Abstract
A cross-sectional study was performed in children 5 through 10 years of age presenting to outpatient clinics in Nyanza Province, Kenya, in which nasal swab and blood specimens were collected during the high malaria transmission season. Patients presenting with malaria-like symptoms within 4 days of fever onset were enrolled in the study. Plasmodium parasitemia was determined by blood smear microscopy. Nasal swabs were screened for a panel of respiratory viruses by polymerase chain reaction. Influenza A, rhinoviruses, and other respiratory viruses were detected in 18%, 26%, and 12% of 197 specimens, respectively. Four of 36 patients with influenza A had a positive malaria blood slide, compared with 20 of 52 patients with rhinovirus. A significant burden of disease caused by influenza A in febrile children during the study period was observed, highlighting the need for further research into the burden of influenza disease in regions where malaria is holoendemic.
Introduction
The importance of malaria infection in children is well appreciated in Africa, but little is known about the burden of viral respiratory disease in malaria-endemic populations. Influenza and other viral respiratory infections are often not distinguishable from other acute febrile diseases in the tropics.1 Limited diagnostic capacity within this region ensures that the etiology of community-acquired pediatric viral infections remains largely unknown. In temperate regions, the limited seasonality of certain respiratory infections enables the diagnosis of specific viral diseases based on clinical syndromes, such as bronchiolitis during respiratory syncytial virus (RSV) outbreaks. In contrast, many respiratory viruses may be endemic or emerge throughout the year in tropical and subtropical regions and the local epidemiology is unpredictable. For example, influenza viruses have been documented to circulate during many months of the year or even throughout the entire year in some areas, whereas these same viruses may predominate during specific seasons in other regions.2–8 Furthermore, although the burden of viral respiratory disease in hospitalized young children in Kenya has been reported in studies using sensitive molecular diagnostic methods, there are few data reported on viral infections in outpatients.9
Given the high incidence of respiratory infection in malaria endemic regions, understanding the overlap between these two causes of disease is critical for informing clinical management and minimizing inappropriate anti-malarial treatment. Sensitive and reliable laboratory techniques that can distinguish respiratory viral infections from malaria are required. In this study, we prospectively evaluated children with a history of fever and symptoms suspicious for malaria who attended outpatient clinics in two sites in western Kenya. We specifically studied children > 5 years of age in whom partial immunity to malaria had been acquired to better appreciate the impact of common community-acquired respiratory viruses in situations of reduced susceptibility to malaria.
Methods
The study was conducted from April through July 2007, during the season of intense malaria transmission. Patients eligible for this study were required to be 5 through 10 years of age, and have experienced acute and recent onset of fever (within 4 days) with suspicion of malaria infection at the time of presentation at the outpatient clinics. This study was part of a cross-sectional study that evaluated etiologies of fever under a protocol approved by the following institutional review boards: Kenya Ethical Review Committee, ERC #1117; Walter Reed Army Institute of Research (WRAIR), HURC #1315; and PATH Research Ethics Committee, HS #358. The participating hospitals were Kombewa Subdistrict Hospital and Kondele Children's Hospital (Kisumu), both in the Nyanza province of Kenya. Participants were informed about the study by the study nurse. Following written informed consent from a parent or guardian, and assent from children > 6 years of age, each child had a nasal-pharyngeal swab obtained, blood drawn, and the child and/or family member answered a short questionnaire administered by a study physician or clinical officer.
Plasmodium infection was determined by light microscopy. Depending on parasite density, thin or thick malaria blood films were read by two blinded experienced microscopists according to published methodology.10 Hemoglobin concentrations were determined by a hematology analyzer (Coulter Counter, Ac.T 5diff CP, Beckman Coulter, USA) on fresh samples taken at the time of clinic visit.
A single nylon flocked nasal swab (Copan Diagnostics, Inc., USA) was inserted midway into the nasal-pharynx of participating children. This swab was then inoculated into 2 mL of viral transport media, the swab removed, and the sample stored at −80°C. Nucleic acid was extracted from samples and tested for RSV; influenza viruses A and B; parainfluenza viruses 1, 2, 3, and 4; human metapneumovirus; adenoviruses; coronaviruses; and rhinoviruses using real-time polymerase chain reaction (PCR) as described previously.11–13 Influenza A viral infections were confirmed by a separate real-time PCR14 assay performed on the same samples in different laboratories, blinded both to each other's results and to microscopy results (data not shown).
