Abstract
Microscopic examination of placental tissue can provide an accurate assessment of malaria infection during pregnancy. In this cross-sectional study of 193 women in Iquitos, Peru, 1.0% and 6.6% had parasites in the peripheral blood as detected by microscopy and polymerase chain reaction, respectively. However, 22% had placental malaria pigment indicating past, subclinical infections. Placental tissues with pigment from 24 cases were matched by gravidity and month of delivery to 24 controls and histopathologically examined. Cases had significantly higher number of monocytes in the intervillous space (44.7 versus 25.5; P = 0.012). Pigmented monocytes in fetal vessels were present in 33.3% of cases. This study demonstrated that subclinical malarial infection occurred frequently in pregnant women and is associated with increased presence of monocytes in the placenta. Pigmented monocytes in fetal vessels suggest parasites can breach the placental barrier and enter the fetal circulation.
Introduction
The epidemiology of malarial infection during pregnancy is often assessed on the basis of peripheral or placental parasitemia at delivery. This assessment for acute infection does not provide an accurate estimate of the exposure experience throughout the duration of pregnancy. Some studies have longitudinally followed-up pregnant women throughout pregnancy with peripheral blood smears. However, peripheral parasitemia often does not correlate with placental malaria infection.1–4 Hemozoin or malaria pigment is a by-product of hemoglobin degradation by Plasmodium spp. that can be used as an indicator of malaria infection in the placenta, and may provide a more accurate estimate of the frequency of malarial infection during pregnancy.4–6
Placental changes caused by malaria infection, including hemozoin deposition and increased monocytes in the intervillous space (IVS), are thought to interfere with fetal development through the impairment of maternal–fetal exchanges by generating hypoxia, and inflammatory reactions.4,7–11 Increased mononuclear infiltrates, have been associated with adverse infant outcomes, specifically low birth weight.12–14 Whether placental parasitemia or host immune responses and local inflammation are mediators of adverse outcomes remains controversial. It has been speculated that severe placental parasitemia occurs and is later suppressed by the onset of the inflammatory infiltration. Therefore, both parasite and host immune factors may play role in malaria-related placental pathology.7
Most studies that have assessed malaria-related placental pathologic changes have been conducted in moderate-to-high transmission settings, such as in sub-Saharan Africa, where Plasmodium falciparum is the dominant infecting species.2,12,13,15 In regions highly endemic for malaria, the prevalence of placental malaria ranges from 30% to 60% and has been associated with increased risk of adverse infant outcomes, particularly in primigravidae.16 In these regions, pregnant women have developed a high level of protective immunity against clinical malaria.17 Therefore, the impact of placental malaria may be different than that in pregnant women living in low-endemicity regions. Additionally, placental pathologic changes caused by P. falciparum may differ from those caused by P. vivax, and few studies have investigated placental malarial infections caused by P. vivax. A study conducted in Thailand demonstrated that P. vivax infection was significantly associated with malaria pigment in the placenta, despite successful clearance of parasitemia by treatment.3
The Amazon region around Iquitos, Peru, provides an opportunity to evaluate placental malaria caused by P. vivax and P. falciparum in a low-transmission setting.18 Previous reports from this region have demonstrated a high frequency of asymptomatic infections, suggesting that protective immunity to clinical malaria is developed despite low transmission.18,19 In the present study, we investigated the prevalence of placental malaria infection in a cross-sectional study of pregnant women at delivery. Subsequently, we assessed histopathologic factors associated with placental infection in a case–control analysis by comparing women with and without placental hemozoin.
Methods
Study design and subject enrollment.
A cross-sectional study with a non-random convenience sampling scheme was conducted at two hospitals in Iquitos: Centro de Salud San Juan and Hospital Apoyo Iquitos. Pregnant women were enrolled at delivery if current residence was in a malaria-endemic community. Trained physicians or obstetricians obtained informed consent and prior to or within 24 hours of delivery completed a detailed epidemiologic questionnaire. Data collected included age, number of pregnancies, number of malaria episodes during her lifetime, and if she experienced any illness during this pregnancy. Previous lifetime malaria is symptomatic clinical malaria reported by the mother and based on her health card that documents the diagnosis (by microscopic detection of parasites) and treatment, both of which are controlled by the Peruvian health system. Therefore, this variable does not indicate any undiagnosed asymptomatic infections. Labor/delivery and infant outcomes data were collected from the patient hospital chart.
Sample collection.
Within 24 hours of delivery, 3 mL of maternal peripheral blood was collected from the enrolled patient by venipuncture into a vacutainer tube containing EDTA. Thick and thin blood smears were prepared for microscopic examination of peripheral parasitemia.
The placenta was processed immediately after delivery when possible (no more than four hours after delivery). Two small (approximately 1 cm3) sections of placenta were removed from the maternal side of the placenta, and the placental blood that pooled in these areas was collected by syringe aspiration into a tube containing EDTA. The tissue sections were then used to make impression smears. A full-thickness section of the placenta was taken approximately one-third of the distance between cord insertion and the edge of the placental disk and placed in Streck tissue fixative (SFT) (Streck Laboratories Inc., Omaha, NE). Full-thickness sections in STF were stored at 4°C. This fixative does not contain formalin and thus prevents possible formation of formalin pigment often misinterpreted as hemozoin (malaria pigment).
Parasite detection and laboratory methods.
