5% nonfat dry milk in Trisbuffered saline) for 1 h at room temperature. The blot was then treated with MRP5 antibody (1:50) overnight at 4°C. After five 10-min washes with TBST (Tris-buffered saline + 0.1% Tween 20), the membrane was treated with secondary antibody in TBST (1:1500 anti-rat IgG-horseradish peroxidase [HRP]) for 1 h. The blots were finally washed three times (15 min each) with TBST. Opti-4CN Western blot amplification kit (Bio-Rad, Herculus, CA) was used to develop the blots.
Immunostaining of human cornea
Human corneas were obtained from Heartland Lion's Eye Bank (Kansas City, MO). The tissues were fixed in 3.7% formalin and embedded in paraffin. These sections were dissected at 5 microns and deparafinized using xylene and ethanol. Antigen retrieval was performed with citrate buffer (pH-6.0) with 0.05% Tween 20 in a 95°C steam bath for 30 min. Sections were rinsed in PBST (phosphate-buffered saline + 0.05% Tween 20) for 5 min on a shaker and then incubated overnight at 4 °C with ABCAM (#M511–54) rat monoclonal antibody (1:100 dilution). Tissues were rinsed three times 15 min each with PBST at 37°C. Then the sections were treated with biotin conjugated donkey anti-rat secondary antibody (1:2500) (BioFX Laboratories, Inc., Owings Mills, MD) followed by horseradish peroxidase–conjugated streptavidin (1:2000) for 20 min. This procedure was followed by PBST wash, as previously mentioned. Finally, the sections were treated with 3,3′-Diaminobenzidine (DAB) for 5 min and counterstained with harris hematoxylin.
Transport of nucleotides across cells
To determine functional activity of MRP5 in corneal epithelial cells, transport studies were performed with PMEA and acyclovir. Transport studies were performed based on the protocol established by Schuetz et al., 1999 with minor modifications. Monolayers of MDCKII-MRP5, SV40-HCEC, rPCEC, and SIRC cultured in 12-well tissue culture plates (Fisher Scientific, PA) were employed. As reported previously, addition of 10-mM sodium azide and 10-mM 2-deoxy-d-glucose to cell monolayer's reduced intracellular ATP by >90% in the first 5 min of incubation but did not affect cell viability.9 The principle behind this study involves preloading drug in to cells by deactivating the efflux transporter (ATP-depleting conditions), washing and then reactivate the transporter with a medium containing glucose. Transport kinetics of MRP5 was observed with and without inhibitors.
Cells were incubated for 60 min with 3H-bis (POM) PMEA (1uCi) and 3H-acyclovir (1uCi) in the presence of 10-mM sodium azide and 2-deoxy-d-glucose. Following 1 h incubation period under normal and ATP-depleting conditions cell viability was determined by trypan blue exclusion test. Cells were examined for viability (unstained) and nonviability/cytotoxicity (stained). Hemocytometer (Hausser scientific, Horsham, PA) was used to count the cells. After incubation drug solution was removed from the wells and cells were washed with 0.16 M NaCl and subsequently incubated for 60 min with Hank's buffered saline solution (HBSS) with glucose, pH-7.2 and HBSS with glucose incorporating the inhibitors. Preparation of HBSS with inhibitors is described in the next section. The supernatant was collected and radioactivity was measured with Beckmann scintillation counter (Beckman Coulter, Inc., CA). The amount of radioactivity in the supernatant represents amount of drug effluxed. Radioactivity was converted to drug concentration based on specific activity of the compound.
Preparation of inhibitor solutions
Indomethacin (10 mg), bimatoprost (1 mg), and latanoprost (1 mg) were dissolved in ethanol (1 mL) to prepare a stock solution and the solution was aliquoted in HBSS to achieve the desired concentrations. Sulfinpyrazone (10 mg) was dissolved in chloroform (1 mL) and aliquoted with HBSS. Organic solvents were employed at ≤0.05% which was same in control. MK-571 was freely soluble in water. Cytotoxicity was measured with CytoTox-ONE Homogeneous Membrane Integrity Assay (Promega, San Luis Obispo, CA) on all the cell lines employed. No cytotoxicity was noticed even at the highest concentrations of inhibitors employed. (data not shown)
Evaluation of active nucleotide transport on freshly excised cornea
New Zealand male albino rabbits (n = 6) were euthanized, Corneas were immediately dissected and transferred to HBSS, pH 7.2. Within approximately 1–2 min, the tissues were successfully mounted on Side-by-Side diffusion cells (type-VSC-1, Crown Glass Company Inc., Somerville, NJ). Side-bi-Side chamber has a donor (apical) and a receiver compartment (basolateral) with the cornea mounted in between. The chamber is maintained at 34°C which is the temperature of cornea exposed to external environment. The donor and receiver compartments were continuously stirred with magnetic console stirrer (Crown Glass Company Inc., Somerville, NJ) to minimize the aqueous boundary layer and to simulate in vivo dynamic conditions. The chambers were filled with 3 mL of HBSS with glucose, pH-7.2 (normal conditions) and with10-mM NaN3 + 10-mM 2-Deoxy-d-Glucose (ATP-depleting conditions) for 30 min. Control chambers were filled with HBSS containing glucose in both apical and basolateral sides. Test chambers are loaded with ATP-depleting medium on apical side and HBSS with glucose on basolateral side. This condition simulates actual in vivo environment, where apical side (corneal surface exposed to external environment) is modulated with ATP-depleting agents. After the specific exposure period, corneas were dismounted, thoroughly washed to remove any residual radioactivity and placed in lysis solution (1% tritonex + 1N NaOH solution) overnight. Later, cornea was thoroughly ground using tissue grind pestle (Fischer scientific, USA) and resuspended in scintillation cocktail (ScintiSafe30) (Fischer scientific, USA) to determine accumulated radioactivity. Pestle was thoroughly swiped with whatmann filter paper and radioactivity determined was added to the initial radioactivity to ensure no superficial loss due to grinding.
Intracellular conversion of bis(POM)PMEA to PMEA
Intracellular conversion of 3H-bis(POM)PMEA to 3H-PMEA within the human and rabbit corneal cells was confirmed using thin layer chromatography (TLC) by the method adopted from Dallas et al., 2004 with modifications. Cells were incubated for 1 h (37°C) under ATP-depleting conditions with 3H-bis(POM)PMEA (0.5 mCi/mL) and then washed twice with 0.16 M NaCl to remove any residual drug. Cells were then incubated in lysis solution overnight. Five microliter of cell lysate, 5 μL donor (3H-bis(POM)PMEA), and 1 μL controls (3H-bis(POM)PMEA and 3H-PMEA) were spotted on a 250-μm thick silica-gel-coated TLC plate (Sigma-Aldrich, St. Louis, MO). Plates were air dried for 1 h and chromatographic separation of 3H-bis(POM)PMEA and 3H-PMEA was performed using the solvent system isopropanol:ammonia:water (7.5:1:1.5 v/v/v). The plates were subsequently air dried for 2 h and sprayed with beta emitter enhancer spray (EN3HANCE) (PerkinElmer, Waltham, MA). TLC plates were exposed to X-ray film and stored at −20°C for 36 h. Care was taken to protect the films from light. The films were developed and the bands obtained were matched to the respective position of the compounds on TLC plates. The areas corresponding to bis(POM)PMEA and PMEA were scrapped and radioactivity was determined by Beckman scintillation counter (Beckman Coulter, Inc., Fullerton, CA). Residual radioactivity from the entire plate is determined and added to the final value to minimize error. Results were expressed as percentage of total intracellular radioactivity recovered (mean ± SD).
