Abstract
Treatment options for cutaneous leishmaniasis in the United States are problematic because the available products are either investigational, toxic, and/or of questionable effectiveness. A retrospective review of patients receiving liposomal amphotericin B through the Walter Reed Army Medical Center for the treatment of cutaneous leishmaniasis during 2007–2009 was conducted. Twenty patients who acquired disease in five countries and with five different strains of Leishmania were treated, of whom 19 received a full course of treatment. Sixteen (84%) of 19 experienced a cure with the initial treatment regimen. Three patients did not fully heal after an initial treatment course, but were cured with additional dosing. Acute infusion-related reactions occurred in 25% and mild renal toxicity occurred in 45% of patients. Although the optimum dosing regimen is undefined and the cost and toxicity may limit widespread use, liposomal amphotericin B is a viable treatment alternative for cutaneous leishmaniasis.
Introduction
Leishmaniasis refers to a broad spectrum of disease caused by protozoan parasites belonging to the genus Leishmania. Cutaneous leishmaniasis (CL) usually occurs in the Old World and is caused by Leishmania major and L. tropica. In the New World, it is caused by L. (Viannia) braziliensis, L. (V). panamensis, and L. mexicana. Infected patients typically have a non-healing ulcer on exposed skin but the diversity of clinical manifestations includes all dermatologic syndromes.1
The treatment of CL in the United States is problematic because there is no U.S. Food and Drug Administration (FDA)–approved drug for this indication. Sodium stibogluconate (Pentostam; Glaxo-Smith-Kline, Brentford, United Kingdom) is the therapy suggested by many experts and is available to clinicians under an Investigational New Drug (IND) protocol from the Centers for Disease Control and Prevention (CDC). For U.S. Department of Defense beneficiaries, Pentostam is available under an IND protocol at the Walter Reed Army Medical Center (WRAMC) in Washington, DC, and more than 500 patients have been treated at WRAMC since 2000. The recommended treatment of moderate-to-severe CL requires daily intravenous infusions (20 doses) and is associated with increased levels of pancreatic enzymes in > 90% of patients, increased levels of liver enzymes in > 50%, and significant arthralgias and myalgias in > 50%.2,3 Rarer side effects include development of herpes zoster infection during or shortly after treatment and electrocardiographic changes.4,5 A safety release from the manufacturer in 2006 warned of particulate matter in the vials (from an interaction with the stopper), and required that the medication be strained through a filter before administration.6
Because Pentostam is not an approved drug in the United States., the administration of Pentostam is only possible under an approved protocol administered under Institutional Review Board oversight and an IND application with the FDA with the attendant documentation for sponsor regulatory oversight. Pentostam can only be given with individual written informed consent, and it is often a time-consuming challenge to explain why Pentostam is the drug of choice for CL and yet not FDA approved for this indication. Because of the rarity of CL in the United States, few clinicians and nurses are experienced with the administration and side effect profile of Pentostam and they may seek expert consultation. For busy clinicians, the combination of the additional documentation burden, written informed consent, an extensive side effect profile that requires close monitoring and inexperience with the drug present significant limitations to the use of Pentostam.
Amphotericin B deoxycholate has been used as a second-line treatment for mucosal leishmaniasis and CL (especially with pentavalent antimony treatment failures) in the New World since the early 1960s. The systemic and renal toxicity, cost, and difficulty of intravenous administration in leishmaniasis-endemic areas prevented more widespread use. The introduction of lipid-associated amphotericin B products with less renal toxicity has enabled more widespread use.
In 1997, the FDA approved liposomal amphotericin B (AmBisome; Astellus Pharam US Inc., Deerfield, IL) for treatment of visceral leishmaniasis in otherwise immunocompetent adults at the dose of 3 mg/kg/day for 7 doses given on days 1–5, 14, and 21 (total dose = 21 mg/kg).7 In a search for a more tolerable therapy for CL, some clinicians have reported success with the use of AmBisome (Table 1).8–18 Most of these reports include only one or small numbers of patients, include persons with immunosuppressive conditions, persons who had shown initial treatment failures with pentavalent antimony, or report efficacy against just one species of Leishmania or from just one geographic region. We report our experience with the use of AmBisome as drug therapy for the treatment of CL in 20 non-immune, immunocompetent returning travelers.