Results
A total of 197 children 5 through 10 years of age were enrolled during 4 months in the rainy season at two clinics in the western Kenya province of Nyanza. The clinical profiles and Plasmodium infection status for this cohort are shown in Table 1. Altogether, 112 viral respiratory infections were detected in 103 of 197 patients (52%) (Table 2), and nine children had two respiratory viruses detected simultaneously, including 5 specimens with both influenza A and rhinovirus; 1 with influenza A and coronavirus; and 1 each with rhinovirus plus coronavirus, human metapneumovirus, or influenza B. Malaria infection with Plasmodium falciparum was detected in 67/197 (34%) patients overall, and in 29/103 (28%) of children with respiratory viral infections. The most common respiratory virus detected was rhinovirus, 52/197 (26.4%). Sixty respiratory virus infections other than rhinoviruses were detected, but influenza A accounted for over half of the infections (N = 36). Of the 36 patients with influenza A infection, only 4 were positive for malaria as determined by microscopy (11%, P = 0.001). In contrast, 20 of the 52 patients with rhinovirus infections were positive for malaria (38%, P = 0.090) (Table 2).
Table 1.
Clinical profiles and malaria infection rates in patients recruited to the study*
| Total number of patients | 197 |
|---|---|
| Age* | 7 years (5, 9) |
| Body temperature* | 38.8°C (38.1, 39.6) |
| Days since onset of fever* | 3 days (1, 4) |
| Percent male | 54% |
| Number of malaria positive by blood smear (%) | 67 (34%) |
| Number of P. falciparum infections (%) | 67 (34%) |
| Number of P. ovale infections | 1 |
| Number of P. malariae infections | 1 |
| Geometric mean parasite density/µL (range) | 23,605 (range: 46–491, 640) |
The 10th and 90th percentiles are shown in brackets on the right of the median values. The episodes of Plasmodium ovale and Plasmodium malariae infections were both co-infections with Plasmodium falciparum.
Table 2.
Co-infections of viral respiratory viruses as determined by real-time polymerase chain reaction (PCR) and Plasmodium falciparum in a cohort of children recruited to the study with symptoms of malaria*
| Viral infections in malaria negative subjects (% total N = 81) | Viral infections in malaria positive subjects (% co-infections N = 31) | Total number of virus infections (% total N = 112) | |
|---|---|---|---|
| Respiratory syncytial virus | 0 | 1 (3%) | 1 (1%) |
| Influenza A | 32 (40%) | 4 (13%) | 36 (32%) |
| Influenza B | 1 (1%) | 1 (3%) | 2 (2%) |
| Parainfluenza 1 | 0 | 0 | 0 |
| Parainfluenza 2 | 2 (2%) | 2 (6%) | 4 (4%) |
| Parainfluenza 3 | 0 | 0 | 0 |
| Parainfluenza 4 | 0 | 0 | 0 |
| Human metapneumovirus | 2 (2%) | 0 | 2 (2%) |
| Adenoviruses | 7 (9%) | 1 (3%) | 8 (7%) |
| Coronaviruses | 5 (6%) | 2 (6%) | 7 (6%) |
| Rhinoviruses | 32 (40%) | 20 (65%) | 52 (46%) |
| Total viral infections | 81 | 31 | 112 |
| Number of subjects with viral infections | 74 | 29 | 103 |
| Total viral infections (rhinovirus excluded) | 49 | 11 | 60 |
| Number of subjects with viral infections (rhinovirus excluded) | 48 | 11 | 59 |
Malaria infection status was defined by blood smear microscopy.
Peripheral blood hemoglobin concentrations in relationship to infection are shown in Figure 1. Hemoglobin concentrations were statistically higher in children with influenza infection as compared with children with malaria infection and no influenza infection (significance determined by a two-tailed Mann–Whitney U test).
Figure 1.