In vivo anterior segment microdialysis
Probe implantation
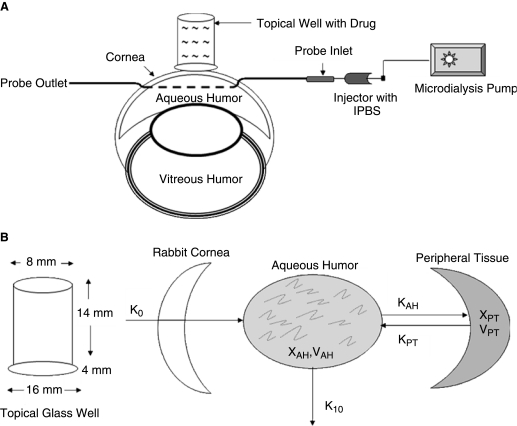
The role of MRP on absorption kinetics of acyclovir was assessed by an in vivo topical well infusion model coupled with aqueous humor microdialysis technique established in our laboratory.3,20 Absorption kinetics of acyclovir across the cornea were measured alone and in the presence of a potent MRP inhibitor, MK-571. Rabbits were given an anesthetic of Ketamine HCl (35 mg/kg) and xylazine (3.5 mg/kg) by intramuscular injection. A diagrammatic representation of ocular microdialysis by a topical well infusion model is shown in Figure 1. Isotonic phosphate buffer saline solution (IPBS – pH, 7.2) was used for the studies.
FIG. 1.
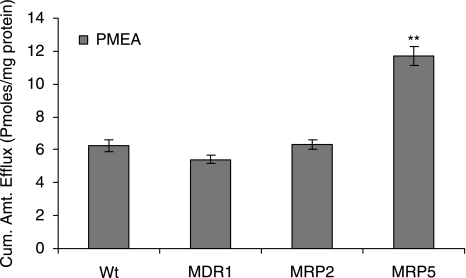
Efflux of active drug PMEA from MDCKII-wt, MDR1, MRP2, and MRP5 cells preloaded with prodrug Bis (POM)-PMEA. HBSS with glucose was used to measure the relative efflux of transporters. Statistical significance was tested by two-factor ANOVA. **P ≤ 0.01 (n = 5).
IPBS was added to fill needles (25 G1). The posterior end of needle was then closed with a plastic cap to prevent the loss of liquid, including the loss of aqueous humor during surgery. The needle was surgically inserted into the anterior side of cornea and carefully penetrated through the aqueous humor and withdrawn from the posterior side of cornea.
The sample-collecting end of the microdialysis probe was inserted into the needle and the needle was withdrawn slowly to ensure placement of the dialyzing membrane probe in the aqueous humor. Extreme care is taken to ensure that there is no damage to the other tissues of the eye (Fig. 2A). The other end of the probe was connected to the syringe needle (3 mL) filled with IPBS which was further connected to a CMA/100 microdialysis pump (Bioanalytical Inc., West Lafayette, IN) which controls the rate of infusion of IPBS through the probe. The flow rate was set at 3.0 μL/min based on standard protocol established in our laboratory. Heart rate and respiratory rate are carefully monitored every 30 min to ensure the animal safety during surgery. Probe implantation was followed by a 2 h stabilization period of the anesthetized animal during which aqueous humor and intra ocular pressure returned to normal.3,20
FIG. 2.
(A) Schematic representation of probe insertion in the anterior segment of the eye. As shown in the figure, dialyzing membrane of the probe was placed in the aqueous humor; probe inlet connected to an IPBS injector connected to a microdialysis pump, which perfuses IPBS at 3 μL/min. Sample was collected at probe outlet. Topical glass well where drug solution is loaded and placed on the cornea with a surgical adhesive as shown. (B) Schematic representation of aqueous humor kinetics of single-dose constant infusion of drug across the cornea. Diagrams drawn based on inspiration from references 2.
Microdialysis
After 2 h, colibri retractors obtained from Spectrum (Stow, OH) were employed to retract the eye lids to provide good exposure of the cornea for the experiment. A topical well was adhered on top of the cornea and after 40 min, 150 μL HBSS containing 14C-erythromycin (10 μCi/mL) alone or in combination with 100 μM MK-571 was added to the well. The wells are monitored for any leakage of drug solution and samples are collected from anterior end of the probe every 20 min. The experiment is designed for 2 h of absorption phase and the drug solution is removed along with the wells after 120 min. The surface of the cornea is washed and aspirated with distilled water to remove any superficial drug solution. Samples are collected from the probe for a 6 h postinfusion period at which time the animals are euthanized following the approved protocol.
In vitro probe calibration
Ocular bioavailability on topical administration is ~5% (ranges from 1% to 10%) for majority of the drugs.1 Probe recovery is performed on all the probes used before and after the animal study. Ten microcurie per milliliter is employed for the in vivo studies, and a 5% of the concentration, that is, 0.5 μCi/mL was employed for the recovery studies. IPBS perfused the probe at 3.0 μL/min and the dialyzing membrane was placed in a solution containing 0.5 μCi/mL of the drug. As indicated by Dey and colleagues, 2003; recovery is considered as the ratio of the concentration of drug obtained in the sample to the concentration present in the solution surrounding the probe.21 The recovery of the drug by microdialysis probe is designated by equation 1.
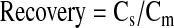 |
(Eq. 1) |
Cs is the concentration in the sample and Cm represents concentration in the medium. Concentration of the drug in aqueous humor (CAH) is calculated based on concentrations obtained in the dialysate sample (Cdl) from equation 2.
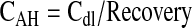 |
(Eq. 2) |
Percent recovery was between 12% and 18% for both acyclovir and erythromycin. Recovery was performed after the in vivo study and MK-571 and GF120918 employed did not alter the recovery. This confirms that the recovery was uniform throughout the duration of study.
Mathematical data treatment
The mathematical treatment was adapted based on the protocol established by Dey et al., 2004 in our laboratory. As the HBSS containing the radioactive drug is loaded in the topical well, it enters the aqueous humor via cornea by passive diffusion. Drug in aqueous humor is distributed to surrounding tissues and the distribution can be reversible. Concentrations of the drug in aqueous humor and surrounding tissues (peripheral) are denoted by XAH and XPT. Rate of infusion across the cornea is represented by K0. First order rate constant for distribution of drug present in the aqueous humor to surrounding tissues and surrounding tissues to the aqueous humor are represented by kAH and kPT. As the drug is eliminated through aqueous outflow, apparent elimination rate constant, which is the first order, is denoted by K10. The absorption, distribution, and elimination are represented as a scheme in Figure 2B.
Designating Y, as aqueous humor and number of surrounding tissues as X, and X ≥ Y, equation 3 can be represented as follows.
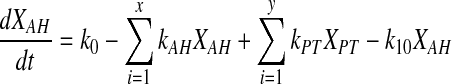 |
(Eq. 3) |
Elimination is considered minimal from the surrounding tissues and is mainly by aqueous out flow pathway. The relationship between rate constant of infusion and concentration of drug in reservoir (CR) is represented as equation 4. VR is the reservoir volume and ka is the first order rate constant for drug absorption.