Standard microscopy of blood and impression smears stained with Giemsa was conducted within 24 hours of sample collection for malaria diagnosis. Thick blood smears were read for parasite detection. For quality assurance, all smears were read by two expert microscopists. Polymerase chain reaction (PCR), as described elsewhere, was subsequently carried out on all samples to detect infections not identifiable by standard microscopy.20
Active and previous placental infection.
Full-thickness sections that were placed in STF were transported to the United States where sections were processed and embedded in paraffin blocks by using standard techniques. Three paraffin sections approximately 5 μm thick were cut and stained with Giemsa, Prussian blue, and hematoxylin and eosin. Giemsa-stained sections were assessed for parasitemia, Prussian blue–stained sections were assessed to differentiate malaria pigment from hemosiderin, a normal by-product of hemoglobin degradation. Although hemozoin and hemosiderin stain brown with the other two stains, hemozoin stains brown and hemosiderin stains bright turquoise blue with Prussian blue stain, enabling differentiation. Hematoxylin and eosin–stained sections were assessed for placental pathologic changes, such as fibrinoid deposition, syncytial damage, cytotrophoblast prominence, and inflammatory infiltrates.
Case–control study design.
Twenty-four controls with no placental hemozoin were matched by gravidity and month of delivery to 24 cases with placental hemozoin deposition to assess malaria associated histopathologic changes. Hematoxylin and eosin–stained sections of all cases and controls were examined for placental changes. Leukocytes, specifically mononuclear and polymorphonuclear cells, were counted in 10 randomly chosen 1,000× microscopic fields of the IVS near the edge of the basal plate of the placenta.
Statistical methods.
Statistical analysis was conducted in SAS version 9.1 software (SAS Institute, Cary, NC). The goals of our statistical analysis were to assess frequency of peripheral and placental malaria, evaluate risk factors associated with placental infection (among all study subjects), and compare histopathologic characteristics (i.e., immune cell counts) between the 24 matched cases and controls. We also evaluated associations between histopathologic characteristics (i.e., immune cell counts) and pregnancy outcomes.
Univariate analysis was conducted to assess frequencies of peripheral infection, acute infection, and past placental infection. Bivariate analysis between categorical variables was conducted using by chi-square or Fisher's exact tests as appropriate. Bivariate analysis to assess the association between placental hemozoin and continuous variables (i.e., age, gravidity, birth weight) was conducted by using the nonparametric Wilcoxon rank sum test.
For the case–control study, a matched analysis was conducted. To compare immune cell counts and pregnancy outcomes between cases and controls, a matched conditional logistic regression was conducted. Report of previous lifetime malaria was selected as a potential confounder for comparison of immune cells because it was a significant risk factor in a previous investigation of serologic responses in pregnant women from this region. An adjusted matched proportional hazard regression analysis was conducted to determine the confounding effect of previous lifetime report of malaria on differences in immune cell counts between cases and controls.
Results
Descriptive frequencies of study population with placental samples.
During April–December 2004, 309 pregnant women were enrolled in the study. Of these women, 193 had peripheral blood and placental tissue samples that fixed properly and were included in this analysis. Median age of 189 women was 22.1 years (range = 14–44 years), and 75 (39.5%) of 190 women were primigravidae with a median gravidity of 2.0 pregnancies (range = 1–13 pregnancies). More than half (55.96%) of the subjects reported having at least one malaria episode during their lifetime and had a median number of lifetime episodes of 1.0 (range = 1–6 episodes). Of those reporting previous lifetime malaria episodes, 86.1% reported P. vivax infections and 35.2% reported P. falciparum infections. The median maternal hematocrit of 160 women was 34.0% (range = 15–45%), and 12.5% of women had anemia at delivery (hematocrit < 30%). Median infant birth weight for 191 newborns was 3,050 grams (range = 1,000–4,010 grams). While a total of 7.9% of the infants had a low birth weight (< 2,500 grams), 21.9% (35 of 164), had a gestational age ≤ 37 weeks as measured by the scoring system of Capurro; the median gestational age was 39 weeks (range = 28–42 weeks).
The frequency of malaria infection as detected by different methods is shown in Table 1. Of 193 study subjects, 1.0% were microscopy-positive for parasites in the peripheral blood, 0.5% in placental blood, and 0.5% in placental tissue impression smears. Positive PCR results were observed in 6.6% of peripheral blood samples and in 5.2% of placental blood samples. Additionally, 8.8% of the women reported a confirmed diagnosis of malaria during the current pregnancy. These women experienced symptoms of malaria, and after diagnosis, by microscopic detection of parasites, received treatment for infection. Diagnosis and treatment were noted on their health cards.
Table 1.
Frequency of malaria infection by different methods of detection and association with placental hemozoin*
| Method of detection | No. (%) (n = 193) | No. positive for hemozoin (P)† |
|---|---|---|
| Microscopy of peripheral blood (n = 192) | 2 (1.04) (1 Pv, 1 Pf) | 1 (P = 0.35) (1 Pf) |
| Microscopy of placental blood (N = 190) | 1 (0.53) (1 Pf) | 1 (P = 0.22) (1 Pf) |
| PCR of peripheral blood (n = 166) | 11 (6.63) (7 Pv, 2 Pf, 1 Pm, 1 Pf/Pm) | 3 (P = 0.72) (2 Pv, 1 Pf/Pm mixed) |
| PCR of placental blood (n = 174) | 9 (5.17) (4 Pv, 4 Pf, 1 Pm) | 3 (P = 0.42) (1 Pv, 2 Pf) |
| Confirmed report of malaria in a pregnancy | 17 (8.81) (13 Pv, 2 Pf, 2 unknown) | 5 (P = 0.54) (2 Pv, 2 Pf, 1 unknown) |
| Placental hemozoin | 43 (22.28) |
Pv = Plasmodium vivax; Pf = P. falciparum; PCR = polymerase chain reaction; Pm = P. malariae.
