Abstract
A number of metabolic changes within the liver occur concurrent with hepatic regeneration. These processes suggest that the administration of an antiestrogen might alter the rate of hepatic regeneration. To examine this question, male Wistar rats were treated with tamoxifen (0.1 mg/rat/day or 1.0 mg/rat/day) or vehicle for three days prior to and after partial hepatectomy, and the anatomic and biochemical process of hepatic regeneration was assessed. Tamoxifen administration caused a dose-dependent decrease in the hepatic cytosolic estrogen receptor activity and, conversely, a dose-dependent increase in cytosolic androgen receptor activity. Despite these changes in baseline hepatic sex steroid receptor status, all receptor activities were comparable between the three groups within 24 hr of partial hepatectomy. Moreover, no differences in any of the the parameters assessing hepatic regeneration following partial hepatectomy were evident: liver–body ratio, ornithine decarboxylase activity, and thymidine kinase activity. This lack of effect of tamoxifen treatment on hepatic regeneration suggests either that estrogens do not play a role in the modulation of liver growth after partial hepatectomy or that, once initiated, the regenerative process per se determines a series of events that regulate hepatocellular sex hormone receptor status independent of extrahepatic stimuli.
Keywords: hepatic regeneration, estrogens, tamoxifen, antiestrogens, hormone receptors
It is established that the mammalian liver has receptors for both androgens (1) and estrogens (2) and that it has a number of sexually dimorphic characteristics (3, 4). Recently it has been shown that during the initial period of hepatic regeneration following two-thirds partial hepatectomy, male rat liver loses many of its male-specific attributes, including a temporary disappearance of its cytosolic androgen receptor content (5). Although the role of hepatic “demasculinization” during regeneration is as yet uncertain, it suggests that agents that either attenuate or accelerate the loss of hepatic sexual dimorphic characteristics or the sex hormone status of the liver might be able to alter either the rate or degree of hepatic regeneration.
To evaluate the effect of hepatic estrogen receptor modulation in the regulation of hepatic regeneration, male rats were pretreated with the triphenyl-ethylene antiestrogen tamoxifen {(Z)-2-[p-(1,2-diphenyl-1-butenyl)-phenoxy]-N,N-dimethylethylamine} for three days prior to and daily after a standard two-thirds partial hepatectomy. The subsequent hepatic regenerative response was assessed with respect to liver growth and the induction of polyamine and DNA synthetic pathways. In addition, both androgen and estrogen receptor activities in hepatic cytosol were measured to investigate the sex hormone receptor status of the liver during regeneration. The liver’s expression of androgenic activity was assessed also by quantitating the alcohol dehydrogenase activity within the liver, as the degradation of this enzyme is known to be accelerated by the presence of androgens (6).
MATERIALS AND METHODS
Animals and Supplies
Adult male inbred Wistar rats (200–350 g) were purchased from Harlan Sprague Dawley (Indianapolis, Indiana). Tamoxifen citrate, manufactured by Stuart Pharmaceuticals (Wilmington, Delaware), was obtained from the Presbyterian University Hospital pharmacy. New England Nuclear (Boston, Massachusetts) was the source for the [14C]ornithine (57.6 mCi/mmol), [3H]estradiol (99 Ci/mmol), [3H]R1881 (87 Ci/mmol), and unlabeled R1881 utilized in these experiments. The tritiated thymidine (5 Ci/mmol) and ACS scintillation fluid used were from Amersham (Arlington Heights, Illinois). The absolute ethanol and DEAE–cellulose paper were from U.S. Industrial Chemicals Co. (Tuscola, Illinois) and BioRad (Richmond, California), respectively. Unlabeled ornithine, pyridoxal phosphate, Tris base, diethylstilbestrol, adenosine triphosphate, sodium molybdate, nicotinamide adenine dinucleotide, and bovine serum albumin were obtained from Sigma Chemical Co. (St. Louis, Missouri). All other chemicals were from Fisher Chemical Co. (Pittsburgh, Pennsylvania).