Table 1.
Published studies using liposomal amphotericin B (AmBisome) for the treatment of cutaneous leishmaniasis*
| Study | Treatment regimen | Total dose of AmBisome (mg/kg) | No. patients evaluated/age/sex | Clinical manifestation | Disease-endemic area resident or returned traveler (non-immune) | Immunocompromised | Prior anti-leishmanial drug treatment | Country in which acquired | Species | Clinical cure | Duration of follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Amato and others8 | 50 mg every 36 h for a total of 300 mg, followed by daily dose of 25 mg | 600 mg (weight NR) | 1/50 years/M | Single ulcer at base of finger | Endemic resident | History of renal transplant and receiving dialysis; diabetes | Oral azithromycin | Brazil | NR | 1/1 | 9 |
| Brown and others9 | 3.125 mg/kg/d or 200 mg/d for 7 days, followed by 200 mg biweekly for 3 weeks | 41 | 1/19 years/M | 6-cm ulcer on left knee and 2 nodules on right hand | Non-immune returned traveler | No | No | Belize | L. (Viannia) braziliensis | 1/1 | 12 |
| Gündüz and others10 | 3 mg/kg/d biweekly for 6 weeks | 36 | 1/60 years/F | 3-year history of extensive facial plaque (leishmanisis recidivans) | Endemic area resident | No | Pentavalent antimony and ketoconazole | Turkey | NR (likely L. tropica) | 0/1, inadequate clinical response | NR |
| Mirzabeigi and others11 | 5 mg/kg/d for 2 months | 300 | 1/50 years/F | Multiple painful nodules on both lower extremities 2 months after transplant surgery (reactivation) | Resident of Bolivia, immigrated to USA | History of renal transplantation and receiving immunosuppressive drugs | No | Bolivia | NR | 1/1 | 12 |
| Paradisi and others12 | 3 mg/kg/d on days 1–5, 14, and 21 | 21 | 1/56 years/M | Three large leg ulcers | Endemic area resident | Type II diabetes; lesions previously treated with topical steroids | Topical paromomycin and itraconazole | Italy | L. infantum | 1/1 | 9 |
| Rapp and others13 | 3 mg/kg/d on days 1–5 and 10 | 18 | 1 | 4-cm crusted lesion on left elbow for 3 years | Non-immune returned traveler | No | Pentavalent antimony and ketoconazole | Djibouti | NR | 1/1 | 18 |
| Rongioletti and others14 | 3 mg/kg/d for 5 days | 15 | 1/82 years/M | Lip swelling for 2 years | Endemic area resident | No | Pentavalent antimony | Italy | L. infantum | 1/1 | 12 |
| Rosal and others15 | 5 mg/kg/d for 10 days | 50 | 1/4 months/M and 1/9 years/M | 3 cm hyperkeratotic nodule cheek; ulcers on jaw and right hand | Endemic area resident; non-immune returned traveler | No | Intralesional pentavalent antimony plus topical imiquimod and amphotercin B; itraconazole | Spain, Bolivia, or Peru | 1 NR, 1 L . (Viannia) braziliensis | 2/2 | 24, NR |
| Solomon and others16 | 3 mg/kg/d on days 1–5 and 10 | 18 | 7, 5/M and 2/F age range = 21–24 years | Upper extremity ulcers (4) and facial ulcers (3) with lymph node enlargement | Non-immune returned travelers | No | 5 pentavalent antimony treatment failures, 2 initial treatment with AmBisome | Bolivia | L. (Viannia) braziliensis | 7/7 | 3–17 |
| Torre-Cisneros and others17 | 1.5 mg/kg/d for 2 weeks, followed by 4 weekly doses of amphotericin B | 31.5 mg AmBisome; 2.5 grams amphotericin B | 1/40 years/F | 0.5-cm erythematous scaling lesion in right ear | Endemic area resident | No | Pentavalent antimony | Spain | NR | 1/1 | NR |
| Perez-Ayala and others18 | 3 mg/kg/d on days 1–5, 14, and 21 | 21 | 1/27 years/M and 1/39 years/F | Two ulcers left thigh; one ulcer lower extremity | Immigrant; non-immune returned traveler | HIV+, No | No | Burkina Faso, French Guiana | NR, L. braziliensis | 0/2 side effects; inadequate clinical response | NR |
NR = not reported; HIV = human immunodeficiency virus.