Bivariate analysis was conducted to determine if placental hemozoin positivity was associated with infection as detected by other methods. The level of significance for P was ≤ 0.05.
In contrast to the low frequency of acute infection, 43 (22.3%) pregnant women had placental hemozoin deposition as detected by histopathologic assessment of full-thickness sections. There was no association between placental hemozoin deposition and evidence of infection detected by the other methods, including a report of malaria in this pregnancy (Table 1). Malaria pigment was most commonly found in the basal plate, fibrinoid, monocytes in the IVS, and necrotic syncytium. Parasites were not found in any of the placental sections.
Malaria pigment deposits in different areas of the same placenta are shown in Figure 1. Pigment deposits in the basal plate on the maternal side of the placenta are shown in Figure 1A, and pigmented maternal monocytes in the IVS near the basal plate are shown in Figure 1B. The woman from whom this placenta was obtained was a primigravidae with no report of previous malaria who had an uncomplicated delivery. The infant birth weight was 2,740 grams, the infant length was 48.5 cm, the gestational age as measured by the procedure of Capurro21 was 39 weeks, and the maternal hematocrit was 40%. The placental pathologic changes of hemozoin deposition in the absence of parasites and evidence or report of clinical manifestations suggest that this woman had an asymptomatic malarial infection during pregnancy that may have self-cleared.
Figure 1.
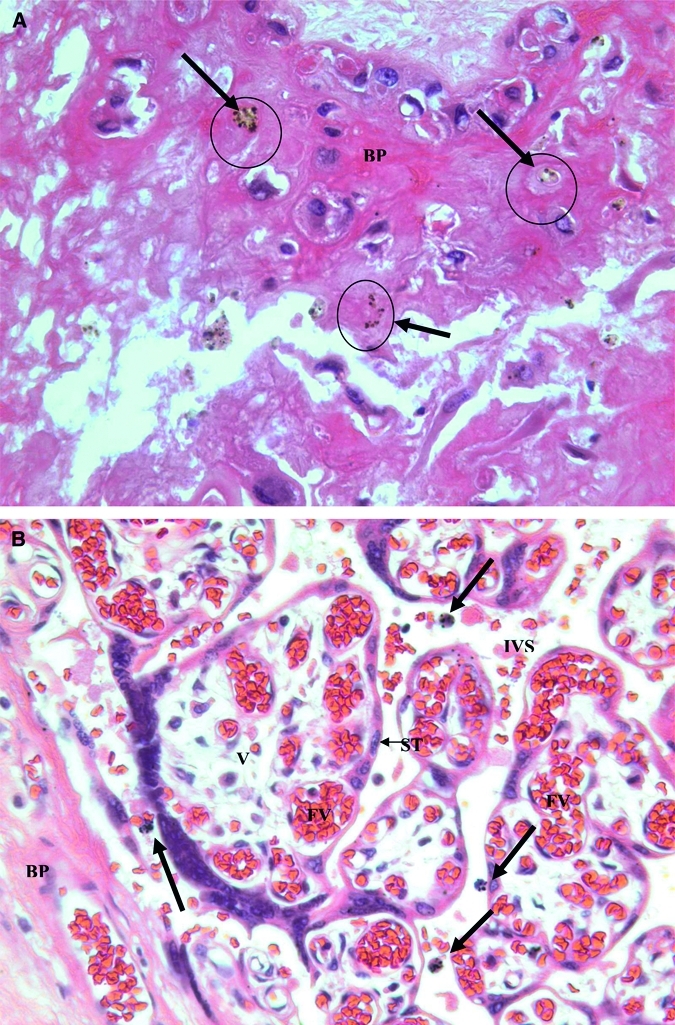
A, Hemozoin deposition (Hz) in a basal plate (BP) of a placenta with evidence of past malaria infection. Malaria pigment or hemozoin appears as brown deposits (arrows). Pigment was confirmed as hemozoin by staining with Prussian blue. The pattern suggests that hemozoin was left in the fibrin of the basal plate after macrophage degeneration. B, Hemozoin deposition in maternal macrophages in the intervillous space. Four pigmented maternal monocytes (bold arrows) are in the intervillous space near the basal plate. BP = basal plate; IVS = intervillous space; V = fetal villi in cross section; ST = syncytiotrophoblast cells; FV = fetal vessels. (Original magnification × 400.)
Characteristics of pregnant women with placental hemozoin compared with those without placental hemozoin.
A risk factor and outcome analysis was conducted to determine whether pregnant women with placental hemozoin differed from pregnant women without hemozoin (Table 2). The 43 pregnant women whose placentas were hemozoin positive demonstrated a trend to be of lower gravidity, and have lower birth weight babies compared with those with no placental hemozoin, but the difference was not statistically significant (Table 2).
Table 2.
