Abstract
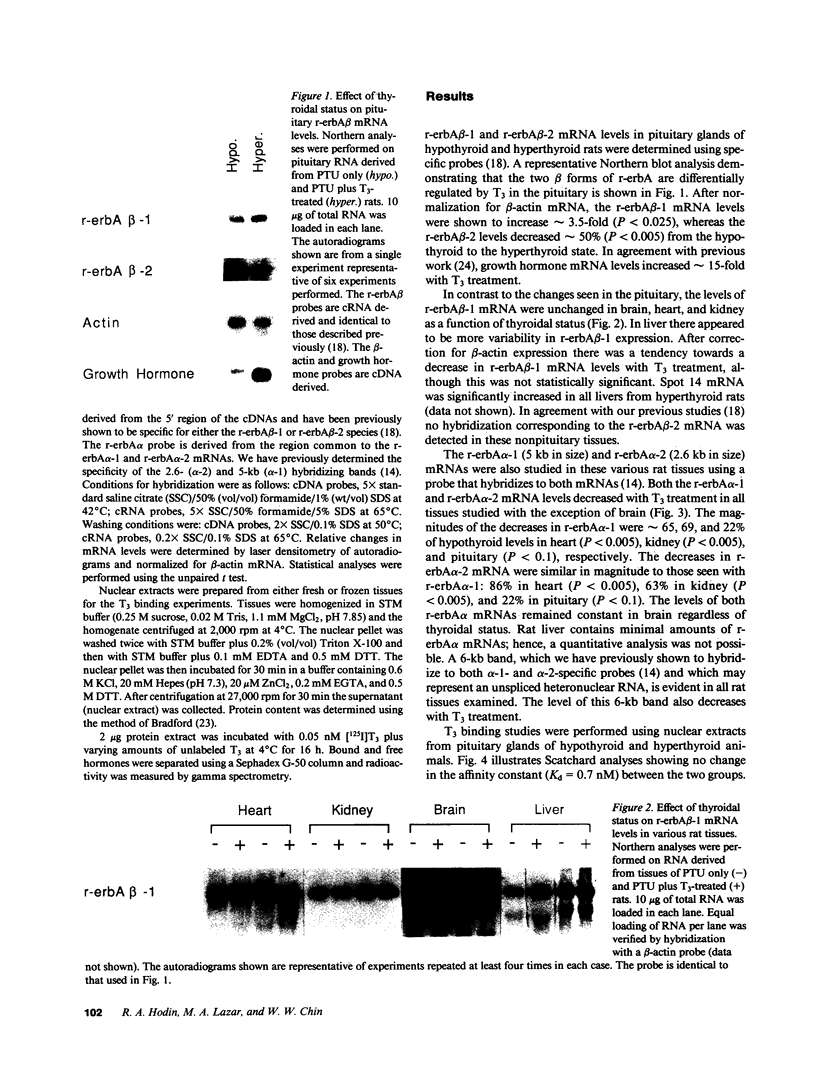
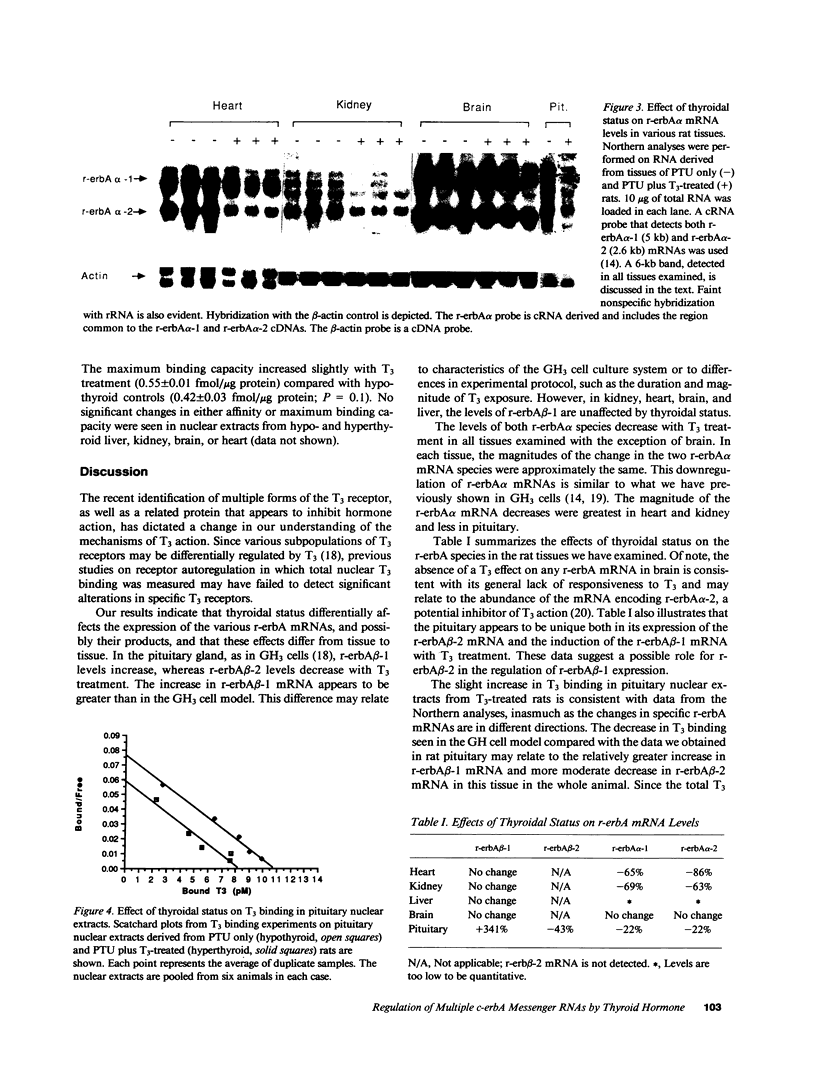
Thyroid hormone (T3) has been shown to regulate the level of its receptor in a number of tissues and cell lines. Recently, proteins encoded by the protooncogene c-erbA have been identified as T3 receptors. In the rat, four c-erbA gene products have been isolated, three of which, r-erbA alpha-1, r-erbA beta-1, and r-erbA beta-2, encode biologically active T3 receptors; the fourth, r-erbA alpha-2, may play an inhibitory role in T3 action. The present work examines the molecular nature of T3 receptor autoregulation using probes specific for each c-erbA mRNA. Rats were rendered hypothyroid with propylthiouracil and then treated with either saline or T3. Northern blot analyses reveal marked tissue-specific and differential regulation of the multiple c-erbA mRNAs by T3. In the pituitary the levels of r-erbA beta-1 mRNA increase, whereas the levels of the pituitary-specific r-erbA beta-2 mRNA decrease with T3 treatment. In heart, kidney, liver, and brain the levels of r-erbA beta-1 are unaffected by thyroidal status. The levels of both r-erbA alpha mRNAs decrease with T3 treatment in all tissues examined except for the brain, where there is no change. In addition, we find that changes in the mRNAs encoding specific subpopulations of T3 receptors do not always parallel changes in total nuclear T3 binding. Differential regulation of the specific c-erbA mRNA species could have important consequences for T3 action.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benbrook D., Pfahl M. A novel thyroid hormone receptor encoded by a cDNA clone from a human testis library. Science. 1987 Nov 6;238(4828):788–791. doi: 10.1126/science.3672126. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Franklyn J. A., Ramsden D. B., Sheppard M. C. Down-regulation of nuclear T3 receptors by thyroid hormones in the rat anterior pituitary. Mol Cell Endocrinol. 1985 May;40(2-3):145–148. doi: 10.1016/0303-7207(85)90169-8. [DOI] [PubMed] [Google Scholar]