Animal Treatment and Tissue Preparation
Male Wistar rats were assigned randomly to the various treatment groups. Animals in the tamoxifen-treated groups were given either 0.1 mg or 1 mg tamoxifen dissolved in a vehicle consisting of 0.2 ml peanut oil–acetone (9: 1) administered subcutaneously beginning 72 hr prior to partial hepatectomy and every 24 hr thereafter until the time of sacrifice. Animals used as placebo controls received 0.2 ml of the identical vehicle administered subcutaneously using the same dosing schedule. A two-thirds partial hepatectomy was performed under light ether anesthesia as described by Higgins and Anderson between 9:00 and 12:00 AM (7). The liver tissue removed at the time of hepatectomy was used to differentiate between the initial effects of the drug and the effects of surgery for the parameters assessed.
At various times up to 72 hr after hepatectomy, animals from the vehicle control group and the two tamoxifen-treated groups were anesthetized with ether and weighed. The liver remnant was removed, weighed, and homogenized in 4 vol of ice-cold buffer consisting of 0.25 M sucrose, 1.5 mM EDTA, 10 mM mercaptoethanol, 150 μM leupeptin, 1 mM benzamidine, and 10 mM Tris HCl (PH 7.4) using a Brinkman Polytron homogenizer. Cytosol was prepared by centrifugation at 103,000 g for 1 hr at 4°C. All cytosolic enzyme assays were performed immediately after preparation of the cytosol.
Sex Steroid Receptor Assays
The activity of the cytosolic estrogen receptors was determined by measuring the specific binding of a saturating concentration of [3H]estradiol (6).
The cytosolic androgen receptor assay used was similar in design to that described for the cytosolic estrogen receptor assay (5). Tritiated R1881, a synthetic androgen, was used as the labeled ligand and unlabeled R1881 was used in the nonspecific binding assays. Triamcinolone acetonide (5 μM) was included in all androgen assays to block binding to the glucocorticoid receptor.
Ornithine Decarboxylase Assay
Ornithine decarboxylase activity was determined by measuring the 14CO2 released from labelled ornithine (9).
Thymidine Kinase Assay
Thymidine kinase activity was determined by measuring the in vitro conversion of thymidine to thymidine phosphate (10).
Alcohol Dehydrogenase Assay
Alcohol dehydrogenase activity was measured spectrophotometrically at 340 nm at ambient temperature by measuring NADH production in a 1-ml reaction mixture consisting of cytosol, 2.8 mM nicotinamide adenine dinucleotide, and 18 mM ethanol in 100 mM Tris HCl buffer (pH 7.2) (11).
Miscellaneous Methods and Procedures
Protein concentrations were determined by the method of Lowry et al (12) with bovine serum albumin used as the standard. Statistical analysis of the data was performed using one-way ANOVA followed by Scheffe’s test through the Abstat program on an IBM PC-XT. A P value of 0.05 or less was considered to be a significant. All results are presented as mean values ± SEM.
RESULTS
Tamoxifen administration for three days caused a significant, dose-dependent reduction in the hepatic cytosolic estrogen receptor activity prior to partial hepatectomy (Figure 1). During the first 6 hr following surgery, the hepatic estrogen receptor activity in the livers from the 0.1 mg/day-treated animals and the vehicle-treated controls decreased further such that there was no difference among the three groups. At 48 hr posthepatectomy, the hepatic estrogen receptor content of the control animals had recovered to a level comparable to that present prior to surgery. In contrast, the hepatic estrogen receptor activity of the animals receiving tamoxifen continued to be markedly reduced through 72 hr.
Fig 1.