Descriptive characteristics of study population by placental hemozoin (n = 193)
| Characteristic | Placental hemozoin+ (n = 43) | Placental hemozoin– (n = 150) | P |
|---|---|---|---|
| Median age, years (n = 189) | 21.01 (n = 42) (SD = 5.41) | 23.31 (n = 147) (SD = 6.82) | 0.43 |
| Median gravidity (n = 190) | 2.00 (n = 43) (SD = 1.54) | 2.00 (n = 147) (SD = 1.90) | 0.058 |
| % Reported previous lifetime malaria | 60.5 | 54.7 | 0.5 |
| Median infant birth weight, grams (n = 191) | 3,020 (n = 41) (SD = 530.63) | 3,088 (n = 150) (SD = 425.71) | 0.09 |
| Median infant gestational age, weeks, by Capurro and others21 (n = 164) | 38 (n = 37) (SD = 2.20) | 39 (n = 127) (SD = 1.93) | 0.19 |
| Median maternal hematocrit, % (n = 160) | 33.0 (n = 36) (SD = 5.13) | 34.0 (n = 124) (SD = 4.27) | 0.68 |
| % Preeclampsia | 4.7 | 8.7 | 0.53* |
| % Urinary tract infections | 7.0 | 0 | 0.01* |
| % Premature rupture of membranes | 7.0 | 5.3 | 0.71* |
P ≤ 0.05, by Fisher's test.
Findings of case–control study.
A case–control study was subsequently designed to determine if placentas with hemozoin deposition had more pathologic changes compared with those who were hemozoin negative. A subset of the hemozoin-positive women was matched to hemozoin-negative controls by gravidity and month of delivery. We were able to match 24 case–control pairs to be included in the case–control analysis. Among these women, none were microscopy positive, two were PCR positive in peripheral blood for P. vivax, two were PCR positive in placental blood, one for P. falciparum and one for P. vivax, and two reported having malaria during this pregnancy. Women who had a confirmed diagnosis of malaria during this pregnancy received treatment at the time of diagnosis, which was documented on their health cards. Fifteen (62.5%) of 24 cases reported at least one previous lifetime infection compared with 11 controls (45.8%).
Leukocyte counts in IVS.
Leukocytes from 10 randomly chosen 1,000× microscopic fields of the IVS near the basal plate were compared between cases and controls (Table 3). Among cases, 10 (41.7%) had pigmented mononuclear cells in the IVS with a mean number of 4.9 pigmented mononuclear cells in 10 1,000× microscopic fields. Cases with placental hemozoin deposition had significantly increased mean number of total mononuclear cells (pigmented and non-pigmented) in the IVS compared with controls (44.7 versus 25.5; P = 0.014) (Table 3). Furthermore, when only non-pigmented monocytes in IVS were compared, cases had significantly increased mean numbers than controls (39.8 versus 25.5; P = 0.037) (Table 3). In contrast, controls had significantly higher mean numbers of PMNs in the IVS compared with cases (Table 3).
Table 3.
Immune cell counts in 10 1,000× microscopic fields by case–control status*
| Cell type | Cases, mean no. counted in 10 fields (n = 24) | Controls, mean no. counted in 10 fields (n = 24) | Unadjusted P | Adjusted P† |
|---|---|---|---|---|
| All mononuclear cells in IVS (non-pigmented plus pigmented) | 44.7 | 25.5 | 0.014 | 0.012 |
| Non-pigmented mononuclear cells in IVS | 39.8 | 25.5 | 0.037 | 0.023 |
| Polymorphonuclear cells in IVS | 0.42 | 1.2 | 0.045 | 0.063 |
IVS = intervillous space. Level of statistical significance was P ≤ 0.05.
Adjusted for report of previous lifetime malaria.
Pigmented monocytes in fetal vessels.
Although much of the hemozoin deposition was seen in the traditionally reported locations including the basal plate, fibrinoid, and mononuclear cells in the IVS, eight (33.3%) of the cases showed pigmented mononuclear cells within fetal vessels (PMFV). The PMFVs were often seen in the larger fetal vessels near the fetal side of the placenta. Two women with PMFVs and other placental histopathologic changes associated with malaria pigment are shown in Figures 2 and 3.
Figure 2.
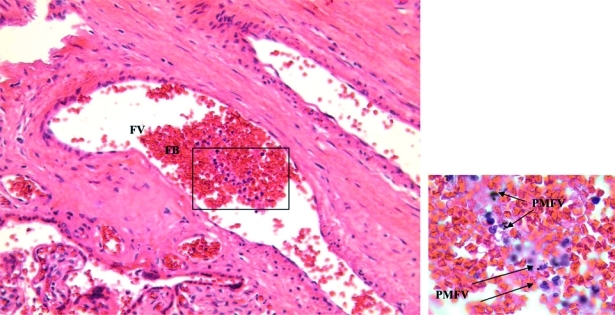
Monocytes and pigmented monocytes in a large fetal vessel in a longitudinal section of a primary stem villous with many monocytes of an infant was born with a congenital malaria infection as confirmed by a Plasmodium vivax–positive peripheral blood smear within 24 hours of delivery. Monocytes in fetal vessel contain malaria pigment (arrows) (original magnification × 200). Inset shows malaria pigment in monocytes (original magnification × 1,000). FV = fetal vessel; FB = fetal blood; PMFV = pigmented monocyte in fetal vessel.
Figure 3.