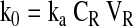 |
(Eq. 4) |
Equation 4 can be further represented as k0 = ka XR.
Equation 3 can be further represented as equation 5.
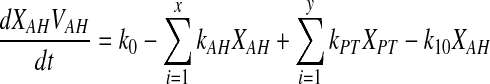 |
(Eq. 5) |
Concentration of drug in the reservoir is very high compared to the concentration in the aqueous humor or tissue compartments. Hence eliminating the insignificant terms, equation 6 can be obtained.
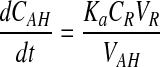 |
(Eq. 6) |
Corneal absorption rate constant is calculated based on equation 7 that is derived from equation 6.
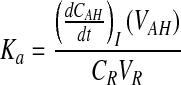 |
(Eq. 7) |
Taken together these equations will allow determination of absorption, distribution, and elimination rate constant (ocular pharmacokinetics) with high accuracy and reliability.
Analytical procedure
Beckman scintillation counter (Model LS 6500; Beckman Instruments Inc., Fullerton, CA) was employed to measure radioactivity with anterior segment microdialysis samples containing 3H-acyclovir.
Data treatment
Data analysis was performed with pharmacokinetic software WinNonlin, v5.0 (Pharsight, CA). Noncompartmental analysis of drug concentrations–time profiles in the aqueous humor was performed according to a noncompartmental model with a constant infusion rate. All the pharmacokinetic parameters were obtained from the analysis. Absorption rate constant (ka) was calculated from equation 6.
Results
MRP5 mRNA and protein expression in corneal epithelial cells
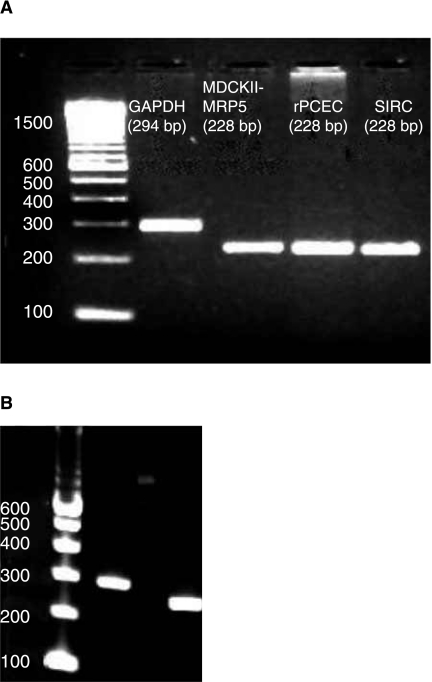
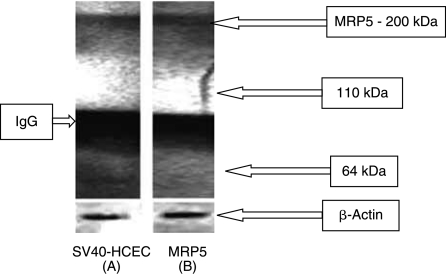
A single specific band at ~228 bp corresponding to MRP5 was observed from RT-PCR conducted on rPCEC, SIRC, and positive control (MDCKII-MRP5). Unique band at ~294 bp for GAPDH (internal control) was also observed (Fig. 3A). RT-PCR on SV40-HCEC also yielded a similar result, that is, a band at ~228 bp. GAPDH band (~294 bp) served as an internal control (Fig. 3B). Sequencing, alignment in Clustal W and comparison with the Genbank sequences indicated that MRP5 from corneal cells is ~95% identical to human MRP5 sequence (Pubmed data base). Western blot analysis with rat monoclonal MRP5 antibodies which react with human epitope detected bands of expected size (~200 kDa) in SV40-HCEC along with positive control (MDCKII-MRP5) (Fig. 4). As antibody was generated from bacterial fusion protein of human MRP 5 containing amino acids 82–168 as immunogen, it was able to bind to human MRP5 protein expressed in SV40-HCEC.
FIG. 3.
(A) RT-PCR confirming the presence of MRP5 in rPCEC, SIRC, MDCKII-MRP5 (positive control), and GAPDH (internal control). A specific band at ~228 bp corresponding to MRP5 can be observed in all cell lines. A band at 294 bp represents internal control. (B) RT-PCR confirming the presence of MRP5 in SV40-HCEC (228 bp) and GAPDH (294 bp).
FIG. 4.
Immunoprecipitation followed by Western blot indicating the presence of MRP5 in membrane fraction of SV40-HCEC and MDCKII-MRP5 (+ve control).
Immunostaining on human cornea
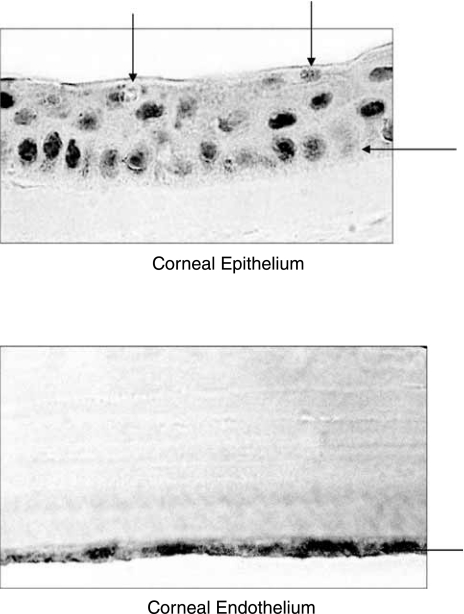
Immunostaining were clearly visible on the apical and basal sides of epithelium. Intense stain can also be noticed on endothelium of human cornea (Fig. 5). Being distributed across the cornea, except stroma, MRP5 can play a significant role in limiting drug absorption across the cornea from topical administration.
FIG. 5.
Immunostaining of MRP5 on human cornea using the M511–54 monoclonal antibody. Specific staining can be observed on both apical and basolateral side of corneal epithelium and endothelium.