Methods
A retrospective chart review was conducted on all patients receiving AmBisome for laboratory-confirmed CL who were either directly managed or managed in consultation with the WRAMC Infectious Disease Service during 2007–2009. Documentation of health care within the Department of Defense is accomplished through a centralized electronic medical record system termed the Armed Forces Health Longitudinal Technology Application system. Patients receiving health care by any Department of Defense health care provider have their encounter documented in the electronic medical record. The study was reviewed and approved by the WRAMC Institutional Review Board.
Laboratory diagnosis was made by lesion sampling (aspirate or scraping) and microscopic visualization of amastigotes, isolation of promastigotes in culture, or detection of Leishmania rRNA by real-time polymerase chain reaction amplification performed in a College of American Pathologists/Clinical Laboratory Improvement Amendments–certified laboratory at the Walter Reed Army Institute of Research (Silver Spring, MD).19 Identification of Leishmania species was performed by using cellulose acetate electrophoresis for isoenzyme analysis and comparison with World Health Organization reference strains.20,21
Treatment with intravenous AmBisome was prescribed at a dose of 3mg/kg/day, with up to 10 doses given within a 21-day period. All patients received premedication with oral acetaminophen (650 mg) and diphenhydramine (50 mg one hour before administration of AmBisome) and intravenous infusion of one liter of physiologic saline. AmBisome was initially infused at a rate of 200 mL/hour. However, because of development of acute infusion–related reactions in two patients, this rate was changed to a dose-escalating infusion (20 mL/hour for 15 minutes, and then 40 mL/hour for 30 minutes, then doubled every 30 minutes until a maximum rate of 200 mL/hour was reached). The infusion duration varied depending on the dose of AmBisome, but lasted approximately two hours.
Efficacy of drug activity was assessed by a review of lesion photographs and/or clinical notes documented in Armed Forces Health Longitudinal Technology Application. Patients who experienced complete healing of their skin lesion(s) after an initial course of AmBisome were scored as cured.
Toxicity of therapy was assessed by a review of the medical record for documentation of adverse events. Nephrotoxicity was assessed by the Common Terminology Criteria (CTC), in which grade 1 indicates values greater than the upper limit of normal (ULN) to 1.5 times the ULN, grade 2 is 1.5–3.0 times the ULN, and grade 3 is > 3.0–6.0 times the ULN.22
Results
Twenty patients with confirmed CL received treatment with AmBisome. The epidemiologic characteristics and outcome of the cohort are shown in Table 2. Two patients had concurrent nasal mucosa and CL (determined by endoscopic nasal biopsies of intranasal lesions) and AmBisome was suggested as the recommended therapy. Three patients had received prior treatment with Pentostam at a dose of 20 mg/kg/day for their disease. One patient had received 20 doses without cure of his lesion, and two patients had adverse events (hepatitis and pancreatitis), which resulted in cessation of therapy after 9 and 4 doses, respectively. The remaining 15 patients chose treatment with AmBisome after a discussion of the risks/benefits and treatment alternatives.
Table 2.
Cohort description and outcome of patients with cutaneous leishmaniasis treated with amphotericin B (AmBisome)
| Characteristic | Result |
|---|---|
| Mean age, years (range) | 29 (19–46) |
| Sex (% male) | 95 |
| Median no. lesions (range) | 1 (1–11) |
| Median maximum lesion size, cm (range) | 2 (1–5) |
| Countries of acquisition (number of cases) | Iraq (5), Afghanistan (5), Peru (1), French Guiana (1), Honduras (2), Columbia (6) |
| Strains of Leishmania recovered (number) | L. (Viannia) braziliensis (3), L. (V.) guyanensis (3), L. (V.) panamensis (4), L. tropica (2), L. major (3) |
| Median total dose of AmBisome for initial treatment course, mg and mg/kg (range) | 1,748 (530–2,670), 21 (10–30) |
| Patients receiving a full treatment course | 19 |
| Patients with a cure after initial treatment regimen | 16/19 (84%) |
| Median cumulative dose of AmBisome prescribed for 3 patients not healing with an initial treatment course, mg and mg/kg (range) | 3,146 (3,000–3,430), 36 (33–42) |
Ten patients acquired their infection in the Old World (5 in Iraq and 5 in Afghanistan). Isoenzyme analysis of five samples identified L. major (3) and L. tropica (2). Ten infections were acquired in the New World (1 in Peru, 1 in French Guiana, 2 in Honduras, and 6 in Columbia). Isoenzyme analysis of samples from these 10 patients identified L. (V.) braziliensis (3), L. (V.) guyanensis (3), and (L). V. panamensis (4).