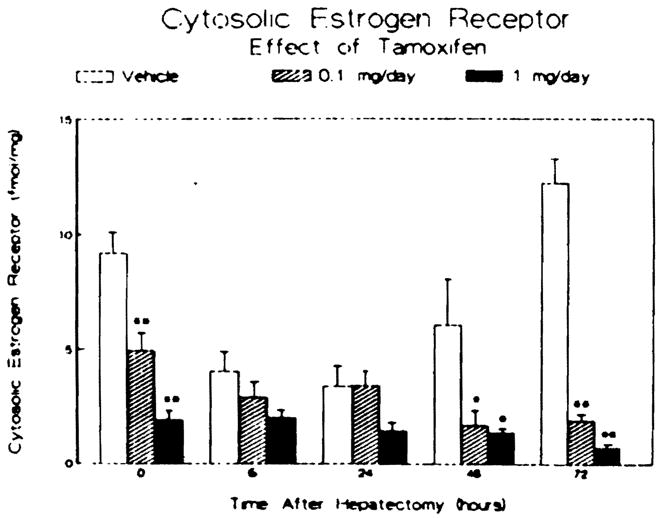
Effect of tamoxifen treatment and partial hepatectomy on hepatic cytosolic estrogen receptor activity. Male rats were treated subcutaneously for three days prior to and daily after partial hepatectomy with vehicle or tamoxifen. Animals were sacrificed at the times noted, and the hepatic cytosolic estrogen receptor activity was determined in femtomoles of specific estrogen bound per milligram cytosolic protein. Bars represent means ± SEM for three to four animals per group. *P < 0.05 vs vehicle-treated controls; **P < 0.01 vs vehicle-treated controls.
The results of study of the androgen receptor activity present in the livers of each group at each time point are shown in Figure 2. Prior to hepatectomy, tamoxifen administration caused a dose-dependent twofold increase in androgen receptor activity in the 1 mg/day-treated animals. Partial hepatectomy produced a significant reduction in the androgen receptor activity within 6 hr in the control and 0.1 mg/day-treated animals. This reduction in androgen receptor activity following partial hepatectomy was delayed in the animals receiving the higher dose of tamoxifen. At 24–72 hr after partial hepatectomy, the androgen receptor in all three groups was reduced but comparable. At 72 hr following surgery, the androgen receptor activity in all three groups was 30% or less of that measured at the time of the partial hepatectomy for each group. As shown in Figure 3, the administration of tamoxifen, at either dose examined, had no effect on alcohol dehydrogenase activity prior to hepatectomy. However. a transient increase in enzyme activity was evident in the two tamoxifen-treated groups at 48 hr after surgery.
Fig 2.
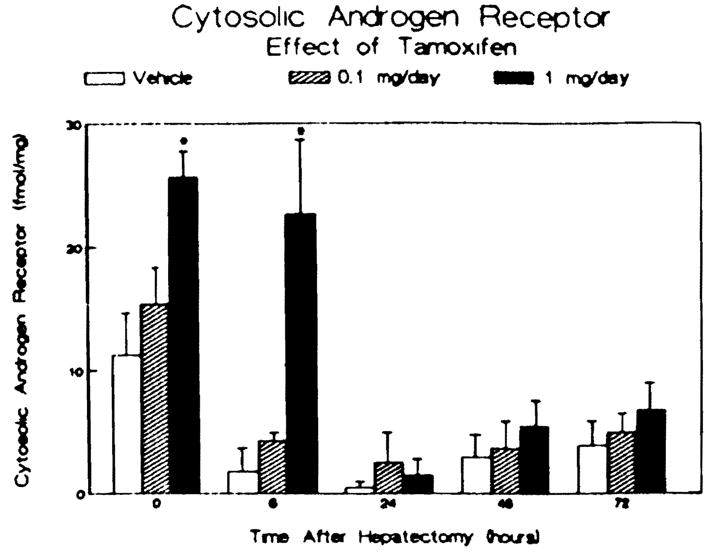
Effect of tamoxifen treatment and partial hepatectomy on hepatic cytosolic androgen receptor activity. Male rats were treated as described in Figure 1. Cytosolic androgen receptor activity is reported as femtomoles of androgen bound per milligram cytosolic protein. Bars represent means ± SEM for three to four animals per group. *p < 0.05 vs vehicle-treated controls.
Fig 3.