Drug efflux by MDCKII-MRP5 cell monolayers
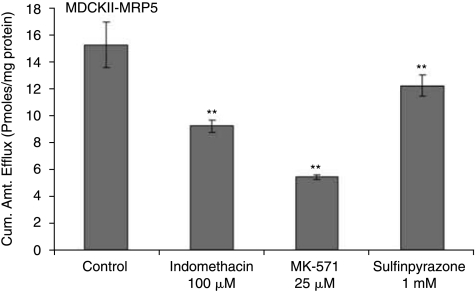
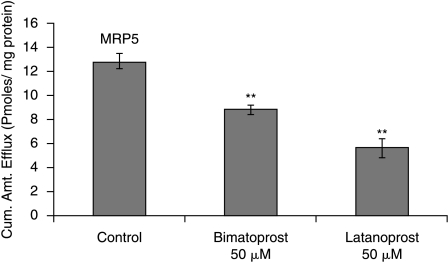
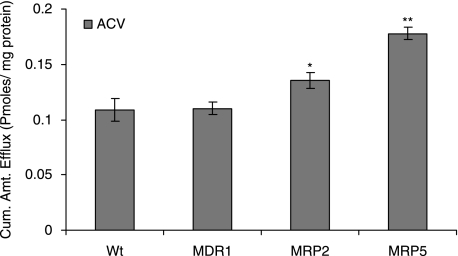
Following a 1 h loading of 3H-bis(POM)PMEA (1 μCi/mL) under ATP-depleting conditions drug efflux was measured from cells by determining the concentration of PMEA released from cytoplasm into the medium. Significant (P < 0.01) decrease in efflux of PMEA was observed in MDCKII-MRP5 cells in the presence of sulfinpyrazone (1 mM), a proven MRP5 modulator (Wijnholds et al., 2000) (11.76 ± 0.76), indomethacin (100 μM) (8.88 ± 0.45) and MK-571 (25 μM) (5.23 ± 0.16) as compared to control (14.70 ± 1.62) pmoles/mg protein (Fig. 6). Efflux of acyclovir from cells diminished significantly in the presence of MK-571 at 25 (0.13 ± 0.01), 50 (0.04 ± 0.005), and 75 μM (0.03 ± 0.001) compared to control (0.16 ± 0.01) pmoles/mg protein (Fig. 7). Drugs for glaucoma suppressed the efflux of PMEA significantly. Bimatoprost (50 μM) (8.85 ± 0.44) and latanoprost (50 μM) (5.65 ± 0.76) significantly lowered the efflux relative to control (12.86 ± 0.67) pmoles/mg protein (Fig. 8).
FIG 6.
Efflux of active drug PMEA from MDCKII-MRP5 cells preloaded with prodrug Bis (POM)-PMEA in the presence and absence of Indomethacin (100 μM), MK-571 (25 μM), and Sulfinpyrazone (1 mM). Statistical significance was examined by two-factor ANOVA. **P ≤ 0.01 (n = 5).
FIG. 7.
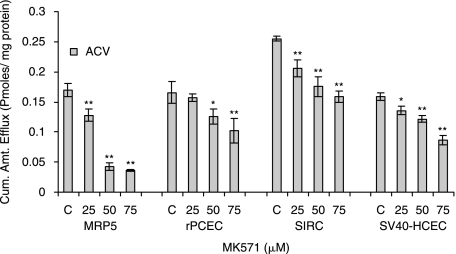
Efflux of acyclovir was measured from MDCKII-MRP5, rPCEC, SIRC, and SV40-HCEC. Monolayers of cells were incubated with 3H-acyclovir under ATP-depleting conditions (10-mM sodium azide; 10-mM 2-deoxy-d-glucose) for 1 h at 37°C. HBSS with glucose was added, with and without 25, 50, 75 μM MK-571, and cumulative amount of acyclovir efflux was determined by measuring the radioactivity in the supernatant. Statistical significance was measured by two-factor ANOVA. **P ≤ 0.01 (n = 5).
FIG. 8.
Efflux of PMEA from MDCKII-MRP5 cells preloaded with Bis (POM)-PMEA under ATP-depleting conditions (10-mM sodium azide; 10-mM 2-deoxy-d-glucose) for 1 h at 37°C. HBSS with glucose was added, with and without bimatoprost, latanoprost, and efflux was determined. Statistical significance was determined by two-factor ANOVA. **P ≤ 0.01 (n = 5).
Efflux studies in SIRC, rPCEC, and SV40-HCEC
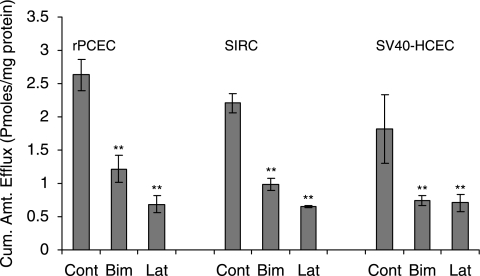
Similar to MDCKII-MRP5, sulfinpyrazone (1 mM and 2.5 mM), Indomethacin (100 μM, 500 μM, and 1000 μM) and MK-571 (50 μM) significantly inhibited the efflux of PMEA (Table 1). PMEA efflux was very low in the presence of strong MRP inhibitor MK-571. Efflux of acyclovir was significantly lower with MK-571 in rPCEC at 25 (0.16 ± 0.006), 50 (0.12 ± 0.01), and 75 μM (0.10 ± 0.02) relative to control (0.16 ± 0.01); SIRC, 25 (0.20 ± 0.004), 50 (0.17 ± 0.01), and 75 μM (0.16 ± 0.009) relative to control (0.25 ± 0.004); SV40-HCEC, 25 (0.13 ± 0.007), 50 (0.12 ± 0.005), and 75 μM (0.08 ± 0.007) compared to control (0.16 ± 0.005) pmoles/mg protein (Fig. 7). Glaucoma-treating drugs also caused a marked lowering in PMEA efflux significantly in rPCEC, that is, bimatoprost (50 μM) (1.21 ± 0.20) and latanoprost (50 μM) (0.68 ± 0.13) relative to control (2.63 ± 0.23); SIRC, bimatoprost (50 μM) (0.98 ± 0.08) and latanoprost (50 μM) (0.64 ± 0.01) compared to control (2.20 ± 0.14); SV40-HCEC, bimatoprost (50 μM) (0.74 ± 0.07) and latanoprost (50 μM) (0.70 ± 0.12) compared to control (1.81 ± 0.51) pmoles/mg protein (Fig. 9).
Table 1.
Efflux of Active Drug PMEA From SIRC, rPCEC, and SV40-HCEC Preloaded With Prodrug Bis (POM)-PMEA in the Presence and Absence of Indomethacin (100 μM, 500 μM, 1000 μM), Sulfinpyrazone (1 mM and 2.5 mM), Glutathione Depleting Agent BSO (50 μM), and Under ATP-Depleting Conditions (10-mM Sodium Azide; 10-mM 2-Deoxy-d-Glucose) Was Shown
| |
Sulfinpyrazone (mM) |
Indomethacin (mM) |
|
|||||
|---|---|---|---|---|---|---|---|---|
| Cont. | 1.0 | 2.5 | Cont. | 0.1 | 0.5 | 1.0 | BSO 50 μM | |
| SIRC | 2.27 ± 0.04 | 1.51 ± 0.21 | 1.24 ± 0.10 | 2.36 ± 0.11 | 1.53 ± 0.06 | 0.90 ± 0.04 | 0.82 ± 0.16 | 2.18 ± 0.08 |
| rPCEC | 2.54 ± 0.32 | 1.12 ± 0.04 | 0.42 ± 0.10 | 1.97 ± 0.19 | 2.02 ± 0.34 | 1.23 ± 0.16 | 0.80 ± 0.23 | 2.38 ± 0.14 |
| SV40-HCEC | 1.78 ± 0.21 | 0.98 ± 0.14 | 0.67 ± 0.08 | 1.65 ± 0.19 | 1.34 ± 0.07 | 1.36 ± 0.20 | 1.22 ± 0.05 | 1.87 ± 0.21 |
Statistical significance was tested by two-factor ANOVA. P ≤ 0.01.
FIG. 9.