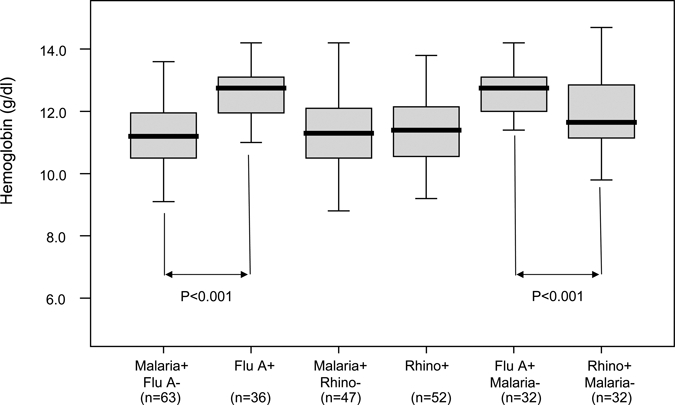
Peripheral blood hemoglobin concentrations and infection status. Malaria infection status was determined by blood smear microscopy; influenza A and rhinovirus infection were determined by polymerase chain reaction (PCR) from nasal swab specimens. Peripheral blood hemoglobin concentrations in patients that have malaria infection but not influenza A infection (Malaria+ Flu A−), all patients that have influenza A infection (Flu A+), patients that have malaria infection but not rhinovirus infection (Malaria+ Rhino−), patients with influenza A infection but no malaria infection (Flu A+, Malaria−), and patients with rhinovirus infection but no malaria infection (Rhino+ Malaria−). The top of the box represents the 75th percentile, the bottom of the box represents the 25th percentile, and the line in the middle represents the 50th percentile. The whiskers (the lines that extend out the top and bottom of the box) represent the highest and lowest values that are not outliers or extreme values.
Discussion
Viral respiratory infections were documented in 103/197 (52%) children attending outpatient clinics in western Kenya with clinical disease consistent with malaria, but importantly, 74 of these children did not have demonstrable malaria parasites by blood smear microscopy. Excluding rhinovirus infection, 48 subjects had respiratory viral infections with undetectable parasitemia with influenza A accounting for 32 of these (67%). This represents a surprisingly high incidence of influenza A infection for a cohort not recruited specifically for upper viral respiratory infections and for children outside the toddler age range. Our study shows that clinical symptoms associated with influenza—such as fever, myalgia, and headache—may mimic those symptoms associated with malaria in this age group and that specific laboratory testing may be required to differentiate these infections. Previous data from Kenya have observed high incidence levels of RSV and influenza in the < 5 age group,9,15–17 but the prevalence and even seasonality of influenza virus infections in much of Africa in other populations and age groups remain largely unknown. The high rates of influenza virus infection documented in this small cross-sectional study from a limited area indicates that the burden of influenza disease is likely to be systematically underestimated in part because of the clinical bias that malaria is responsible for much of the febrile disease during childhood.
Rhinovirus was the most common respiratory virus detected in this study. This virus is becoming increasingly recognized as a major cause of hospitalization for acute respiratory disease in children of all ages in industrialized countries,18 and an important pathogen associated with fever in children, leading to outpatient visits and exacerbation of reactive airway disease. The burden of febrile disease related to rhinovirus in Africa is not well described19 in large part because the diagnosis of rhinovirus infection was problematic before the advent of sensitive real-time PCR techniques. We note the high prevalence of rhinovirus detection in our sample, but acknowledge that rhinovirus shedding as detected by PCR methods may be prolonged, and that viral detection by PCR is not necessarily proof of acute infection.20 By comparison, detection of other respiratory viruses such as influenza or RSV by PCR generally is an indicator of recent disease and long-term, persistent shedding of these parmyxo- and myxoviruses is generally uncommon except in profoundly immunosuppressed patients. The RSV was not a major contributor to viral infection in our cohort despite our use of a sensitive testing method, although our period of testing was relatively short and may have missed the RSV season. Furthermore, RSV is likely to be more common in younger children than the population targeted in this study.9,16,17
Very few data are available regarding the overlap of influenza A and malaria in the age group we evaluated.1 Most data have emanated from travelers returning from malaria-endemic countries where malaria infections are often attributed to influenza because of the similarity of the clinical syndromes.21–23 An extensive influenza epidemiological study performed in Senegal from 1996 through 1998 suggests that the peak acute respiratory infection and influenza periods occur during the hot and rainy season (June–September) in western Africa.3 In this same study, the majority of patients presenting with acute respiratory infection were < 15 years of age, although influenza A was isolated equally from all age groups. Our specimens were collected during the rainy and peak malaria transmission season, corresponding to the peak season for influenza transmission observed in other tropical countries and in a vulnerable age group.2–7 However, the high incidence of influenza, particularly in those children who did not have laboratory evidence of malaria, was unexpected.
