Abstract
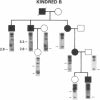
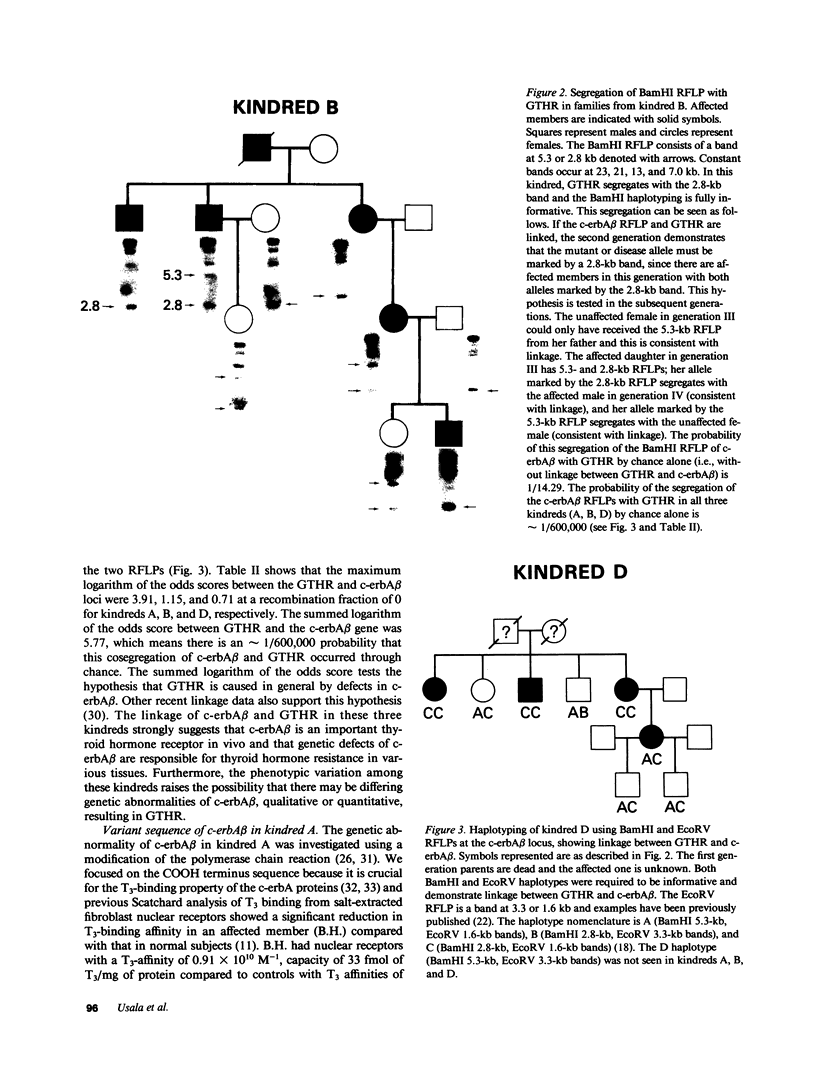
Generalized thyroid hormone resistance (GTHR) is a disorder of thyroid hormone action that we have previously shown to be tightly linked to one of the two thyroid hormone receptor genes, c-erbA beta, in a single kindred, A. We now show that in two other kindreds, B and D, with differing phenotypes, there is also linkage between c-erbA beta and GTHR. The combined maximum logarithm of the odds score for all three kindreds at a recombination fraction of 0 was 5.77. In vivo studies had shown a triiodothyronine (T3)-binding affinity abnormality in nuclear receptors of kindred A, and we therefore investigated the defect in c-erbA beta in this kindred by sequencing a major portion of the T3-binding domain in the 3'-region of fibroblast c-erbA beta cDNA and leukocyte c-erbA beta genomic DNA. A base substitution, cytosine to adenine, was found at cDNA position 1643 which altered the proline codon at position 448 to a histidine. By allelic-specific hybridization, this base substitution was found in only one allele of seven affected members, and not found in 10 unaffected members of kindred A, as expected for a dominant disease. Also, this altered base was not found in kindreds B or D, or in 92 random c-erbA beta alleles. These results and the fact that the mutation is predicted to alter the secondary structure of the crucial T3-binding domain of the c-erbA beta receptor suggest this mutation is an excellent candidate for the genetic cause of GTHR in kindred A. Different mutations in the c-erbA beta gene are likely responsible for the variant phenotypes of thyroid hormone resistance in kindreds B and D.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Accili D., Frapier C., Mosthaf L., McKeon C., Elbein S. C., Permutt M. A., Ramos E., Lander E., Ullrich A., Taylor S. I. A mutation in the insulin receptor gene that impairs transport of the receptor to the plasma membrane and causes insulin-resistant diabetes. EMBO J. 1989 Sep;8(9):2509–2517. doi: 10.1002/j.1460-2075.1989.tb08388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bercu B. B., Haupt R., Johnsonbaugh R., Rodbard D. The pulse wave arrival time (QKd interval) in normal children. J Pediatr. 1979 Nov;95(5 Pt 1):716–721. doi: 10.1016/s0022-3476(79)80717-9. [DOI] [PubMed] [Google Scholar]
- Bradley D. J., Young W. S., 3rd, Weinberger C. Differential expression of alpha and beta thyroid hormone receptor genes in rat brain and pituitary. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7250–7254. doi: 10.1073/pnas.86.18.7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T. R., Lubahn D. B., Wilson E. M., Joseph D. R., French F. S., Migeon C. J. Deletion of the steroid-binding domain of the human androgen receptor gene in one family with complete androgen insensitivity syndrome: evidence for further genetic heterogeneity in this syndrome. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8151–8155. doi: 10.1073/pnas.85.21.8151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Qasba P. K. Alkaline transfer of DNA to plastic membrane. Biochem Biophys Res Commun. 1984 Jul 18;122(1):340–344. doi: 10.1016/0006-291x(84)90480-7. [DOI] [PubMed] [Google Scholar]