- Hamada S., Nakamura H., Nanno M., Imura H. Tri-iodothyronine-induced increase in rat liver nuclear thyroid-hormone receptors associated with increased mitochondrial alpha-glycerophosphate dehydrogenase activity. Biochem J. 1979 Aug 15;182(2):371–375. doi: 10.1042/bj1820371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervas F., Morreale de Escobar G., Escobar Del Rey F. Rapid effects of single small doses of L-thyroxine and triiodo-L-thyronine on growth hormone, as studied in the rat by radioimmunoassy. Endocrinology. 1975 Jul;97(1):91–101. doi: 10.1210/endo-97-1-91. [DOI] [PubMed] [Google Scholar]
- Hodin R. A., Lazar M. A., Wintman B. I., Darling D. S., Koenig R. J., Larsen P. R., Moore D. D., Chin W. W. Identification of a thyroid hormone receptor that is pituitary-specific. Science. 1989 Apr 7;244(4900):76–79. doi: 10.1126/science.2539642. [DOI] [PubMed] [Google Scholar]
- Izumo S., Mahdavi V. Thyroid hormone receptor alpha isoforms generated by alternative splicing differentially activate myosin HC gene transcription. Nature. 1988 Aug 11;334(6182):539–542. doi: 10.1038/334539a0. [DOI] [PubMed] [Google Scholar]
- Kalinyak J. E., Dorin R. I., Hoffman A. R., Perlman A. J. Tissue-specific regulation of glucocorticoid receptor mRNA by dexamethasone. J Biol Chem. 1987 Aug 5;262(22):10441–10444. [PubMed] [Google Scholar]
- Koenig R. J., Lazar M. A., Hodin R. A., Brent G. A., Larsen P. R., Chin W. W., Moore D. D. Inhibition of thyroid hormone action by a non-hormone binding c-erbA protein generated by alternative mRNA splicing. Nature. 1989 Feb 16;337(6208):659–661. doi: 10.1038/337659a0. [DOI] [PubMed] [Google Scholar]
- Koenig R. J., Warne R. L., Brent G. A., Harney J. W., Larsen P. R., Moore D. D. Isolation of a cDNA clone encoding a biologically active thyroid hormone receptor. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5031–5035. doi: 10.1073/pnas.85.14.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar M. A., Chin W. W. Regulation of two c-erbA messenger ribonucleic acids in rat GH3 cells by thyroid hormone. Mol Endocrinol. 1988 Jun;2(6):479–484. doi: 10.1210/mend-2-6-479. [DOI] [PubMed] [Google Scholar]
- Lazar M. A., Hodin R. A., Darling D. S., Chin W. W. Identification of a rat c-erbA alpha-related protein which binds deoxyribonucleic acid but does not bind thyroid hormone. Mol Endocrinol. 1988 Oct;2(10):893–901. doi: 10.1210/mend-2-10-893. [DOI] [PubMed] [Google Scholar]
- McDonnell D. P., Mangelsdorf D. J., Pike J. W., Haussler M. R., O'Malley B. W. Molecular cloning of complementary DNA encoding the avian receptor for vitamin D. Science. 1987 Mar 6;235(4793):1214–1217. doi: 10.1126/science.3029866. [DOI] [PubMed] [Google Scholar]