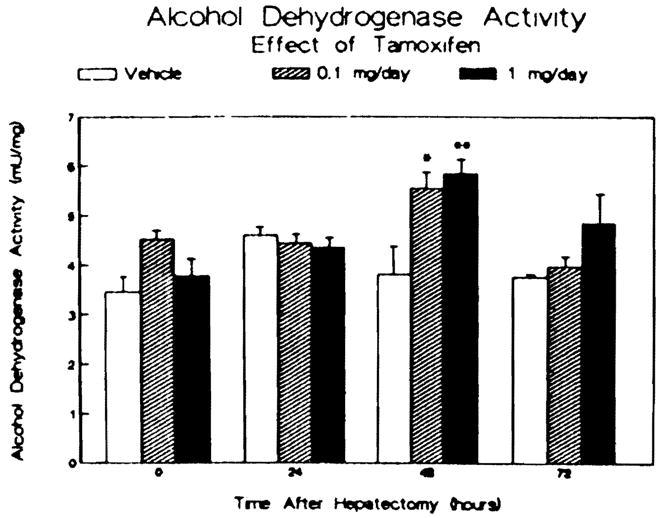
Effect of tamoxifen treatment and partial hepatectomy on hepatic alcohol dehydrogenase activity. Data are reported as milliunits alcohol dehydrogenase activity per milligram cytosolic protein. The bars represent means ± SEM for three to four animals per group. *p < 0.05 vs vehicle-treated controls. **P < 0.01 vs vehicle-treated controls.
Despite the changes in the cytosolic steroid receptor activities following tamoxifen administration, the rate of hepatic growth after two-thirds partial hepatectomy, presented in Figure 4, was not affected. Between 6 and 72 hr following hepatectomy, there was a twofold increase in the liver–body ratio in both the tamoxifen-treated animals and the vehicle-treated controls. There was no difference in the liver–body ratio among the three groups of animals at any time point examined. Furthermore, there was no difference in the protein concentrations of the liver remnants removed at the various time points among the controls and the tamoxifen-treated groups (data not shown), indicating that any hepatic edema or steatosis occurring during the regenerative process was comparable in all three groups.
Fig 4.
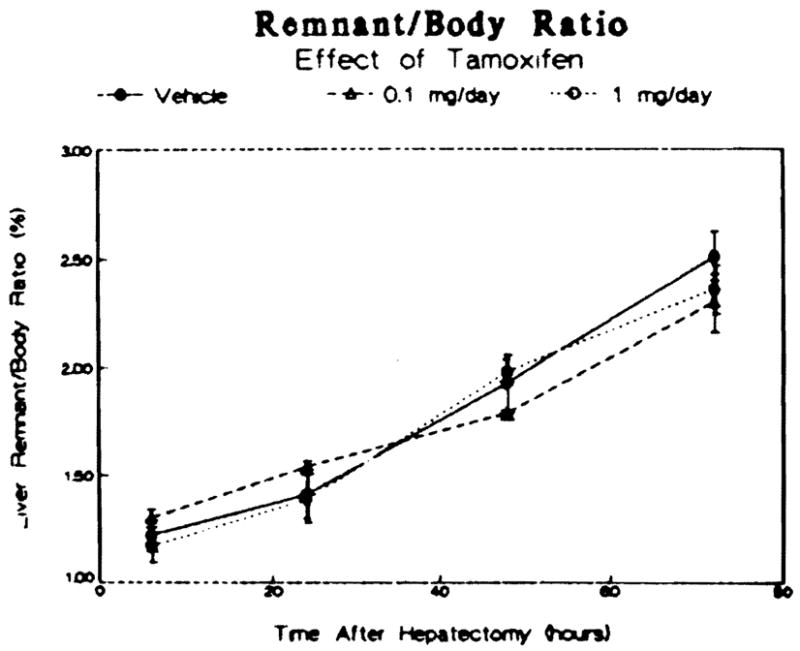
Effect of tamoxifen treatment on the rate of liver growth after partial hepatectomy in male rats. Data are reported as a ratio of the liver weight to the body weight at the indicated times and are means ± SEM for three to four animals per group.
Because an increase in polyamine synthesis has been shown to be an important aspect of the initiation of hepatic regeneration (13), the effects of tamoxifen treatment on the activity of hepatic ornithine decarboxylase, the rate-limiting factor in polyamine synthesis, was assessed. Prior to hepatectomy, tamoxifen administration had no effect on ornithine decarboxylase activity (Figure 5, baseline). Partial hepatectomy caused a significant increase in enzyme activity within the liver in all three groups (Figure 5, peak) 6 hr after surgery, the time that maximal induction of the enzyme activity has been shown to occur (9, 13). There was no difference in the extent of induction among the three groups.
