Abstract
Because the activation of telomerase is a relatively early event in the progression of cervical carcinogenesis, the expression of the human telomerase RNA gene, TERC, has the potential to serve as a biomarker for both the diagnosis and prognosis of cervical neoplasias. In total, 83 research centers participated in the study, and 7786 patients were enrolled. TERC amplification was detected using a dual-color fluorescence in situ hybridization (FISH) probe set, and these results were compared with cytological and histological results, testing for high-risk human papillomavirus (HPV) DNA (n = 2316 for the HPV DNA test), as well as patient age. TERC amplification was found to be increased in more advanced cases of cervical carcinogenesis. Moreover, a Youden's index value and the area under the receiver operating characteristic (ROC) curve were also calculated for samples with TERC amplification and found to be higher than the same values calculated for both cytology and high-risk HPV analyses of the same samples. With regard to cytological ASCUS and LSIL findings, the combination of HPV + TERC testing showed the potential to provide effective triaging to detect CIN2+. Therefore, TERC amplification represents a valuable genetic biomarker, which in combination with an evaluation of cytology or HPV testing, can achieve higher sensitivity and specificity in distinguishing high-grade cervical lesions and invasive cancers from low-grade lesions compared with conventional methods.
Cervical cancer is the second most common cancer in women, and in 2005, more than 250,000 deaths worldwide were the result of cervical cancer.1 Because ∼80% of all cervical cancer cases are diagnosed in developing countries, in China, there are ∼135,000 new cases and 50,000 deaths each year due to this disease.2 Without improvements in the treatments available, the number of cases of cervical cancer are projected to rise by almost 25% over the next 10 years. Therefore, to decrease the incidence and mortality associated with cervical cancer, effective approaches are needed to facilitate the screening and diagnosis of cervical precancerous lesions, especially high-grade lesions.
The use of cervical cytopathologic examinations has reduced the incidence of cervical cancer in many countries,3,4 however the low sensitivity and high variability of this method has limited its significance.5 With human papillomavirus (HPV) being the main cause of cervical cancer, detection of HPV DNA has become an important supplement to cytologic evaluations performed during cervical cancer screenings.6,7 However, most women who are infected with HPV eliminate the virus and do not progress to high-grade disorders, thereby decreasing the specificity of HPV DNA tests for the detection of cervical cancer.8
The infection and integration of HPV into epithelial cells are key steps in the induction of malignant alterations in cervical cancer; however, there are additional genomic events that also occur.9 One of the events associated with cervical cancer is an increase in copy number of the long arm of chromosome 3 (3q).10 Gain of 3q in cervical cancer has been mapped to 3q26-27, a region which contains the human TERC gene that encodes the template of telomerase RNA.11 Because telomerase activation is a relatively early event in cervical carcinogenesis, telomerase activity and expression of its components may represent valuable biomarkers for the diagnosis and prognosis of patients with cervical neoplasia.12
Previous studies have shown that detection of TERC amplification might be a valuable biomarker for the detection of cervical lesions.13,14,15 We have also investigated this issue since 2007 and have found that detection of TERC amplification by fluorescence in situ hybridization (FISH) has the potential to be an effective approach for the differential diagnosis of cervical disorders.16,17 Despite this work, the association between TERC amplification and cervical cytopathology, histopathology, HPV infection status, and patient age remains unclear. Importantly, the sensitivity and specificity of detecting TERC amplification remains unclear. Therefore, in this study, TERC amplification, in combination with cytopathology and HPV testing, was performed to detect cervical disorders, and these results were compared with histopathological evaluations of the samples. Moreover, the clinical usefulness of detecting TERC amplification to identify patients with cervical precancerous lesions was investigated.
Materials and Methods
Study Centers and Patients
In total, 7786 women from 83 research centers in China were recruited for this multicenter study, and informed consent was obtained from each patient before enrollment. The centers involved represent hospitals located in various parts of China, including 29 provinces, autonomous regions, and municipalities. All of the hospitals included were considered tier 1 and had the capacity to perform liquid-based thin-layer cytopathologic evaluations, colposcopy, biopsies, histopathological examinations, and FISH analysis, and were found to be consistent in their analyses. In addition, 1033 women included in this study were also previously analyzed in another study.16
The women included in this study ranged in age from 18 to 93 years (mean, 39.7 ± 9.7) and visited an outpatient clinic between June 2007 and May 2009. These patients either underwent routine screening or were returning for evaluation based on an abnormal cervical cytology result, HPV results, or to have symptoms of increased leucorrhea discharge or postcoital bleeding examined. All patients were checked to exclude acute pelvic infection and pregnancy, and liquid-based, thin-layer, cytopathologic examinations were performed using ThinPrep (Cytyc Corp, Boxborough, MA) or AutoCyte (AutoCyte, Burlington, NC) systems. FISH analysis was also performed using a TERC-specific probe in blinded assays. A colposcopy, biopsy, and histopathological examination were conducted as needed. For some patients, high-risk HPV DNA was detected using the Digene Hybrid Capture 2 method (HC2) (Qiagen, Gaithersburg, MD) depending on the institution at which the patient was treated. This project was approved by the ethical committee of Peking University People's Hospital, which played the leading role in directing this research.
Cervical Cytopathologic Evaluation
Cervical cells were collected from the sqaumocolumnar conjunction of the patient's cervix using a sampling brush and the cells were stored in Thin-prep or Autocyte cytologic test reserving fluid. Cells were subsequently prepared on slides and screened by cytotechnologists. Grading of all 7786 cases was performed according to the 2001 revision of the Bethesda system3 and included the following: cases negative for intraepithelial lesion or malignancy (NILM; 1958 cases, 25.1%), cases of benign cellular changes (29 cases, 0.4%), atypical squamous cells of undetermined significance (ASCUS; 1985 cases, 25.5%), atypical squamous cells that cannot exclude a high-grade lesion (ASC-H; 87 cases, 1.1%), low-grade squamous intraepithelial lesions (LSIL; 1824 cases, 23.4%), high-grade squamous intraepithelial lesions (HSIL; 1900 cases, 24.4%), and atypical glandular cells (AGC; 3 cases, 0.04%). For the purpose of this study, calculations of sensitivity, specificity, and positive and negative predictive values were performed with a positive cytologyic test defined as a result > = HSIL.
Cervical Histopathological Examination
Colposcopy was performed on 6726 patients who had a cytology result of ASCUS or greater, had a positive high-risk HPV result, or had apparent symptoms. All of the colposcopists and histopathologists received uniform training before the start of this study, with colposcopists following a standardized protocol. Four-quadrant biopsy, or abnormal-appearing biopsy areas, were collected and biopsy specimens were reported using terminology associated with the grading of cervical intraepithelial neoplasia.18 To avoid discrepancies among different centers as much as possible, questionable slides were delivered to the central laboratory in Beijing for a final decision.
High-Risk HPV DNA Test
There were 25 hospitals in the study that were able to perform high-risk HPV DNA tests using the HC2 method. HPV samples were obtained from 2316 women at the same time that cytology specimens were collected, according to the HC2 protocol. Levels of HPV DNA >1 pg/ml were considered a positive result.