In a study investigating the interactions between malaria and influenza in patients of all ages from a malaria holoendemic region (Rufigi, Tanzania), it was suggested that influenza A infection and measles infection repressed parasitemia.24 Of interest, serology using complement fixation was used to diagnose influenza infection. This study also noted that all children under the age of 10 with influenza reported fever as a clinical symptom. We observed that patients with influenza A infection were least likely to have levels of parasitemia detectable by microscopy in our study. However, most of these patients had sub-microscopic levels of parasitemia as determined by reverse transcription-PCR (RT-PCR) (unpublished results, Waitumbi JN). Additionally, we observed that hemoglobin levels in patients with influenza A were significantly higher than in patients who were blood smear positive for malaria and who were negative for influenza A (P > 0.001) (Figure 1). This implies that children with influenza, even with possible malaria, have influenza as their primary disease, and not malaria. This is in contrast to observations in patients infected with rhinovirus. Interestingly, patients infected with influenza A and without microscopic evidence of malaria also had a significantly higher concentration of hemoglobin than blood smear negative, rhinovirus-positive patients, possibly because of the role of other co-infections in the latter population (Figure 1 and unpublished data, Waitumbi JN).
The data presented in this study are constrained by the short recruitment period (April–July 2007) and the age group (5–10 years of age). Future studies should include the higher malaria risk group of children from birth through 4 years of age in whom both the incidence and severity of both viral respiratory diseases and malaria infection is higher.
Our data suggest that, in this population of outpatients presenting with recent acute febrile illness, children with influenza A may have a distinct syndrome from malaria that is characterized by absence of malaria parasites and a much higher hemoglobin level than that seen in children with malaria or the ubiquitous rhinovirus. Understanding the overlap between respiratory viral infection and malaria in disease presentation is critical toward providing more accurate case management as well as assessing the potential impact of influenza vaccine programs in these regions. Further research into co-infection and interactions between malaria and viral respiratory disease is warranted.
Disclaimer: The views expressed by the authors do not necessarily reflect the views of the funding agency.
Footnotes
Financial support: Support for this project was provided through funding from the Bill & Melinda Gates Foundation's Grand Challenges in Global Health Initiative under grant number 37884, “A Point-of-Care Diagnostic System for the Developing World.” This grant was awarded to the University of Washington (UW)-led consortium: Dr. Paul Yager, PI (UW) and collaborators at Micronics, Inc.; Epoch Biosciences, a division of Wescor, Inc. in the EliTech Group; PATH; and the Stayton Group (UW).
Disclosure: This work has been published with the permission of the director of the Kenya Medical Research Institute.
Authors' addresses: John N. Waitumbi, Samuel B. Anyona, Joseph N. Koros, Mark E. Polhemus, Walter Reed Project/Kenya Medical Research Institute, Kisumu, Kenya, E-mails: JWaitumbi@wrp-ksm.org, sbonuke@gmail.com, jkoros@wrp-ksm.org, and mark.polhemus@conus.army.mil. Jane Kuypers, Department of Laboratory Medicine, University of Washington, Seattle, WA, E-mail: kuypers@u.washington.edu. Jay Gerlach, Matthew Steele, Kathleen M. Neuzil, Gonzalo J. Domingo, PATH, Seattle, WA, E-mails: jgerlach@path.org, msteele@path.org, kneuzil@path.org, and gdomingo@path.org. Janet A. Englund, Department of Pediatrics, University of Washington, Children's Hospital and Regional Medical Center, Seattle, WA, E-mail: janet.englund@seattlechildrens.org.