Fig 5.
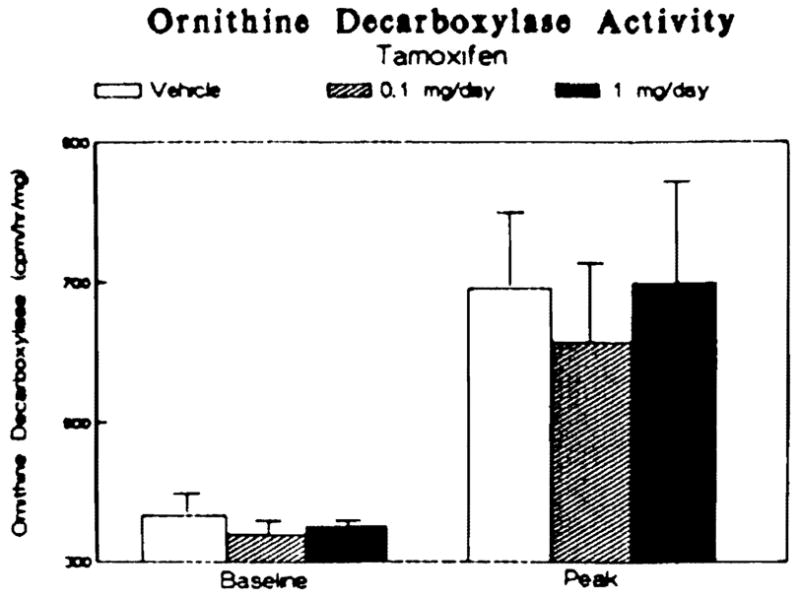
Effect of tamoxifen treatment on the induction of hepatic ornithine decarboxylase activity following partial hepatectomy. Data are presented as counts per minute 14CO2 released per hour per milligram cytosolic protein at the time of hepatectomy (baseline) and 6 hr thereafter (peak). Bars represent means ± SEM for three to four animals per group.
During hepatic regeneration, an increase in DNA synthesis, initiated by an increase in hepatic thymidine kinase activity, occurs. Tamoxifen pretreatment had no effect on thymidine kinase activity prior to or following partial hepatectomy (Figure 6, time zero). Induction of enzyme activity induced by partial hepatectomy peaked at 48 hr at similar levels of activity in all three groups. No difference among the tamoxifen-treated and vehicle-treated animals with respect to the time of peak activity or the level of activity at any time point assessed was found.
Fig 6.
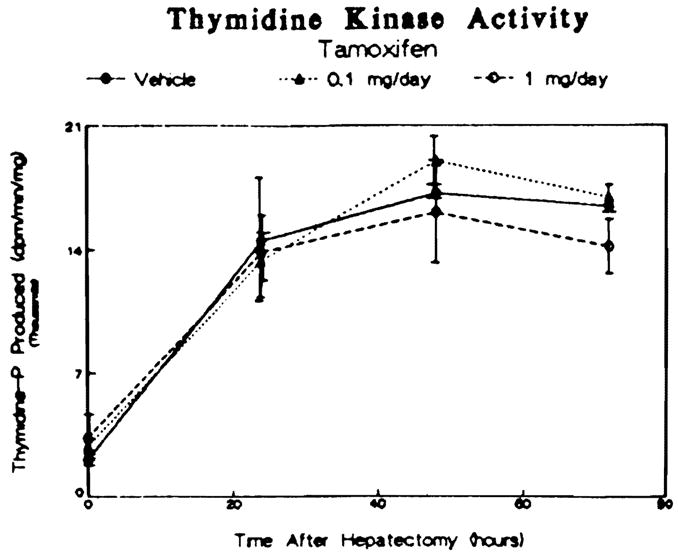
Effect of tamoxifen treatment on hepatic thymidine kinase activity induced by partial hepatectomy in male rats. Data are presented as disintegrations per minute thymidine phosphate produced per minute per milligram cytosolic protein and are reported as means ± SEM for three to four animals per group.
