Abstract
The clays consumed by geophagous individuals contain large quantities of aluminum, a known neurological and hematological toxin. This is the first study to evaluate the risk of aluminum poisoning in geophagous individuals. Blind determinations of plasma and urinary aluminum concentrations were carried out in 98 anemic geophagous pregnant women and 85 non-anemic non-geophagous pregnant women. Aluminum concentrations were significantly higher (P < 0.0001) in the geophagous anemic women than in the controls, with odds ratios of 6.83 (95% confidence interval [CI] = 2.72–19.31) for plasma concentrations (13.92 ± 14.09 μg/L versus 4.95 ± 7.11 μg/L) and 5.44 (95% CI = 2.17–14.8) for urinary concentrations (92.83 ± 251.21 μg/L versus 12.11 ± 23 μg/L). The ingested clay is the most likely source of this overexposure to aluminum. If confirmed, the clinical consequences of this absorption for pregnant women and their offspring should be explored.
Introduction
Geophagy, or the ingestion of earth, is a universal, ancestral practice that is particularly common in populations of African origin and disadvantaged social classes.1–3 A close link between this practice and iron-deficient anemia has been clearly established.3–8 The compulsive consumption of non-nutritional substances, or pica, is most prevalent during pregnancy.
The population of West French Guiana consists mostly of the descendants of black slaves. In this population, which has a low socioeconomic status, geophagy is on the increase, particularly among pregnant women, and this has led to an increase in the incidence of severe iron-deficient anemia during pregnancy.9
A number of studies have highlighted the presence of aluminum in the clays consumed by geophagous individuals, but no study has yet explored the possibility of aluminum absorption in the context of geophagy.5,6,10,11 The results of animal studies suggest that most of the aluminum ingested is potentially toxic to the mother and the developing brain of the fetus.12
We tested the hypothesis of an overexposure to aluminum associated with geophagy. We carried out a prospective transverse study in 2009, in which we determined blood and urinary aluminum concentrations blindly in pregnant women displaying chronic clay consumption behavior identified on the basis of the characteristics of their anemia and also in non-geophagous pregnant women as controls.
Methods
Study type and population.
This transverse study was carried out in 2009 on pregnant women consulting at St. Laurent du Maroni (a town in French Guiana on the border with Suriname) at mother-and-child centers, the offices of doctors in private practice, or the maternity unit of West French Guiana Hospital (CHOG). The reference maternity unit has a catchment population of 70,000 inhabitants, and it carried out 2,317 deliveries in the year of the study.
Based on the experiences of the medical teams and the findings of published studies, we decided to construct the geophagy group in two stages. Because the regular consumption of clay is associated with anemia, we decided to focus on women with severe anemia who remained severely anemic over the period corresponding to two blood samples as a means of selecting only women who regularly consumed earth.3–8 We then selected only those women who had no other identified cause of anemia and who admitted that they regularly consumed earth. The inclusion criterion for the geophagy group was being a pregnant woman with a hemoglobin (Hb) concentration of ≤ 85 g/L on the most recent hemogram obtained during the current pregnancy. Data concerning family situation, obstetric history, and clay consumption (Tables 1 and 2) were collected on inclusion. An examination focusing on anemia and renal function was carried out and included hemogram, hemoglobin electrophoresis, and determinations of red blood cell counts, ferritinemia, reactive protein C concentration, soluble transferrin receptor levels, haptoglobin concentration, lactate dehydrogenase activity, free and conjugated bilirubin concentrations, pyruvate kinase and glucose-6-phosphodehydrogenase (G6PD) activities, vitamin B12 and erythrocyte folate concentrations, creatinemia, uremia, natremia, blood lead concentration, and parasitological analysis of stools. On the same day, blood and urine samples were collected for the determination of aluminum concentrations. Because the patients were not living in a zone of endemic malaria, screening for blood parasites was not systematically carried out. The criteria for exclusion from the geophagy group were a hemoglobin concentration exceeding 90 g/L on the hemogram of the evaluation, the identification of a cause of anemia other than geophagy, and an absence of geophagy.