FISH Analysis
After the liquid-based cytological preparations were completed, the remaining cervical cells were used for FISH analysis. Briefly, cells were centrifuged at 1600 × g for 6 minutes, washed twice with 1× PBS, then incubated with collagenase B at 37°C for 20 minutes. Samples were then centrifuged again, incubated with deionized water at 37°C for 30 minutes, centrifuged, and cells were resuspended in 5 ml 0.075 mol/L KCl. After another incubation for 20 minutes at 37°C, cells were fixed at RT for 10 minutes with 5 ml methanol-acetic acid (3:1), centrifuged, then smeared with 1–2 ml methanol-acetic acid onto slides pretreated with 3-aminopropyltriethoxysilane.
The dual-color FISH probe set was produced and provided by GP Medical Technologies, Ltd. (Beijing, China). This probe set had previously been approved by the State Food and Drug Administration of China and accepted for clinical application in China. Chromosome 3q26.3 is the binding site for the TERC probe, and details of the probe set and its conditions have previously been described.16,17 Before hybridization, slides were subjected to pepsin digestion and dehydrated in an ethanol series. The slides and probe mixture were denatured separately in 70% formamide/2× standard saline citrate (SSC) at 73 ± 1°C for 5 minutes. After hybridization in a humid environment overnight at 37°C, slides were washed with 50% formamide/2× SSC, 2× SSC, and 2× SSC/0.1% NP40, then counterstained with 4,6-diamidino-2-phenylindole (DAPI).
Hybridized slides were evaluated using a fluorescent microscope by uniformly trained personnel. Slides were analyzed independently by two observers at each center, and at least 100 nuclei were evaluated for each specimen. The signal ratio of CSP3 to TERC at 2:2 in a cell indicated a normal signal pattern, whereas ratios of 2:3, 2:4, 2:5, 3:3, 4:4, and so on, represented abnormal signal patterns. Therefore, an abnormal signal pattern was recorded if more than two TERC signals were observed. For each medical center, the mean and three times SD of the percentage of nuclei with a combination of all of the abnormal signal patterns possible were calculated as the cut-off value. Cervical epithelial cells from 20 individuals with normal cytopathologic examinations and negative HPV tests were used to establish the cut-off value. For samples positive for TERC amplification, the percentage of nuclei having a combination of abnormal signal patterns should be more than the cut-off value. In this study, the mean cut-off value determined for all of the participating centers was 6.4 ± 2.3%. Correspondingly, 6.4% was established as the standard cut-off value for interpreting the testing results from all study centers.
Statistical Analysis
All of the data collected were entered in a database by a data-entry agency (Likang, Beijing, China) and analyzed uniformly. The relationship between TERC amplification, HPV analyses, as well as cytology and histology diagnoses, were evaluated using the χ2 test. Spearman and Kendall's coefficients, as well as Kappa values, were also calculated. To account for a combination of increased sensitivity and decreased specificity, a Youden's index value (Y = sensitivity + specificity − 1.0) was calculated to compare the overall accuracy of each method.19,20
All analyses were performed using SPSS 13.0 (SPSS, Chicago, IL), and receiver operating characteristic (ROC) analysis was conducted using STATA 9.0 (StataCorp LP, College Station, TX). P values less than 0.05 were considered statistically significant.
Results
Of the 7786 cases of cervical samples evaluated, TERC amplification rate in cases with different cytological results was shown in Table 1. For the ASCUS, LSIL, ASC-H, and HSIL cases, a pairwise significant difference was detected (P < 0.01 in each case). Of the 6726 cases where colposcopy and histopathological evaluations were performed, TERC amplification rate in cases with different histopathological results was shown in Table 2. Among the cases labeled as normal, with inflammation, as cervical intraepithelial neoplasia (CIN)1–3, and as squamous cervical cancer (SCC), significant differences in each pairwise comparison were detected (P < 0.01 in each case). Interestingly, the number of abnormal cells present and the complexity of the abnormal signal patterns observed increased with the severity of the cervical disorders evaluated (Figure 1, A–D).
Table 1.
TERC Amplification Rate in Cases with Different Cytological Results (n = 7786)
| Cytological results | TERC amplification rate, % (cases) |
|---|---|
| NILM | 10.1 (198/1958) |
| Benign cellular change | 31.0 (9/29) |
| ASCUS | 34.5 (684/1985) |
| LSIL | 43.1 (786/1824) |
| ASC-H | 60.9 (53/87) |
| HSIL | 82.0 (1558/1900) |
| AGC | 100 (3/3) |
Table 2.
TERC Amplification Rate in Cases with Different Histopathological Results (n = 6726)
| Histopathological results | TERC amplification rate, % (cases) |
|---|---|
| Normal | 6.2 (11/178) |
| Inflammatory | 10.5 (113/1079) |
| CIN1 | 20.8 (428/2054) |
| CIN2 | 68.6 (952/1387) |
| CIN3 | 82.4 (1162/1410) |
| SCC | 94.9 (556/586) |
| Glandular carcinoma | 87.5 (28/32) |
Figure 1.
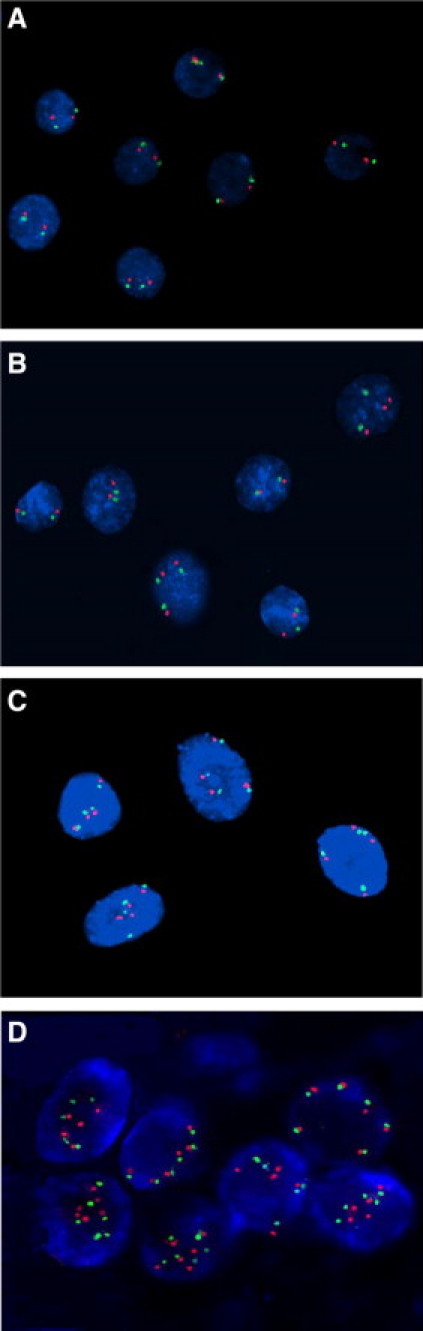
Representative immunofluorescence images of different cell types stained for TERC and CSP3. A: Normal cells with a CSP3 (green):TERC (red) signal ratio of 2:2. B: A few CIN1 cells with 2:3 or 3:3 signal patterns. C: CIN3 cells exhibiting a 4:4 signal pattern. D: SCC cells with 5:5 and 6:6 signal patterns. Magnification, ×1000.