- Damm K., Thompson C. C., Evans R. M. Protein encoded by v-erbA functions as a thyroid-hormone receptor antagonist. Nature. 1989 Jun 22;339(6226):593–597. doi: 10.1038/339593a0. [DOI] [PubMed] [Google Scholar]
- Drabkin H., Kao F. T., Hartz J., Hart I., Gazdar A., Weinberger C., Evans R., Gerber M. Localization of human ERBA2 to the 3p22----3p24.1 region of chromosome 3 and variable deletion in small cell lung cancer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9258–9262. doi: 10.1073/pnas.85.23.9258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman B. M., Yang C. R., Au M., Casanova J., Ghysdael J., Samuels H. H. A domain containing leucine-zipper-like motifs mediate novel in vivo interactions between the thyroid hormone and retinoic acid receptors. Mol Endocrinol. 1989 Oct;3(10):1610–1626. doi: 10.1210/mend-3-10-1610. [DOI] [PubMed] [Google Scholar]
- Gershengorn M. C., Weintraub B. D. Thyrotropin-induced hyperthyroidism caused by selective pituitary resistance to thyroid hormone. A new syndrome of "inappropriate secretion of TSH". J Clin Invest. 1975 Sep;56(3):633–642. doi: 10.1172/JCI108133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskowitz I. Functional inactivation of genes by dominant negative mutations. Nature. 1987 Sep 17;329(6136):219–222. doi: 10.1038/329219a0. [DOI] [PubMed] [Google Scholar]
- Horowitz Z. D., Yang C. R., Forman B. M., Casanova J., Samuels H. H. Characterization of the domain structure of chick c-erbA by deletion mutation: in vitro translation and cell transfection studies. Mol Endocrinol. 1989 Jan;3(1):148–156. doi: 10.1210/mend-3-1-148. [DOI] [PubMed] [Google Scholar]
- Hughes M. R., Malloy P. J., Kieback D. G., Kesterson R. A., Pike J. W., Feldman D., O'Malley B. W. Point mutations in the human vitamin D receptor gene associated with hypocalcemic rickets. Science. 1988 Dec 23;242(4886):1702–1705. doi: 10.1126/science.2849209. [DOI] [PubMed] [Google Scholar]
- Ichikawa K., Hughes I. A., Horwitz A. L., DeGroot L. J. Characterization of nuclear thyroid hormone receptors of cultured skin fibroblasts from patients with resistance to thyroid hormone. Metabolism. 1987 Apr;36(4):392–399. doi: 10.1016/0026-0495(87)90214-9. [DOI] [PubMed] [Google Scholar]
- Izumo S., Mahdavi V. Thyroid hormone receptor alpha isoforms generated by alternative splicing differentially activate myosin HC gene transcription. Nature. 1988 Aug 11;334(6182):539–542. doi: 10.1038/334539a0. [DOI] [PubMed] [Google Scholar]
- Kaplan M. M., Swartz S. L., Larsen P. R. Partial peripheral resistance to thyroid hormone. Am J Med. 1981 May;70(5):1115–1121. doi: 10.1016/0002-9343(81)90885-8. [DOI] [PubMed] [Google Scholar]
- Koenig R. J., Lazar M. A., Hodin R. A., Brent G. A., Larsen P. R., Chin W. W., Moore D. D. Inhibition of thyroid hormone action by a non-hormone binding c-erbA protein generated by alternative mRNA splicing. Nature. 1989 Feb 16;337(6208):659–661. doi: 10.1038/337659a0. [DOI] [PubMed] [Google Scholar]