- Mitsuhashi T., Tennyson G. E., Nikodem V. M. Alternative splicing generates messages encoding rat c-erbA proteins that do not bind thyroid hormone. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5804–5808. doi: 10.1073/pnas.85.16.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M. B., Zilz N. D., McCreary N. L., MacDonald M. J., Towle H. C. Isolation and characterization of rat cDNA clones for two distinct thyroid hormone receptors. J Biol Chem. 1988 Sep 5;263(25):12770–12777. [PubMed] [Google Scholar]
- Murthy P. V., Banovac K., McKenzie J. M. Hypothyroidism-induced changes in triiodothyronine binding to nuclei and cytosol-binding proteins in rat liver. Endocrinology. 1978 Apr;102(4):1129–1136. doi: 10.1210/endo-102-4-1129. [DOI] [PubMed] [Google Scholar]
- Nakai A., Seino S., Sakurai A., Szilak I., Bell G. I., DeGroot L. J. Characterization of a thyroid hormone receptor expressed in human kidney and other tissues. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2781–2785. doi: 10.1073/pnas.85.8.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okret S., Poellinger L., Dong Y., Gustafsson J. A. Down-regulation of glucocorticoid receptor mRNA by glucocorticoid hormones and recognition by the receptor of a specific binding sequence within a receptor cDNA clone. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5899–5903. doi: 10.1073/pnas.83.16.5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saceda M., Lippman M. E., Chambon P., Lindsey R. L., Ponglikitmongkol M., Puente M., Martin M. B. Regulation of the estrogen receptor in MCF-7 cells by estradiol. Mol Endocrinol. 1988 Dec;2(12):1157–1162. doi: 10.1210/mend-2-12-1157. [DOI] [PubMed] [Google Scholar]
- Samuels H. H., Stanley F., Shapiro L. E. Dose-dependent depletion of nuclear receptors by L-triiodothyronine: evidence for a role in induction of growth hormone synthesis in cultured GH1 cells. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3877–3881. doi: 10.1073/pnas.73.11.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels H. H., Stanley F., Shapiro L. E. Modulation of thyroid hormone nuclear receptor levels by 3,5,3'-triiodo-L-thyronine in GH1 cells. Evidence for two functional components of nuclear-bound receptor and relationship to the induction of growth hormone synthesis. J Biol Chem. 1977 Sep 10;252(17):6052–6060. [PubMed] [Google Scholar]