In 2316 cases tested for high-risk HPV DNA, 75.7% (1754/2316) were positive, including 27.3% (12/44) of histologically normal specimens, 42.2% (144/341) of inflammatory specimens, 70.5% (560/794) of CIN1 cases, 88.1% (406/461) of CIN2 cases, 93.9% (490/522) of CIN3 cases, 92.7% (140/151) of SCC cases, and 66.7% (2/3) of glandular carcinoma cases. Overall, the positive rate for TERC amplification was 57.2% (1003/1754) for HPV-positive cases, and only 18.9% (106/562) for HPV-negative cases, and this difference was statistically significant (P < 0.001). Moreover, a positive correlation between TERC amplification and detection of HPV was observed and associated with a Spearman correlation coefficient of 0.329 (P < 0.001).
To better understand the capacity for different methods to evaluate high-grade cervical lesions (ie, CIN2/3) and invasive cancers, the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of cytology, high-risk HPV detection, and TERC amplification analyses were calculated (Table 3). High-risk HPV DNA testing was associated with the highest levels of sensitivity (91.3%), followed by TERC amplification (80.7%) and cytology analyses (53.9%). However, in terms of specificity, cytology evaluations were the best (93.3%), followed by TERC amplification (83.8%) and high-risk HPV DNA analyses (39.3%), with significant differences detected between each (P < 0.05). The PPVs of TERC amplification (82.8%) and cytology analyses (88.6%) were not statistically different, although both were significantly higher than that of high-risk HPV testing (59.2%). Moreover, the NPV of TERC amplification analysis (81.9%) was similar to that of high-risk HPV testing (82.4%), yet higher than that for cytological evaluations (67.7%). Regarding the analysis according to Youden's index and the area under the ROC curve, detection of TERC amplification had the highest values (see also Figure 2). A kappa value was also calculated to determine the uniformity of the three methods in evaluating the histological classification of each sample, and detection of TERC amplification was found to have the highest value.
Table 3.
Sensitivity, Specificity, Positive Prediction Value (PPV), Negative Prediction Value (NPV), and Area under ROC Curve of Cytology (≥HSIL), High-Risk HPV, and TERC Amplification Analyses for the Diagnosis of High-Grade Cervical Lesions and Invasive Cancer (n = 2316)
| Characteristic | Cytology | High-risk HPV | TERC |
|---|---|---|---|
| Sensitivity, % (95%CI) | 53.9 (51.0–56.8) | 91.3 (89.7–92.9) | 80.7 (78.4–83.0) |
| Specificity, % (95%CI) | 93.3 (91.9–94.7) | 39.3 (36.5–42.1) | 83.8 (81.7–85.9) |
| PPV, % (95%CI) | 88.6 (86.2–91.0) | 59.2 (56.9–61.5) | 82.8 (80.6–85.0) |
| NPV, % (95%CI) | 67.7 (65.5–70.0) | 82.4 (79.2–85.5) | 81.9 (79.7–84.0) |
| Youden's index, % (95%CI) | 47.2 (44.0–50.4) | 30.6 (27.3–33.8) | 64.5 (61.4–67.6) |
| Area under ROC curve, % (95%CI) | 73.6 (71.5–75.7) | 65.3 (63.0–67.5) | 82.3 (80.5–84.1) |
| Kappa value, % (95%CI) | 47.5 (44.2–50.8) | 30.3 (27.0–33.6) | 64.6 (61.5–67.7) |
Figure 2.
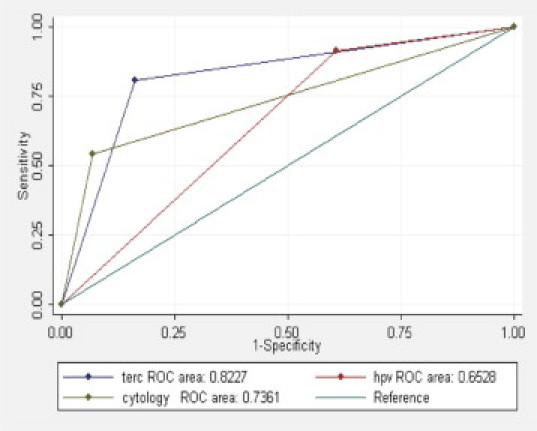
ROC curves for detection of CIN2+ by cytology, high-risk HPV DNA testing, and TERC amplification analysis.
The correlation between TERC amplification and patient age was determined. Four age groups were established that included patients of 18–29 years (353 cases), 30–49 years (1679 cases), 50–64 years (263 cases), and 65+ years (21 cases). The positive rate of TERC amplification was found to increase with patient age and was associated with a Kendall's correlation coefficient of 0.070 (P < 0.001). The ability of different methods to detect high-grade cervical lesions in different age groups is shown in Table 4 and Figure 3(A–C). TERC amplification provided the highest sensitivity for patients 30–64 years old (81.6–82.0%), whereas the best specificity and NPV were associated with patients 18–29 years old (88.7% and 85.3%, respectively). The highest PPV was detected for patients older than 65 years old (91.7%). Within the liquid-based cytology examinations, the highest specificity was associated with patients 18–29 years old (95.5%), and the highest PPV was detected for patients 30–49 years old (89.6%). For samples tested for high-risk DNA, very good levels of sensitivity were associated with the 18- to 64-year-old group (91.0–91.6%), and the highest NPV was detected for the 18- to 29-year-old group (85.7%). However, there was no statistically significant difference between each pairwise comparison.
Table 4.
Cytology (≥HSIL), High-Risk HPV, and TERC Amplification Analyses in Association with Different Age Groups for the Detection of High-Grade Cervical Lesions and Invasive Cancer (n = 2316)
| Cytology |
High-risk HPV |
TERC |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | 18–29 | 30–49 | 50–64 | 65+ | 18–29 | 30–49 | 50–64 | 65+ | 18–29 | 30–49 | 50–64 | 65+ |
| Sensitivity, % (95%CI) | 38.2 (66.5–81.6) | 56.3 (53.0–59.6) | 54.1 (45.3–62.9) | 50.0 (23.8–76.2) | 91.6 (86.9–96.4) | 91.5 (89.6–93.3) | 91.0 (85.9–96.1) | 78.6 (57.1–100.0) | 74.0 (66.5–81.6) | 81.6 (79.0–84.2) | 82.0 (75.1–88.8) | 78.6 (57.1–100.0) |
| Specificity, % (95%CI) | 95.5 (92.8–98.2) | 93.0 (91.2–94.7) | 92.2 (87.8–96.6) | 85.7 (59.8–100.0) | 29.7 (23.7–35.7) | 41.3 (37.9–44.7) | 43.3 (35.1–51.4) | 28.6 (0–62.0) | 88.7 (84.6–92.9) | 82.1 (79.4–84.7) | 85.8 (80.1–91.6) | 85.7 (59.8–100.0) |
| PPV, % (95%CI) | 83.3 (73.9–92.8) | 89.6 (87.0–92.1) | 85.7 (77.9–93.5) | 87.5 (64.6–100.0) | 43.5 (37.6–49.3) | 62.6 (60.0–65.3) | 58.1 (51.1–65.1) | 68.8 (46.0–91.5) | 79.5 (72.3–86.7) | 83.0 (80.5–85.6) | 83.3 (76.7–90.0) | 91.7 (76.0–100.0) |
| NPV, % (95%CI) | 72.4 (67.2–77.5) | 66.4 (63.7–69.2) | 69.9 (63.3–76.5) | 46.2 (19.1–73.3) | 85.7 (77.9–93.5) | 81.9 (78.1–85.6) | 84.7 (76.4–93.0) | 40.0 (0–82.9) | 85.3 (80.7–89.9) | 80.6 (77.9–83.3) | 84.6 (78.7–90.5) | 66.7 (35.9–97.5) |
| Youden's index, % (95%CI) | 33.7 (24.9–42.4) | 49.3 (45.5–53.0) | 46.3 (36.4–56.2) | 35.7 (0–72.6) | 21.3 (13.7–29.0) | 32.8 (28.9–36.6) | 34.2 (24.6–43.9) | 7.1 (0–46.9) | 62.8 (54.2–71.4) | 63.7 (60.0–67.4) | 67.8 (58.9–76.7) | 64.3 (30.6–98.0) |
Figure 3.