DISCUSSION
Male liver has been shown to lose many of its male-specific characteristics during the process of hepatic regeneration that follows partial hepatectomy (5). The present study was designed to examine whether the antiestrogen tamoxifen would interfere with the regenerative process and alter the rate or extent of the resultant hepatic regeneration. In these experiments, male rats were treated for three days prior to partial hepatectomy with either tamoxifen or the vehicle used to solubilize the drug and then subjected to a standard two-thirds partial hepatectomy.
Previously published data support the hypothesis that estrogens and antiestrogens may play a role in the initiation of hepatic regeneration (5). Moreover estrogens have been shown to act as promoters of neoplasia in rats (14). Thus an increased incidence of hepatomas has been reported in women using oral contraceptives (15). Estrogen administration also has been shown to induce increases in liver mass, thymidine kinase activity, mitotic index, and [3H]thymidine incorporation into DNA (16, 17). Conversely, chronic tamoxifen administration results in a reduction in liver mass, although the liver–body ratio is not altered.
The role of estrogens in hepatic regeneration after partial hepatectomy has been incompletely investigated to date. Partial hepatectomy leads to an increase in serum estradiol levels and hepatic estrogen receptor content in untreated male rats (5). Experiments on treatment with exogenous estrogens, however, have given conflicting results. In a study by Mukundan and Bamji (17), administration of the combination of ethynyl estradiol and estradiol diacetate, two drugs found in oral contraceptives, to female rats was shown to inhibit liver growth during the first 24 hr following partial hepatectomy and to delay the expected increase in [3H]thymidine incorporation into DNA. In contrast, Fisher et al (16) have reported that female rats treated with 17β-estradiol achieve greater liver weights seven days after hepatectomy than do vehicle-treated controls. It is difficult to compare directly the results of these two experiments because of the many differences between the studies, including age of the rats at the time of hepatectomy, the strain of rat used, the type of estrogen used, the duration of treatment prior to hepatectomy, and the parameters that were assessed after hepatectomy.
Even less information is available on the effect of antiestrogens on hepatic regeneration. Chronic administration of a low dose of tamoxifen to male rats has been shown by one group of investigators to have no effect on liver growth following partial hepatectomy (18). Furthermore, these same investigators found that the acute administration of a higher dose of tamoxifen also had no effect on liver growth, although they observed a delay in the expected increase in the mitotic index following tamoxifen treatment (18).
The present study extends these earlier studies with respect to the sex of the rat used, the dosage range of drug examined, and the number of parameters assessed, including sex steroid receptor activity (18). Another difference between the present study and the earlier study is the use of hepatic thymidine kinase activity as a measure of DNA synthesis rather than the mitotic index. The variance for thymidine kinase activity is less than that for the mitotic index, and a good correlation between the two measures has been established by several investigators (10).
In the present study, treatment with tamoxifen at two different doses a full log unit apart had no effect on the rate of liver growth observed after partial hepatectomy. The similar levels of hepatic ornithine decarboxylase activity in treated and control animals further supports the lack of an effect of tamoxifen on hepatic regeneration. Moreover, the time course and peak levels of DNA synthesis stimulated by hepatectomy and assessed by thymidine kinase activity measurements were not different between the three treatment groups.
The observed lack of effect of tamoxifen or hepatic regeneration cannot be attributed to some inability of the drug to interact with the sex hormone receptor systems within the liver. In the present study, tamoxifen administration, prior to partial hepatectomy, caused a dose-dependent decrease in the hepatic cytosolic estrogen receptor activity. This finding is consistent with prior reports in which antiestrogen treatment reduced hepatic cytosolic estrogen receptor content, presumably by interfering with receptor cycling (19). Tamoxifen also alters androgen-sensitive systems and, in the present study, produced a dose-dependent increase in hepatic cytosolic androgen receptor activity prior to hepatectomy and a transient increase in the activity of the androgen-sensitive enzyme alcohol dehydrogenase 48 hr following surgery.
Interestingly, within 24 hr after the initiation of hepatic regeneration, all tamoxifen-induced changes in the activities of both sex steroid receptors were abolished. One explanation for this observation is that tamoxifen might not have been converted to its more active 4-hydroxytamoxifen derivative, as well as several other metabolites of the drug by hepatic microsomal enzymes (20), because partial hepatectomy is known to produce an obligate reduction in the liver’s microsomal mixed function oxidase activity. This would have reduced its effective biologic activity below a threshold level required for an effect upon regeneration to have been observed. The fact that the doses of tamoxifen used ranged over a full log unit makes this possibility less likely, however.