Efflux of PMEA from rPCEC, SIRC, and SV40-HCEC preloaded with Bis (POM)-PMEA under ATP-depleting conditions (10 mM sodium azide; 10 mM 2-deoxy-d-glucose) for 1 h at 37°C. HBSS with glucose was added, with and without bimatoprost, latanoprost, and efflux was determined. Statistical significance was examined by two-factor ANOVA. **P ≤ 0.01 (n = 5).
Role of glutathione and ATP
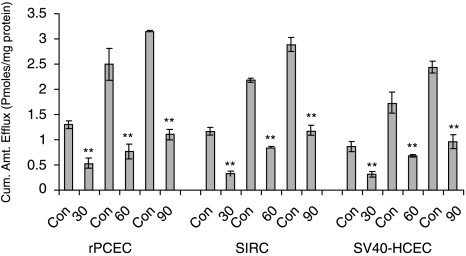
Treatment with 50-μM BSO for 24 h has been reported to deplete glutathione levels by >90% (Dallas et al., 2004). BSO treatment did not show significant decrease in PMEA efflux in the cell lines employed (Table 1). Depletion of ATP by 10-mM sodium azide and 10-mM 2-deoxy-d-glucose resulted in significant decrease in PMEA efflux compared to control (Fig. 10). Efflux was low at all time points (30, 60, and 90 min) compared to control in rPCEC, SIRC, and SV40-HCEC. In rPCEC efflux under ATP-depleting conditions at 30 min was (0.35 ± 0.11) versus control (1.39 ± 0.21), at 60 min (0.50 ± 0.03) versus control (2.54 ± 0.32), at 90 min (1.23 ± 0.19) versus control (3.24 ± 0.26) pmoles/mg protein. In SIRC, the efflux at 30 min under ATP-depleting conditions was (0.28 ± 0.17) versus control (1.23 ± 0.12), at 60 min (0.40 ± 0.15) versus control (2.27 ± 0.04), at 90 min (1.05 ± 0.18) versus control (3.12 ± 0.021) pmoles/mg protein. Similarly, in SV40-HCEC, efflux under ATP-depleting conditions was at 30 min (0.21 ± 0.10) versus control (0.96 ± 0.11), at 60 min (0.60 ± 0.02) versus control (1.78 ± 0.21), and at 90 min was (0.98 ± 0.23) versus control (2.46 ± 0.12) pmoles/mg protein. Figure 10 clearly illustrates that ATP depletion suppressed the efflux significantly at all time points.
FIG. 10.
Time-dependant efflux of active drug PMEA from SIRC, rPCEC, and SV40-HCEC preloaded with prodrug Bis (POM)-PMEA under ATP-depleting conditions (10 mM sodium azide; 10 mM 2-deoxy-d-glucose). ATP depletion suppressed PMEA efflux significantly at all time points. Statistical significance was examined by two-factor ANOVA. **P ≤ 0.01 (n = 5).
Active nucleotide efflux pump on freshly excised cornea
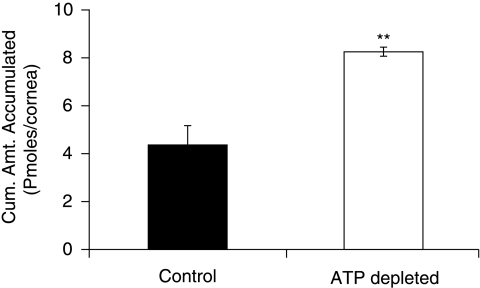
Cumulative amount of PMEA accumulated in freshly excised rabbit cornea under ATP-depleting conditions was significantly higher (8.25 ± 0.16) compared to normal conditions (HBSS with glucose) (4.32 ± 0.86) pmoles/cornea (Fig. 11).
FIG. 11.
Cumulative amount of PMEA accumulated in freshly excised rabbit cornea mounted on a side-by-side diffusion chamber under normal conditions and under ATP-depleting conditions at 34°C. Statistical significance was tested by two-factor ANOVA. **P ≤ 0.01.
Conversion of 3H-bis(POM)PMEA to PMEA human and rabbit corneal epithelial cells
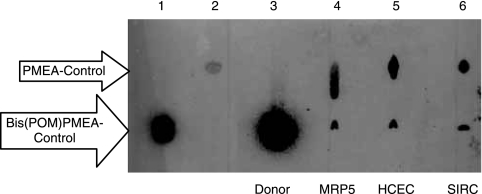
Lipophilic prodrug bis(POM)PMEA is metabolized in the cell cytoplasm to PMEA which is a substrate for MRP5. This mechanism is verified in both human and rabbit corneal epithelial cells. X-ray film developed after TLC and autoradiography revealed representative bands for bis(POM)PMEA and PMEA recovered from cell lysates of SV40-HCEC (lane 5), SIRC (lane 6), and MDCKII-MRP5 (lane 3) (Fig. 12). Control bands for bis(POM)PMEA (lane 1), PMEA (lane 2), and donor band for bis(POM)PMEA (lane 3) were also detected. At zero time point, no conversion of bis(POM)PMEA was observed as demonstrated in lane 3. Also, no intracellular radioactivity was detected. After 1 h incubation with 3H-bis(POM)PMEA under ATP-depleting conditions, ~93% of the radioactivity in the supernatant is identified as bis(POM)PMEA and ~5% corresponds to PMEA. However intracellularly in MDCKII-MRP5, SV40-HCEC, and SIRC, ~80% of the radioactivity is associated with PMEA and ~15% with bis(POM)PMEA (lanes 4, 5, 6). Remaining ~5% of intracellular radioactivity may represent mono(POM)PMEA (intermediate formed during the conversion of bis(POM)PMEA to PMEA) and several PMEA phosphorylated metabolites (i.e., PMEA monophosphate and PMEA diphosphate).9 Similar result was obtained with rPCEC (data now shown). Lower concentrations of these metabolites were detected after bis(POM)PMEA administration in human lymphoid cell lines.22,23 As demonstrated by TLC/autoradiography, conversion of inactive prodrug Bis(POM)PMEA to active MRP5 substrate PMEA occurs in the cell cytoplasm of human and rabbit corneal epithelial cells causing active drug efflux.
FIG. 12.
TLC separation of PMEA and bis(POM)PMEA shown by autoradiography. MDCKII-MRP5, SV40-HCEC, and SIRC were loaded with 3H-bis(POM)PMEA and radioactivity present in the cellular extracts was quantified. Lane 1, bis(POM)PMEA standard; Lane 2, PMEA standard; Lane 3, donor, intracellular conversion of bis(POM)PMEA to PMEA in 60 min; Lane 4, MRP5; Lane 5, SV40-HCEC; Lane 6, SIRC.
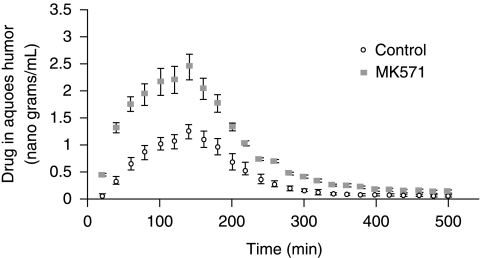
In vivo ocular absorption of 3H-acyclovir in the presence of MRP inhibitor (MK571)
Pharmacokinetic analysis revealed a significant elevation in the absorption of acyclovir across the cornea in presence of 100 μM MK-571 (Table 2). Cmax, AUC0-α, and ka increased ~2 fold with the inhibition of MRP. Even the Clast, the last measured concentration in aqueous humor is significantly higher (0.15 ± 0.01) than control (0.05 ± 0.02) μg/mL. Highest aqueous humor concentration was observed at 140 min and drug concentrations in aqueous humor were superior at all time points when MK-571 was employed (Fig. 13). Based on the in vitro substrate specificity data (Fig. 14), predominantly MRP5 and to a certain extent MRP2 appear to play a critical role in acyclovir efflux.