The initial treatment regimen for AmBisome was 3 mg/kg/day for a median number of 7 doses (range = 2–10 doses) and a median total dose of 1,748 mg (range = 530–2670 mg). By milligrams/kilogram, the median total treatment dose was 21 mg/kg (range = 6–30 mg/kg). Thirteen patients (65%) had documentation of at least one adverse reaction to AmBisome. Five (25%) patients experienced infusion-related reactions, with development of chest pain, dyspnea, flank pain, flushing, and/or urticaria. For one patient, AmBisome was stopped after two doses because of these side effects, and the remaining patients tolerated further infusions. Some evidence of renal toxicity developed in 9 (45%) patients; 8 had a grade 1 toxicity value and 1 had a grade 2 toxicity value. Creatinine levels were either normalized (2 patients) or were returning to normal (4 patients) at time of discharge from medical care. Three patients did not return for follow-up laboratory testing.
Excluding one patient who received only two doses of AmBisome secondary to an acute infusion-related reaction and who then received treatment with Pentostam, 19 patients were included in the efficacy analysis. Median length of follow-up was 4 months (range = 1–27 months). Sixteen (84%) patients experienced a cure of their skin lesion after receiving an initial treatment course of AmBisome. Unfortunately, lack of standardized follow-up time points (caused by re-deployment of patients to combat zones or delay in responding to the queries of the investigators) prevented determination of a time to cure after administration of Ambisome. An illustrative example of a patient with L. (V.) braziliensis who experienced a cure after receiving seven doses of AmBisome is shown in Figure 1.
Figure 1.
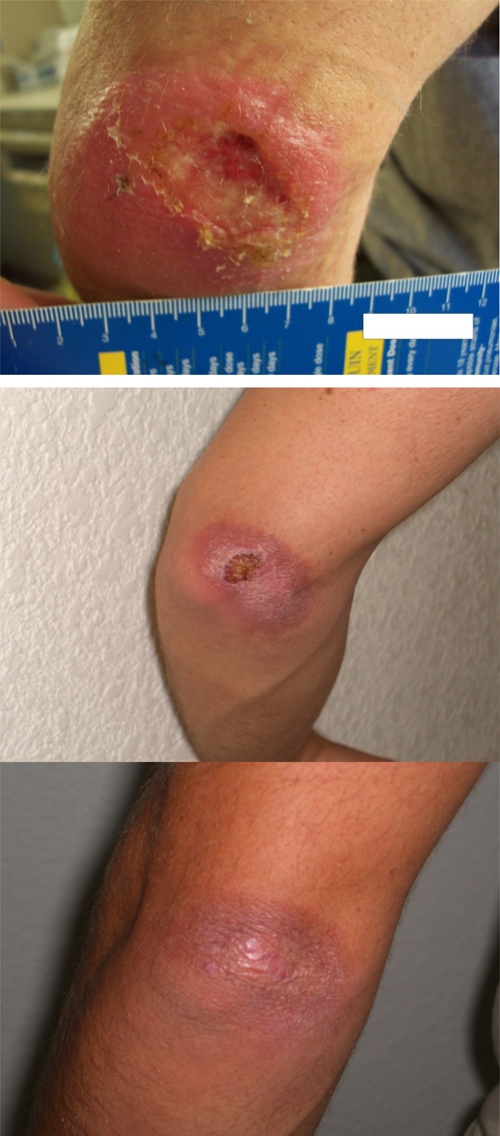
Patient with cutaneous leishmaniasis caused by Leishmania (Viannia) braziliensis baseline and three weeks and five months after treatment with seven doses of AmBisome. This figure appears in color at www.ajtmh.org.