Alternatively, endogenous regulatory factors associated with hepatic regeneration may be more potent than tamoxifen in the modulation of sex hormone receptor activity within the liver, although a mechanism by which regeneration might control sex hormone receptor activity is not yet known. Another possibility is that a putative humoral growth-stimulating factor(s) though to be released from regenerating liver (21, 22) might modulate hepatic sex steroid receptor activity. Yet another possibility is that these same growth factors might interact with extrahepatic regulatory systems such as the hypothalamic–pituitary axis (23, 24), which then modulates both the regenerative process and the hepatic content of sex hormone receptors. The data from the present studies do not allow us to discriminate among these various hypotheses.
In summary, the results of the present study demonstrate that antiestrogens interact with the hepatic sex steroid receptor systems, and, despite the changes in cytosolic androgen and estrogen receptor activity caused by tamoxifen preadministration, no change in the hepatic regenerative response to a two-thirds partial hepatectomy was observed in male rats.
Acknowledgments
This work was supported in part by the Veteran’s Administration and grants from NIAAA AA06601, NIAAA AA04425, and NIDDK AM 32556.
References
- 1.Roy AK, Milan BS, McMinn DM. Androgen receptor in rat liver: Hormonal and developmental regulation of the cytosoplasmic receptor and its correlation with the androgen-dependent synthesis of alpha2u-globulin. Biochim Biophys Acta. 1974;354:213–232. doi: 10.1016/0304-4165(74)90008-7. [DOI] [PubMed] [Google Scholar]
- 2.Chamness GC, Costlow ME, McGuire WL. Estrogen receptor in rat liver and its dependence on prolactin. Steroids. 1975;26:363–371. doi: 10.1016/0039-128x(75)90081-1. [DOI] [PubMed] [Google Scholar]
- 3.Bardin CW, Catteral JS. Testosterone: A major determinant of extragenital sexual dimorphism. Science. 1981;211:1285–1294. doi: 10.1126/science.7010603. [DOI] [PubMed] [Google Scholar]
- 4.Gustafsson JA, Mode A, Norstedt G, Skett P. Sex steroid induced changes in hepatic enzymes. Ann Rev Physiol. 1983;45:51–60. doi: 10.1146/annurev.ph.45.030183.000411. [DOI] [PubMed] [Google Scholar]
- 5.Francavilla A, Eagon PK, DiLeo A, et al. Sex hormone-related functions in regenerating male rat liver. Gastroenterology. 1986;91:1263–1270. doi: 10.1016/s0016-5085(86)80026-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mezey E, Potter JJ. Effect of castration of the turnover of rat liver alcohol dehydrogenase. Biochem Pharmacol. 1985;34:369–372. doi: 10.1016/0006-2952(85)90045-0. [DOI] [PubMed] [Google Scholar]
- 7.Higgins EM, Anderson RM. Experimental pathology of liver I Restoration of liver of the white rat following partial surgical removal. Arch Pathol. 1931;12:186–202. [Google Scholar]
- 8.Eagon PK, Fisher SE, Imhoff AF, et al. Estrogen-binding proteins of male rat liver: Influences of hormonal changes. Arch Biochem Biophys. 1980;201:486–499. doi: 10.1016/0003-9861(80)90537-8. [DOI] [PubMed] [Google Scholar]
- 9.McGowan JA, Fausto A. Ornithine decarboxylase activity and the onset of deoxyribonucleic acid synthesis in regenerating liver. Biochem J. 1978;170:123–127. doi: 10.1042/bj1700123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kahn D, Stadler J, Terblanche J, van Hoorn-Hickman R. Thymidine kinase: An inexpensive index of liver regeneration in a large animal model. Gastroenterology. 1980;79:907–911. [PubMed] [Google Scholar]
