Abstract
Severe Plasmodium vivax malaria in adults has been reported from Bikaner (northwestern India) but the reports on children are scanty. This prospective study was done on 303 admitted children of malaria. The diagnosis was done by peripheral blood smear and rapid diagnostic test. Further confirmation of severe P. vivax monoinfection was done by polymerase chain reaction (PCR). The proportion of P. falciparum, P. vivax, and mixed (P. falciparum and P. vivax) infection was 61.01%, 33.99%, and 4.95%, respectively. Severe disease was present in 49.5% (150/303) children with malaria, with the risk greatest among P. vivax monoinfection (63.1% [65/103]) compared with P. falciparum, either alone (42.7% [79/185]; odds ratio [OR] = 2.3 [95% confidence interval (CI) = 1.40–3.76], P = 0.001) or mixed infections (40% [6/15]; OR = 2.57 [95% CI = 0.88–7.48]). In children < 5 years of age, the proportion of severe malaria attributable to P. vivax rose to 67.4% (31/46) compared with 30.4% (14/46) of P. falciparum (OR = 4.7 [95% CI = 2.6–8.6], P < 0.0001) and 2.2% (1/46) of mixed infection (OR = 92 [95% CI = 24.6–339.9], P < 0.0001). The proportion of patients having severe manifestations, which included severe anemia, thrombocytopenia, cerebral malaria, acute respiratory distress syndrome, hepatic dysfunction, renal dysfunction, abnormal bleeding was significantly high in association with P. vivax monoinfection in 0–5 year age group, while the same was significantly high in association with P. falciparum monoinfection in 5–10 year age group. Similarly P. vivax monoinfection had greatest propensity to cause multiorgan dysfunction in 0–5 year age group (34.1% [17/41], P < 0.0001) in comparison to P. falciparum monoinfection, which had similar propensity in 5–10 year age group (36.8% [35/95], P = 0.039). Plasmodium vivax monoinfection was almost equally serious to cause significant mortality in comparison to P. falciparum (case fatality rate of severe P. vivax was 3.9% versus 3.2% of severe P. falciparum malaria; P = 1.0). This study reaffirms the evidence of severe P. vivax malaria in children in Bikaner.
Introduction
Malaria is endemic in the tropics and subtropics causing 247 million infections worldwide and 3.3 billion world's population were at risk in 2006 causing nearly a million deaths of which 88% occurred in sub-Saharan African children < 5 years of age.1 The burden of malaria in Southeast Asia has been underappreciated, despite recent evidence suggesting that the continent contributes almost 40% of the world's malaria.2 India contributes 77% of the total malaria in Southeast Asia and about 95% of the population of moderate to high risk of malaria in SEA Region is living in India.1,3,4 In sub-Saharan Africa the overwhelming majority of malaria-associated morbidity and mortality occurs with Plasmodium falciparum infections. However, Plasmodium vivax accounts for 50% of the malaria prevalence in Asia, and yet the morbidity associated with this infection and its spectrum of disease is largely ignored. Most of the research and published literature on malaria focus on P. falciparum and much less on P. vivax,5,6 which is caused by the very high burden of mortality attributed to the falciparum species in Africa.7
Although P. vivax is widely regarded as benign, its propensity to recur is increasingly recognized by clinicians in endemic areas to result in appreciable disease, particularly in young children.8,9 Moreover, most of the published literature consists of case reports or small descriptive clinical series on severe P. vivax lacking denominators.9–11 The relative contribution of P. vivax versus P. falciparum to severe morbidity has not been properly assessed, except for one study from Thailand, which found very little severe disease and no death caused by P. vivax.12 It is only recently that severe disease caused by P. vivax has received more attention after the recent publications from India,13–16 Indonesia,17,18 and Papua New Guinea.19
The previous reports on severe P. vivax malaria in children from Indonesia and Papua New Guinea did not attempt to classify the whole spectrum of severe manifestations of malaria but restricted to anemia, coma, and respiratory distress only and also did not rule out other associated comorbid conditions.18,19 However, in this prospective, hospital-based clinical observational study, we tried to investigate the patients for a whole spectrum of severe illness and attempt was also made to be reasonably sure to rule out possibilities of other comorbid conditions in a scientific manner. We also performed polymerase chain reaction (PCR) confirmation of all the patients of severe P. vivax malaria.
Material and Methods
Study site.
This prospective study was carried out at the Department of Pediatrics, Sardar Patel Medical College and Associated Group of Hospitals, Bikaner, Rajasthan, India from August 2007 to November 2008. The prevalence of admitted febrile patients in the post rainy season is almost 32% of the total admission, whereas the rest of the year it remains about 3–4%. Hospital guideline requires blood film examination for all the patients presenting with history of fever.
Study procedures.
This study was conducted on admitted children of malaria in which the diagnosis was made by peripheral blood smear (PBF) and rapid diagnostic test (RDT). Categorization of severe malaria and treatment was done according to World Health Organization (WHO) guidelines (2000).20 The confirmation of severe P. vivax malaria was done by PCR. The study plan was approved by the hospital research committee and a written consent by parents was mandatory. There was a study team of researchers, which included faculty members of departments of Pediatrics and Medicine along with full-time research workers working on different projects of malaria. All informations were collected on a standard proforma, which was common for adult and pediatric patients.
Selection criteria.
Pediatric malaria patients with severe manifestations and evidence of asexual phase of malaria parasite in PBF or positive RDT were included in the study.
Exclusion criteria.
Children whose parents refused to give the written consent or had other concurrent illness were not included in the study.
Laboratory procedures.
Diagnostic methods used to detect malaria parasites were conventional thick and thin PBFs, stained with Giemsa stain and examined under oil immersion. The slide was considered negative when there were no parasites in the 100 high-power field. The RDTs were based on detection of specific Plasmodium antigen, Lactate dehydrogenase (OptiMal test; Diamed AG, Cressier sur Morat, Switzerland) and histidine-rich protein-2 (Falcivax test; Zephyr Biomedical System, Goa, India). Apart from PBF and RDT, other laboratory investigations, which were done in all the patients of severe malaria, included complete blood count, platelet count, bleeding time, clotting time, blood glucose, blood urea, serum creatinine, serum bilirubin (total and direct), serum aspartate aminotranferase (AST), serum alanine aminotransferase (ALT), serum alkaline phosphatase, complete urine analysis, electrocardiogram, and appropriate blood test to rule out typhoid fever (widal test), leptospirosis (enzyme immunoassay for the differential detection of immunoglobulin G [IgG] and IgM antibodies), infectious mononucleosis (monospot test), and dengue infection (differential detection of IgG and IgM antibodies).
Depending upon the clinical manifestations, other specific tests included like reticulocyte count and peripheral blood smear examination for type of anemia; prothrombin time for bleeding diathesis; skigram chest, serum electrolytes, and arterial blood gas analysis for ARDS; fundus examination, cerebrospinal fluid (CSF) examination, computerized tomography (CT) of the head and electroencephalography (EEG) for cerebral malaria (CM); ultrasonography of whole abdomen and specific test for hepatitis B and C in hepatic dysfunction and jaundice; and glucose-6-phosphate dehydrogenase (G6PD) enzyme level (kinetic method: G-SIX Kit, Crest Biosystems, Goa) for hemolysis. Blood culture was taken on brain–heart infusion broth in every child who was having continuous high grade fever > 101°F for more than 24 hours after admission.
The PCR confirmation was done in all the children having severe manifestations with evidence of P. vivax monoinfection on PBF and/or RDT. The PCR studies were targeted against the 18S ribosomal RNA gene of the parasite and used 1 genus-specific 5¢ primer and 2 species-specific 3¢ primers in the same reaction mixture. Some of the primer sequences were modified for this study:
-
1.
5¢–ATCAGCTTTTGATGTTAGGGT ATT–3¢, genus specific;
-
2.
5¢–TAACAAGGACTTCCAAGC–3¢, P. vivax specific; and
-
3.
5¢–GCTCAAAGATACAAATATAAGC–3¢, P. falciparum specific.
Each sample was subjected to a minimum of four rounds of PCR with various template amounts to eliminate overlooking P. falciparum co-infection.