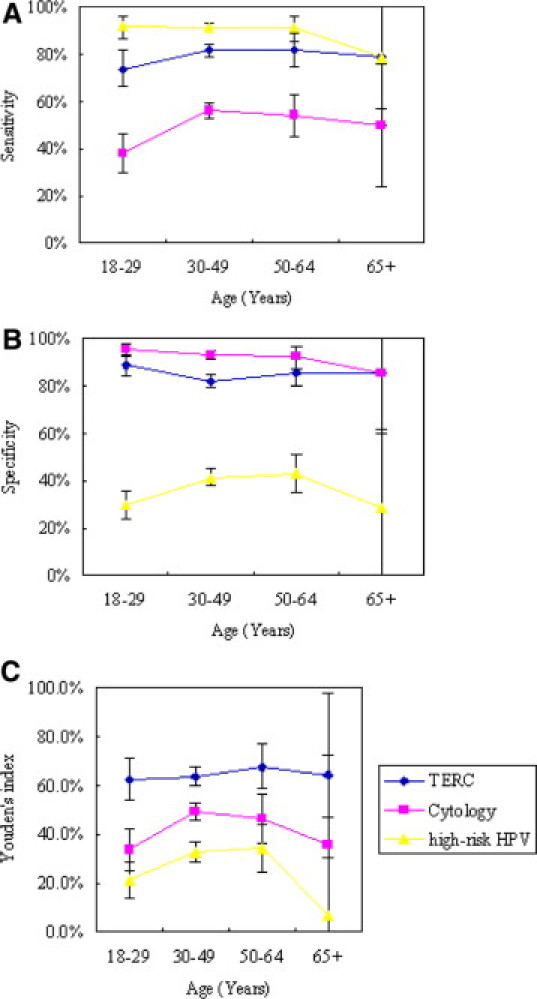
Graphs of the sensitivity (A), specificity (B), and Youden's index values (C) calculated for the methods of cytology, high-risk HPV DNA detection, and TERC amplification analyses for the detection of high-grade cervical lesions and invasive cancer in different age groups. Bars represent 95% confidence intervals.
Finally, the capacity for TERC amplification, in combination with detection of high-risk HPV DNA, to identify histological CIN2+ or CIN3+ patients among cytological ASCUS and LSIL cases was analyzed. Of the 660 patients with cytological ASCUS and 601 patients with LSIL, all underwent an HPV test, colposcopy-directed biopsy, and TERC amplification examination. Of these, 196 (29.7%) samples were verified to be histological CIN2+, 97 (14.7%) samples were CIN3+ in cytological ASCUS, and 233 (38.8%) samples were verified to be histological CIN2+, 105 (17.5%) samples were CIN3+ in cytological LSIL women. The sensitivity of detecting CIN2+ or CIN3+ by TERC amplification was lower than that for HPV testing in both ASCUS and LSIL cases, yet the specificity associated with the detection of TERC amplification was higher than that for HPV testing (at least P < 0.05). The NPV of TERC testing was also found to be similar to that of HPV testing (P > 0.05), whereas if TERC and HPV testing were performed on the same sample, the specificity and NPV of these methods for detecting CIN2+ was significantly higher than for either test alone (at least P < 0.05). Moreover, no significant decrease in specificity was observed (Table 5). Similarly, the specificity associated with detecting CIN3+ by assaying both TERC amplification and HPV DNA was significantly higher than performing either test alone (at least P < 0.05). Moreover, the NPV for the combined testing was higher than that for detection of TERC amplification (P < 0.05) yet was similar to the NPV of HPV testing alone (P > 0.05, Table 6).
Table 5.
Sensitivity, Specificity, and NPV of High-Risk HPV, TERC Amplification, and High-Risk HPV + TERC Amplification Analyses for the Diagnosis of CIN2+ in Cytologic ASCUS and LSIL Women
| Characteristic | Cytology (n) | High-risk HPV | TERC | High-Risk HPV + TERC |
|---|---|---|---|---|
| Sensitivity, % (95%CI) | ASCUS (660) | 89.3 (85.0–93.6) | 75.5† (69.5–81.5) | 96.4† (93.8–99.0) |
| LSIL (601) | 95.3 (92.6–98.0) | 75.5† (70.0–81.1) | 99.1* (98.0–100.0) | |
| Specificity, % (95%CI) | ASCUS (660) | 45.5 (40.9–50.0) | 86.9† (83.8–89.9) | 41.6 (37.1–46.1) |
| LSIL (601) | 23.4 (19.1–27.7) | 78.5† (74.3–82.7) | 21.2 (17.0–25.4) | |
| NPV, % (95%CI) | ASCUS (660) | 91.0 (87.3–94.6) | 89.4 (86.5–92.2) | 96.5* (94.0–99.1) |
| LSIL (601) | 88.7 (82.4–95.0) | 83.5 (79.6–87.4) | 97.5* (94.1–100.0) |
Statistically different from high-risk HPV test
P < 0.05,
P < 0.01.
Table 6.
Sensitivity, Specificity, and NPV of High-Risk HPV, TERC Amplification, and High-Risk HPV + TERC Amplification Analyses for the Diagnosis of CIN3+ in Cytologic ASCUS and LSIL Women
| Characteristic | Cytology (n) | High-risk HPV | TERC | High-risk HPV + TERC |
|---|---|---|---|---|
| Sensitivity, % (95%CI) | ASCUS (660) | 92.8 (87.6–97.9) | 82.5* (74.9–90.0) | 99.0* (97.0–100.0) |
| LSIL (601) | 96.2 (92.5–99.9) | 81.0† (73.4–88.5) | 100.0* (100.0–100.0) | |
| Specificity, % (95%CI) | ASCUS (660) | 40.0 (35.9–44.0) | 77.1† (73.6–80.6) | 35.4 (31.4–39.3) |
| LSIL (601) | 18.8 (15.3–22.2) | 65.7† (61.6–69.9) | 16.1 (12.9–19.4) | |
| NPV, % (95%CI) | ASCUS (660) | 97.0 (94.8–99.2) | 96.2 (94.5–98.0) | 99.5‡ (98.5–100.0) |
| LSIL (601) | 95.9 (91.9–99.8) | 94.2 (91.8–96.7) | 100.0‡ (100.0–100.0) |
Statistically different from high-risk HPV test
Statistically higher than TERC amplification
P < 0.05,
P < 0.01.