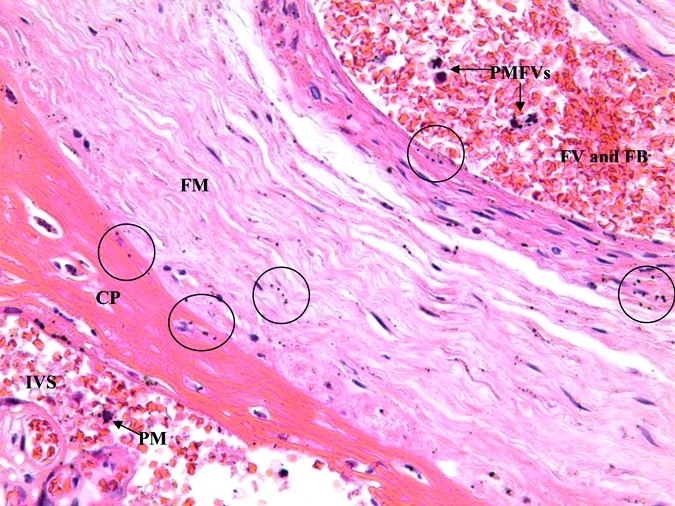
Hemozoin deposition in chorionic plate, fetal membranes, and pigmented monocyte in fetal vessel of an infant born with a congenital malaria infection as confirmed by a Plasmodium vivax–positive peripheral blood smear within 24 hours of delivery. The intervillous space (IVS) contains a pigmented maternal monocyte (PM). The fetal membrane (FM) contains deposits of hemozoin denoted by circled areas. The large fetal vessel (FV) filled with fetal blood (FB) at the top contains four fetal monocytes that contain malaria pigment (PMFV). CP = chorionic plate. (Original magnification × 400.)
A 200× magnification in Figure 2 shows a large fetal vessel containing fetal blood and numerous mononuclear cells. The inset (boxed area) at 1,000× magnification in Figure 2 shows that many of these mononuclear cells contain malaria pigment. This placenta was from a woman with no evidence of symptomatic malarial infection during pregnancy by microscopy, PCR, or report. She had three previous malaria episodes in her lifetime, two caused by P. vivax infections and one caused by a P. falciparum infection. Infant birth weight was 3,620 grams, infant length was 50 cm, gestational age was 37 weeks, and maternal hematocrit was 33%. The placental pathologic changes for this case suggest that not only did this woman experience an asymptomatic malarial infection, but also that the parasite may have crossed the placental barrier and entered the fetal circulation.
A case with malaria pigment in various locations on the fetal side of the placenta is shown in Figure 3. This figure shows the maternal IVS of the placenta, the fetal membrane, and a large fetal vessel full of fetal blood and some mononuclear cells. Malaria pigment was in all three areas: a maternal pigmented mononuclear cell in the IVS, hemozoin deposits in the fetal membrane, and PMFVs in the fetal vessel. This mother was diagnosed with P. vivax infection earlier in her pregnancy. She has had five previous malaria episodes in her lifetime; three caused by P. vivax and 2 caused by P. falciparum. The infant was congenitally infected with P. vivax as determined by microscopic detection of P. vivax parasites in the infant's peripheral blood within 24 hours of delivery while the mother and infant were still in the hospital. The infant birth weight was 3,020 grams, gestational age was 40 weeks, and maternal hematocrit was 35%. The infant subsequently died of neonatal sepsis.
Relationship of placental hemozoin and mononuclear cell counts with maternal and infant outcomes.
Although a greater proportion of cases delivered infants with low birth weights (16.7% versus 8.3% in controls) there was no significant difference in birth weight between cases (2,847 grams) and controls (3,053 grams) (P = 0.22). Similarly, more cases had infants with decreased gestational age, as measured by the procedure of Capurro,21 (38.1%) than controls (25.0%), but no significant differences in mean gestational age were detected (37.6 weeks versus 37.9 weeks; P = 0.85). Mean levels of infant birth weight, infant gestational age, and maternal hematocrit between cases and controls are shown in Table 4; differences were not statistically significant.
Table 4.
Relationship of placental hemozoin and monocyte counts with infant and maternal outcomes (n = 48)
| Group | Mean infant birth weight, grams | P | Mean infant gestational age, weeks (n = 41) | P | Mean maternal hematocrit, % (n = 45) | P |
|---|---|---|---|---|---|---|
| Cases with hemozoin (n = 24) | 2,847 | 0.22* | 37.6 (n = 21) | 0.85* | 33.9 (n = 22) | 0.90* |
| Controls without hemozoin (n = 24) | 3,053 | 37.9 (n = 20) | 33.6 (n = 23) |
By matched conditional logistic regression, SAS procedure Proc Phreg.
Discussion
The present study investigated the prevalence of placental malaria infection in a cross-sectional study of pregnant women in the Amazon region of Peru, an area of low malaria endemicity with transmission of P. vivax and P. falciparum. A case–control study was designed to investigate the placental histopathologic changes associated with placental infection, and whether these changes were associated with adverse maternal and infant outcomes.
In this investigation, a low frequency of acute, active infection was detected. However, a high proportion of pregnant women enrolled in this study (22.3%) had malaria hemozoin deposition in their placentas. On the basis of the widely accepted criteria for pathologic classification of placental malaria of Bulmer and others,5,6 this finding is indicative of a past chronic infection during pregnancy.5,6 The presence of hemozoin in the absence of patent parasitemia or patient report of clinical symptoms suggests that most women with placental hemozoin experienced subclinical malaria infections during pregnancy. Interestingly, the presence of placental malaria pigment was not significantly associated with infection detected by any of the other methods (Table 2). This finding further suggests that women with evidence of malarial infection detected by placental hemozoin only were able to successfully clear parasites without developing clinical disease.
Little is known about the extent and distribution of placental hemozoin deposition caused by P. vivax infection. Therefore, it is difficult to determine which species caused the hemozoin deposition in each of the cases. One study in Thailand demonstrated that women infected with P. falciparum had increased amounts of and more widely distributed placental hemozoin deposition than women infected with P. vivax,22 but there was no difference in the amount and distribution of pigmented maternal monocytes in the IVS between the two species. However, pregnant women in this study in Thailand were treated upon detection of infection. Therefore, treatment may have precluded observation of the true placental pathologic changes caused by P. vivax. In the current study, most infections that were detected by microscopy, PCR, and patient report in pregnant women were caused by P. vivax. Thus, a substantial proportion of the placental hemozoin demonstrated in this investigation may have been caused by P. vivax infections.