Table 1.
Clinical characteristics of the geophagous and control women
| Characteristics | Geophagy group n (%) or mean ± SD | Control group n (%) or mean ± SD | P value |
|---|---|---|---|
| Age (years) | 26 ± 7.3 | 27 ± 6.4 | 0.42* |
| < 20 (n [%]) | 22 (22.5%) | 11 (14.5%) | |
| 20–29 (n [%]) | 44 (45%) | 38 (51%) | |
| > 30 (n [%]) | 32 (32.5%) | 26 (34.5%) | |
| Number of previous pregnancies | |||
| 0 (n [%]) | 19 (19.5%) | 11 (14.5%) | 0.71* |
| 1–2 (n [%]) | 29 (29.5%) | 23 (30.5%) | |
| ≥ 3 (n [%]) | 50 (51%) | 41 (55%) | |
| Number of days between last delivery and current pregnancy | 867 ± 948 | 866 ± 867 | 0.99* |
| Number of closely spaced pregnancies (difference < 365 days) | 13 (13%) | 17 (24%) | 0.45* |
| Timing of first assessment (weeks of amenorrhea) | 28 ± 7 | 19 ± 10 | < 0.001* |
| Timing of inclusion (weeks of amenorrhea) | 31 ± 6 | 29 ± 7 | 0.044* |
| Access to tap water | 38 (53.5%) | 30 (55%) | < 0.50* |
| Consumption of beverages from metal cans | |||
| Never | 24 (37.5%) | 12 (24%) | 0.43* |
| Monthly | 4 (6%) | 7 (14%) | |
| Weekly | 20 (31.5%) | 21 (42%) | |
| Daily | 16 (25%) | 10 (20%) | |
Probability value for Pearson's χ2 test.
Table 2.
Interview concerning pemba consumption habits (N = 71)
| Variable | n (%) |
|---|---|
| Type of consumption (N = 71) | |
| Occasional | 15 (21%) |
| Regular | 56 (79%) |
| Amount consumed during pregnancy (N = 71) | |
| 100 g per day or more | 12 (17%) |
| 500 g per week | 18 (25%) |
| 500 g per month | 16 (23%) |
| 500 g or less throughout the pregnancy | 19 (27%) |
| Not determined | 6 (8%) |
| Mode of consumption (N = 71) | |
| Alone | 34 (47%) |
| With water | 21 (30%) |
| With soda or juice | 2 (3%) |
| With beer | 8 (11%) |
| With milk | 4 (6%) |
| Mixed with food or plants | 2 (3%) |
| Would like help with withdrawal (N = 71) | 69 (97%) |
| Free comments (N = 38) | |
| It's bad for my health. | 10 (26%) |
| It's bad for my baby. | 9 (24%) |
| A health worker told me to stop. | 9 (24%) |
| My family is advising me to stop. | 6 (16%) |
The control group consisted of pregnant women with an Hb concentration of at least 105 g/L on the most recent hemogram since the start of the pregnancy who did not consume clay. The same medical history data were collected as for the geophagy group. For the clinical examination, only the hemogram and determinations of ferritinemia, vitamin B12 concentration, erythrocyte folate concentration, and urinary and blood concentrations of aluminum were carried out. We excluded from this group all women for whom a hemogram carried out as part of this study revealed an Hb concentration below 100 g/L.
Written or oral (for illiterate women) consent was obtained from the women included in the study or from their legal guardian (for minors), and the women were free to decide whether to complete the questionnaire. This protocol was approved by the institutional review board of the CHOG. Samples were analyzed blindly by the laboratories.
Laboratory techniques.
Some of the analyses were carried out by the polyvalent laboratory of the CHOG. More specialized analyses (determinations of plasma and urinary aluminum concentrations, blood lead concentrations, G6PD and erythrocyte pyruvate kinase levels, and soluble transferrin receptors) were carried out by the Pasteur Cerba laboratory (Saint-Ouen l'Aumône, France).