All of the clinical syndromes were classified according to WHO (2000) criteria20 for severe malaria except for multiorgan dysfunctions (MODs), which has not been described properly by WHO for pediatric malaria. Most of the studies had considered the involvement of two or more than two organs to take it as MODs.16
Pre-admission drugs included antibiotics and antipyretics. Antimalarial treatment was given in the hospital according to WHO guideline (artisunate).
Results
During the study period (August 2007–November 2008) 800 children with fever were admitted into the hospital. Out of which peripheral blood film examination and/or rapid diagnostic test were positive for malaria in 331 (41.4%) children. Twenty-eight children were not included in the study because of evidence of concomitant illness in 8 patients (measles 2, chickenpox 2, dengue 1, aplastic anemia 1, leukemia 1, and infectious mononucleosis 1) and unable to get written consent in 16 children and problems related to confirmation of species in 4 children. Thus, the subsequent analysis was done in 303 children. The age-stratified composition of different species of malaria was P. vivax monoinfection 33.9% (103/303) (children aged 0–5 years 42.3% [41/97]; in 5–10 years 30.1% [44/146]; > 10 years 30% [18/60]) compared with P. falciparum monoinfection 61.01% (185/303) (children aged 0–5 years 51.5% [50/97]; in 5–10 years 65.1% [95/146]; > 10 years 66.7% [40/60]) and mixed (Pf + Pv) infection 4.95% (15/303) (children aged 0–5 years 6.2% [6/97]; in 5–10 years 4.8% [7/146]; > 10 years 3.3% [2/60]) (Figure 1). The male/female child ratio was 70.6% versus 29.4%. However, the proportion of female children was higher in P. vivax infections (33% [34/103]) compared with P. falciparum infections (27.1% [50/185]; odds ratio [OR] = 1.3 [95% confidence interval (CI) = 0.79–2.24], P = 0.352).
Figure 1.
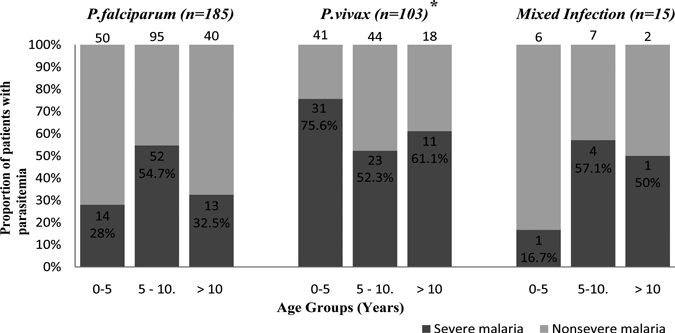
For each age group the number on the top of the bar represents the total number of children with the species of infection. *Confirmed by polymerase chain reaction (PCR) examination.
Severe malaria.
Out of the 303 children with malaria, 150 (49.5%) were having severe disease according to WHO criteria (2000). Although 52.7% (79/150) of severe malaria was caused by P. falciparum, the risk was greater among children with P. vivax infection (63.1% [65/103]) than in those with P. falciparum infection alone (42.7% [79/185], OR = 2.3 [95% CI = 1.40–3.76], P = 0.001) (Figure 1, Table 1). The number of P. vivax malaria children were significantly greater in 0–5 year age group (75.7% [31/41], OR = 2.55 [95% CI = 1.08–6.02], P = 0.038) compared with P. falciparum infection, which had more numbers of severe cases in 5–10 year age group (54.7% [52/95]; OR = 2.82 [95% CI = 1.54–5.16], P = 0.001). The proportion of children with severe disease varied with age, and this relationship differed among different species. The proportion of female children having severe malaria was significantly higher in P. vivax infections (41.5% [27/65]) compared with P. falciparum infections (25.3% [20/79]; OR = 2.1 [95% CI = 1.0–4.2], P = 0.05). This predominance of female children with severe P. vivax infection was more apparent from 0–5 year age group (57.1% [4/7]) and reaching 80% (12/15) in 5–10 year age group and 100% (11/11) in > 10 year age group. The peripheral blood film of patients of severe vivax malaria showed predominantly trophozoites and the density of parasites was 7,600–60,000/mm3 (mean ± SD = 29855.38 ± 12846.77/mm3). The details of individual severe manifestations in different age groups according to Pv and Pf monoinfection and mixed infections are depicted in Table 2 and Figure 2.
Table 1.
The distribution of children according to species and age groups
| Category | Age group in years | Total | ||||||
|---|---|---|---|---|---|---|---|---|
| 0–5 | 5–10 | > 10 | ||||||
| N | % | N | % | n | % | n | % | |
| Total malaria patients | 97 | 146 | 60 | 303 | ||||
| Plasmodium falciparum monoinfection | 50 | 95 | 40 | 185 | ||||
| Plasmodium vivax monoinfection | 41 | 44 | 18 | 103 | ||||
| Mixed (Pf + Pv) infection | 6 | 7 | 2 | 15 | ||||
| Total severe malaria patients | 46 | 47.4 | 79 | 54.2 | 25 | 41.7 | 150 | 49.5 |
| Severe P. falciparum monoinfection | 14 | 28 | 52 | 54.7 | 13 | 32.5 | 79 | 42.7 |
| Severe P. vivax monoinfection* | 31 | 75.6 | 23 | 52.3 | 11 | 61.1 | 65 | 63.1 |
| Severe mixed (Pf + Pv) infection | 1 | 16.7 | 4 | 57.1 | 1 | 50 | 6 | 40 |
Confirmed by polymerase chain reaction (PCR) examination.
Table 2.
The distribution of organ dysfunction in different species
| Markers of severity | P. falciparum monoinfection | P. vivax monoinfection | Mixed (Pf + Pv) infection | Total | ||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | n | % | |
| All patients | 185 | 103 | 15 | 303 | ||||
| Severe illness | 79 | 42.7 | 65† | 63.1 | 6 | 40 | ||
| Anemia alone | 16 | 8.6 | 18 | 17.5 | 1 | 6.6 | 37 | 12.2 |
| Thrombocytopenia alone | 5 | 2.7 | 9 | 8.7 | 4 | 26.7 | 19 | 6.3 |
| Cerebral malaria alone | 3 | 1.6 | 2 | 1.9 | 0 | 0 | 8 | 2.6 |
| ARDS* alone | 2 | 1.1 | 1 | 0.9 | 0 | 0 | 7 | 2.3 |
| Hepatic dysfunction alone | 3 | 1.6 | 2 | 1.9 | 0 | 0 | 7 | 2.3 |
| Renal dysfunction alone | 1 | 0.5 | 1 | 0.9 | 0 | 0 | 4 | 1.3 |
| Abnormal bleeding alone | 1 | 0.5 | 1 | 0.9 | 0 | 0 | 3 | 0.9 |
| Multiorgan dysfunction | 48 | 25.9 | 31 | 30.1 | 1 | 6.6 | 65 | 21.4 |
ARDS = acute respiratory distress syndrome.
Confirmed by polymerase chain reaction (PCR) examination.
Figure 2.
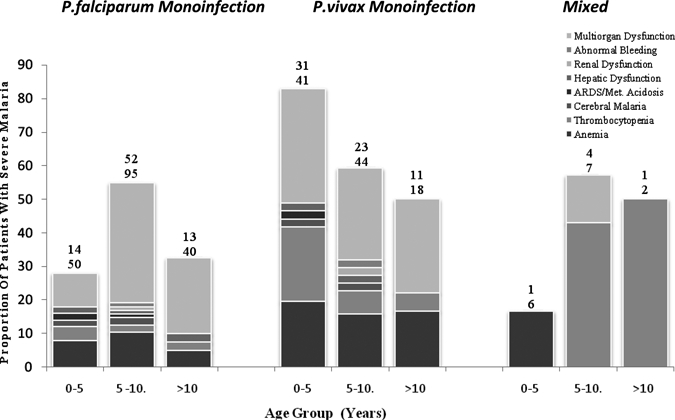
For each age group the upper number above each column represents the total number of children with severe disease (numerator), and the lower number is the total number of children with the species of infection (denominator).