- Sap J., Muñoz A., Damm K., Goldberg Y., Ghysdael J., Leutz A., Beug H., Vennström B. The c-erb-A protein is a high-affinity receptor for thyroid hormone. Nature. 1986 Dec 18;324(6098):635–640. doi: 10.1038/324635a0. [DOI] [PubMed] [Google Scholar]
- Schussler G. C., Orlando J. Fasting decreases triiodothyronine receptor capacity. Science. 1978 Feb 10;199(4329):686–688. doi: 10.1126/science.204004. [DOI] [PubMed] [Google Scholar]
- Shupnik M. A., Gordon M. S., Chin W. W. Tissue-specific regulation of rat estrogen receptor mRNAs. Mol Endocrinol. 1989 Apr;3(4):660–665. doi: 10.1210/mend-3-4-660. [DOI] [PubMed] [Google Scholar]
- Silva J. E., Larsen P. R. Contributions of plasma triiodothyronine and local thyroxine monodeiodination to triiodothyronine to nuclear triiodothyronine receptor saturation in pituitary, liver, and kidney of hypothyroid rats. Further evidence relating saturation of pituitary nuclear triiodothyronine receptors and the acute inhibition of thyroid-stimulating hormone release. J Clin Invest. 1978 May;61(5):1247–1259. doi: 10.1172/JCI109041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svec F. Glucocorticoid receptor regulation. Life Sci. 1985 Jun 24;36(25):2359–2366. doi: 10.1016/0024-3205(85)90339-x. [DOI] [PubMed] [Google Scholar]
- Thompson C. C., Weinberger C., Lebo R., Evans R. M. Identification of a novel thyroid hormone receptor expressed in the mammalian central nervous system. Science. 1987 Sep 25;237(4822):1610–1614. doi: 10.1126/science.3629259. [DOI] [PubMed] [Google Scholar]
- Weinberger C., Thompson C. C., Ong E. S., Lebo R., Gruol D. J., Evans R. M. The c-erb-A gene encodes a thyroid hormone receptor. Nature. 1986 Dec 18;324(6098):641–646. doi: 10.1038/324641a0. [DOI] [PubMed] [Google Scholar]
- de The H., Marchio A., Tiollais P., Dejean A. Differential expression and ligand regulation of the retinoic acid receptor alpha and beta genes. EMBO J. 1989 Feb;8(2):429–433. doi: 10.1002/j.1460-2075.1989.tb03394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Overbeck K., Lemarchand-Béraud T. Modulation of thyroid hormone nuclear receptor levels by L-triiodothyronine (T3) in the rat pituitary. Mol Cell Endocrinol. 1983 Dec;33(2-3):281–292. doi: 10.1016/0303-7207(83)90173-9. [DOI] [PubMed] [Google Scholar]