The most significant finding of this study is the presence of PMFVs in 33.3% of the cases. This is the first time that PMFVs have been reported in association with placental malaria. It is traditionally believed that because placental malaria is caused by maternal infection, malaria-related placental lesions and changes are limited to the maternal side of the placenta, such as the basal plate, IVS, and villous surface.10 Placental changes on the fetal side of the placenta or in the primarily fetal structures, such as the amniochorionic membrane, have rarely been reported. The reason for this finding may be that most studies assessing malaria-related placental histopathologic changes analyze small biopsy sections from the maternal side of the placenta instead of full-thickness sections. This limited analysis does not enable an assessment of the side of the placenta nearest the fetus where most larger fetal vessels are visible. However, other studies that examined full-thickness sections, in which the frequency of acute and past placental infection was high, also did not report PMFVs.10,13,23
Traditionally, it is believed that parasites cannot breach the placental barrier and enter the fetal circulation. Although oxygen, nutrients, and antibodies are transplacentally transferred, maternal and fetal circulations are not in direct contact in the placenta. Thus, an infected erythrocyte would not cross into the fetal circulation without placental damage. The observation of PMFVs in this study challenges this widely accepted theory and indicates that fetal exposure to parasites occurs in utero. Transplacental fetal infection in utero was reported in Ceylon, and was observed to cause fetal death in several cases.24 Moreover, a more recent study in rhesus monkeys, in which pregnant mothers were infected with P. coatneyi found an infected fetal erythrocyte within a fetal vessel. This baby subsequently became congenitally infected with malaria 80 days after birth, which indicated that fetal exposure to malaria can be manifested several months after birth.25 Another study that assessed paired maternal and cord blood samples from Kenya showed that 57% of infected paired samples had discordant malaria strains, suggesting that transplacental transfer of P. falciparum-infected erythrocytes occurs.26 Thus, congenital malaria infection by in utero exposure may occur more frequently than previously believed.
The question could arise as whether increased monocytes seen in fetal vessels are of maternal or fetal origin. Although transplacental transfer of maternal leukocytes has been reported, the frequency and number of cells crossing the placental barrier are usually low, unlike the large numbers detected in fetal vessels in the current study, making it unlikely that these cells are maternal.27 Additionally, it has been established that strong maternal inflammatory responses can result in adverse pregnancy outcomes, including spontaneous abortion, intrauterine growth restriction, and preterm delivery.28 Therefore, it would be expected that transplacental transfer of an inflammatory response would have resulted in more severe adverse outcomes than was observed in this investigation. Thus, it is likely that the monocytes and PMFVs are of fetal origin and are indicative of a fetal immune response against the malaria parasite. This notion is also supported by previous reports of the presence of IgM in cord blood (which does not cross the placental barrier) against malarial antigens.29–33 A study in Cameroon found that 14% of infants experienced in utero infection and produced IgM responses to as many as 25 different P. falciparum antigens.29 Thus, our findings are consistent in that the fetus mounts an immune response that includes phagocytic uptake of parasites, potentially followed by a more specific humoral response. Therefore, it is conceivable that the PMFVs detected in this study are of fetal origin developed in response to infected erythrocytes that crossed into the fetal circulation. Immunofluorescent assays to determine whether fetal markers are expressed on PMFVs would be needed to confirm the origin of PMFVs.
There are important limitations in the current study. First, the sample size of the current study was low and may have precluded detection of significant associations, particularly in the case–control analysis. Second, assessment of mononuclear cell counts only on the maternal side of the placenta is not optimal, particularly in light of the findings of PMFVs near the fetal side. Histopathologic assessment was not extended to the fetal side, so as not to bias the results of the study through selective evaluation of placentas with evidence of PMFVs. We believe that histopathologic assessment of the fetal side, including mononuclear cell counts on the fetal side and in fetal vessels, can be highly informative and should be evaluated in subsequent studies.
In conclusion, this study demonstrated an increased frequency of placental hemozoin deposition in the absence of current parasitemia and report of clinical symptoms, suggesting that subclinical malaria infections caused by P. vivax and P. falciparum are common in pregnant women living in malaria-endemic areas of Iquitos, Peru. Patients with hemozoin had significantly increased mononuclear cells in the IVS than controls. Most importantly, this is the first report of the presence of PMFVs of the placenta. This phenomenon indicates that in utero exposure and true congenital infection may occur frequently in this region, and that the fetus may be capable of mounting a pro-inflammatory immune response necessary for effective clearance of parasites.
Footnotes
Financial support: This study was supported by the Gorgas Memorial Institute, the Centers for Disease Control and Prevention (grant UR3/CCU 418652 to Falgunee K. Parekh), by RO1 grant Al064831 from the National Institute of Health/National Institute of Allergy and Infectious Disease, and the American Society of Tropical Medicine and Hygiene.