P < 0.05.
Discussion
Most patients with CIN1 regress spontaneously, whereas a few patients persist or advance to high-grade lesions. Therefore, from a clinical management point of view, it is crucial to accurately identify patients with high-grade lesions (CIN2/3) to appropriately treat them as soon as possible to prevent the progression to carcinoma. Despite the success of cytologic screenings provided as a public health service to reduce the incidence and mortality of cervical cancer, a single cytologic testing is relatively insensitive, poorly reproducible, and frequently yields equivocal results.21 Because more than 95% of cervical cancer biopsies contain oncogenic HPV,22 an HPV test represents a very sensitive method for discerning lesions that have low versus high risk for progression. However, the low specificity associated with HPV testing has limited its clinical application.8 Therefore, the search for more effective biomarkers and treatment strategies for managing cervical disorders remains.
In 2003, Heselmeyer-Haddad et al21 first studied the genomic amplification of TERC in 57 thin-layer cervical cytologic slides and found that 63% of HSIL (CIN2) lesions and 76% of HSIL (CIN3) lesions contained extra copies of chromosome 3q. Several authors subsequently proposed that amplification of chromosome 3q was associated with higher grade cervical disorders, with 0–15.4% of 3q amplifications detected in normal cytology slides, 13% in ASCUS slides, 0 to 70% in LSIL slides, 25% in ASC-H slides, and 62.5 to 100% in HSIL slides.13,14,15,21 Correspondingly, these data suggested that TERC amplification could be used to screen for cervical lesions. Although the results of these reports were relatively consistent, most of the values varied greatly, probably due to the small sample size (<70 cases) analyzed in each of these studies.
We previously reported that the positive rate of TERC amplification in an analysis of 1033 cases increased with progression of severity of cervical lesions.16 These cases are included in this study where we present the largest sample size to date analyzing the role of TERC amplification. TERC amplification was detected in 10.1% of patients with cytological NILM, in 31.0% of patients with benign cellular changes, in 34.5% of patients with ASCUS, in 43.1% of patients with LSIL, in 60.9% of patients with ASC-H, and in 82.0% of cases with HSIL. These results further confirm that the positive rate of TERC amplification increases with more advanced cervical disorders.
Because histopathological evaluation is considered the gold standard for determining the presence of cervical lesions,23 TERC amplification was also compared with histological analysis in this study. Detection of TERC amplification produced a similar pattern of identification as cytological evaluations, with TERC amplification detected in 6.2% of normal cases, 10.5% of inflammatory cases, 20.8% of CIN1 cases, 68.6% of CIN2 cases, 82.4% of CIN3 cases, and 94.5% of invasive cancers. These results clearly demonstrate that the proportion of cases with TERC amplification increased with the grade of lesion analyzed, especially between CIN1 and CIN2 cases (20.8% versus 68.6%, respectively). Furthermore, these results strongly support the use of TERC amplification in combination with cytological and histopathological evaluations for the differential diagnosis of low-grade versus high-grade cervical disorders.
The transition of an episomal virus to an integrated oncogenic virus is a risk associated with the progression of cervical dysplasia to invasive cancer and can lead to genomic instability including TERC amplification.11 In the present study, the rate of TERC amplification was found to be much higher in HPV-positive cases than in HPV-negative cases, and the presence of TERC amplification corresponded with the presence of HPV infection. Because TERC amplification represents an aspect of genomic instability that may develop in the early stages of cancer, it would be predicted to be more specific than the detection of HPV in evaluating morphological changes as a result of cervical disorders.16 In the present study, both the specificity and PPV of TERC amplification for the diagnosis of high-grade cervical lesions were higher than the values associated with the detection of high-risk HPV DNA. Given the similar NPV value and higher PPV value associated with TREC amplification analysis versus high-risk HPV DNA detection, the former may have an advantage in monitoring the recovery or recurrence of cervical lesions posttherapy.
The Youden's index value and area under the ROC curve calculated for samples with TERC amplification were higher than the same values calculated for cytology and high-risk HPV analyses. These results imply that detection of TERC gene amplification by FISH could improve the sensitivity and specificity of diagnosing high-grade cervical lesions and invasive cancer, and this would be consistent with other recent reports.24 Furthermore, detection of TERC amplification has been shown to be more consistent with histopathological diagnoses at the highest Kappa value. In the literature, individual studies have evaluated cytology using different thresholds. We also calculated the accuracy of the cytology method by using ASCUS as the cut-off point. As a result, the specificity decreased dramatically to 21.6% despite the level of sensitivity increasing to 93.1%. Overall, the combined sensitivity and specificity levels associated with the cytology analysis using HSIL as a threshold was higher than the sensitivity and specificity associated with ASCUS as a cut-off value. Based on these data, we selected HSIL as the cut-off point for this study, and the accuracy of liquid-based cytology was found to be similar to that reported in Europe and North America, with a sensitivity for detecting high-grade lesions ranging from 42.2–56.4%, and a specificity ranging from 96.3–99.1%.5,25 Therefore, from a clinical standpoint, it appears that detection of TERC amplification, in conjunction with cytology and/or high-risk HPV DNA testing, can improve the detection of high-grade cervical lesions and invasive cancer.
When the positive rate of TERC amplification was compared with patient age, increased levels of amplification were associated with older patients, and this is consistent with the observation that the severity and invasiveness of cervical diseases increases with age. TERC amplification had the highest sensitivity for patients between the ages of 30–64 years, thereby suggesting that detection of TERC amplification could improve the detection of moderate and severe cervical precancerous lesions in women of child-bearing age, or closely after the onset of menopause. TERC amplification had the highest specificity for the 18- to 29-year-old women, which could be helpful for avoiding overtreatment on young women in this age group. In contrast, Cuzik et al5 found that the sensitivity of HPV testing was uniformly high for all ages of women during cervical screening, and the sensitivity of cytology examination was better for women older than 50 years old, with the specificity for both methods increasing with patient age. In this study, the sensitivity of HPV testing and the specificity of cytology examination were both high for patients between 18 and 64 years old, however, both values dropped greatly when the women evaluated were greater than 65 years old. Interestingly, however, detection of TERC amplification maintained good sensitivity and specificity for patients 65 years and older. Similarly, the Youden's index value for detection of TERC amplification was consistently high for all age groups, while the highest Youden's index value for cytology was associated with the 30–49 age group, and with HPV testing in the 50–64 age group. To our knowledge, this study may represent the first study to evaluate the association between TERC amplification and patient age.
