Abstract
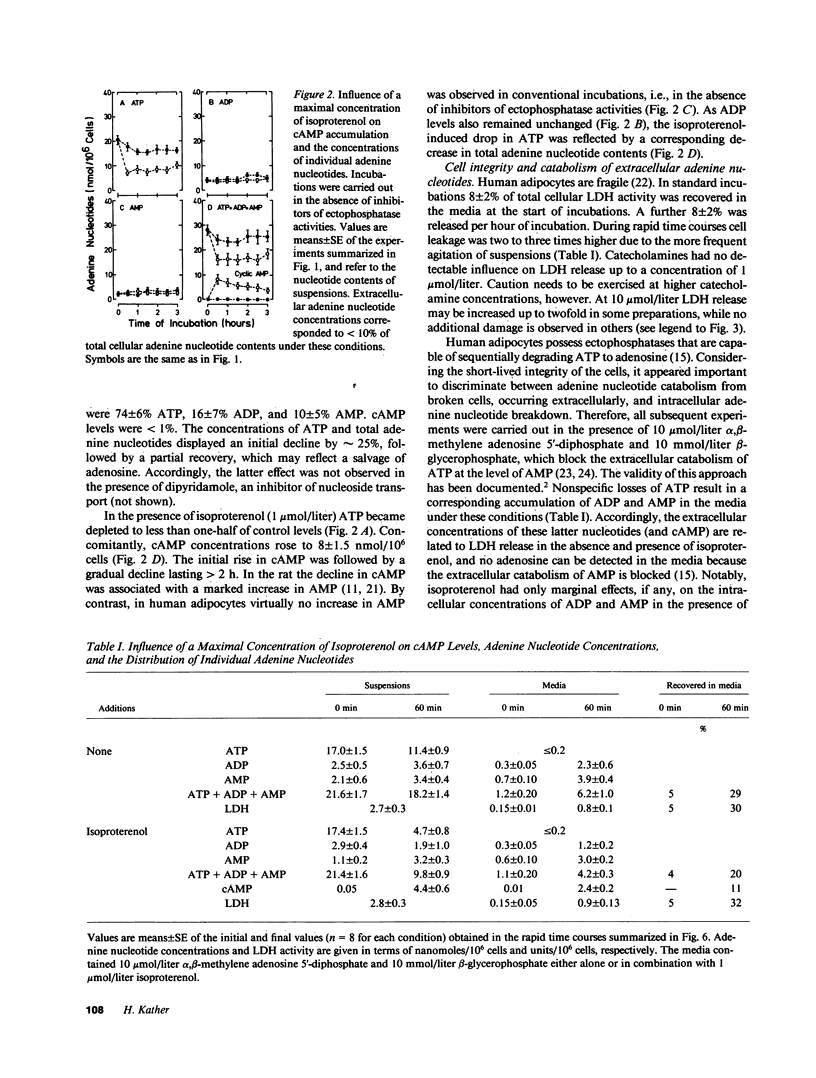
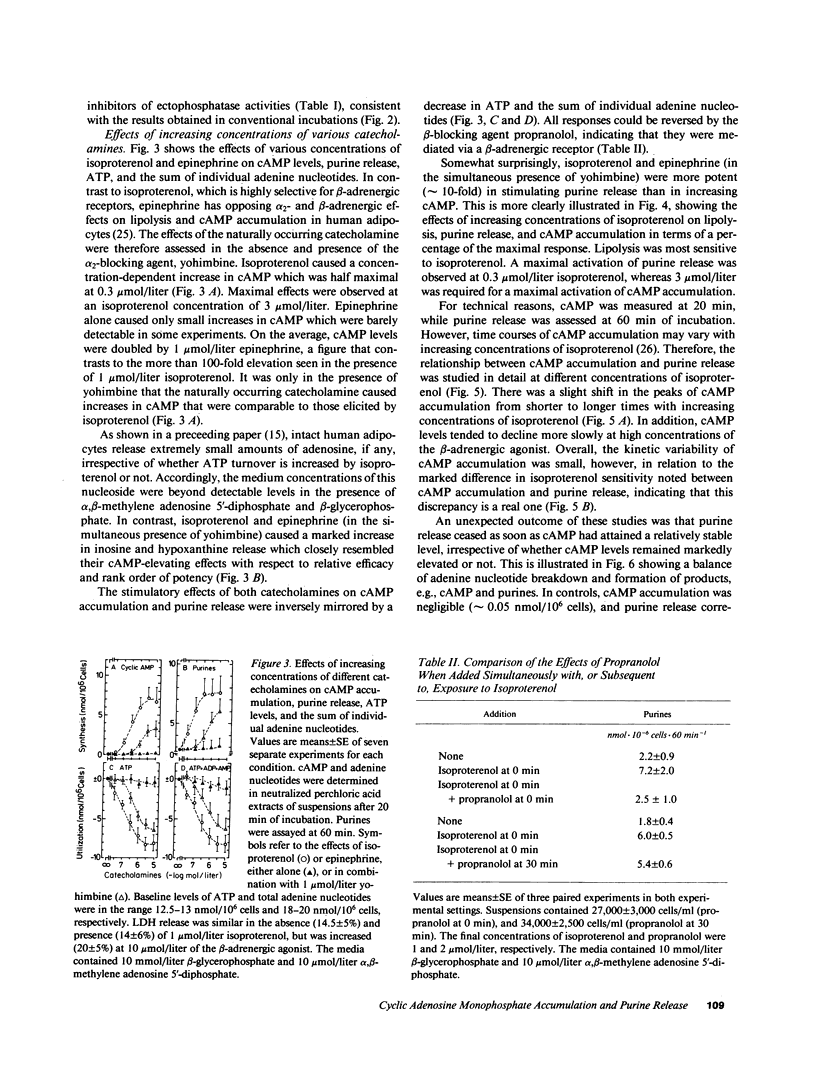
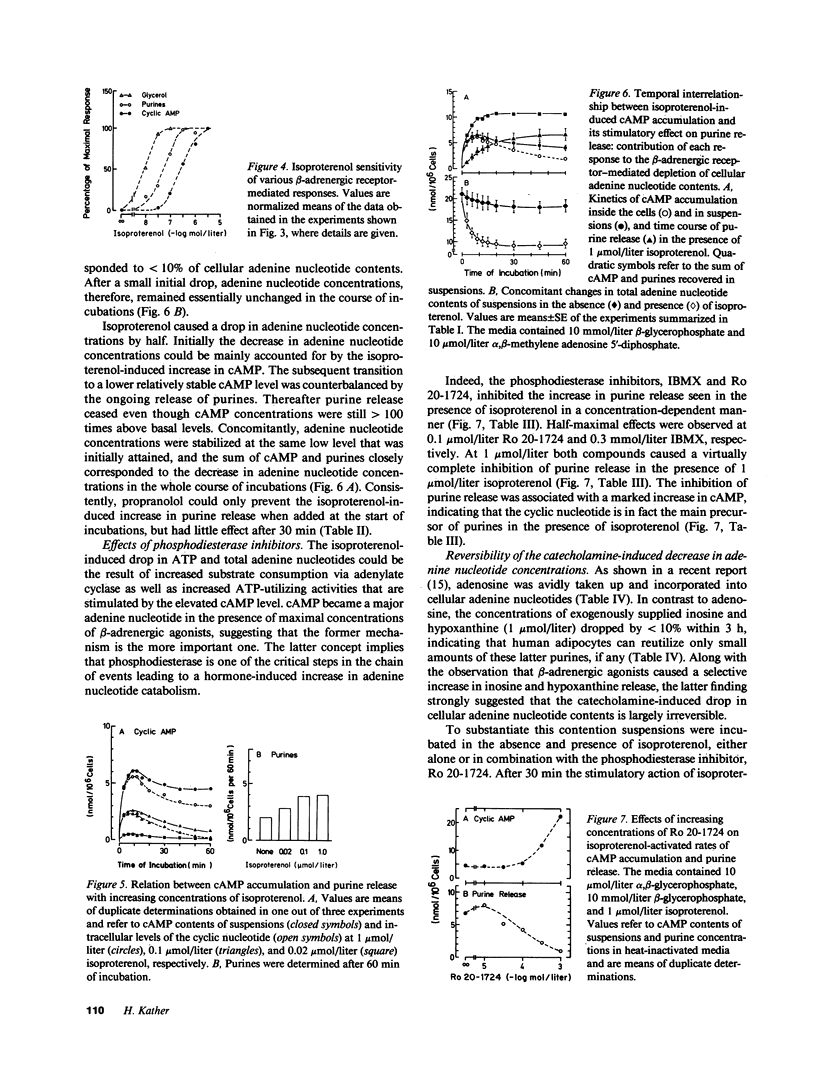
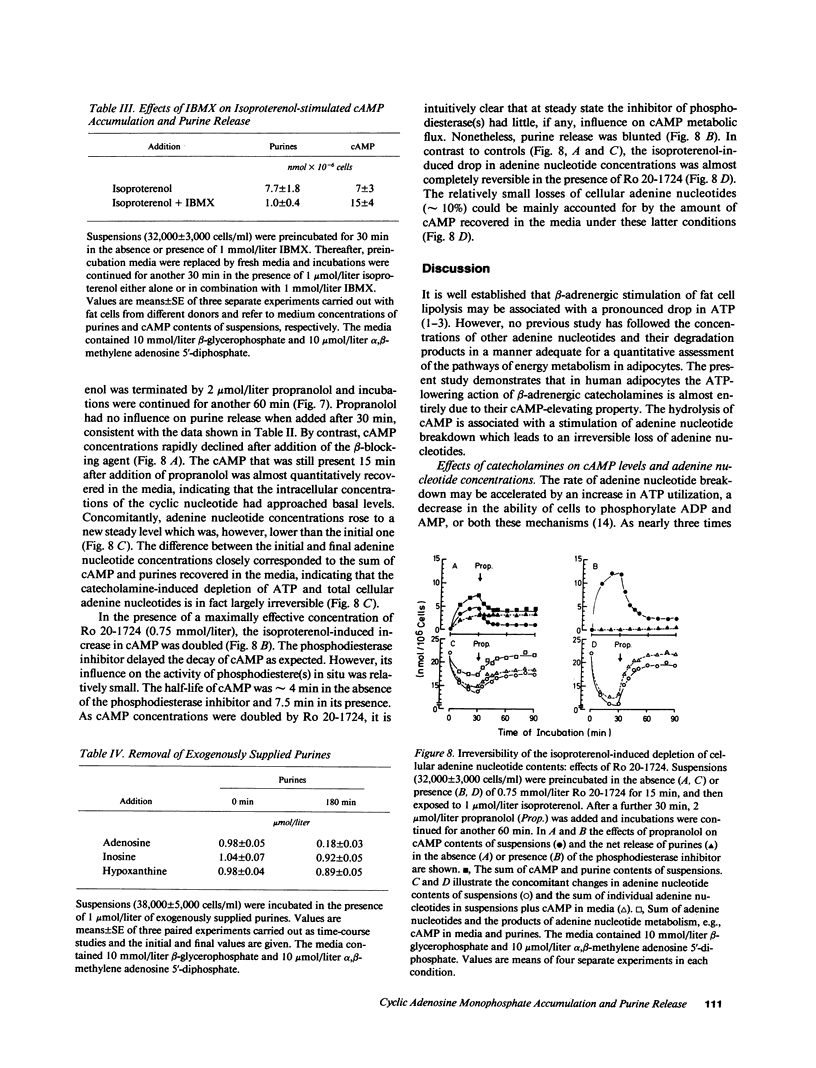
The effects of beta-adrenergic agonists on ATP utilization and adenine nucleotide breakdown in human adipocytes were examined. The catecholamine-induced increase in cAMP was associated with an enhancement of adenine nucleotide catabolism resulting in an increase in release of inosine and hypoxanthine which can not be reutilized for adenine nucleotide synthesis. Therefore, one-third of total cellular adenine nucleotides were irreversibly lost in the presence of 1 mumol/liter isoproterenol. The catecholamine-induced increase in purine release could be blocked by phosphodiesterase inhibitors, suggesting that cAMP is the main precursor of purines in the presence of beta-adrenergic agonists. However, epinephrine (in the simultaneous presence of the alpha 2-adrenergic blocking agent, yohimbine) and isoproterenol were 10 times more potent in stimulating purine release than in elevating cAMP. In addition, purine release ceased when cAMP was still markedly increased, suggesting a compartmentation of the cyclic nucleotide and/or involvement of the hormone-sensitive, low Km cAMP phosphodiesterase. The results document that white fat cells have an enormous potential for dissipating energy, and demonstrate that the pathway involving cAMP formation and hydrolysis constitutes the principle route of adenine nucleotide catabolism in the presence of beta-adrenergic agonists.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angel A., Desai K. S., Halperin M. L. Reduction in adipocyte ATP by lipolytic agents: relation to intracellular free fatty acid accumulation. J Lipid Res. 1971 Mar;12(2):203–213. [PubMed] [Google Scholar]
- Arch J. R., Ainsworth A. T., Cawthorne M. A., Piercy V., Sennitt M. V., Thody V. E., Wilson C., Wilson S. Atypical beta-adrenoceptor on brown adipocytes as target for anti-obesity drugs. Nature. 1984 May 10;309(5964):163–165. doi: 10.1038/309163a0. [DOI] [PubMed] [Google Scholar]