Severe anemia (hemoglobin < 5 g/dL) was present in 81% (64/79), 75.4% (49/65), and 33.3% (2/6) children having P. falciparum, P. vivax, and mixed infections, respectively. Among children in 0–5 year age group this proportion was 26% (13/50), 75.6% (31/41), and 16.7% (1/6), whereas in 5–10 year age group it was 44.2% (42/95), 22.7% (10/44), and 14.3% (1/7) and in > 10 year age group it was 22.5% (9/40), 44.4% (8/18), and 0%, respectively. Thus, children in 0–5 year age group had more predilection for severe P. vivax monoinfection (OR = 9.3 [95% CI = 3.68–23.46], P < 0.0001), whereas children in 5–10 year age group were more predilicted to P. falciparum monoinfection (OR = 2.88 [95% CI = 1.33–6.22], P = 0.007). In children of severe anemia caused by P. vivax malaria, mean ± SD hemoglobin level was 4.142 ± 0.662 mg % and mean reticulocyte count was 1.1 ± 0.422% (normal range 0.5–1.5%). Type of anemia was normocytic normochromic in 35 (71.43%) and microcytic hypochromic in 14 (28.57%) children (OR = 6.3 [95% CI = 3.39–11.52], P < 0.0001). In children of severe P. vivax malaria, mean ± SD level of total leukocyte count was 9743.1372 ± 4710.839 cells/mm3.
Thrombocytopenia (platelet count < 1,00,000/µL) was present in 60.8% (48/79), 61.5% (40/65), and 83.3% (5/6) children having P. falciparum, P. vivax, and mixed infections, respectively. Among children in 0–5 year age group this proportion was 18% (9/50), 61% (25/41), and 50% (3/6), whereas in 5–10 year age group it was 34.7% (33/95), 18.2% (8/44), and 14.3% (1/7) and in > 10 year age group it was 15% (6/40), 38.9% (7/18), and 50% (1/2), respectively. Thus, children in 0–5 year age group had more predilection for severe P. vivax monoinfection (OR = 5.73 [95% CI = 2.36–13.91], P < 0.0001), whereas children in 5–10 year age group were more predilicted to P. falciparum monoinfection (OR = 2.48 [95% CI = 1.09–5.63], P = 0.035). In children of severe P. vivax malaria, thrombocytopenia was found in 40 (61.54%) children with mean ± SD platelet count 54175 ± 21256.537/µL and minimum platelet count of 13,000/µL. Platelet count < 20,000/µL were present in 3 (7.5%); between 20,000/µL and 50,000/µL in 12 (30%) and between 50,000/µL and 1,000,000/µL in 25 (62.5%) children. Out of these 40 children, bleeding manifestations were present only in 7 children (10.77%) in the form of epistaxis (57.1% [4/7]) and hematemesis (42.9% [3/7]). All of these 7 children required platelet transfusion.
Cerebral malaria was present in 21.5% (17/79) and 13.9% (9/65) children having P. falciparum and P. vivax infections, respectively. Among children in 0–5 year age group this proportion was 20% (10/50) and 12.2% (5/41), whereas in 5–10 year age group it was 4.2% (4/95) and 6.8% (3/44) and in > 10 year age group it was 7.5% (3/40) and 5.6% (1/18), respectively. Thus, risk of CM was not different in different species and in different age groups. There were a total 9 children (13.85%) of cerebral malaria caused by P. vivax infection with Blantyre coma scale ≤ 2. Multiple convulsions were present in 77.8% (7/9) children and bilateral upper motor neuron (UMN) lesion signs in 66.7% (6/9). Four (44.4%) children presented with semidilated pupil reacting to light and 3 (33.3%) children had pallor optic disc in fundus examination. No children had evidence of hemorrhage and papilledema in fundus examination. The CSF examination, CT scan of head, and EEG were unremarkable in all the children. Out of these 9 children, 4 (44.4%) had multiorgan derangement. Comparing the rate of each neurological sign between P. vivax and P. falciparum severe malaria, multiple convulsions were as frequent in both species (22% for both, χ2 = 0, P = 0.96); and coma (4% versus 2%, respectively, χ2 = 1.25, P = 0.26).
Respiratory distress was present in 17.7% (14/79) and 10.8% (7/65) children having P. falciparum and P. vivax infections, respectively. Among children in 0–5 year age group this proportion was 2% (1/50) and 14.6% (6/41), whereas in 5–10 year age group it was 12.6% (12/95) and 2.3% (1/44) and in > 10 year age group it was 2.5% (1/40) and 0% (0/18), respectively. Thus, children in 0–5 year age group had more predilection for severe P. vivax monoinfection (OR = 9.43 [95% CI = 1.4–61.59], P = 0.039), whereas children in 5–10 year age group were more predilicted to P. falciparum monoinfection (OR = 7.23 [95% CI = 1.16–44.31], P = 0.034). In arterial blood gas analysis of severe P. vivax children, PaO2 (mean value 91.1 mm/Hg) and O2 saturation (mean value 93.4%) was low in 28.6% cases showing hypoxemia. All the patients had a lower level of PaCO2 with mean level of 25.6 mm/Hg caused by hyperventilation and CO2 washout showing respiratory compensation for metabolic acidosis. The mean arterial pH was 7.14 with mean bicarbonate level 12.1 mmol/L and mean base excess level 17.9 mmol/L. The metabolic acidosis was presented clinically as vomiting (7/7 [100%]), diarrhea (3/7 [42.9%]), and dehydration (7/7 [100%]). None of the children showed hypoglycemia, and urinary sugar and ketone bodies were also not present in any case.
Hepatic dysfunction was present in 44.3% (35/79), 26.2% (17/65), and 16.7% (1/6) children having P. falciparum, P. vivax, and mixed infections, respectively. Among children in 0–5 year age group this proportion was 16% (8/50), 36.6% (15/41), and 16.7% (1/6), whereas in 5–10 year age group it was 25.3% (24/95), 2.3% (1/44), and 0% and in > 10 year age group it was 7.5% (3/40), 5.6% (1/18), and 0% (0/2), respectively. Thus, children in 0–5 year age group had more predilection for severe P. vivax monoinfection (OR = 3.01 [95% CI = 1.18–7.70], P = 0.031), whereas children in 5–10 year age group were more predilicted to P. falciparum monoinfection (OR = 16.9 [95% CI = 2.79–100.90], P < 0.0001). Out of the 17 children of hepatic dysfunction in severe P. vivax monoinfection mean ± SD level of serum bilirubin was 4.829 ± 2.229 mg/dL. Mild jaundice (3–5 mg/dL) was present in 12 children (70.6%), whereas moderate jaundice (5–10 mg/dL) in 4 (23.5%) and severe jaundice (> 10 mg/dL) was present in only 1 (5.9%) child. Jaundice was predominantly the conjugated type (82.4% [14/17]) with mean level of conjugated bilirubin of 2.953 ± 2.243 mg/dL. The mean level of AST (537.471 ± 776.373 IU/L) was higher than that of ALT (484.059 ± 699.020 IU/L). Total protein and serum albumin level was also decreased with mean level 4.512 ± 0.507 gm/dL and 2.364 ± 0.609 gm/dL, respectively. The mean level of serum alkaline phosphatase (SAP) in children having severe P. vivax monoinfection and hepatic dysfunction was 602.706 ± 237.263 IU/L. On clinical examination it was associated with hepatosplenomegaly (47%), splenomegaly (17.6%), and hepatomegaly (5.9%), which was also confirmed by ultrasonography of abdomen without any evidence of parenchymal lesion. Six (35.3%) children were having signs of hepatic encephalopathy, four in grade II and two in grade I.
Renal dysfunction was present in 30.4% (24/79) and 15.4% (10/65) children having P. falciparum and P. vivax infections, respectively. Among children in 0–5 year age group this proportion was 6% (3/50) and 19.5% (8/41), whereas in 5–10 year age group it was 14.7% (14/95) and 2.3% (1/44) and in > 10 year age group it was 15% (6/40) and 5.6% (1/18), respectively. Thus, children in 0–5 year age group had more predilection for severe P. vivax monoinfection (OR = 4.28 [95% CI = 1.14–15.92], P = 0.049), whereas children in 5–10 year age group were more predilicted to P. falciparum monoinfection (OR = 8.64 [95% CI = 1.40–52.57], P = 0.020). Out of the 10 children of renal dysfunction in severe P. vivax monoinfection the mean level of blood urea and serum creatinine was 193.9 ± 47.153 mg/dL and 3.680 ± 0.653 mg/dL, respectively. The mean serum potassium level was 5.13 ± 0.967 meq/L. Seven (70%) children were having oliguric renal failure, whereas 3 (30%) were of nonoliguric renal failure. Urine examination showed granular cast (50%), pus cell (50%), microscopic hematuria (20%), albuminurea (20%), and hemoglobinuria (10%). Ultrasonography of abdomen showed normal kidneys without any parenchymal lesion.