References
- 1.Yazdanbakhsh M, Kremsner PG. Influenza in Africa. PLoS Med. 2009;6:e1000182. doi: 10.1371/journal.pmed.1000182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beckett CG, Kosasih H, Ma'roef C, Listiyaningsih E, Elyazar IR, Wuryadi S, Yuwono D, McArdle JL, Corwin AL, Porter KR. Influenza surveillance in Indonesia: 1999–2003. Clin Infect Dis. 2004;39:443–449. doi: 10.1086/422314. [DOI] [PubMed] [Google Scholar]
- 3.Dosseh A, Ndiaye K, Spiegel A, Sagna M, Mathiot C. Epidemiological and virological influenza survey in Dakar, Senegal: 1996–1998. Am J Trop Med Hyg. 2000;62:639–643. doi: 10.4269/ajtmh.2000.62.639. [DOI] [PubMed] [Google Scholar]
- 4.Moura FE, Perdigao AC, Siqueira MM. Seasonality of influenza in the tropics: a distinct pattern in northeastern Brazil. Am J Trop Med Hyg. 2009;81:180–183. [PubMed] [Google Scholar]
- 5.Nguyen HL, Saito R, Ngiem HK, Nishikawa M, Shobugawa Y, Nguyen DC, Hoang LT, Huynh LP, Suzuki H. Epidemiology of influenza in Hanoi, Vietnam, from 2001 to 2003. J Infect. 2007;55:58–63. doi: 10.1016/j.jinf.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Wong CM, Chan KP, Hedley AJ, Peiris JS. Influenza-associated mortality in Hong Kong. Clin Infect Dis. 2004;39:1611–1617. doi: 10.1086/425315. [DOI] [PubMed] [Google Scholar]
- 7.Zaman RU, Alamgir AS, Rahman M, Azziz-Baumgartner E, Gurley ES, Sharker MA, Brooks WA, Azim T, Fry AM, Lindstrom S, Gubareva LV, Xu X, Garten RJ, Hossain MJ, Khan SU, Faruque LI, Ameer SS, Klimov AI, Luby SP. Influenza in outpatient ILI case-patients in national hospital-based surveillance, Bangladesh, 2007–2008. PLoS ONE. 2009;4:e8452. doi: 10.1371/journal.pone.0008452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brooks WA, Goswami D, Rahman M, Nahar K, Fry AM, Balish A, Iftekharuddin N, Azim T, Xu X, Klimov A, Bresee J, Bridges C, Luby S. Influenza is a major contributor to childhood pneumonia in a tropical developing country. Pediatr Infect Dis J. 2010;29:216–221. doi: 10.1097/INF.0b013e3181bc23fd. [DOI] [PubMed] [Google Scholar]
- 9.Berkley JA, Munywoki P, Ngama M, Kazungu S, Abwao J, Bett A, Lassauniere R, Kresfelder T, Cane PA, Venter M, Scott JA, Nokes DJ. Viral etiology of severe pneumonia among Kenyan infants and children. JAMA. 2010;303:2051–2057. doi: 10.1001/jama.2010.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohrt C, Obare P, Nanakorn A, Adhiambo C, Awuondo K, O'Meara WP, Remich S, Martin K, Cook E, Chretien JP, Lucas C, Osoga J, McEvoy P, Owaga ML, Odera JS, Ogutu B. Establishing a malaria diagnostics centre of excellence in Kisumu, Kenya. Malar J. 2007;6:79. doi: 10.1186/1475-2875-6-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuypers J, Wright N, Ferrenberg J, Huang ML, Cent A, Corey L, Morrow R. Comparison of real-time PCR assays with fluorescent-antibody assays for diagnosis of respiratory virus infections in children. J Clin Microbiol. 2006;44:2382–2388. doi: 10.1128/JCM.00216-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuypers J, Martin ET, Heugel J, Wright N, Morrow R, Englund JA. Clinical disease in children associated with newly described coronavirus subtypes. Pediatrics. 2007;119:e70–e76. doi: 10.1542/peds.2006-1406. [DOI] [PubMed] [Google Scholar]