Three patients (16%) required additional doses of AmBisome for cure of their lesions. The median total dose of AmBisome given during the initial treatment course was 1,750 mg (range = 1,260–2,670 mg) for those patients who were cured with one course of AmBisome and 1,745 mg (range = 1,250–2,002 mg) for those who required a second course. Of those patients who required a second course, one patient with L. (V.) braziliensis acquired in Honduras initially experienced healing of his single skin lesion (4 × 2.5 cm) after 5 doses totaling 1,250 mg (doses 6 and 7 were not given because of development of gastroenteritis), but subsequent re-ulceration at the same site developed approximately 6 weeks after his treatment course. The patient was re-treated with a seven-dose regimen of AmBisome (re-treatment dose = 1,750 mg [21 mg/kg], with a cumulative dose of 3,000 mg [36 mg/kg]) with subsequent clinical cure. Two patients with L. (V.) panamensis acquired in Columbia were treated with seven doses of AmBisome each (total doses = 1,745 mg and 2,002 mg [21 mg/kg]) with transient improvement in their solitary lesions (1 × 1 cm and 2 × 2 cm). Both lesions failed to completely heal, but were successfully cured with seven and four dose re-treatment courses of AmBisome (cumulative doses = 3,430 mg [42 mg/kg] and 3,146 mg [33 mg/kg], respectively) approximately 10 weeks and 7 months, respectively, after the initial treatment course. One patient refused more than four additional doses of AmBisome because of infusion-related reactions, but nonetheless experienced healing of his lesion.
Discussion
Our results demonstrate that AmBisome is an efficacious treatment for CL. Although encompassing a small number of patients, this study represents the largest published cohort of patients treated with AmBisome for CL to date. In addition, our study encompasses greater diversity than prior reports and describes disease acquired in five countries with five different strains of Leishmania. Sixteen (84%) of 19 patients were cured with an initial course of AmBisome. The 16% failure rate and the fact that the three failures were successfully treated with a second course of AmBisome suggests that the optimum dosing regimen for treatment of CL with AmBisome is still undefined.
In this study, administration of AmBisome was associated with mild-to-moderate toxicity; thus, this drug should be administered with caution. Acute infusion–related reactions associated with liposomal amphotericin B have been described, and a recent review reported a frequency of 20%, which is similar to the rate observed in this study.23 Nephrotoxocity is a recognized complication of therapy with AmBisome, and 45% of patients in our study experienced some evidence of renal dysfunction as measured by Common Terminology Criteria. Fortunately, renal dysfunction among our cohort was mild and transient. However, careful monitoring of renal function during therapy is prudent.
A direct comparison between AmBisome and the gold standard treatment of Pentostam was not conducted as part of this study, but previous studies have reported similar cure rates with Pentostam (depending on the patient population and geographic locale). Toxicity was observed with the administration of AmBisome, but Pentostam has its own host of recognized side-effects. A prospective, randomized controlled study comparing AmBisome and Pentostam would be required to more clearly establish the strength and quality of the evidence base to support a recommendation to use AmBisome in non-immune returned travelers. However, the low numbers of patients in the United States receiving CL each year would necessitate a long-term, multi-site, and multi-national effort that would be costly and unlikely to be undertaken. Given our results, one could argue that such a study is not needed because AmBisome, although not the panacea for CL, appears to be efficacious for CL caused by several different infecting strains.
Unfortunately, the expense (pharmacy cost of $821 per 218-mg dose or approximately $6,500 for the median dose in our cohort of 1,748 mg) and potential toxicity of AmBisome argue that an improved therapy for CL is still needed, with perhaps an inexpensive, well-tolerated oral regimen as the goal. Because AmBisome is a costly off-label prescription in the United States, some insurance and health care plans may be reluctant to reimburse for the cost of treatment. However, alternatives are limited. Pentostam is provided free of charge to requesting civilian physicians by the Centers for Disease Control and Prevention and to Department of Defense beneficiaries by the U.S. Army, but this drug carries the cost of regulatory oversight and has defined toxicities, and there are increasing reports of therapeutic failures with pentavalent antimonial drugs.24,25 In addition, although Pentostam can be administered in the outpatient setting, some providers may not have access to an infusion clinic, necessitating hospitalization for approximately three weeks. This cost and that of laboratory toxicity testing add to the expense of Pentostam. Oral agents (such as miltefosine and azole antifungal medications) have variable efficacy, may not be readily available, or are not FDA approved for a CL indication. Local destructive therapies (such as cryotherapy and the Thermomed® device) (Thermosurgery Technologies, Inc., Phoenix, AZ) are, in the authors' opinion, unsuitable for large lesions. Other lipid-associated amphotericin B products have extremely limited data for use in CL in humans, and animal data have demonstrated variable efficacy among the different products.26 There is also a single case report of a treatment failure for CL with amphotericin B lipid complex.27 At this time, there is insufficient information to recommend any other lipid-associated amphotericin B formulation for treatment of CL.