- Barber R., Goka T. J. Hypothesis: cyclic AMP turnover in S49 cells. J Cyclic Nucleotide Res. 1981;7(6):353–361. [PubMed] [Google Scholar]
- Beavo J. A. Multiple isozymes of cyclic nucleotide phosphodiesterase. Adv Second Messenger Phosphoprotein Res. 1988;22:1–38. [PubMed] [Google Scholar]
- Brooks B., Arch J. R., Newsholme E. A. Effects of hormones on the rate of the triacylglycerol/fatty acid substrate cycle in adipocytes and epididymal fat pads. FEBS Lett. 1982 Sep 20;146(2):327–330. doi: 10.1016/0014-5793(82)80945-9. [DOI] [PubMed] [Google Scholar]
- Butcher R. W. Phosphodiesterase after twenty years: an introduction. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;16:1–12. [PubMed] [Google Scholar]
- Chung F. Z., Weber H. W., Appleman M. M. Extensive but reversible depletion of ATP via adenylate cyclase in rat adipocytes. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1614–1617. doi: 10.1073/pnas.82.6.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb C. E., Beth A. H., Corbin J. D. Purification and characterization of an inactive form of cAMP-dependent protein kinase containing bound cAMP. J Biol Chem. 1987 Dec 5;262(34):16566–16574. [PubMed] [Google Scholar]
- Degerman E., Belfrage P., Newman A. H., Rice K. C., Manganiello V. C. Purification of the putative hormone-sensitive cyclic AMP phosphodiesterase from rat adipose tissue using a derivative of cilostamide as a novel affinity ligand. J Biol Chem. 1987 Apr 25;262(12):5797–5807. [PubMed] [Google Scholar]
- Engfeldt P., Arner P., Ostman J. Studies on the regulation of phosphodiesterase in human adipose tissue in vitro. J Clin Endocrinol Metab. 1983 Mar;56(3):501–506. doi: 10.1210/jcem-56-3-501. [DOI] [PubMed] [Google Scholar]