Table 2.
Pharmacokinetic Parameters After a Single-Dose Infusion of 3H-Acyclovir (10 mci/ml) in the Absence and Presence of MK-571 (50 mM).
| Drug and/or Inhibitor | Acyclovir | Acyclovir + 100 μM MK571 |
|---|---|---|
| AUCo−α (μg.min/mL) | 215.43 ± 35.20 | 466.28 ± 25.35 |
| Cmax (μg/mL) | 1.19 ± 0.15 | 2.64 ± 0.16 |
| Ka | 7.28 × 10−4 | 13.31 × 10−4 |
| K10 × 103 (min−1) | 5.37 ± 1.87 | 8.02 ± 1.42 |
As evident from the data, Cmax and AUC0-α values of acyclovir were significantly elevated in the presence of MK-571 relative to control. Corneal absorption rate constant (ka) significantly increased in the presence of MK-571 leading to higher Cmax and AUC0-α values relative to control. Data is expressed as mean ± S.D. (n = 4).
FIG. 13.
Scatter plot representing the amount of 3H-acyclovir present in the aqueous humor in the presence and absence of MK-571. Amount of acyclovir was significantly higher in the presence of MK-571 compared to control. Statistical significance was tested by two-factor ANOVA. **P ≤ 0.01 and data is expressed as mean ± S.D. (n = 4).
FIG. 14.
Efflux of drug was measured from MDCKII-wt, MDR1, MRP2, and MRP5 cells preloaded with 3H-acyclovir under ATP-depleting conditions (10 mM sodium azide; 10 mM 2-deoxy-d-glucose) for 1 h at 37°C. HBSS with glucose was added and comparative efflux of transporters was determined. Statistical significance was examined by two-factor ANOVA. **P ≤ 0.01 (n = 5).
Discussion
Expression of ATP-binding cassette transporters on human cornea and their role on transport processes of various drugs has not been explored. Also, there are contradicting reports on the expression of ABC transporters on human cornea.25 Dey and colleagues, 2003 and Karla and colleagues, 2007 confirmed the expression of Pgp, MRP2, and MRP1 on human corneal cells. Presence of MRP3 on corneal cells was also reported from our laboratory.24 Becker et al., 2007 contradicted the expression of Pgp and MRP2 on human cornea, but indicated the expression of MRP2 in human corneal epithelial cells. However, a recent study by Zhang et al., 2008 on quantitative expression of ABC transporters revealed that intact human cornea expresses MRP1>MRP3>MDR1=MRP2. This study confirms our earlier reports on molecular expression of ABC transporters in cornea. Relative role of individual transporters on ocular drug disposition is yet to be established.
Our results demonstrate for the first time the presence and localization of MRP5 on human cornea. Efflux data on human and rabbit corneal epithelial cells demonstrate rapid efflux of acyclovir and PMEA. As shown in Figure 8, bimatoprost and latanoprost, the two widely used antiglaucoma drugs currently on the market are found to be excellent substrates for MRP5. Figure 9 shows the inhibition of PMEA efflux in rabbit and human corneal epithelial cells by both bimatoprost and latanoprost. With later compound marketed under the trade name Xalatan by Pfizer appears to be a better substrate for MRP5 than bimatoprost marketed as Lumigan by Allergan. This is evident from the results in all three cell lines, that is, MDCKII-MRP5, rPCEC, and SIRC. However, in SV40-HCEC bimatoprost and latanoprost were equally effective in lowering the efflux of PMEA. Efflux data in Table 1 shows that PMEA efflux is more predominant in rPCEC and SIRC as compared to SV40-HCEC. This result indicates relatively higher expression levels of MRP5 in rPCEC and SIRC when relative to SV40-HCEC. Latanoprost at 50 μM was sufficient to saturate the MRP5 efflux in SV40-HCEC. However, PMEA efflux decreased by >50% in rPCEC, SIRC, and SV40-HCEC. Glaucoma drug formulations with added efflux pump inhibitors might provide better clinical outcome. However, an in vivo analysis on the efficacy of transporter in modulating drug absorption of these compounds and a comprehensive clinical analysis on cytotoxicity along with inhibitors should be studied in humans. In vivo pharmacokinetic parameters in male New Zealand rabbits provide exciting results with a ~2 fold increase in Cmax and AUC0-α of acyclovir in the presence of MK-571. Acyclovir can be employed for the treatment of ocular herpes keratitis.
However, it is found to have a low ocular bioavailability when administered topically.26 A 3% acyclovir ointment applied 5 times a day did not treat herpes keratitis.27 Efflux-mediated resistance of acyclovir absorption in the cornea is a quiet unexpected result. In vitro efflux studies and in vivo microdialysis results reveal that MRP5 along with MRP2 can play a vital role in imparting drug resistance to ocular acyclovir absorption. As shown in Figure 14, both MRP5 and MRP2 can efflux the acyclovir but acyclovir seems to be a better substrate for MRP5 than MRP2. MDR1 did not play a role in acyclovir efflux. Results from Figure 7, indicates strong suppression in acyclovir efflux in MRP5 over expressing cells with higher concentration of MK-571 (50 and 75 μM). Dose-dependant suppression in acyclovir efflux was also noticed in rPCEC, SIRC, and SV40-HCEC. It can be concluded that both MRP5 and MRP2 (MRP5 > MRP2) can play a critical role in conferring resistance to the absorption of acyclovir across cornea. MK-571 was employed in the in vivo studies as it is the only specific MRP inhibitor currently available in the market. It was found to not interact with other efflux pumps, such as Pgp.32 As MRP is a relatively new class of efflux pump, there is no specific inhibitor available for individual members of MRP family. Employing the cells which over express the specific MRP transporters or using the gene knockout mice are the methods suggested in the literature.32,33 Hence, we procured the MRP overexpressing cells (MDCKII-MRP1, MDCKII-MRP2, and MDCKII-MRP5) from Dr. Piet Borst (Netherlands cancer institute, Amsterdam, Netherlands). MDCKII-MRP5 cells along with other MRP-transfected cells were used to delineate the role of various MRPs in ocular drug resistance.
The probe recovery was below ~10% with PMEA and hence it was not employed in in vivo pharmacokinetics studies. However, to measure the role of MRP5 on resistance to PMEA, we performed transport studies on freshly excised rabbit cornea using side-by-side diffusion cells. The study indicated a ~2-fold increase in corneal accumulation of PMEA with ATP-depleting medium. Reid G et al. indicated that PMEA is not a substrate for ABC transporters, Pgp (or) breast cancer resistance protein (BCRP). Also MRP1–3 was found not to play a role in PMEA efflux.9,28 As demonstrated in Figure 1, only MRP5 played a significant role in the PMEA efflux. Neither MRP2 nor MDR1 participated in the efflux of PMEA.