- Koenig R. J., Warne R. L., Brent G. A., Harney J. W., Larsen P. R., Moore D. D. Isolation of a cDNA clone encoding a biologically active thyroid hormone receptor. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5031–5035. doi: 10.1073/pnas.85.14.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V., Chambon P. The estrogen receptor binds tightly to its responsive element as a ligand-induced homodimer. Cell. 1988 Oct 7;55(1):145–156. doi: 10.1016/0092-8674(88)90017-7. [DOI] [PubMed] [Google Scholar]
- Lazar M. A., Hodin R. A., Darling D. S., Chin W. W. Identification of a rat c-erbA alpha-related protein which binds deoxyribonucleic acid but does not bind thyroid hormone. Mol Endocrinol. 1988 Oct;2(10):893–901. doi: 10.1210/mend-2-10-893. [DOI] [PubMed] [Google Scholar]
- Magner J. A., Petrick P., Menezes-Ferreira M. M., Stelling M., Weintraub B. D. Familial generalized resistance to thyroid hormones: report of three kindreds and correlation of patterns of affected tissues with the binding of [125I] triiodothyronine to fibroblast nuclei. J Endocrinol Invest. 1986 Dec;9(6):459–470. doi: 10.1007/BF03346968. [DOI] [PubMed] [Google Scholar]
- Menezes-Ferreira M. M., Eil C., Wortsman J., Weintraub B. D. Decreased nuclear uptake of [125I]triiodo-L-thyronine in fibroblasts from patients with peripheral thyroid hormone resistance. J Clin Endocrinol Metab. 1984 Dec;59(6):1081–1087. doi: 10.1210/jcem-59-6-1081. [DOI] [PubMed] [Google Scholar]
- Mitsuhashi T., Tennyson G. E., Nikodem V. M. Alternative splicing generates messages encoding rat c-erbA proteins that do not bind thyroid hormone. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5804–5808. doi: 10.1073/pnas.85.16.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller D. E., Flier J. S. Detection of an alteration in the insulin-receptor gene in a patient with insulin resistance, acanthosis nigricans, and the polycystic ovary syndrome (type A insulin resistance). N Engl J Med. 1988 Dec 8;319(23):1526–1529. doi: 10.1056/NEJM198812083192306. [DOI] [PubMed] [Google Scholar]
- Muñoz A., Zenke M., Gehring U., Sap J., Beug H., Vennström B. Characterization of the hormone-binding domain of the chicken c-erbA/thyroid hormone receptor protein. EMBO J. 1988 Jan;7(1):155–159. doi: 10.1002/j.1460-2075.1988.tb02795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer J. H. Thyroid hormone action at the cellular level. Science. 1979 Mar 9;203(4384):971–979. doi: 10.1126/science.218285. [DOI] [PubMed] [Google Scholar]
- Ott J. A computer program for linkage analysis of general human pedigrees. Am J Hum Genet. 1976 Sep;28(5):528–529. [PMC free article] [PubMed] [Google Scholar]
- Refetoff S., DeWind L. T., DeGroot L. J. Familial syndrome combining deaf-mutism, stuppled epiphyses, goiter and abnormally high PBI: possible target organ refractoriness to thyroid hormone. J Clin Endocrinol Metab. 1967 Feb;27(2):279–294. doi: 10.1210/jcem-27-2-279. [DOI] [PubMed] [Google Scholar]