Recently, determination of TERC amplification by FISH was found to serve as a persistence-progression indicator in LSIL cases,26 and the NPV of TERC amplification during the development of CIN2/3 reached as high as 93–100% after an average follow-up period of 12–41 months.27 In this study, the combination of detecting TERC amplification and testing for high-risk HPV DNA helped to triage ASCUS and LSIL cytologic findings. For example, the sensitivity of detecting CIN2+ using HPV + TERC testing was 96.4% in the ASCUS cytologic group and 99.1% in the LSIL group, with NPV values of 96.5% and 97.5%, respectively. Furthermore, all of the above figures were significantly higher for the combined testing approach than for detection using either approach alone. For an ASCUS cytologic diagnosis, HC2 testing for high-risk HPV types has previously been shown to be a sensitive triage method for the detection of CIN2+.28 In our results, detection of TERC amplification further increased the sensitivity and NPV of HC2 without decreasing its specificity to detect CIN2+ in high-risk women. With regard to cytologic LSIL findings, neither a single HPV test, nor repeat cytology, has previously been shown to provide useful triage.29 However, in this study, the combination of HPV + TERC testing showed the potential to provide effective triaging and would thus reduce the referral of LSIL cases for colposcopy. Furthermore, regarding the detection of CIN3+ cases, the combination of HPV + TERC testing appears to have few advantages compared with HPV test both in cytologic ASCUS and LSIL groups, and although the sensitivity resulting from the combination of these two methods increased significantly, the NPV only rose slightly and did not reach statistical significance.
There is a very high rate of spontaneous regression associated with CIN1, therefore management of CIN1 is recommended to include cytological follow-ups at defined intervals according to the 2006 consensus guidelines of the American Society for Colposcopy and Cervical Pathology (ASCCP).30 However, not all cases of CIN1 will regress. It has been observed that a small number of cases will actually progress, and based on data of an ASCUS/LSIL Triage Study (ALTS), ∼12% of women who have CIN1 or less have a risk of developing CIN2/3 within 2 years.31 Correspondingly, it is of great importance to distinguish transforming CIN1 lesions from naturally regressing CIN1 lesions. While some studies have reported that detection of TERC amplification could predict the progression of CIN1/2 cases to CIN3 with a predictive sensitivity of 100%,13 428/2054 (20.8%) cases of CIN1 in this study exhibited TERC amplification. Additional evaluations are being performed to identify the patients of this group who are experiencing more aggressive biological behavior of their CIN1 cases, and then the predictive value of TERC amplification in relation to disease progression can be evaluated.
It is important to note, however, that this study enrolled a hospital-based population of high-risk women and was not representative of the general population. Therefore, while the incidence of CIN2+ was >40% in the 2316 patients whose data were used to calculate the NPV, this is much higher than the previously reported rate of 8%.32,33,34 This would explain the low NPV of HPV testing to detect CIN2+ (ie, 82.4%). Furthermore, the results of this study may be more applicable to high-risk women undergoing consultation rather than primary cervical screenings.
In summary, FISH can be performed on cervical exfoliated cells preserved in Thin-prep or Autocyte reserving fluid after the preparation of thin-layer cytopathologic slides without the need for additional collection of samples. As such, this would prevent the need to obtain additional samples for analysis. Detection of TERC amplification in cervical cells using the FISH technique was also shown to be a valuable method in combination with cytology and/or HPV testing for the diagnosis of cervical disorders. The superiority of this approach was evidenced by the fact that TERC amplification detection was associated with higher sensitivity and specificity in distinguishing high-grade cervical lesions and invasive cancers from low-grade lesions in patients ranging in age from 18 to 65 years. Clinically, detection of TERC amplification has the potential to help distinguish between cases of CIN1 and CIN2, as well as to triage cytological ASCUS and LSIL patients to avoid unnecessary colposcopy procedures. However, additional prospective studies will be needed to confirm these results.
Acknowledgements
We thank all 83 research centers for providing their study data, and the center names and experts in charge of each center were as follows:
Affiliated Hospital of Guangdong Medical College (Yu Zhong-Hua); Affiliated Hospital of NingXia Medical College (Wei Jun); Affiliated Hospital of Zunyi Medical College (Sun Li-Jun); Beijing Anzhen Hospital (Li Bin); Beijing Friendship Hospital (Wang Jian-Jie); Beijing Military General Hospital (Ding Hua-Ye); Beijing Obstetrics and Gynecology Hospital (Wang Shu-Yu); Beijing Tiantan Hospital (Kang Xi-Xiong); Beijing Union Hospital (Yang Jia-Xin); Benxi Center Hospital (Huang Li-Qiang); Cancer Center of Sun Yat-sen University (Shao Jian-Yong); Cancer Hospital of Chinese Academy of Medical Sciences (Wu Ling-Ying); Central Hospital of Taizhou (Li Zhao-Yun); China-Japan Friendship Hospital (Bian Mei-Lu); Chinese PLA General Hospital (Li Ya-Li); Daping Hospital of The Third Military Medical University (Li Li); Dongguan People's Hospital (Lu Yi-Sheng); First Affiliated Hospital of Medical College of Xi'an Jiaotong University (Li Xu); First Hospital of China Medical University (Wu Guang-Ping); First Hospital of Jilin University (Wang Mei); First Hospital of Zhengzhou University (Qiao Yu-Huan); Fourth Hospital of China Medical University (Wei Li); Fourth Hospital of Hebei Medical University (Cheng Jian-Xin); Fudan University Shanghai Cancer Center (Wu Xiao-Hua); Fujian Provincial Maternity and Child Hospital (Dai Li-Yu); Fuzhou General Hospital of Nanjing Military Command (Yu Ying-Hao); Gansu Provincial Maternity and Child Care Hospital (Shi Qing-Fang); General Hospital of Tianjin Medical University (Xue Feng-Xia); Guangdong People's Hospital (Liu Yan-Hui); Haikou People's Hospital (Huang Shou-Guo); Hainan People's Hospital (Fu Sheng-Di); Henan Province Tumor Hospital (Wang Li); Jinan Maternal and Child Care Hospital (Qiao Yun-Bo); Maternal and Child Health Hospital of Dalian (Wu Xiong-Fei); Maternal and Child Health Hospital of Inner Mongolia Autonomous Region (Ji Xiao-Ping); Maternal and Child Health Hospital of Jiangxi (Li Long-Yu); Maternity and Child Care Hospital of Shanxi (Zhou Yong-An); Nanfang Hospital of Southern Medical University (Wang Qian); Nanjing Maternity and Child Heath Care Hospital (Xu Zheng-Feng); Peking University First Hospital (Liao Qin-Ping); Peking University People's Hospital (Wei Li-Hui); Peking University Shenzhen Hospital (Wu Rui-Fang); Peking University Third Hospital (Geng Li); Qilu Hospital of Shandong University (Kong Bei-Hua); Qingdao Municipal Hospital (Wang Xia); Second Hospital of Jilin University (Cui Man-Hua); Second Hospital of Tianjin Medical University (Yin Li-Rong); Second Military Medical University Changhai Hospital (Zhu Ming-Hua); Shanghai Jiaotong University Affiliated First People's Hospital (Wan Xiao-Ping); Sichuan Provincial People's Hospital (Yang Zheng-Lin); Southwest Hospital of The Third Military Medical University (Fu Wei-ling); The 181th Hospital of PLA (Sui Wei-Guo); The Affiliated Hospital of Medical College of Qingdao University (Dai Shu-Zhen); The Affiliated Hospital of Wuhan University of Science and Technology (Yin Ling); Women's Hospital School of Medicine Zhejiang University (Xie Xing); The Affiliated People's Hospital of Wuhan University (Luo Ruo-Yu); The Affiliated Provincial Hospital of Anhui Medical University (Ling Bin); The Affiliated Tumor Hospital of Hrrbin Medical University (Lou Ge); The Afflicted Hospital of Hainan Medical College (Jin Song); The First Affiliated Hospital of Dalian Medical University (Shi Hong); The First Affiliated Hospital of Fujian Medical University (Zhang Sheng); The First Affiliated Hospital of Inner Mongolia Medical College (Song Jing-Hui); The First Affiliated Hospital of Jinan University (Luo Xin); The First Affiliated hospital of NangHua University (Xie Wan-Yu); The First Hospital of Lanzhou University (Jin Ping); The First Hospital of Shanxi Medical University (Wu Su-Hui); The First Hospital of Xinjiang Medicine University (Ding Yan); The First People s Hospital of Yunnan Province (Zhu Bao-Sheng); The General Hospital of Jinan Military Area (Yin Ge-Ping); The Gynecology and Obstetrics Hospital of Fudan University (Feng You-Ji); The People's Hospital of Hunan Province (Li Zhuo-Ri); The Second Affiliated Hospital of Dalian Medical University (Zou Zhong-Wen); The Second Affiliated Hospital of Kunming Medical University (Yang Hong-Ying); The Second People's Hospital of Zhuhai City (Cao Xiao-Zhe); The Third Affiliated Hospital of Sun Yat-sen University (Jin Yi); The Third Affiliated Hospital of Suzhou University (Wu Chang-Ping); The Tumor Hospital of HuNan Province (Zeng Liang); Third Affiliated Hospital of Zhengzhou University (Zhang Zhan); Tumor Hospital of Xinjiang Medical University (Liu Kai-Jiang); Union Hospital Affiliated to Tongji Medical College of Huazhong University of Science and Technology (Wang Ze-Hua); West China Second Hospital of Sichuan University (Wang He); Xiangya Hospital of Central South University (Zhang Yi); and Zhuhai People's Hospital (Ren Da-Hong).