Authors' addresses: Falgunee K. Parekh, 20408 Shore Harbour Drive, #P, Germantown, MD 20874. Billie B. Davison, 300 Reine Street, Mandeville, LA 70471. Dionicia Gamboa, Instituto de Medicina Tropical Alexander von Humboldt and Departmento de Bioquimica, Biologia Molecular y Farmacologia, Facultad de Ciencias y Filosofia, Universidad Peruana Cayetano Heredia, Lima, Peru. Jean Hernandez, Laboratorio de Investigacion en Productos Naturales Antiparasitoarios de la Amazonia, Pasaje Los Paujiles S/N, AA.HH, Nuevo San Lorenzo Iquitos, Peru. OraLee H. Branch, 341 E. 25th Street, OPH-210, New York, NY 10010.
References
- 1.Anchang-Kimbi JK, Achidi EA, Nkegoum B, Sverremark-Ekstrom E, Troye-Blomberg M. Diagnostic comparison of malaria infection in peripheral blood, placental blood and placental biopsies in Cameroonian parturient women. Malar J. 2009;8:126. doi: 10.1186/1475-2875-8-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rogerson SJ, Mkundika P, Kanjala MK. Diagnosis of Plasmodium falciparum malaria at delivery: comparison of blood film preparation methods and of blood films with histology. J Clin Microbiol. 2003;41:1370–1374. doi: 10.1128/JCM.41.4.1370-1374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGready R, Davison BB, Stepniewska K, Cho T, Shee H, Brockman A, Udomsangpetch R, Looareesuwan S, White NJ, Meshnick SR, Nosten F. The effects of Plasmodium falciparum and P. vivax infections on placental histopathology in an area of low malaria transmission. Am J Trop Med Hyg. 2004;70:398–407. [PubMed] [Google Scholar]
- 4.Matteelli A, Caligaris S, Castelli F, Carosi G. The placenta and malaria. Ann Trop Med Parasitol. 1997;91:803–810. doi: 10.1080/00034989760563. [DOI] [PubMed] [Google Scholar]
- 5.Bulmer JN, Rasheed FN, Francis N, Morrison L, Greenwood BM. Placental malaria. I. Pathological classification. Histopathology. 1993;22:211–218. doi: 10.1111/j.1365-2559.1993.tb00110.x. [DOI] [PubMed] [Google Scholar]
- 6.Bulmer JN, Rasheed FN, Morrison L, Francis N, Greenwood BM. Placental malaria. II. A semi-quantitative investigation of the pathological features. Histopathology. 1993;22:219–225. doi: 10.1111/j.1365-2559.1993.tb00111.x. [DOI] [PubMed] [Google Scholar]
- 7.Leopardi O, Naughten W, Salvia L, Colecchia M, Matteelli A, Zucchi A, Shein A, Muchi JA, Carosi G, Ghione M. Malaric placentas. A quantitative study and clinico-pathological correlations. Pathol Res Pract. 1996;192:892–898. doi: 10.1016/S0344-0338(96)80068-9. discussion 899–900. [DOI] [PubMed] [Google Scholar]
- 8.Ordi J, Ismail MR, Ventura PJ, Kahigwa E, Hirt R, Cardesa A, Alonso PL, Menendez C. Massive chronic intervillositis of the placenta associated with malaria infection. Am J Surg Pathol. 1998;22:1006–1011. doi: 10.1097/00000478-199808000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Galbraith RM, Fox H, Hsi B, Galbraith GM, Bray RS, Faulk WP. The human materno-foetal relationship in malaria. II. Histological, ultrastructural and immunopathological studies of the placenta. Trans R Soc Trop Med Hyg. 1980;74:61–72. doi: 10.1016/0035-9203(80)90012-7. [DOI] [PubMed] [Google Scholar]
- 10.Walter PR, Garin Y, Blot P. Placental pathologic changes in malaria. A histologic and ultrastructural study. Am J Pathol. 1982;109:330–342. [PMC free article] [PubMed] [Google Scholar]
- 11.Yamada M, Steketee R, Abramowsky C, Kida M, Wirima J, Heymann D, Rabbege J, Breman J, Aikawa M. Plasmodium falciparum associated placental pathology: a light and electron microscopic and immunohistologic study. Am J Trop Med Hyg. 1989;41:161–168. doi: 10.4269/ajtmh.1989.41.161. [DOI] [PubMed] [Google Scholar]
- 12.Menendez C, Ordi J, Ismail MR, Ventura PJ, Aponte JJ, Kahigwa E, Font F, Alonso PL. The impact of placental malaria on gestational age and birth weight. J Infect Dis. 2000;181:1740–1745. doi: 10.1086/315449. [DOI] [PubMed] [Google Scholar]
- 13.Rogerson SJ, Pollina E, Getachew A, Tadesse E, Lema VM, Molyneux ME. Placental monocyte infiltrates in response to Plasmodium falciparum malaria infection and their association with adverse pregnancy outcomes. Am J Trop Med Hyg. 2003;68:115–119. [PubMed] [Google Scholar]
- 14.Watkinson M, Rushton DI. Plasmodial pigmentation of placenta and outcome of pregnancy in West African mothers. Br Med J (Clin Res Ed) 1983;287:251–254. doi: 10.1136/bmj.287.6387.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shulman CE, Marshall T, Dorman EK, Bulmer JN, Cutts F, Peshu N, Marsh K. Malaria in pregnancy: adverse effects on haemoglobin levels and birthweight in primigravidae and multigravidae. Trop Med Int Health. 2001;6:770–778. doi: 10.1046/j.1365-3156.2001.00786.x. [DOI] [PubMed] [Google Scholar]