Abnormal bleeding was present in 17.7% (14/79), 10.8% (7/65), and 16.7% (1/6) children having P. falciparum, P. vivax, and mixed infections, respectively. Among children in 0–5 year age group this proportion was 2% (1/50), 14.6% (6/41), and 0%, whereas in 5–10 year age group it was 12.6% (12/95), 2.3% (1/44), and 0% and in > 10 year age group it was 2.5% (1/40), 0% (0/18), and 50% (1/2), respectively. Thus, children in 0–5 year age group had more predilection for severe P. vivax monoinfection (OR = 9.43 [95% CI = 1.4–61.59], P = 0.039), whereas children in 5–10 year age group were more predilicted to P. falciparum monoinfection (OR = 7.23 [95% CI = 1.16–44.31], P = 0.034). Out of the 7 children of bleeding manifestation in severe P. vivax monoinfection 57.1% (4/7) had epistaxis and 42.9% (3/7) had hematemesis. Prothrombin time was increased in only 42.9% (3/7) children with mean value of 31 seconds (control time 16 seconds). Bleeding time and clotting time were normal in all the children. All the children having abnormal bleeding in this study had thrombocytopenia (mean platelet count 58142.85 cells/c.mm).
Hemoglobinuria was present in 4 (5.1% [4/79]) children of severe P. falciparum infection (all were in 5–10 year age group) and 2 (3.1% [2/65]) children of severe P. vivax infection (both were in 0–5 year age group; 1 child expired in 4 days with hepatic and renal dysfunction and another recovered in 5 days). Glucose-6-phosphate dehydrogenase enzyme level was normal in all the children. Shock was present in 2.5% (2/79) children of severe P. falciparum infection (both were in 5–10 year age group) and 3.1% (2/65) children of severe P. vivax infection (both were in 0–5 year age group). One (1.5% [1/65]) case had pulmonary edema with P. vivax malaria presenting with multiorgan dysfunction as anemia (Hb 3.6 gm %), thrombocytopenia (platelet 54,000/µL), metabolic acidosis (pH 7.1; HCO3– 11.2 mmol/L), respiratory distress and renal failure (S. Creatinine 4.8 mg/dL). The child was managed according to WHO guidelines but expired after 3 days of admission. Dyselectrolytemia was present in 15.2% children of severe P. falciparum infection, 9.2% children of severe P. vivax infection, and 16.7% children of severe mixed infection. In children of severe P. vivax malaria, mean ± SD level of Na+ was 125.28 ± 6.185 mmol/L, K+ was 4.8 ± 0.482 mmol/L, iCa++ was 0.99 ± 0.239 mmol/L, and HCO3− was 12.1 ± 0.844 mmol/L.
Multiorgan dysfunction (involvement of two or more than two organs) was present in 60.8% (48/79), 47.7% (31/65), and 16.7% (1/6) children having P. falciparum, P. vivax, and mixed infections, respectively. Among children in 0–5 year age group this proportion was 10% (1/50), 41.5% (17/41), and 0%, whereas in 5–10 year age group it was 36.8% (35/95), 20.5% (9/44), and 14.3% (1/7) and in > 10 year age group it was 20% (8/40), 27.8% (5/18), and 0% (0/2), respectively. Thus, children in 0–5 year age group had more predilection for severe P. vivax monoinfection (OR = 7.23 [95% CI = 2.45–21.11], P < 0.0001), whereas children in 5–10 year age group were more predilicted to P. falciparum monoinfection (OR = 2.39 [95% CI = 1.08–5.29], P = 0.039).
Malnutrition was associated in 62% (49/79), 69.2% (45/65), and 50% (3/6) children having P. falciparum, P. vivax, and mixed infection, respectively, whereas diarrhea and/or vomiting were present in 25.3% (20/79), 32.3% (21/65), and 33.3% (2/6) children having P. falciparum, P. vivax, and mixed infection, respectively.
Mortality.
A total of 10 (3.3%) children with malaria died in which 60% of deaths occurred within 72 hours and 40% of deaths thereafter. The case-fatality rate in children with P. vivax monoinfection was 3.9% (4/103) and that in children with P. falciparum was (3.2% [6/185]), overall P = 0.749. Severe malaria in children had a risk of death of 6.7% (10/150) in which P. vivax and P. falciparum both species attributed for 6.2% (4/65) and 7.6% (6/79), respectively, overall P = 0.749. Overall mortality was significantly higher in 0–5 year age group (7.2% [7/97]) compared with > 5 year age group (1.5% [3/206]) (OR = 5.26 [95% CI = 1.45–19.09], P = 0.014) but there was no significant difference in mortality in respect of sex (male 3.3% [7/214]; female 3.4% [3/89], P = 1.0) (Table 3).
Table 3.
Factors associated with mortality in different species*
| Category | Risk factor | Percent mortality rate (n/total n) | OR [95% CI]; P value |
|---|---|---|---|
| Species of infection | Plasmodium falciparum monoinfection | 3.2 (6/185) | 0.83 (0.25–0.80); P = 0.749 |
| Plasmodium vivax monoinfection† | 3.9 (4/103) | ||
| Mixed (Pf + Pv) infection | 0 (0/15) | ||
| Age | Overall | 3.3 (10/303) | 5.26 (1.45–19.09); P = 0.014 |
| 0–5 years | 7.2 (7/97) | ||
| 5–10 years | 1.5 (3/146) | ||
| > 10 years | 0 (0/60) | ||
| Markers of severity | Cerebral malaria alone | 60 (3/5) | 17.79 (2.98–105.53); P = 0.008 |
| Multiorgan dysfunction | 8.8 (7/80) | ||
| Sex | Females | 3.4 (3/89) | 1.03 (0.28–3.76); P = 1.0 |
| Males | 3.3 (7/214) |
OR = odds ratio; CI = confidence interval.
Confirmed by polymerase chain reaction (PCR) examination.
Major predictors of death in the children population.
Major predictors of death in this study emerged as cerebral malaria alone (60% [3/5]) and multiorgan dysfunction (8.8% [7/80]). Shock and spontaneous bleeding were excluded from this analysis because of the very small number of children in each group. In addition, we observed a clear overlap among these two major predictors of death. In children of severe P. falciparum infection, 34 had MODs without CM, 14 had MODs with CM, and 3 had CM only. The major predictor of death in these children was cerebral malaria (66.7% [2/3]). Although MODs without CM had mortality of 5.9% (2/34), it increased to 14.3% (2/14) when concomitant CM was present. Similarly in children of severe P. vivax malaria, 24 had MODs without CM, 7 had MODs with CM, and 2 had CM only. Mortality was highest in CM only subgroup (50% [1/2]), whereas MODs without CM subgroup had mortality of 4.2% (1/24), which increased to 28.6% (2/7) when concomitant CM was present (Figure 3).
Figure 3.
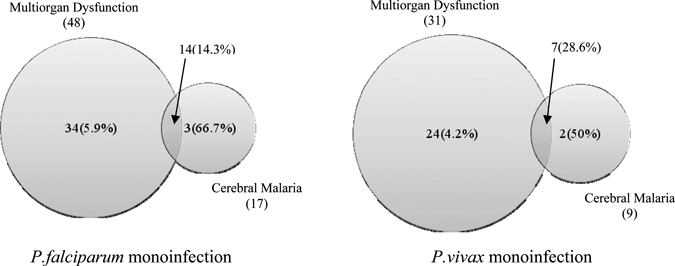
Major predictors of death in severe malaria in difference species. (Total numbers are given with mortality in percentage in parenthesis. The sizes of the circles are not to scale.)
Malnutrition, diarrhea and/or vomiting, and dyselectrolytemia were associated in 66.7%, 33.3%, and 83.3% mortality in severe P. falciparum malaria, respectively, whereas in severe P. vivax malaria these factors were associated in 75%, 25%, and 100% mortality, respectively.