The principal difficulty in aluminum determinations is the risk of contamination during the pre-analytical phase. We were, therefore, careful to ensure that plasma and urine samples were collected in strictly identical conditions for the two groups. For blood samples, we used ethylenediaminetetraacetic acid (EDTA) K2 vacuum tubes (reference VF076SDK; Terumo Venosafe, Laboratoire Terumo France SA, Guyancourt, France) in accordance with the instructions of the manufacturer. Plasma aluminum concentration was determined by atomic absorption spectroscopy (AAnalyst; Perkin Elmer Inc., Waltham, MA). Urine samples were collected at the laboratory in polystyrene flasks. They were then subject to inductively coupled plasma mass spectrometry (ICP-MS; Agilent 7500; Agilent Technologies Inc., Massy, France).
Hemograms and red blood cell counts were determined on whole-blood samples (EDTA tubes) on the basis of multiangular diffraction and flow cytometry (Cell-Dyn 3200; Abbott France, Rungis, France). Hemoglobin electrophoresis was carried out with the Hemoglobin Testing System using the Bthal short program (Bio-Rad Laboratories Inc., Marne la Coquette, France) on whole-blood samples (EDTA tubes). Biochemical measures (total bilirubin, conjugated bilirubin, free bilirubin, lactose dehydrogenase, ferritin, reactive C protein, haptoglobin, urea, creatinine, and sodium and potassium levels) were determined on heparin-treated plasma or serum (haptoglobin) samples with a Dimension Xpand Plus machine (Dade Behring Inc., Courbevoie, France). Vitamin B12 and B9 concentrations (erythrocyte folate levels) were determined by Microparticle Enzyme Immunoassay chemiluminescence (AxSYM; Abbot Diagnostics Inc., Chicago, IL). G6PD and erythrocyte pyruvate kinase activities were determined by enzyme assays on whole-blood samples (EDTA tubes). Plasma soluble transferring receptor levels were determined by immunoturbimetry. Blood lead concentrations were determined on whole-blood samples (EDTA tubes) by electrothermic atomic absorption spectrometry.
Five samples of white clay, known locally as pemba, were obtained from various sources (market sellers) and stored in transparent polystyrene tubes. They were analyzed in September 2008 by IPC-MS in the pharmacokinetics and clinical toxicology laboratory of Professor Jean-Pierre Goullé at Le Havre Hospital, Le Havre Cedex, France.
Statistical analysis.
The data were analyzed with STAT 9.0 software (College Station, TX). Student t tests were used for comparisons of continuous variables, with non-parametric Mann and Whitney tests used for those that were not normally distributed. For qualitative variables, we carried out Pearson's χ2 tests. Spearman's rank correlation analysis was applied to each group to test whether there was a direct, confounding relationship between aluminemia and hemoglobulinemia. Odds ratios (OR) were calculated with 95% confidence intervals (CI). A significance threshold of 5% was used. The normal range for aluminum concentrations used for reference was that generally used for non-dialyzed subjects by the laboratory analyzing the samples.
Results
Analysis of the pemba samples.
The five samples of pemba tested displayed the characteristic metal composition of clays, with a predominance of aluminum (15,340 μg/g of sample) and silicon (10,201 μg/g of sample) (Table 3). Calcium and iron were also present but at concentrations an order of magnitude lower (2,565 μg/g of sample and 1,080 μg/g of sample, respectively).
Table 3.