Footnotes
Supported by a Scientific Research grant from the Ministry of Health of the People's Republic of China (WKJ 2007-3-001).
J.J. and L.-H.W. contributed equally to this study.
References
- 1.World Health Organization Reproductive Health and Research . Chronic Diseases and Health Promotion. Comprehensive cervical cancer control: a guide to essential practice. World Health Organization; Geneva: 2006. pp. 3–4. [PubMed] [Google Scholar]
- 2.Wei LH. Excessiveness and insufficiency in the diagnosis and treatment of cervical lesions. Chin J Clin Obstet Gynecol. 2009;10:83–84. [Google Scholar]
- 3.Solomon D, Davey D, Kurman R, Moriarty A, O'Connor D, Prey M, Raab S, Sherman M, Wilbur D, Wright T, Jr, Young N, Forum Group Members Bethesda 2001 Workshop: The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA. 2002;287:2114–2119. doi: 10.1001/jama.287.16.2114. [DOI] [PubMed] [Google Scholar]
- 4.Wright TC, Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D. 2006 ASCCP-Sponsored Consensus Conference: 2006 consensus guidelines for the management of women with abnormal cervical screening tests. J Low Genit Tract Dis. 2007;11:201–222. doi: 10.1097/LGT.0b013e3181585870. [DOI] [PubMed] [Google Scholar]
- 5.Cuzick J, Clavel C, Petry KU, Meijer CJ, Hoyer H, Ratnam S, Szarewski A, Birembaut P, Kulasingam S, Sasieni P, Iftner T. Overview of the European and North American studies on HPV testingin primary cervical cancer screening. Int J Cancer. 2006;119:1095–1101. doi: 10.1002/ijc.21955. [DOI] [PubMed] [Google Scholar]
- 6.Naucler P, Ryd W, Törnberg S, Strand A, Wadell G, Elfgren K, Rådberg T, Strander B, Johansson B, Forslund O, Hansson BG, Rylander E, Dillner J. Human papillomavirus and papanicolaou tests to screen for cervical cancer. N Engl J Med. 2007;357:1589–1597. doi: 10.1056/NEJMoa073204. [DOI] [PubMed] [Google Scholar]
- 7.Qiao YL, Sellors JW, Eder PS, Bao Y, Lim JM, Zhao FH, Weigl B, Zhang WH, Peck RB, Li L, Chen F, Pan QJ, Lorincz AT. A new HPV-DNA test for cervical-cancer screening in developing regions: a crosssectional study of clinical accuracy in rural China. Lancet Oncol. 2008;9:929–936. doi: 10.1016/S1470-2045(08)70210-9. [DOI] [PubMed] [Google Scholar]
- 8.Castle PE, Wacholder S, Lorincz AT, Scott DR, Sherman ME, Glass AG, Rush BB, Schussler JE, Schiffman M. A prospective study of high-grade cervical neoplasia risk among human papillomavirus-infected women. J Natl Cancer Inst. 2002;94:1406–1414. doi: 10.1093/jnci/94.18.1406. [DOI] [PubMed] [Google Scholar]
- 9.Münger K, Baldwin A, Edwards KM, Hayakawa H, Nguyen CL, Owens M, Grace M, Huh K. Mechanisms of human papillomavirus-induced oncogenesis. J Virol. 2004;78:11451–11460. doi: 10.1128/JVI.78.21.11451-11460.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirchhoff M, Rose H, Petersen BL, Maahr J, Gerdes T, Philip J, Lundsteen C. Comparative genomic hybridization reveals non-random chromosomal aberrations in early preinvasive cervical lesions. Cancer Genet Cytogenet. 2001;129:47–51. doi: 10.1016/s0165-4608(01)00424-1. [DOI] [PubMed] [Google Scholar]
- 11.Hopman AH, Theelen W, Hommelberg PP, Kamps MAF, Herrington CS, Morrison LE, Speel EJ, Smedts F, Ramaekers FC. Genomic integration of oncogenic HPV and gain of the human telomerase gene TERC at 3q26 are strongly associated events in the progression of uterine cervical dysplasia to invasive cancer. J Pathol. 2006;210:412–419. doi: 10.1002/path.2070. [DOI] [PubMed] [Google Scholar]
- 12.Zheng PS, Iwasaka T, Zhang ZM, Pater A, Sugimori H. Telomerase activity in Papanicolaou smear-negative exfoliated cervical cells and its association with lesions and oncogenic human papillomaviruses. Gynecol Oncol. 2000;77:394–398. doi: 10.1006/gyno.2000.5779. [DOI] [PubMed] [Google Scholar]
- 13.Heselmeyer-Haddad K, Sommerfeld K, White NM, Chaudhri N, Morrison LE, Palanisamy N, Wang ZY, Auer G, Steinberg W, Ried T. Genomic amplification of the human telomerase gene (TERC) in Pap smears predicts the development of cervical cancer. Am J Pathol. 2005;166:1229–1238. doi: 10.1016/S0002-9440(10)62341-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caraway NP, Khanna A, Dawlett M, Guo M, Guo N, Lin E, Katz RL. Gain of the 3q26 region in cervicovaginal liquid-based pap preparations is associated with squamous intraepithelial lesions and squamous cell carcinoma. Gynecol Oncol. 2008;110:37–42. doi: 10.1016/j.ygyno.2008.01.040. [DOI] [PubMed] [Google Scholar]
- 15.Ramsaroop R, Oei P, Ng D, Kumar N, Cotter PD. Cervical intraepithelial neoplasia and aneusomy of TERC: assessment of liquid-based cytological preparations. Diagn Cytopathol. 2009;37:411–415. doi: 10.1002/dc.21007. [DOI] [PubMed] [Google Scholar]
- 16.Tu Z, Zhang A, Wu R, Jiang J, Li Y, Wulan N, Li J, Zhang Y, Li Y, Chen Z, Wei L. Genomic amplification of the human telomerase RNA gene for differential diagnosis of cervical disorders. Cancer Genet Cytogenet. 2009;191:10–16. doi: 10.1016/j.cancergencyto.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Jiang J, Tu Z, Zhang G, Li JR, Zhao LJ, Zhao C, Cui SH, Li XP, Chen Z, Wei LH. Evaluation of genomic amplification of the human telomerase RNA component gene in the screening of cervical lesions. Chin J Obstet Gynecol. 2008;43:849–853. [PubMed] [Google Scholar]