- Fain J. N. Effect of lipolytic agents on adenosine and AMP formation by fat cells. Biochim Biophys Acta. 1979 Jun 21;573(3):510–520. doi: 10.1016/0005-2760(79)90225-x. [DOI] [PubMed] [Google Scholar]
- Fain J. N., Shepherd R. E. Free fatty acids as feedback regulators of adenylate cyclase and cyclic 3':5'-AMP accumulation in rat fat cells. J Biol Chem. 1975 Aug 25;250(16):6586–6592. [PubMed] [Google Scholar]
- Gettys T. W., Vine A. J., Simonds M. F., Corbin J. D. Activation of the particulate low Km phosphodiesterase of adipocytes by addition of cAMP-dependent protein kinase. J Biol Chem. 1988 Jul 25;263(21):10359–10363. [PubMed] [Google Scholar]
- Goldberg N. D., Walseth T. F., Eide S. J., Krick T. P., Kuehn B. L., Gander J. E. Cyclic AMP metabolism in intact platelets determined by 18O incorporation into adenine nucleotide alpha-phosphoryls. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;16:363–379. [PubMed] [Google Scholar]
- Gordon E. L., Pearson J. D., Slakey L. L. The hydrolysis of extracellular adenine nucleotides by cultured endothelial cells from pig aorta. Feed-forward inhibition of adenosine production at the cell surface. J Biol Chem. 1986 Nov 25;261(33):15496–15507. [PubMed] [Google Scholar]
- Hales C. N., Luzio J. P., Siddle K. Hormonal control of adipose-tissue lipolysis. Biochem Soc Symp. 1978;(43):97–135. [PubMed] [Google Scholar]
- Hammond V. A., Johnston D. G. Substrate cycling between triglyceride and fatty acid in human adipocytes. Metabolism. 1987 Apr;36(4):308–313. doi: 10.1016/0026-0495(87)90199-5. [DOI] [PubMed] [Google Scholar]
- Heindel J. J., Cushman S. W., Jeanrenaud B. Cell-associated fatty acid levels and energy-requiring processes in mouse adipocytes. Am J Physiol. 1974 Jan;226(1):16–24. doi: 10.1152/ajplegacy.1974.226.1.16. [DOI] [PubMed] [Google Scholar]
- Hoffman B. B., Prokocimer P., Thomas J. M., Vagelos R., Chang H., Reaven G. M. Cellular tolerance to adenosine receptor-mediated inhibition of lipolysis: altered adenosine 3',5'-monophosphate metabolism and protein kinase activation. Endocrinology. 1989 May;124(5):2434–2442. doi: 10.1210/endo-124-5-2434. [DOI] [PubMed] [Google Scholar]