Discussion
Although P. vivax malaria is considered to be benign malaria with a very low case-fatality ratio, it may still cause a severe and debilitating febrile illness as in P. falciparum malaria, especially in children.1,20 Studies from Asia and the Pacific region have shown that P. vivax malaria accounts for a substantial proportion of hospitalized patients.12,21–24 The dominant paradigm of P. vivax being a benign infection has been challenged recently by studies from India,16 Indonesia,17,18 Papua New Guinea,19 and Pakistan,25 which documented hospitalization and severe disease including deaths in patients of P. vivax monoinfection. Population-based studies in Venezuela have also showed a rising trend in deaths associated with P. vivax malaria, particularly in children.26 There are many case reports on various clinical manifestations of severe P. vivax malaria in children from different parts of the world including India,27–37 but in most of these studies the diagnosis was made by PBF and/or RDT without molecular diagnostic confirmation (PCR). Thus, there are always chances of species misidentification and missing the mixed infection thereby lacking authenticity.
The aim of the present clinic-epidemiological study from Bikaner (north western part of India) is to describe the evidence of severe P. vivax monoinfection in children in which the diagnosis was confirmed by PCR to overcome the problem of results obtained by PBF and/or RDT along with the description of clinical spectrum of severe malaria in children with P. vivax (Pv), P. falciparum (Pf) monoinfection, and mixed Plasmodia (Pf + Pv) infection. This prospective study on severe malaria included 303 patients of malaria in which severe disease was present in 150 (49.5%) patients, with a greater risk among patients infected with P. vivax (63.1%) than in those with P. falciparum (42.7%) (OR = 2.3). However, the case fatality rates of 3.9% of patients with P. vivax monoinfection did not differ significantly from the 3.2% mortality among patients with P. falciparum monoinfection.
In the 0–5 year age group children, we observed more patients of severe manifestations in P. vivax monoinfection as compared with P. falciparum monoinfection. These data on severe P. vivax malaria are in line with observations of a prospective cohort study from Indonesia and Papua New Guinea.18,19 These observations were also consistent with data from a passive morbidity surveillance system in another community in Papua New Guinea.38,39 Another longitudinal study from Papua New Guinean children reported that by 9 years of age, children have acquired almost complete clinical immunity to P. vivax, whereas the acquisition of immunity to symptomatic P. falciparum malaria remained incomplete.40 Similar differences in rates of immune acquisition have also been described in Vanuatu where both species coexisted.41 These observations suggest the role of different mechanisms of immunity in these malaria species and the potential reasons may include key biologic differences between the two species, particularly in relation to red blood cell invasion, adhesion or sequestration in vascular beds, and the nature of variant surface antigens.40
Although the ratio of males to females was approximately equal in children with all species, there was a consistent preponderance of females children with severe malaria (57.3% [51/89]), which was sequentially greater with increasing age of children (0–5 year age group 44.4% [8/18]; 5–10 year age group 53.2% [25/47]; and > 10 year age group 70.8% [17/24]). The severity of malaria in female children was greater in P. vivax monoinfection (79.4% [27/34]) compared with P. falciparum monoinfection (40% [20/50]; OR = 5.79 [95% CI = 2.15–15.50], P < 0.0001). The reason for this observation is not clear. Because the symptoms of uncomplicated P. vivax and P. falciparum malaria are similar, the chances of treatment seeking behavior or referral biases are unlikely.42 The effect was maximal after adolescence and may reflect the lower starting hemoglobin in women compared with men, and therefore a greater propensity to severe anemia in response to P. vivax. Although sex hormones including dehydroepiandrosterone (DHEAS) have been linked to reduce post-pubertal risk in P. falciparum infection, it is possible that female sex hormones confer less protection against P. vivax malaria.43 However, neither of these explanations would adequately account for these observations.
Severe anemia was the major manifestation of severe malaria in this study. The percentage of children with severe anemia caused by P. vivax malaria was highest in the 0–5 year age group (75.6%), whereas the same in P. falciparum infection occurred in the 5–10 year age group (44.2%). This observation of 0–5 year age group's susceptibility of severe anemia by P. vivax malaria is consistent with the observations of studies from Indonesia18 and Venezuela.44 The type of anemia in severe P. vivax monoinfection was predominantly normocytic normochromic type and this observation was similar to the earlier reports.45,46
Although variable degrees of reduction in circulating platelet count are consistently reported in the different types of malaria, the earlier observations found that thrombocytopenia is quite rare in P. vivax malaria, but recently it has been reported in P. vivax monoinfection in children from many parts of the world including India.27–34 In this study thrombocytopenia was found in 61.54% children of severe P. vivax malaria, which are comparable to earlier studies.44,47 The bleeding manifestations were present only in 10.77% patients and these findings are also consistent with earlier studies.48,49 Recently, severe thrombocytopenia with bleeding from skin and mucosal surfaces have been reported in children with P. vivax malaria.30,34 In acute malaria infection, platelets are found to be hypersensitive and there is increased concentrations of platelet-specific proteins such as beta thromboglobulin (βTG), platelet factor 4 (PF4), thromboxane A2, and prostacyclin. It has also been postulated that these hypersensitive (hyperactive) platelets will enhance hemostatic responses, and that may be the reason of bleeding episodes in acute malarial infections.50
Cerebral malaria is the most lethal entity of severe malaria and children are more prone than other susceptible groups.20 Although P. falciparum is the usual pathogen, but recently P. vivax is also reported as an emerging pathogen to cause this severe disease in children.16–19,33,35–37,51 There were 9 (13.85%) children of cerebral malaria caused by P. vivax monoinfection in this study out of which 4 (44.4%) had multiorgan derangement. One child of multiorgan dysfunction presented with shock, metabolic acidosis, and anemia and expired after 24 hours of admission, whereas the remaining 8 children recovered in a mean duration of 5.5 days with no residual neurological sequelae.
The appreciable burden of respiratory distress associated with P. vivax malaria suggests that it is more frequent than suggested by the isolated case reports in the literature. Such reports have previously described mostly nonfatal syndromes in pauci-immune adults caused by acute lung injury.52 In African children with P. falciparum, respiratory distress is associated with metabolic acidosis and/or concurrent pneumonia.53,54 However, the etiology of P. vivax-associated respiratory distress in Asia is unknown and will require detailed clinical studies to determine the relative contributions of acute lung injury, possible pulmonary parasite sequestration, acidosis, and coinfections.55 In an earlier study in children, metabolic acidosis caused by P. vivax malaria was associated with 9% morbidity and 6% mortality.56 Another study from India recently reported 7.2% cases of metabolic acidosis caused by P. vivax in Indian children but blood gas analysis was not done.16 There were a total 7 (10.76%) children of metabolic acidosis caused by P. vivax infection in this study, which was commonly associated with hypoglycemia, severe anemia, acute renal failure, and refractory circulatory failure. All the children had multiorgan dysfunction and 2 (28.6%) children expired while the rest recovered in mean duration of 6 days. Plasmodium vivax monoinfection has also been associated with acute respiratory distress syndrome (ARDS)/noncardiogenic pulmonary edema, a disease process previously thought to occur only in malaria caused by P. falciparum.55 Our study included 1 (1.5%) case of pulmonary edema with P. vivax malaria presenting with multiorgan dysfunction.
Liver abnormalities are a relatively common finding in severe P. falciparum malaria.20 Although transient derangement of liver function is a common feature of childhood malaria, but can also progress to significant deterioration in P. vivax monoinfection.57 The occurrence of predominantly conjugated hyperbilirubinemia and raised liver enzymes indicative of “malarial hepatitis” (82.4%) in this study is similar to observations in adults reporting 85.7% patients having conjugated hyperbilirubinemia.57 These findings suggested that besides hemolysis, cholestasis and hepatocellular injury is an important factor for causing jaundice. The mean duration of recovery of jaundice in this study was 7.3 days as reported earlier in adults.58 The increase in ALT, AST, and SAP in this study has also been reported by other workers.57,59 The total protein and serum albumin level in children with severe P. vivax malaria observed in this study also correlates with the observation of other workers.60 In an earlier clinical study on P. vivax malaria in children, it was reported to be associated with hepatosplenomegaly (40.3%), splenomegaly (11.3%), and hepatomegaly (4.8%).61 In this study we also observed the same in 47%, 17.6%, and 5.9% of patients, respectively. According to WHO, apart from jaundice, signs of hepatic dysfunction are unusual and clinical signs of liver failure such as asterexis or liver flaps are never seen unless there is concomitant viral hepatitis.20 However, in recent years many reports with definite evidence of hepatic encephalopathy with malaria have been reported in adults from different parts of the world, including India.62,63 In this study out of 17 children of hepatic dysfunction, 6 (35.3%) had hepatic encephalopathy, four of them in grade II and rest two in grade I. Out of these 17 children of hepatic dysfunction, 15 (88.2%) had multiorgan involvement. One child with hepatic encephalopathy grade II and multiorgan dysfunction including renal failure, anemia, and hemoglobinurea expired after 4 days of admission.