Bis(POM)-PMEA enters cells rapidly by passive diffusion.29 Rapid uptake of bis(POM)-PMEA by different cell types and its fast conversion to PMEA which is an excellent substrate for nucleotide efflux transporter makes it an attractive candidate to study efflux mechanism. Following bis(POM)-PMEA loading in cells overexpressing MRP5, 98% of prodrug is converted to PMEA.7,30 TLC/autoradiography data in Figure 12 proved the conversion of bis(POM)-PMEA to PMEA in both human and rabbit corneal epithelial cells. This finding further confirms the expression of a nucleotide efflux transporter in cornea. MRP5 belongs to the class of ATP-dependant transporters and Figure 10 clearly depicts time-dependant suppression in PMEA efflux with the use of ATP-depleting medium. Efflux was low at all the time points (30, 60, 90 min) relative to control. As indicated from data in Table 1, 50-μM BSO did not alter the efflux of PMEA. Our results agree with Dallas et al., 2004 who demonstrated that 25-μM BSO did not alter the transport of PMEA by MRP5. Also, Wielinga et al., 2003 indicated that GSH depletion did not alter the efflux of cGMP/cAMP and cyclic nucleotides by MRP5. These results are consistent with earlier reports about the role of glutathione in MRP5-mediated efflux. Various cell lines were found to express MRP5, and in vivo, MRP5 has been detected in the majority of tissues of the body except the eye.31,34,35. Human urinary-genital tract smooth muscle, epithelial cells of blood vessels, uterus and urethra, syncytiotrophoblasts and fetal cells of placenta, astrocytes and pyramidal neurons of brain, cadiomyocytes, endothelial cells, and smooth muscle cells of heart have all been found to abundantly express MRP5.34–39
For the first time, we have demonstrated functional and molecular expression of a nucleotide efflux transporter (MRP5) in human and rabbit cornea. Also, for the first time, we have demonstrated that acyclovir, a widely used antiviral agent and glaucoma drugs (bimatoprost and latanoprost) are excellent substrates for MRP5 and are effluxed by corneal epithelial cells. Overall distribution and accumulation of various classes of drugs in anterior segment of eye might depend on their relative substrate specificity for the efflux pumps. Incorporation of one or a combination of inhibitors in ophthalmic formulation can be an effective strategy to improve ocular bioavailability. In light of new efflux transporters like MRP5, extensive research needs to be performed to delineate relative roles of these transporters in ocular drug disposition.
Acknowledgments
We thank Dr. Piet Borst for contributing MRP5-transfected cell line for this project. This work was presented in a preliminary format to NIH, AAPS, ARVO, and National Eye Foundation. The work was supported by NIH 05R01EY009171–14 and Sara Morrison grant from UMKC School of Medicine.
Disclosure Statement
The authors report no conflict of interest.
References
- 1.Kawazu K. Nakamura M. Ota A. Characterization of cyclosporin A transport in cultured rabbit corneal epithelial cells: P-glycoprotein transport activity and binding to cyclophilin. Invest. Ophthalmol. Vis. Sci. 1999;40:1738–1744. [PubMed] [Google Scholar]
- 2.Dey S. Patel J. Anand B.S., et al. Molecular evidence and functional expression of P-glycoprotein (MDR1) in human and rabbit cornea and corneal epithelial cell lines. Invest. Ophthalmol. Vis. Sci. 2003;44:2909–2918. doi: 10.1167/iovs.02-1142. [DOI] [PubMed] [Google Scholar]
- 3.Dey S. Gunda S. Mitra A.K. Pharmacokinetics of erythromycin in rabbit corneas after single-dose infusion: Role of P-glycoprotein as a barrier to in vivo ocular drug absorption. J. Pharmacol. Exp. Ther. 2004;311:246–255. doi: 10.1124/jpet.104.069583. [DOI] [PubMed] [Google Scholar]
- 4.Karla P.K. Pal D. Quinn T., et al. Molecular evidence and functional expression of a novel drug efflux pump (ABCC2) in human corneal epithelium and rabbit cornea and its role in ocular drug efflux. Int. J. Pharm. 2006;84:53–60. doi: 10.1016/j.ijpharm.2006.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karla P.K. Pal D. Mitra A.K. Molecular evidence and functional expression of multidrug resistance associated protein (MRP) in rabbit corneal epithelial cells. Exp. Eye. Res. 2007;84:53–60. doi: 10.1016/j.exer.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 6.Bosrt P. Dewolf C. Van De Wetering K. Multidrug resistance associated proteins 3, 4, and 5. Eur. J. Physiol. 2007;453:661–673. doi: 10.1007/s00424-006-0054-9. [DOI] [PubMed] [Google Scholar]
- 7.Borst P. Evers R. Kool M., et al. A family of drug transporters: The multidrug resistance-associated proteins. J. Natl. Cancer Inst. 2000;92:1295–1302. doi: 10.1093/jnci/92.16.1295. [DOI] [PubMed] [Google Scholar]
- 8.Zhang T. Xiang C.D. Gale D., et al. Drug transporter and CYP P450 mRNA expression in human ocular barriers: Implications for ocular drug disposition. Drug Metab. Dispos. 2008;36:1300–1307. doi: 10.1124/dmd.108.021121. [DOI] [PubMed] [Google Scholar]
- 9.Dallas S. Schlichter L. Bendayan R. Multidrug resistance protein (MRP) 4- and MRP 5-mediated efflux of 9-(2-phosphonylmethoxyethyl)adenine by microglia. J. Pharmacol. Exp. Ther. 2004;309:1221–1229. doi: 10.1124/jpet.103.063966. [DOI] [PubMed] [Google Scholar]
- 10.Pratt S. Chen V. Perry W.I., III, et al. Kinetic validation of the use of carboxydichlorofluorescein as a drug surrogate for MRP5-mediated transport. Eur. J. Pharm. Sci. 2006;27:524–532. doi: 10.1016/j.ejps.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Polcicova K. Biswas P.S. Benerjee K., et al. Herpes keratitis in the absence of anterograde transport of virus from sensory ganglia to the cornea. Proc. Natl. Acad. Sci. USA. 2005;102:11462–11467. doi: 10.1073/pnas.0503230102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hughes P.M. Krishnamooorthy R. Mitra A.K. Effect of acylation on the ocular disposition of acyclovir. I: Synthesis, physicochemical properties, and antiviral activity of 2′-esters. J. Ocul. Pharmacol. 1993;9:287–297. doi: 10.1089/jop.1993.9.287. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz G.S. Holland E.J. Oral acyclovir for the management of herpes simplex virus keratitis in children. Ophthalmology. 2000;107:278–282. doi: 10.1016/s0161-6420(99)00052-4. [DOI] [PubMed] [Google Scholar]