- Honnor R. C., Dhillon G. S., Londos C. cAMP-dependent protein kinase and lipolysis in rat adipocytes. II. Definition of steady-state relationship with lipolytic and antilipolytic modulators. J Biol Chem. 1985 Dec 5;260(28):15130–15138. [PubMed] [Google Scholar]
- Kather H., Bieger W., Michel G., Aktories K., Jakobs K. H. Human fat cell lipolysis is primarily regulated by inhibitory modulators acting through distinct mechanisms. J Clin Invest. 1985 Oct;76(4):1559–1565. doi: 10.1172/JCI112137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kather H. Purine accumulation in human fat cell suspensions. Evidence that human adipocytes release inosine and hypoxanthine rather than adenosine. J Biol Chem. 1988 Jun 25;263(18):8803–8809. [PubMed] [Google Scholar]
- Kather H., Simon B. Adrenoceptor of the alpha 2-subtype mediating inhibition of the human fat cell adenylate cyclase. Eur J Clin Invest. 1981 Apr;11(2 Suppl 1):111–114. doi: 10.1111/j.1365-2362.1981.tb02047.x. [DOI] [PubMed] [Google Scholar]
- Kather H., Simon B. Effects of salbutamol and butoxamine on the human fat cell adenylate cyclase. Horm Metab Res. 1980 Dec;12(12):695–697. doi: 10.1055/s-2007-999234. [DOI] [PubMed] [Google Scholar]
- Kather H., Wieland E., Waas W. Chemiluminescent determination of adenosine, inosine, and hypoxanthine/xanthine. Anal Biochem. 1987 May 15;163(1):45–51. doi: 10.1016/0003-2697(87)90091-1. [DOI] [PubMed] [Google Scholar]
- Leibel R. L., Hirsch J. A radioisotopic technique for analysis of free fatty acid reesterification in human adipose tissue. Am J Physiol. 1985 Jan;248(1 Pt 1):E140–E147. doi: 10.1152/ajpendo.1985.248.1.E140. [DOI] [PubMed] [Google Scholar]
- Lönnroth P., Jansson P. A., Fredholm B. B., Smith U. Microdialysis of intercellular adenosine concentration in subcutaneous tissue in humans. Am J Physiol. 1989 Feb;256(2 Pt 1):E250–E255. doi: 10.1152/ajpendo.1989.256.2.E250. [DOI] [PubMed] [Google Scholar]