Renal manifestations of malaria were present in 10 (15.4%) children of severe P. vivax malaria in this study. Although renal involvement is more common in P. falciparum malaria, there have been reports of acute renal failure, electrolyte abnormality, abnormal urinary sediment, and increased urinary protein excretion in P. vivax malaria in children recently.27,28,32,64,65 An earlier study from India reported that P. vivax accounted for 30% cases of renal impairment with decreased endogenous creatinine clearance with acute malaria.65 Our results are also comparable with these studies. Out of these 10 children of renal dysfunction, 3 (30%) expired because of multiorgan dysfunction. Our observation of ARF in 15.4% children may not show the true prevalence but definitely provides evidence of this entity in P. vivax monoinfection.
Activation of coagulation cascade occurs even in mild malaria but the degree of its activation is proportional to the disease severity. Consumption coagulopathy is usually of low grade but in a small proportion of cases it may become clinically significant.66,67 There were a total 7 (10.8%) children of abnormal bleeding caused by P. vivax in this study. Six (85.7%) children had multiorgan dysfunction. Six children recovered in mean duration of 7 days and 1 (14.3%) expired in 2 days with multiorgan dysfunction.
Two (3.1%) children of P. vivax malaria were having hemoglobinuria in this study. Glucose-6-phosphate dehydrogenase enzyme level was normal in both. One child expired in 4 days with hepatic and renal dysfunction and the other recovered at the end of 5 days. In literature there are very few case reports of hemoglobinuria in P. vivax infection. Two patients with P. vivax malaria were reported from the United Kingdom having marked intravascular hemolysis and hemoglobinuria despite normal levels of glucose-6-phosphate dehydrogenase activity in blood.68
Although multiorgan dysfunction with circulatory shock has also been reported with P. vivax monoinfection, they are rare in children.20 In this study 2 (3.1%) children of P. vivax presented with shock. One child, 9 years of age, had cerebral malaria; anemia, and metabolic acidosis with shock and expired within 24 hours of admission. Another patient, 12 years of age, presented with anemia, bleeding, jaundice, metabolic acidosis, and diarrhea/vomiting with shock. The patient recovered in 10 days after successful treatment. Two cases of P. vivax malaria with toxic shock were reported from the Republic of Korea.69 Both showed disseminated intravascular coagulation with marked thrombocytopenia, oliguric renal failure, and pulmonary edema. Another case of shock with ARDS was reported from the United Kingdom recently.70 In our study, 31 (47.7%) children of severe P. vivax malaria had multiorgan dysfunction, whereas 48 (60.8%) of P. falciparum had MODs.
The mortality in severe P. vivax malaria was recorded in 6.15% (4/65) children, whereas the same in severe P. falciparum was observed in 7.59% (6/79) children. Thus, P. vivax infection was found to be almost equally serious to cause significant mortality in comparison to P. falciparum. Most of the earlier published literature consists of few death reports or small descriptive clinical series lacking denominators. Recent study from Papua, Indonesia reported 1.6% and 2.2% case fatality rate caused by P. vivax and P. falciparum, respectively.18
All these observations of this study definitely indicate that a significant proportion of severe malaria morbidity is caused by P. vivax monoinfection in this region where both these species coexist. With calls for increased efforts to eliminate malaria internationally, it will be important to ensure that P. vivax receives appropriate attention. The burden and severity of P. vivax in different regions requires further study and with the availability of the P. vivax genome, it will be possible to compare isolates associated with severe/uncomplicated disease from different geographical regions.71 This study reaffirms the evidence of severe malaria associated with P. vivax monoinfection in children in Bikaner having almost similar spectrum to P. falciparum monoinfection. These observations provide an impetus to study the different issues related to severe P. vivax malaria including underlying pathogenesis of severe disease.
Acknowledgment
The American Society of Tropical Medicine and Hygiene (ASTMH) assisted with publication expenses.
Footnotes
Authors' addresses: Dhanpat Kumar Kochar, Department of Medicine, Kothari Medical and Research Institute, S.P. Medical College, Bikaner, Rajasthan, India, E-mails: drdkkochar@yahoo.com or drdkkochar@indiatimes.com. Gajanand Singh Tanwar, Poonam Chand Khatri, and Ghanshyam Singh Sengar, Department of Pediatrics, S.P. Medical College, Bikaner, Rajasthan, India, E-mails: gajanand.tanwar@gmail.com or drgstanwar@gmail.com, gajanand.tanwar@gmail.com, and gajanand.tanwar@gmail.com. Sanjay Kumar Kochar, Anjana Gupta, Abhishek Kochar, Sheetal Middha, and Jyoti Acharya, Department of Medicine, S.P. Medical College, Bikaner, Rajasthan, India, E-mails: drskkochar@rediffmail.com, anjanagupta@in.com, abhi_kochar@hotmail.com, sheetal_bikaner@yahoo.com, and jyotiacharya2@gmail.com. Vishal Saxena, Deepak Pakalapati, Shilpi Garg, and Ashish Das, Birla Institute of Technology and Sciences, Pilani, Rajasthan, India, E-mails: vishalsaxena@bits-pilani.ac.in, adas@bits-pilani.ac.in, adas@bits-pilani.ac.in, and adas@bits-pilani.ac.in or ashdas28@gmail.com.
References
- 1.World Health Organization . World Malaria Report 2008. Geneva, Switzerland: World Health Organization; 2008. pp. 9–15. [Google Scholar]
- 2.Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434:214–217. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar A, Valecha N, Jain T, Dash AP. Burden of malaria in India: retrospective and prospective view. Am J Trop Med Hyg. 2007;77((6 Suppl)):69–78. [PubMed] [Google Scholar]
- 4.World Health Organization Malaria: Disease Burden in SEA Region. 2009. http://www.searo.who.int/EN/Section10/Section21/Section340_4018.htm Available at. Accessed October 15, 2009.