- 14.Ghosh S. Jhanji V. Lamoureux E., et al. Acyclovir therapy in prevention of recurrent herpetic keratitis following penetrating keratoplasty. Am. J. Ophthalmol. 2008;145:198–202. doi: 10.1016/j.ajo.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Ray A.S. Cihlar T. Robinson K.L., et al. Mechanism of active renal tubular efflux of tenofovir. Antimicrob. Agents Chemother. 2008;50:3297–3304. doi: 10.1128/AAC.00251-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wielinga P.R. VanderHeijden I. Reid G., et al. Characterization of the MRP4- and MRP5-mediated transport of cyclic nucleotides from intact cells. J. Biol. Chem. 2008;278:17664–17671. doi: 10.1074/jbc.M212723200. [DOI] [PubMed] [Google Scholar]
- 17.Araki-Sasaki K. Ohashi Y. Sasabe T., et al. An SV40-immortalized human corneal epithelial cell line and its characterization. Invest. Ophthalmol. Vis. Sci. 1995;36:614–621. [PubMed] [Google Scholar]
- 18.Sugarawa M. Nakanishi T. Fei Y.J. Cloning of an amino acid transporter with functional characteristics and tissue expression pattern identical to that of system A. J. Biol. Chem. 2000;275:16473–16477. doi: 10.1074/jbc.C000205200. [DOI] [PubMed] [Google Scholar]
- 19.Schuetz J.D. Connelly M.C. Sun D., et al. MRP4: A previously unidentified factor in resistance to nucleoside-based antiviral drugs. Nat. Med. 1999;5:1048–1051. doi: 10.1038/12487. [DOI] [PubMed] [Google Scholar]
- 20.Macha S. Mitra A.K. Ocular pharmacokinetics of cephalosporins using microdialysis. J. Ocul. Pharmacol. Ther. 2001;17:485–498. doi: 10.1089/108076801753266866. [DOI] [PubMed] [Google Scholar]
- 21.Zetterstrom T. Vernet L. Ungerstedt U., et al. Purine levels in the intact rat brain. Studies with an implanted perfused hollow fibre. Neurosci. Lett. 1982;29:111–115. doi: 10.1016/0304-3940(82)90338-x. [DOI] [PubMed] [Google Scholar]
- 22.Srinivas R.V. Robbins B.L. Connelly M.C., et al. Metabolism and in vitro antiretroviral activities of bis(pivaloxymethyl)prodrugs of acyclic nucleoside phosphonates. Antimicrob. Agents Chemother. 1993;37:2247–2250. doi: 10.1128/aac.37.10.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robbins B.L. Greenhaw J. Connelly M., et al. Metabolic pathways for activation of the antiviral agent 9-(2 phosphonylmethoxyethyl)adenine in human lymphoid cells. Antimicrob. Agents Chemother. 1995;39:2304–2308. doi: 10.1128/aac.39.10.2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karla P.K. Pal D. Mitra A.K. Molecular evidence and functional expression of multi drug resistance-associated protein (MRP) in human corneal epithelium, rabbit cornea and evaluation of its role using a specific inhibitor (AAPS–abstract 000373) 2006.
- 25.Becker U. Ehrhardt C. Daum N., et al. Expression of ABC-transporters in human corneal tissue and the transformed cell line, HCE-T. J. Ocul. Pharmacol. Ther. 2007;23:172–181. doi: 10.1089/jop.2006.0095. [DOI] [PubMed] [Google Scholar]
- 26.Tak R.V. Pal D. Gao H., et al. Transport of acyclovir ester prodrugs through rabbit cornea and SIRC-rabbit corneal epithelial cell line. J. Pharm. Sci. 2001;90:1505–1515. doi: 10.1002/jps.1101. [DOI] [PubMed] [Google Scholar]
- 27.Trousdale M.D. Dunkel E.C. Nesburn A.B. Effect of acyclovir on acute and latent herpes simplex virus infections in the rabbit. Invest. Ophthalmol. Vis. Sci. 1980;19:1336–1341. [PubMed] [Google Scholar]
- 28.Lee K. Klein-Szanto A.J. Kruh G.D. Analysis of the MRP4 drug resistance profile in transfected NIH3T3 cells. J. Natl. Cancer Inst. 2000;92:1934–1940. doi: 10.1093/jnci/92.23.1934. [DOI] [PubMed] [Google Scholar]
- 29.Hatse S. De Clercq E. Balzarini J. Enhanced 9-(2 phosphonylmethoxyethyl)adenine secretion by a specific, indomethacinsensitive efflux pump in a mutant 9–2(phosphonylmethoxyethyl)adenine-resistant human erythroleukemia K562 cell line. Mol. Pharmacol. 1998;54:907–917. doi: 10.1124/mol.54.5.907. [DOI] [PubMed] [Google Scholar]
- 30.Reid G. Wielinga P. Zelcer N., et al. Characterization of the transport of nucleoside analog drugs by the human multidrug resistance proteins MRP4 and MRP5. Mol. Pharmacol. 2003;63:1094–1103. doi: 10.1124/mol.63.5.1094. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y. Schuetz J.D. Elmquist W.F., et al. Plasma membrane localization of multidrug resistance-associated protein homologs in brain capillary endothelial cells. J. Pharmacol. Exp. Ther. 2004;311:449–455. doi: 10.1124/jpet.104.068528. [DOI] [PubMed] [Google Scholar]
- 32.Gekeler V. Ise W. Sanders K.H., et al. The leukotriene LTD4 receptor antagonist MK571 specifically modulates MRP associated multidrug resistance. Biochem. Biophys. Res. Commun. 1995;208:345–352. doi: 10.1006/bbrc.1995.1344. [DOI] [PubMed] [Google Scholar]
- 33.Tian X. Swift B. Zamek-Gliszczynski M.J., et al. Impact of basolateral multidrug resistance-associated protein (Mrp) 3 and Mrp4 on the hepatobiliary disposition of fexofenadine in perfused mouse livers. Drug Metab. Dispos. 2008;36:911–915. doi: 10.1124/dmd.107.019273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borst P. De Wolf C. Van de Wetering K. Multidrug resistance-associated proteins 3, 4, and 5. Pflugers Arch. 2007;453:661–673. doi: 10.1007/s00424-006-0054-9. [DOI] [PubMed] [Google Scholar]
- 35.Kool M. De Haas M. Scheffer G.L., et al. Analysis of expression of cMOAT (MRP2), MRP3, MRP4, and MRP5, homologues of the multidrug resistance-associated protein gene (MRP1), in human cancer cell lines. Cancer Res. 1997;57:3537–3547. [PubMed] [Google Scholar]
- 36.Dazert P. Meissner K. Vogelgesang S., et al. Expression and localization of the multidrug resistance protein 5 (MRP5/ABCC5), a cellular export pump for cyclic nucleotides, in human heart. Am. J. Pathol. 2003;163:1567–1577. doi: 10.1016/S0002-9440(10)63513-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meyer Z. Schwabedissen H.E. Grube M. et al. Expression, localization, and function of MRP5 (ABCC5), a transporter for cyclic nucleotides, in human placenta and cultured human trophoblasts: Effects of gestational age and cellular differentiation. Am. J. Pathol. 2005;166:39–48. doi: 10.1016/S0002-9440(10)62230-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nies A.T. Spring H. Thon W.F., et al. Immunolocalization of multidrug resistance protein 5 in the human genitourinary system. J. Urol. 2002;167:2271–2275. [PubMed] [Google Scholar]
- 39.Wijnholds J. Mol C.A. Van Deemter L., et al. Multidrug-resistance protein 5 is a multispecific organic anion transporter able to transport nucleotide analogs. Proc. Natl. Acad. Sci. USA. 2000;97:7476–7481. doi: 10.1073/pnas.120159197. [DOI] [PMC free article] [PubMed] [Google Scholar]