- 18.Buckley CH, Butler EB, Fox H. Cervical intraepithelial neoplasia. J Clin Pathol. 1982;35:1–13. doi: 10.1136/jcp.35.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 20.Ferreccio C, Bratti MC, Sherman ME, Herrero R, Wacholder S, Hildesheim A, Burk RD, Hutchinson M, Alfaro M, Greenberg MD, Morales J, Rodriguez AC, Schussler J, Eklund C, Marshall G, Schiffman M. A comparison of single and combined visual, cytologic, and virologic tests as screening strategies in a region at high risk of cervical cancer. Cancer Epidemiol Biomarkers Prev. 2003;12:815–823. [PubMed] [Google Scholar]
- 21.Heselmeyer-Haddad K, Janz V, Castle PE, Chaudhri N, White N, Wilber K, Morrison LE, Auer G, Burroughs FH, Sherman ME, Ried T. Detection of genomic amplification of the human telomerase gene (TERC) in cytologic specimens as a genetic test for the diagnosis of cervical dysplasia. Am J Pathol. 2003;163:1405–1416. doi: 10.1016/S0002-9440(10)63498-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.zur Hausen H. Papillomaviruses in the causation of human cancers - a brief historical account. Virology. 2009;384:260–265. doi: 10.1016/j.virol.2008.11.046. [DOI] [PubMed] [Google Scholar]
- 23.Malpica A, Matisic JP, Niekirk DV, Crum CP, Staerkel GA, Yamal JM, Guillaud MH, Cox DD, Atkinson EN, Adler-Storthz K, Poulin NM, Macaulay CA, Follen M. Kappa statistics to measure interrater and intrarater agreement for 1790 cervical biopsy specimens among twelve pathologists: qualitative histopathologic analysis and methodologic issues. Gynecol Oncol. 2005;99:S38–S52. doi: 10.1016/j.ygyno.2005.07.040. [DOI] [PubMed] [Google Scholar]
- 24.Andersson S, Sowjanya P, Wangsa D, Hjerpe A, Johansson B, Auer G, Gravitt PE, Larsson C, Wallin KL, Ried T, others Detection of genomic amplification of the human telomerase gene TERC, a potential marker for triage of women with HPV-positive, abnormal Pap smears. Am J Pathol. 2009;175:1831–1847. doi: 10.2353/ajpath.2009.090122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mayrand MH, Duarte-Franco E, Rodrigues I, Walter SD, Hanley J, Ferenczy A, Ratnam S, Coutlée F, Franco EL. Canadian Cervical Cancer Screening Trial Study Group: Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357:1579–1588. doi: 10.1056/NEJMoa071430. [DOI] [PubMed] [Google Scholar]
- 26.Alameda F, Espinet B, Corzo C, Munoz R, Bellosillo B, Lloveras B, Pijuan L, Gimeno J, Salido M, Solé F, Carreras R, Serrano S. 3q26 (hTERC) gain studied by fluorescence in situ hybridization as a persistence-progression indicator in low-grade squamous intraepithelial lesion cases. Hum Pathol. 2009;40:1474–1478. doi: 10.1016/j.humpath.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 27.Jalali GR, Herzog TJ, Dziura B, Walat R, Kilpatrick MW. Amplification of the chromosome 3q26 region shows high negative predictive value for nonmalignant transformation of LSIL cytologic finding. Am J Obstet Gynecol. 2010;202:581.e1–581.e5. doi: 10.1016/j.ajog.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 28.Solomon D, Schiffman M, Tarone R, ALTS Study group Comparison of three management strategies for patients with atypical squamous cells of undetermined significance: baseline results from a randomized trial. J Natl Cancer Inst. 2001;93:293–299. doi: 10.1093/jnci/93.4.293. [DOI] [PubMed] [Google Scholar]
- 29.Sherman ME, Schiffman M, Cox JT. Atypical Squamous Cells of Undetermined Significance/Low-Grade Squamous Intraepithelial Lesion Triage Study Group. Effects of age and human papilloma viral load on colposcopy triage: data from the randomized Atypical Squamous Cells of Undetermined Significance/Low-Grade Squamous Intraepithelial Lesion Triage Study (ALTS) J Natl Cancer Inst. 2002;94:102–107. doi: 10.1093/jnci/94.2.102. [DOI] [PubMed] [Google Scholar]
- 30.Wright TC, Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D. 2006 American Society for Colposcopy and Cervical Pathology-sponsored Consensus Conference: 2006 consensus guidelines for the management of women with cervical intraepithelial neoplasia or adenocarcinoma in situ. Am J Obstet Gynecol. 2007;197:340–345. doi: 10.1016/j.ajog.2007.07.050. [DOI] [PubMed] [Google Scholar]
- 31.Cox JT, Schiffman M, Solomon D. ASCUS-LSIL Triage Study (ALTS) Group: Prospective follow-up suggests similar risk of subsequent cervical intraepithelial neoplasia grade 2 or 3 among women with cervical intraepithelial neoplasia grade 1 or negative colposcopy and directed biopsy. Am J Obstet Gynecol. 2003;188:1406–1412. doi: 10.1067/mob.2003.461. [DOI] [PubMed] [Google Scholar]
- 32.Shlay JC, Dunn T, Byers T, Barón AE, Douglas JM., Jr Prediction of cervical intraepithelial neoplasia grade 2–3 using risk assessment and human papillomavirus testing in women with atypia on papanicolaou smears. Obstet Gynecol. 2000;96:410–416. doi: 10.1016/s0029-7844(00)00907-8. [DOI] [PubMed] [Google Scholar]
- 33.Wu S, Meng L, Wang S, Ma D. A comparison of four screening methods for cervical neoplasia. Int J Gynaecol Obstet. 2005;91:189–193. doi: 10.1016/j.ijgo.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 34.Bhatla N, Dar L, Patro AR, Kumar P, Kriplani A, Gulati A, Iyer VK, Mathur SR, Sreenivas V, Shah KV, Gravitt PE. Can human papillomavirus DNA testing of self-collected vaginal samples compare with physician-collected cervical samples and cytology for cervical cancer screening in developing countries? Cancer Epidemiol. 2009;33:446–450. doi: 10.1016/j.canep.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]