- Manganiello V., Degerman E., Elks M. Selective inhibitors of specific phosphodiesterases in intact adipocytes. Methods Enzymol. 1988;159:504–520. doi: 10.1016/0076-6879(88)59050-x. [DOI] [PubMed] [Google Scholar]
- May J. M. Triacylglycerol turnover in large and small rat adipocytes: effects of lipolytic stimulation, glucose, and insulin. J Lipid Res. 1982 Mar;23(3):428–436. [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Reed R. G. Location of long chain fatty acid-binding sites of bovine serum albumin by affinity labeling. J Biol Chem. 1986 Nov 25;261(33):15619–15624. [PubMed] [Google Scholar]
- Rothwell N. J., Stock M. J. A role for brown adipose tissue in diet-induced thermogenesis. Nature. 1979 Sep 6;281(5726):31–35. doi: 10.1038/281031a0. [DOI] [PubMed] [Google Scholar]
- Schwabe U., Ebert R., Erbler H. C. Adenosine release from isolated fat cells and its significance for the effects of hormones on cyclic 3',5'-AMP levels and lipolysis. Naunyn Schmiedebergs Arch Pharmacol. 1973;276(2):133–148. doi: 10.1007/BF00501186. [DOI] [PubMed] [Google Scholar]
- Schwabe U., Ebert R. Stimulation of cyclic adenosine 3',5'-monophosphate accumulation and lipolysis in fat cells by adenosine deaminase. Naunyn Schmiedebergs Arch Pharmacol. 1974;282(1):33–44. doi: 10.1007/BF00647401. [DOI] [PubMed] [Google Scholar]
- Spielmann H., Jacob-Müller U., Schulz P. Simple assay of 0.1-1.0 pmol of ATP, ADP, and AMP in single somatic cells using purified luciferin luciferase. Anal Biochem. 1981 May 1;113(1):172–178. doi: 10.1016/0003-2697(81)90061-0. [DOI] [PubMed] [Google Scholar]
- Wilson C., Wilson S., Piercy V., Sennitt M. V., Arch J. R. The rat lipolytic beta-adrenoceptor: studies using novel beta-adrenoceptor agonists. Eur J Pharmacol. 1984 May 4;100(3-4):309–319. doi: 10.1016/0014-2999(84)90007-4. [DOI] [PubMed] [Google Scholar]