- 5.Sina B. Focus on Plasmodium vivax. Trends Parasitol. 2002;18:287–289. doi: 10.1016/s1471-4922(02)02329-2. [DOI] [PubMed] [Google Scholar]
- 6.Baird JK. Neglect of Plasmodium vivax malaria. Trends Parasitol. 2007;23:533–539. doi: 10.1016/j.pt.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 7.Hay SI, Guerra CA, Tatem AJ, Noor AM, Snow RW. The global distribution and population at risk of malaria: past, present, and future. Lancet Infect Dis. 2004;4:327–336. doi: 10.1016/S1473-3099(04)01043-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mendis K, Sina BJ, Marchesini P, Carter R. The neglected burden of Plasmodium vivax malaria. Am J Trop Med Hyg. 2001;64:97–106. doi: 10.4269/ajtmh.2001.64.97. [DOI] [PubMed] [Google Scholar]
- 9.Price RN, Tjitra E, Guerra CA, Yeung S, White NJ, Anstey NM. Vivax malaria: neglected and not benign. Am J Trop Med Hyg. 2007;77:79–87. [PMC free article] [PubMed] [Google Scholar]
- 10.Baird JK. Neglect of Plasmodium vivax malaria. Trends Parasitol. 2007;23:533–539. doi: 10.1016/j.pt.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 11.Tan LK, Yacoub S, Scott S, Bhangani B, Jacobs M. Acute lung injury and other serious complications of Plasmodium vivax malaria. Lancet Infect Dis. 2008;8:449–454. doi: 10.1016/S1473-3099(08)70153-1. [DOI] [PubMed] [Google Scholar]
- 12.Luxemburger C, Ricci F, Nosten F, Raimond D, Bathet S, White NJ. The epidemiology of severe malaria in an area of low transmission in Thailand. Trans R Soc Trop Med Hyg. 1997;91:256–262. doi: 10.1016/s0035-9203(97)90066-3. [DOI] [PubMed] [Google Scholar]
- 13.Kochar D, Saxena V, Singh N, Kochar S, Kumar V, Das A. Plasmodium vivax malaria. Emerg Infect Dis. 2005;11:132–134. doi: 10.3201/eid1101.040519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kochar DK, Pakalapati D, Kochar SK, Sirohi P, Khatri MP, Kochar A, Das A. An unexpected cause of fever and seizures. Lancet. 2007;370:908. doi: 10.1016/S0140-6736(07)61417-2. [DOI] [PubMed] [Google Scholar]
- 15.Kochar DK, Das A, Kochar SK, Saxena V, Sirohi P, Garg S, Kochar A, Khatri MP, Gupta V. Severe Plasmodium vivax malaria: a report on serial cases from Bikaner in northwestern India. Am J Trop Med Hyg. 2009;80:194–198. [PubMed] [Google Scholar]
- 16.Tripathy R, Parida S, Das L, Mishra DP, Tripathy D, Das MC, Chen H, Maguire JH, Panigrahi P. Clinical manifestations and predictors of severe malaria in Indian children. Pediatrics. 2007;120:e454–e460. doi: 10.1542/peds.2006-3171. [DOI] [PubMed] [Google Scholar]
- 17.Barcus MJ, Basri H, Picarima H, Manyakori C, Sekartuti, Elyazar I, Bangs MJ, Maguire JD, Baird JK. Demographic risk factors for severe and fatal vivax and falciparum malaria among hospital admissions in northeastern Indonesian Papua. Am J Trop Med Hyg. 2007;77:984–991. [PubMed] [Google Scholar]
- 18.Tjitra E, Anstey NM, Sugiarto P, Warikar N, Kenangalem E, Karyana M, Lampah DA, Price RN. Multidrug-resistant Plasmodium vivax associated with severe and fatal malaria: a prospective study in Papua, Indonesia. PLoS Med. 2008;5:e128. doi: 10.1371/journal.pmed.0050128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Genton B, Acremont VD, Rare L, Baea K, Reeder JC, Alpers MP, Müller I. Plasmodium vivax and mixed infections are associated with severe malaria in children: a prospective cohort study from Papua New Guinea. PLoS Med. 2008;5:e127. doi: 10.1371/journal.pmed.0050127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WHO Severe falciparum malaria. Trans R Soc Trop Med Hyg. 2000;94((1Suppl)):S1–S90. [PubMed] [Google Scholar]
- 21.Gopinathan VP, Subramanian AR. Vivax and falciparum malaria seen at an Indian service hospital. J Trop Med Hyg. 1986;89:51–55. [PubMed] [Google Scholar]
- 22.Maitland K, Williams TN, Peto TE, Day KP, Clegg JB. Absence of malaria-specific mortality in children in an area of hyperendemic malaria. Trans R Soc Trop Med Hyg. 1997;91:562–566. doi: 10.1016/s0035-9203(97)90026-2. [DOI] [PubMed] [Google Scholar]
- 23.Vannaphan S, Saengnetswang T, Suwanakut P, Kllangbuakong A, Klinnak W. The epidemiology of patients with severe malaria who died at the Hospital for Tropical Diseases, 1991–2004. Southeast Asian J Trop Med Public Health. 2005;36:385–389. [PubMed] [Google Scholar]
- 24.Carrara VI, Sirilak S, Thonglairuam J, Rojanawatsirivet C, Proux S. Deployment of early diagnosis and mefloquine-artesunate treatment of falciparum malaria in Thailand: the Tak Malaria Initiative. PLoS Med. 2006;3:e183. doi: 10.1371/journal.pmed.0030183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beg MA, Sani N, Mehraj V, Jafri W, Khan MA. Comparative features and outcomes of malaria at a tertiary care hospital in Karachi, Pakistan. Int J Infect Dis. 2008;12:37–42. doi: 10.1016/j.ijid.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez-Morales AJ, Benitez JA, Arria M. Malaria mortality in Venezuela: focus on deaths due to Plasmodium vivax in children. J Trop Pediatr. 2008;54:94–101. doi: 10.1093/tropej/fmm074. [DOI] [PubMed] [Google Scholar]
- 27.Kaur D, Wasir V, Gulati S, Bagga A. Unusual presentation of Plasmodium vivax malaria with severe thrombocytopenia and acute renal failure. J Trop Pediatr. 2007;53:210–212. doi: 10.1093/tropej/fml092. [DOI] [PubMed] [Google Scholar]
- 28.Devidayal, Jabbar Z, Kumar S, Singh M. Acute renal failure and severe thrombocytopenia in a young boy with vivax malaria: case report. J. Pediatr Infect Dis. 2008;3:145–147. [Google Scholar]
- 29.Shah I. Chloroquine resistant vivax malaria in an infant: a report from India. J Vector Borne Dis. 2008;45:176–177. [PubMed] [Google Scholar]
- 30.Thapa R, Biswas B, Mallick D, Sardar S, Modak S. Childhood Plasmodium vivax malaria with severe thrombocytopenia and bleeding manifestations. J Pediatr Hematol Oncol. 2009;31:758–759. doi: 10.1097/MPH.0b013e3181b7eb12. [DOI] [PubMed] [Google Scholar]
- 31.Zaki SA. Chloroquine resistance vivax malaria in an infant: a report from India. J Vector Borne Dis. 2009;46:83. [PubMed] [Google Scholar]
- 32.Saharan S, Kohli U, Lodha R, Sharma A, Bagga A. Thrombotic microangiopathy associated with Plasmodium vivax malaria. J Pediatric Nephrology. 2009;24:623–624. doi: 10.1007/s00467-008-0945-4. [DOI] [PubMed] [Google Scholar]
- 33.Harish R, Gupta S. Plasmodium vivax malaria presenting with severe thrombocytopenia, cerebral complication and hydrocephalus. Indian J Pediatr. 2009;76:551–552. doi: 10.1007/s12098-009-0087-0. [DOI] [PubMed] [Google Scholar]
- 34.Bhatia V, Bhatia J. Severe thrombocytopenia with bleeding manifestations in two children secondary to Plasmodium vivax. Platelets. 2010;21:307–309. doi: 10.3109/09537100903518278. Letter to the editor: 1–3; doi: 10.3109/09537100903518278 [PMID: 20178400] [DOI] [PubMed] [Google Scholar]
- 35.Thapa R, Patra V, Kundu R. P. vivax cerebral malaria. Indian Pediatr. 2007;44:433–434. [PubMed] [Google Scholar]
- 36.Taksande AM, Vilhekar KY. Cerebellar malaria due to Plasmodium vivax in a child. Iran J Parasitol. 2008;3:48–50. [Google Scholar]
- 37.Thapa R, Ranjan R, Patra VS, Chakrabartty S. Childhood cerebral vivax malaria with pancytopenia. J Pediatr Hematol Oncol. 2009;31:116–117. doi: 10.1097/MPH.0b013e318186855a. [DOI] [PubMed] [Google Scholar]
- 38.Genton B, Al-Yaman F, Beck HP, Hii J, Mellor S, Rare L, Ginny M, Smith T, Alpers MP. The epidemiology of malaria in the Wosera area, East Sepik Province, Papua New Guinea, in preparation for vaccine trials. II. Mortality and morbidity. Ann Trop Med Parasitol. 1995;89:377–390. doi: 10.1080/00034983.1995.11812966. [DOI] [PubMed] [Google Scholar]