- 16.Ismaili J, van der Sande M, Holland MJ, Sambou I, Keita S, Allsopp C, Ota MO, McAdam KP, Pinder M. Plasmodium falciparum infection of the placenta affects newborn immune responses. Clin Exp Immunol. 2003;133:414–421. doi: 10.1046/j.1365-2249.2003.02243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Desai M, ter Kuile FO, Nosten F, McGready R, Asamoa K, Brabin B, Newman RD. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis. 2007;7:93–104. doi: 10.1016/S1473-3099(07)70021-X. [DOI] [PubMed] [Google Scholar]
- 18.Branch O, Casapia WM, Gamboa DV, Hernandez JN, Alava FF, Roncal N, Alvarez E, Perez EJ, Gotuzzo E. Clustered local transmission and asymptomatic Plasmodium falciparum and Plasmodium vivax malaria infections in a recently emerged, hypoendemic Peruvian Amazon community. Malar J. 2005;4:27. doi: 10.1186/1475-2875-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parekh FK, Hernandez JN, Krogstad DJ, Casapia WM, Branch OH. Prevalence and risk of Plasmodium falciparum and P. vivax malaria among pregnant women living in the hypoendemic communities of the Peruvian Amazon. Am J Trop Med Hyg. 2007;77:451–457. [PMC free article] [PubMed] [Google Scholar]
- 20.Rubio JM, Benito A, Berzosa PJ, Roche J, Puente S, Subirats M, Lopez-Velez R, Garcia L, Alvar J. Usefulness of seminested multiplex PCR in surveillance of imported malaria in Spain. J Clin Microbiol. 1999;37:3260–3264. doi: 10.1128/jcm.37.10.3260-3264.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Capurro H, Konichezky S, Fonseca D, Caldeyro-Barcia R. A simplified method for diagnosis of gestational age in the newborn infant. J Pediatr. 1978;93:120–122. doi: 10.1016/s0022-3476(78)80621-0. [DOI] [PubMed] [Google Scholar]
- 22.Brabin BJ, Romagosa C, Abdelgalil S, Menendez C, Verhoeff FH, McGready R, Fletcher KA, Owens S, D'Alessandro U, Nosten F, Fischer PR, Ordi J. The sick placenta-the role of malaria. Placenta. 2004;25:359–378. doi: 10.1016/j.placenta.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 23.Matteelli A, Donato F, Shein A, Muchi JA, Abass AK, Mariani M, Leopardi O, Maxwell CA, Carosi G. Malarial infection and birthweight in urban Zanzibar, Tanzania. Ann Trop Med Parasitol. 1996;90:125–134. doi: 10.1080/00034983.1996.11813036. [DOI] [PubMed] [Google Scholar]
- 24.Wickramasuriya GA. Some observations on malaria occurring in association with pregnancy. J Obstet Gynaecol Br Empire. 1935;42:816–834. [Google Scholar]
- 25.Davison BB, Cogswell FB, Baskin GB, Falkenstein KP, Henson EW, Krogstad DJ. Placental changes associated with fetal outcome in the Plasmodium coatneyi/rhesus monkey model of malaria in pregnancy. Am J Trop Med Hyg. 2000;63:158–173. doi: 10.4269/ajtmh.2000.63.158. [DOI] [PubMed] [Google Scholar]
- 26.Malhotra I, Mungai P, Muchiri E, Kwiek JJ, Meshnick SR, King CL. Umbilical cord-blood infections with Plasmodium falciparum malaria are acquired antenatally in Kenya. J Infect Dis. 2006;194:176–183. doi: 10.1086/505150. [DOI] [PubMed] [Google Scholar]
- 27.Lo YM, Lo ES, Watson N, Noakes L, Sargent IL, Thilaganathan B, Wainscoat JS. Two-way cell traffic between mother and fetus: biologic and clinical implications. Blood. 1996;88:4390–4395. [PubMed] [Google Scholar]
- 28.Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF, III, Petraglia F. Inflammation and pregnancy. Reprod Sci. 2009;16:206–215. doi: 10.1177/1933719108329095. [DOI] [PubMed] [Google Scholar]
- 29.Xi G, Leke RG, Thuita LW, Zhou A, Leke RJ, Mbu R, Taylor DW. Congenital exposure to Plasmodium falciparum antigens: prevalence and antigenic specificity of in utero-produced antimalarial immunoglobulin M antibodies. Infect Immun. 2003;71:1242–1246. doi: 10.1128/IAI.71.3.1242-1246.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Achidi EA, Salimonu LS. Malaria parasitaemia and immunoglobulin levels in paired maternal-cord sera from south western Nigeria. Afr J Med Sci. 1997;26:167–170. [PubMed] [Google Scholar]
- 31.Chizzolini C, Trottein F, Bernard FX, Kaufmann MH. Isotypic analysis, antigen specificity, and inhibitory function of maternally transmitted Plasmodium falciparum-specific antibodies in Gabonese newborns. Am J Trop Med Hyg. 1991;45:57–64. doi: 10.4269/ajtmh.1991.45.57. [DOI] [PubMed] [Google Scholar]
- 32.Deloron P, Dubois B, Le Hesran JY, Riche D, Fievet N, Cornet M, Ringwald P, Cot M. Isotypic analysis of maternally transmitted Plasmodium falciparum-specific antibodies in Cameroon, and relationship with risk of P. falciparum infection. Clin Exp Immunol. 1997;110:212–218. doi: 10.1111/j.1365-2249.1997.tb08319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Egwunyenga OA, Ajayi JA, Duhlinska-Popova DD. Transplacental passage of Plasmodium falciparum and seroevaluation of newborns in northern Nigeria. J Commun Dis. 1995;27:77–83. [PubMed] [Google Scholar]