- Refetoff S. Syndromes of thyroid hormone resistance. Am J Physiol. 1982 Aug;243(2):E88–E98. doi: 10.1152/ajpendo.1982.243.2.E88. [DOI] [PubMed] [Google Scholar]
- Rösler A., Litvin Y., Hage C., Gross J., Cerasi E. Familial hyperthyroidism due to inappropriate thyrotropin secretion successfully treated with triiodothyronine. J Clin Endocrinol Metab. 1982 Jan;54(1):76–82. doi: 10.1210/jcem-54-1-76. [DOI] [PubMed] [Google Scholar]
- Sakurai A., Nakai A., DeGroot L. J. Expression of three forms of thyroid hormone receptor in human tissues. Mol Endocrinol. 1989 Feb;3(2):392–399. doi: 10.1210/mend-3-2-392. [DOI] [PubMed] [Google Scholar]
- Samuels H. H., Forman B. M., Horowitz Z. D., Ye Z. S. Regulation of gene expression by thyroid hormone. J Clin Invest. 1988 Apr;81(4):957–967. doi: 10.1172/JCI113449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sap J., Muñoz A., Damm K., Goldberg Y., Ghysdael J., Leutz A., Beug H., Vennström B. The c-erb-A protein is a high-affinity receptor for thyroid hormone. Nature. 1986 Dec 18;324(6098):635–640. doi: 10.1038/324635a0. [DOI] [PubMed] [Google Scholar]
- Sheer D., Sheppard D. M., le Beau M., Rowley J. D., San Roman C., Solomon E. Localization of the oncogene c-erbA1 immediately proximal to the acute promyelocytic leukaemia breakpoint on chromosome 17. Ann Hum Genet. 1985 Jul;49(Pt 3):167–171. doi: 10.1111/j.1469-1809.1985.tb01690.x. [DOI] [PubMed] [Google Scholar]
- Tennyson G. E., Sabatos C. A., Higuchi K., Meglin N., Brewer H. B., Jr Expression of apolipoprotein B mRNAs encoding higher- and lower-molecular weight isoproteins in rat liver and intestine. Proc Natl Acad Sci U S A. 1989 Jan;86(2):500–504. doi: 10.1073/pnas.86.2.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. C., Evans R. M. Trans-activation by thyroid hormone receptors: functional parallels with steroid hormone receptors. Proc Natl Acad Sci U S A. 1989 May;86(10):3494–3498. doi: 10.1073/pnas.86.10.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. C., Weinberger C., Lebo R., Evans R. M. Identification of a novel thyroid hormone receptor expressed in the mammalian central nervous system. Science. 1987 Sep 25;237(4822):1610–1614. doi: 10.1126/science.3629259. [DOI] [PubMed] [Google Scholar]
- Tsai S. Y., Carlstedt-Duke J., Weigel N. L., Dahlman K., Gustafsson J. A., Tsai M. J., O'Malley B. W. Molecular interactions of steroid hormone receptor with its enhancer element: evidence for receptor dimer formation. Cell. 1988 Oct 21;55(2):361–369. doi: 10.1016/0092-8674(88)90059-1. [DOI] [PubMed] [Google Scholar]
- Usala S. J., Bale A. E., Gesundheit N., Weinberger C., Lash R. W., Wondisford F. E., McBride O. W., Weintraub B. D. Tight linkage between the syndrome of generalized thyroid hormone resistance and the human c-erbA beta gene. Mol Endocrinol. 1988 Dec;2(12):1217–1220. doi: 10.1210/mend-2-12-1217. [DOI] [PubMed] [Google Scholar]
- Weinberger C., Thompson C. C., Ong E. S., Lebo R., Gruol D. J., Evans R. M. The c-erb-A gene encodes a thyroid hormone receptor. Nature. 1986 Dec 18;324(6098):641–646. doi: 10.1038/324641a0. [DOI] [PubMed] [Google Scholar]