The limitations of our data reflect the demographic characteristic of our adult military population (19 of 20 male patients). Thus, we have data for only one female patient and no children. In addition, the U.S. military population is otherwise healthy, well nourished, and immunocompetent. The efficacy of all drug treatments for immuncompromised patients is likely to be lower.
Faced with a multitude of less-than-optimal choices, we suggest the following strategy for the treatment of CL in otherwise healthy non-immune returning travelers. For patients with small lesions (< 1 cm) caused by strains of Leishmania that often self-heal (such as L. mexicana and L. major), the best therapy may be no therapy because the risks of treatment may outweigh the benefits.1 Local treatments such as cryotherapy, the Thermomed® device, and intra-lesional Pentostam (as practiced at some locations outside the United States) may be appropriate for limited disease. Therapy with Pentostam or AmBisome could be offered to patients with cosmetically concerning lesions (e.g., on the face), or with strains of Leishmania that self-heal slowly or that can metastasize (such as L. tropica and L. (V). braziliensis). Our experience suggests that AmBisome be considered as an option for initial therapy in patients requiring systemic therapy for CL. The optimal regimen (daily dose and schedule) for each infecting strain of Leishmania has not been determined, but the current FDA-approved regimen for the treatment of visceral leishmaniasis in immunocompetent patients of 3 mg/kg/day on days 1–5, 14, and 21 (21 mg/kg) is a reasonable starting point for most patients. The day 14 and 21 dose may not be logistically feasible for some patients, and administering the total dose of 21 mg/kg over a shorter period can be considered.
Acknowledgments
We thank Kelly Hummer, Cyrilla Smalls, and Angela Tyler for their outstanding nursing support.
Disclaimer: The views expressed in this article are those of the authors and do not reflect the official policy of the Department of Army, the Department of Defense, or the US Government.
Footnotes
Authors' addresses: Glenn Wortmann, Michael Zupor, Roseanne Ressner, Susan Fraser, Josh Hartzell, and Amy Weintrob, Infectious Diseases Clinic, Washington, DC, E-mails: glenn.wortmann@us.army.mil, michael.zapor@us.army.mil, rose.ressner@us.army.mil, susan.fraser@us.army.mil, joshua.hartzell@us.army.mil, and amy.weintrob@us.army.mil. Joseph Pierson, Guthrie Army Health Clinic, Fort Drum, NY, E-mail: joe.c.pierson@afghan.swa.army.mil. Alan Magill, Division of Experimental Therapeutics, Walter Reed Army Institute of Research, Silver Spring, MD, E-mail:alan.magill@us.army.mil.
References
- 1.Magill A. In: Principles and Practices of Infectious Diseases. Seventh edition. Mandell GL, Bennett JE, Dolin R, editors. Volume 2. Philadelphia, PA: Churchill Livingstone Elsevier; 2010. pp. 3463–3480. (Leishmania species: visceral (kala-azar), cutaneous, and mucosal leishmaniasis). [Google Scholar]
- 2.Aronson NE, Wortmann GW, Johnson SC, Jackson JE, Gasser RA, Magill AJ, Endy TP, Coyne PE, Grogl M, Benson PM, Beard JS, Tally JD, Gambel JM, Kreutzer RD, Oster CN. Safety and efficacy of intravenous sodium stibogluconate in the treatment of leishmaniasis: recent U.S. military experience. Clin Infect Dis. 1998;27:1457–1464. doi: 10.1086/515027. [DOI] [PubMed] [Google Scholar]
- 3.Wortmann G, Miller RS, Oster C, Jackson J, Aronson N. A randomized, double-blind study of the efficacy of a 10- or 20- day course of sodium stibogluconate for treatment of cutaneous leishmaniasis in United States military personnel. Clin Infect Dis. 2002;35:261–267. doi: 10.1086/341406. [DOI] [PubMed] [Google Scholar]
- 4.Wortmann GW, Aronson NE, Byrd JC, Grever MR, Oster CN. Herpes zoster and lymphopenia associated with sodium stibogluconate therapy for cutaneous leishmaniasis. Clin Infect Dis. 1998;27:509–512. doi: 10.1086/514689. [DOI] [PubMed] [Google Scholar]
- 5.Lawn SD, Armstrong M, Chilton D, Whitty CJ. Electrocardiographic and biochemical adverse effects of sodium stibogluconate during treatment of cutaneous and mucosal leishmaniasis among returned travelers. Trans R Soc Trop Med Hyg. 2006;100:264–269. doi: 10.1016/j.trstmh.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 6.Drug Alert MHRA. 2006. https://www.cas.dh.gov.uk/ViewandAcknowledgment/ViewAlert.aspx?AlertID=100876 Available at. Accessed March 15, 2010.