- 13.Lu X, Holloway B, Dare RK, Kuypers J, Yagi S, Williams JV, Hall CB, Erdman DD. Real-time reverse transcription-PCR assay for comprehensive detection of human rhinoviruses. J Clin Microbiol. 2008;46:533–539. doi: 10.1128/JCM.01739-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Elden LJ, Nijhuis M, Schipper P, Schuurman R, van Loon AM. Simultaneous detection of influenza viruses A and B using real-time quantitative PCR. J Clin Microbiol. 2001;39:196–200. doi: 10.1128/JCM.39.1.196-200.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bulimo WD, Garner JL, Schnabel DC, Bedno SA, Njenga MK, Ochieng WO, Amukoye E, Magana JM, Simwa JM, Ofula VO, Lifumo SM, Wangui J, Breiman RF, Martin SK. Genetic analysis of H3N2 influenza A viruses isolated in 2006–2007 in Nairobi, Kenya. Influenza Other Respir Viruses. 2008;2:107–113. doi: 10.1111/j.1750-2659.2008.00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hazlett DT, Bell TM, Tukei PM, Ademba GR, Ochieng WO, Magana JM, Gathara GW, Wafula EM, Pamba A, Ndinya-Achola JO. Viral etiology and epidemiology of acute respiratory infections in children in Nairobi, Kenya. Am J Trop Med Hyg. 1988;39:632–640. doi: 10.4269/ajtmh.1988.39.632. [DOI] [PubMed] [Google Scholar]
- 17.Nokes DJ, Ngama M, Bett A, Abwao J, Munywoki P, English M, Scott JA, Cane PA, Medley GF. Incidence and severity of respiratory syncytial virus pneumonia in rural Kenyan children identified through hospital surveillance. Clin Infect Dis. 2009;49:1341–1349. doi: 10.1086/606055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller EK, Lu X, Erdman DD, Poehling KA, Zhu Y, Griffin MR, Hartert TV, Anderson LJ, Weinberg GA, Hall CB, Iwane MK, Edwards KM. Rhinovirus-associated hospitalizations in young children. J Infect Dis. 2007;195:773–781. doi: 10.1086/511821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peltola V, Ruuskanen O. Editorial commentary: respiratory viral infections in developing countries: common, severe, and unrecognized. Clin Infect Dis. 2008;46:58–60. doi: 10.1086/524020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wright PF, Deatly AM, Karron RA, Belshe RB, Shi JR, Gruber WC, Zhu Y, Randolph VB. Comparison of results of detection of rhinovirus by PCR and viral culture in human nasal wash specimens from subjects with and without clinical symptoms of respiratory illness. J Clin Microbiol. 2007;45:2126–2129. doi: 10.1128/JCM.02553-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson ME, Weld LH, Boggild A, Keystone JS, Kain KC, von Sonnenburg F, Schwartz E. Fever in returned travelers: results from the GeoSentinel Surveillance Network. Clin Infect Dis. 2007;44:1560–1568. doi: 10.1086/518173. [DOI] [PubMed] [Google Scholar]
- 22.Askling HH, Lesko B, Vene S, Berndtson A, Bjorkman P, Blackberg J, Bronner U, Follin P, Hellgren U, Palmerus M, Ekdahl K, Tegnell A, Struwe J. Serologic analysis of returned travelers with fever, Sweden. Emerg Infect Dis. 2009;15:1805–1808. doi: 10.3201/eid1511.091157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clark IA, Alleva LM, Mills AC, Cowden WB. Pathogenesis of malaria and clinically similar conditions. Clin Microbiol Rev. 2004;17:509–539. doi: 10.1128/CMR.17.3.509-539.2004. table. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rooth IB, Bjorkman A. Suppression of Plasmodium falciparum infections during concomitant measles or influenza but not during pertussis. Am J Trop Med Hyg. 1992;47:675–681. doi: 10.4269/ajtmh.1992.47.675. [DOI] [PubMed] [Google Scholar]