- 39.Mueller I. Differential acquisition of immunity to P. vivax and P. falciparum in Papua New Guinean children. Vivax Malaria Research III: 2009 and Beyond, 2009 Abstract Book 2009 [Google Scholar]
- 40.Michon P, Cole-Tobian JL, Dabod E, Schoepflin S, Igu J, Susapu M, Tarongka N, Zimmerman PA, Reeder JC, Beeson JG, Schofield L, King CL, Mueller I. The risk of malarial infections and disease in Papua New Guinean children. Am J Trop Med Hyg. 2007;76:997–1008. [PMC free article] [PubMed] [Google Scholar]
- 41.Maitland K, Williams AI, Bennett NM, Newbold C, Peto TE, Viji J, Timothy R, Clegg A, Weatherall DJ. The interaction between Plasmodium falciparum and P. vivax in children on Espiritu Santo Island, Vanuatu. Trans R Soc Trop Med Hyg. 1996;90:614–620. doi: 10.1016/s0035-9203(96)90406-x. [DOI] [PubMed] [Google Scholar]
- 42.Luxemburger C, Thwai KL, White NJ, Webster HK, Kyle DE. The epidemiology of malaria in a Karen population on the western border of Thailand. Trans R Soc Trop Med Hyg. 1996;90:105–111. doi: 10.1016/s0035-9203(96)90102-9. [DOI] [PubMed] [Google Scholar]
- 43.Kurtis JD, Mtalib R, Onyango FK, Duffy PE. Human resistance to Plasmodium falciparum increases during puberty and is predicted by dehydroepiandrosterone sulfate levels. Infect Immun. 2001;69:123–128. doi: 10.1128/IAI.69.1.123-128.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodríguez-Morales AJ, Sánchez E, Vargas M, Piccolo C, Colina R, Arria M. Anemia and thrombocytopenia in children with Plasmodium vivax malaria. J Trop Pediatr. 2006;52:49–51. doi: 10.1093/tropej/fmi069. [DOI] [PubMed] [Google Scholar]
- 45.Facer CA. Infection and Hematology. Oxford, UK: Butterworth Heinemann Ltd; 1994. pp. 259–294. (Hematological aspect of malaria). [Google Scholar]
- 46.Beals PF. Anemia in malaria control: a practical approach. Ann Trop Med Parasitol. 1997;91:713–718. doi: 10.1080/00034989760446. [DOI] [PubMed] [Google Scholar]
- 47.Jamal A, Memon IA, Latif F. The association of Plasmodium vivax malaria with thrombocytopenia in febrile children. Pak Pediatr J. 2007;31:85–89. [Google Scholar]
- 48.Ladhani S, Lowe B, Cole AO, Kowuondo K, Newton CR. Changes in white blood cells and platelets in children with falciparum malaria: relationship to disease outcome. Br J Haematol. 2002;119:839–847. doi: 10.1046/j.1365-2141.2002.03904.x. [DOI] [PubMed] [Google Scholar]
- 49.Abro AH, Ustadi AM, Younis NJ, Abdou AS, Hamed DA, Saleh AA. Malaria and hematological changes. Pak J Med Sci. 2008;24:287–291. [Google Scholar]
- 50.Bashwari LA, Mandil AA, Bahnassy AA, Alshamsi MA, Bukhari HA. Epidemiological profile of malaria in a university hospital in the eastern region of Saudi Arabia. Saudi Med J. 2001;22:133–138. [PubMed] [Google Scholar]
- 51.Ozen M, Gungor S, Atambay M, Daldal N. Cerebral malaria owing to Plasmodium vivax: case report. Ann Trop Paediatr. 2006;26:141–144. doi: 10.1179/146532806X107494. [DOI] [PubMed] [Google Scholar]
- 52.Tan LK, Yacoub S, Scott S, Bhagani S, Jacobs M. Acute lung injury and other serious complications of Plasmodium vivax malaria. Lancet Infect Dis. 2008;8:449–454. doi: 10.1016/S1473-3099(08)70153-1. [DOI] [PubMed] [Google Scholar]
- 53.Bejon P, Berkley JA, Mwangi T, Ogada E, Mwangi I. Defining childhood severe falciparum malaria for intervention studies. PLoS Med. 2007;4:e251. doi: 10.1371/journal.pmed.0040251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marsh K, Forster D, Waruiru C, Mwangi I, Winstanley M. Indicators of life-threatening malaria in African children. N Engl J Med. 1995;332:1399–1404. doi: 10.1056/NEJM199505253322102. [DOI] [PubMed] [Google Scholar]
- 55.Anstey NM, Handojo T, Pain MC, Kenangalem E, Tjitra E. Lung injury in vivax malaria: pathophysiological evidence for pulmonary vascular sequestration and posttreatment alveolar-capillary inflammation. J Infect Dis. 2007;195:589–596. doi: 10.1086/510756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murphy SC, Breman JG. Gaps in the childhood malaria burden in Africa: cerebral malaria, anemia, respiratory distress, and hypoglycemia. Am J Trop Med Hyg. 2001;64:57–67. doi: 10.4269/ajtmh.2001.64.57. [DOI] [PubMed] [Google Scholar]
- 57.Patwari A, Aneja S, Berry AM, Ghosh S. Hepatic dysfunction in childhood malaria. Arch Dis Child. 1979;54:139–141. doi: 10.1136/adc.54.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kochar DK, Singh P, Agarwal P, Kochar SK, Pokharna R, Sareen PK. Malarial hepatopathy. J Assoc Physicians India. 2003;51:1069–1072. [PubMed] [Google Scholar]
- 59.Sadun EH, Williams JS, Martin LK. Serum biochemical changes in malarial infections in man, chimpanzees, and mice. Mil Med. 1966;131:1094–1110. [PubMed] [Google Scholar]
- 60.Tangpukdee N, Thanachartwet V, Krudsood S, Luplertlop N, Pornpininworakij K, Chalermrut K, Phokham S, Kano S, Looareesuwan S, Wilairatana P. Minor liver profile dysfunctions in Plasmodium vivax, P. malariae and P. ovale patients and normalization after treatment. Korean J Parasitol. 2006;44:295–302. doi: 10.3347/kjp.2006.44.4.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bhave SY, Joshi SV, Warad V, Dhar HL. Hepatic and renal dysfunction in childhood malaria. BHJ. 2005;47:225. [Google Scholar]
- 62.Premaratna R, Gunatilake AK, Desilva NR, Tilakaratney Y, Fonreka MM, de Silva HJ. Severe hepatic dysfunction associated with falciparum malaria. Southeast Asian J Trop Med Public Health. 2001;32:70–72. [PubMed] [Google Scholar]
- 63.Kochar DK, Agarwal P, Kochar SK, Jain R, Rawat N, Pokharna RK, Kachhawa S, Srivastava T. Hepatocyte dysfunction and hepatic encephalopathy in Plasmodium falciparum malaria. Q J Med. 2003;96:505–512. doi: 10.1093/qjmed/hcg091. [DOI] [PubMed] [Google Scholar]
- 64.Sharma A, Khanduri U. How benign is benign tertian malaria? J Vector Borne Dis. 2009;46:141–144. [PubMed] [Google Scholar]
- 65.Ahmad SH, Danish T, Faridi MM, Ahmad AJ, Fakhir S, Khan AS. Renal function in acute malaria in children. J Trop Pediatr. 1989;35:291–294. doi: 10.1093/tropej/35.6.291. [DOI] [PubMed] [Google Scholar]
- 66.Takaki K, Aoki T, Akeda H, Kajiwara T, Honda S, Maeda Y. A case of Plasmodium vivax malaria with findings of DIC. Kansenshogaku Zasshi. 1991;65:488–492. doi: 10.11150/kansenshogakuzasshi1970.65.488. [DOI] [PubMed] [Google Scholar]
- 67.Lakhkar BB, Babu S, Shenoy V. DIC in vivax malaria. Indian Pediatr. 1996;33:972–973. [PubMed] [Google Scholar]
- 68.Lawn SD, Krishna S, Jarvis JN, Joet T, Macallan DC. Case reports: pernicious complications of benign tertian malaria. Trans R Soc Trop Med Hyg. 2003;97:551–553. doi: 10.1016/s0035-9203(03)80024-x. [DOI] [PubMed] [Google Scholar]
- 69.Song JY, Park CW, Jo YM, Kim JY, Yoon HJ. Two cases of P. vivax malaria resembling toxic shock. Am J Trop Med Hyg. 2007;77:609–611. [PubMed] [Google Scholar]
- 70.Kumar S, Melzer M, Dodds P, Watson J. P. vivax malaria complicated by shock and ARDS. Scand J Infect Dis. 2007;39:255–256. doi: 10.1080/00365540600904787. [DOI] [PubMed] [Google Scholar]
- 71.Feng X, Carlton JM, Joy DA, Mu J, Furuya T. Single-nucleotide polymorphisms and genome diversity in Plasmodium vivax. Proc Natl Acad Sci USA. 2003;100:8502–8507. doi: 10.1073/pnas.1232502100. [DOI] [PMC free article] [PubMed] [Google Scholar]