- 7.Meyerhoff A. U.S. Food and Drug Administration approval of AmBisome (liposomal amphotericin B) for treatment of visceral leishmaniasis. Clin Infect Dis. 1999;28:42–48. doi: 10.1086/515085. [DOI] [PubMed] [Google Scholar]
- 8.Amato VS, Rabello A, Rotondo-Silva A, Kono A, Maldonado T, Alves I, Floeter-Winter L, Neto V, Shikanai-Yasuda M. Successful treatment of cutaneous leishmaniasis with lipid formulations of amphotericin B in two immunocompromised patients. Acta Trop. 2004;92:127–132. doi: 10.1016/j.actatropica.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Brown M, Noursadeghi M, Boyle J, Davidson RN. Successful liposomal amphotericin B treatment of Leishmania braziliensis cutaneous leishmaniasis. Br J Dermatol. 2005;153:203–205. doi: 10.1111/j.1365-2133.2005.06670.x. [DOI] [PubMed] [Google Scholar]
- 10.Gündüz K, Afsar S, Ayhan S, Kandiloglu A, Turel A, Filiz E, Ok U. Recidivans cutaneous leishmaniasis unresponsive to liposomal amphotericin B (AmBisome) J Eur Acad Dermatol Venereol. 2000;14:11–13. doi: 10.1046/j.1468-3083.2000.00004.x. [DOI] [PubMed] [Google Scholar]
- 11.Mirzabeigi M, Farooq U, Baraniak S, Dowdy L, Ciancio G, Vincek V. Reactivation of dormant cutaneous Leishmania infection in a kidney transplant patient. J Cutan Pathol. 2006;33:701–704. doi: 10.1111/j.1600-0560.2006.00532.x. [DOI] [PubMed] [Google Scholar]
- 12.Paradisi A, Capizzi R, Zampetti A, Proietti I, De Simone C, Feliciani C, Amerio P. Atypical multifocal cutaneous leishmaniasis in an immunocompetent patient treated by liposomal amphotericin B. J Infect. 2005;51:e261–e264. doi: 10.1016/j.jinf.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Rapp C, Imbert P, Darie H, Simon F, Gros P, Debord T, Roue R. Liposomal amphotericin B treatment of cutaneous leishmaniasis contracted in Djibouti and resistant to meglumine antimoniate. Bull Soc Pathol Exot. 2003;96:209–211. [PubMed] [Google Scholar]
- 14.Rongioletti F, Cannata GE, Parodi A. Leishmaniasis due to L. infantum presenting as macrocheilitis and responding to liposomal amphotericin B. Eur J Dermatol. 2009;19:281–282. doi: 10.1684/ejd.2009.0652. [DOI] [PubMed] [Google Scholar]
- 15.Del Rosal T, Artigao FB, Miguel MJ, de Lucas R, Del Castillo F. Successful treatment of childhood cutaneous leishmaniasis with liposomal amphotericin B: report of two cases. J Trop Pediatr. 2010;56:122–124. doi: 10.1093/tropej/fmp073. [DOI] [PubMed] [Google Scholar]
- 16.Solomon M, Baum S, Barzilai A, Pavlotsky F, Trau H, Schwartz E. Liposomal amphotericin B in comparison to sodium stibogluconate for cutaneous infection due to Leishmania braziliensis. J Am Acad Dermatol. 2007;56:612–616. doi: 10.1016/j.jaad.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 17.Torre-Cisneros J, Prada JL, Villanueva JL, Valverde F, Sanchez-Guijo P. Successful treatment of antimony-resistant cutaneous leishmaniasis with liposomal amphotericin B. Clin Infect Dis. 1994;18:1024–1025. doi: 10.1093/clinids/18.6.1024. [DOI] [PubMed] [Google Scholar]