- 11.Crow KE, Cornell NW, Veech RL. The rate of ethanol metabolism in isolated hepatocytes. Alcoholism Clin Exp Res. 1977;1:43–47. doi: 10.1111/j.1530-0277.1977.tb05765.x. [DOI] [PubMed] [Google Scholar]
- 12.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 13.Luk GD. Essential role of polyamine metabolism in hepatic regeneration Inhibition of deoxyribonucleic acid synthesis and protein synthesis and tissue regeneration by difluoromethylornithine in the rat. Gastroenterology. 1986;90:1261–1267. doi: 10.1016/0016-5085(86)90394-x. [DOI] [PubMed] [Google Scholar]
- 14.Wanless R, Medline A. Role of estrogens as promoters of hepatic neoplasia. Lab Invest. 1982;46:313–320. [PubMed] [Google Scholar]
- 15.Edmondson HA, Henderson B, Benton B. Liver cell adenomas associated with use of oral contraceptives. N Engl J Med. 1976;294:470–472. doi: 10.1056/NEJM197602262940904. [DOI] [PubMed] [Google Scholar]
- 16.Fisher B, Gunduz N, Saffer EA, Zheng S. Relation of estrogen and its receptor to rat liver growth and regeneration. Cancer Res. 1984;44:2410–2415. [PubMed] [Google Scholar]
- 17.Mukundan MA, Bamji MS. Liver regeneration in oral contraceptive treated female rats—effects of moderate malnutrition. Horm Metab Res. 1984;16:641–645. doi: 10.1055/s-2007-1014872. [DOI] [PubMed] [Google Scholar]
- 18.Mouriquand P, Mouriquand J, Louis D, Beriel H, Louis J, Mermet MA, Mouriquand C. Les effets du Tamoxifene sur la regeneration hepatique apres hepatectomie partielle experimentale. Lyon Chir. 1985;81:221–225. [Google Scholar]
- 19.Clark JH, Anderson JN, Peck EJ., Jr Estrogen receptor–anti-estrogen complex: Atypical binding by uterine nuclei and effects on uterine growth. Steroids. 1973;22:707–718. doi: 10.1016/0039-128x(73)90118-9. [DOI] [PubMed] [Google Scholar]
- 20.Robertson DW, Katzenellenbogen JA, Long DJ, Rorke EA, Katzenellenbogen BS. Tamoxifen antiestrogens A comparison of the activity, pharmacokinetics, and metabolic activation of the cis and trans isomers of tamoxifen. J Ster Biochem. 1982;16:1–13. doi: 10.1016/0022-4731(82)90137-6. [DOI] [PubMed] [Google Scholar]
- 21.Terblanche J, Porter KA, Starzl TE, Moore J, Patzelt L, Hayashida N. Stimulation of hepatic regeneration after partial hepatectomy by infusion of a cytosol extract from regenerating dog liver. Surg Gynecol Obstet. 1980;151:538–544. [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen DM, Jaspan JB, Polonsky KS, Lever EG, Moosa AR. Pancreatic hormone profiles and metabolism posthepatectomy in the dog Evidence for a hepatotrophic role of insulin, glucagon, and pancreatic polypeptide. Gastroenterology. 1984;87:679–687. [PubMed] [Google Scholar]
- 23.Gustafsson JA, Eneroth P, Hokfelt T, Mode A, Norstedt G. Studies on the hypothalamo-pituitary axis: A novel concept in regulation of steroid and drug metabolism. In: Langer M, Chiandussi L, Chopra IJ, Martini L, editors. The Endocrines and the Liver. London: Academic Press; 1984. pp. 9–34. [Google Scholar]
- 24.Mode A, Norstedt G, Simic B, Eneroth P, Gustafsson JA. Continuous infusion of growth hormone feminizes hepatic steroid metabolism in the rat. Endocrinology. 1981;108:2103–2108. doi: 10.1210/endo-108-6-2103. [DOI] [PubMed] [Google Scholar]
- 25.Kneifel MA, Katzenellenbogen BS. Comparative effects of estrogen and antiestrogen on plasma renin substrate levels and hepatic estrogen receptors in the rat. Endocrinology. 1981;108:545–552. doi: 10.1210/endo-108-2-545. [DOI] [PubMed] [Google Scholar]