- 18.Pérez-Ayala A, Norman F, Pérez-Molina JA, Herrero JM, Monge B, López-Vélez R. Imported leishmaniasis: a heterogeneous group of diseases. J Travel Med. 2009;16:395–401. doi: 10.1111/j.1708-8305.2009.00341.x. [DOI] [PubMed] [Google Scholar]
- 19.Wortmann G, Sweeney C, Houng HS, Aronson N, Stiteler J, Jackson J, Ockenhouse C. Rapid diagnosis of leishmaniasis by fluorogenic polymerase chain reaction. Am J Trop Med Hyg. 2001;65:583–587. doi: 10.4269/ajtmh.2001.65.583. [DOI] [PubMed] [Google Scholar]
- 20.Kreutzer RD, Christensen HA. Characterization of Leishmania spp. by isozyme electrophoresis. Am J Trop Med Hyg. 1980;29:199–208. doi: 10.4269/ajtmh.1980.29.199. [DOI] [PubMed] [Google Scholar]
- 21.Kreutzer RD, Souraty N, Semko ME. Biochemical identities and differences among Leishmania species and subspecies. Am J Trop Med Hyg. 1987;36:22–32. doi: 10.4269/ajtmh.1987.36.22. [DOI] [PubMed] [Google Scholar]
- 22.National Cancer Institute . Common Terminology Criteria for Adverse Events (CTCAE) Bethesda, MD: 2006. https://webapps.ctep.nci.nih.gov/webobjs/ctc/webhelp/Common_Terminology_Criteria_for_Adverse_Events_CTCAE_v3.htm Available at. Accessed April 22, 2010. [Google Scholar]
- 23.Roden MM, Nelson LD, Knudsen TA, Jarosinki PF, Starling JM, Shiflett SE, Calis K, DeChristoforo R, Donowitz GR, Buell D, Walsh TJ. Triad of acute infusion-related reactions associated with liposomal amphotericin B: analysis of clinical and epidemiological characteristics. Clin Infect Dis. 2003;36:1213–31220. doi: 10.1086/374553. [DOI] [PubMed] [Google Scholar]
- 24.Llanos-Cuentas A, Tulliano G, Auaujo-Castillo R, Miranda-Verastegui C, Santamaria-Castrellon G, Ramirez L, Lazo M, de Doncker S, Boelaert M, Robays J, Dujarkin J, Arevalo J, Chappuis F. Clinical and parasite species risk factors for pentavalent antimonial treatment failure in cutaneous leishmanisis in Peru. Clin Infect Dis. 2008;46:223–231. doi: 10.1086/524042. [DOI] [PubMed] [Google Scholar]
- 25.Hadighi R, Boucher P, Khamesipour A, Meamar AR, Roy G, Ouellette M, Mohebali M. Glucantime-resistant Leishmania tropica isolated from Iranian patients with cutaneous leishmaniasis are sensitive to alternative antileishmania drugs. Parasitol Res. 2007;101:1319–1322. doi: 10.1007/s00436-007-0638-0. [DOI] [PubMed] [Google Scholar]
- 26.Yardley C, Croft SL. A comparison of the activities of three amphotericin B lipid formulations against experimental visceral and cutaneous leishmaniasis. Int J Antimicrob Agents. 2000;13:243–248. doi: 10.1016/s0924-8579(99)00133-8. [DOI] [PubMed] [Google Scholar]
- 27.Wortmann GW, Fraser SL, Aronson NE, Davis C, Miller RS, Jackson JD, Oster CN. Failure of amphotericin B lipid complex in the treatment of cutaneous leishmaniasis. Clin Infect Dis. 1998;26:1006–1007. doi: 10.1086/517634. [DOI] [PubMed] [Google Scholar]