ICP-MS analysis of the five pemba samples: results in micrograms per gram
| Sample 1 | Sample 2 | Sample 3 | Sample 4 | Sample 5 | |
|---|---|---|---|---|---|
| Aluminum | 11,493 | 20,788 | 13,640 | 13,121 | 17,956 |
| Silicon | 10,893 | 10,648 | 10,951 | 11,344 | 7,171 |
| Calcium | 3,205 | 1,965 | 2,481 | 1,807 | 3,368 |
| Iron | 456 | 1,518 | 1,731 | 977 | 720 |
| Titanium | 202 | 411 | 225 | 524 | 271 |
| Barium | 54.6 | 32.1 | 36.1 | 35.3 | 55.8 |
| Zinc | 28.1 | 18.9 | 23.6 | 22.1 | 31.5 |
| Magnesium | 23 | 39 | 28 | 35 | 30 |
| Boron | 14 | 12 | 13 | 9 | 9 |
| Selenium | 7.4 | 6.8 | 10 | 6.4 | 9.66 |
| Vanadium | 7.1 | 15.9 | 8 | 15.6 | 12.9 |
| Lead | 5.61 | 9.12 | 9.28 | 4.53 | 9.65 |
| Gallium | 3.69 | 9.2 | 6.01 | 7.06 | 6.28 |
| Lanthanum | 2.32 | 6.84 | 3.11 | 3.45 | 4.31 |
| Tungsten | 2.23 | 0.14 | 0.10 | 0.09 | 0.28 |
| Strontium | 1.56 | 4.44 | 1.46 | 2.06 | 1.9 |
| Manganese | 1.02 | 1.72 | 1 | 1.44 | 2.06 |
| Nickel | 0.97 | 2.50 | 0.98 | 2.13 | 2.66 |
| Germanium | 0.79 | 1.20 | 1.31 | 1.06 | 1.20 |
| Others* | < 1 | < 1 | < 1 | < 1 | < 1 |
Others include tin, rubidium, lithium, cobalt, mercury, silver, molybdenum, thallium, palladium, uranium, gadolinium, copper, tellurium, beryllium, cadmium, antimony, arsenic, platinum, and bismuth.
Characteristics of two groups.
In 2009, 103 severely anemic (Hb < 85 g/L) geophagous pregnant women agreed to participate in the study (Table 1). We included 98 of these women in the final study population. The other five women were excluded, because a higher Hb concentration was obtained on the hemogram carried out as part of the study protocol. During this period, we retained 75 of 93 controls initially identified. The women excluded from the control group displayed a decrease in Hb concentration on the hemogram carried out for the protocol. The mean age of the patients was 27 ± 6.9 years, with no difference in the age structure of the populations of women in the two groups. Just over one-half of the women (51% of the anemic women and 55% of the controls; P = 0.71) had more than three children. Seventeen control women and 13 anemic women (P = 0.45) had had pregnancies very close together (less than 1 year between the last delivery and the start of the next pregnancy). The onset of anemia was generally toward the end of the first 6 months of pregnancy, whereas the control women were generally to be recruited at about 18 weeks of amenorrhea (P < 0.001). More than one-half of the pregnant women had no access to a reliable source of drinking water and drank rainwater or water from small water courses, with no difference between the two groups for this factor (P = 0.5).
Biological characteristics of the two groups.
Mean Hb concentration on the hemogram at inclusion was 67 ± 9.8 mg/L for the geophagous patients and 112 ± 8.8 mg/L for the controls (P < 0.001) (Table 4 and Figures 1 and 2). The red blood cell populations of the two groups had different characteristics, with microcytosis (mean globular volume of 66 ± 8.8 μm3 for the anemic women versus 82.7 ± 5.7 μm3 for the controls; P < 0.001) and hypochromia (corpuscle Hb concentration of 27.9 ± 2.4% in the anemic women versus 33.2 ± 2% in the controls; P < 0.001) lower in the anemic women. Ferritinemia was also lower in these patients than in controls (6.6 ± 4.8 versus 17.6 ± 23; P < 0.001), whereas vitamin B12 (172.7 ± 73.1 versus 186.6 ± 85.5; P = 0.25) and erythrocyte folate concentrations (689.1 ± 360.3 versus 689.1 ± 566.5; P = 0.89) were similar in the two groups. Detailed investigation of the geophagous women confirmed the non-regenerative, iron-deficient nature of the anemia (ferritinemia = 6.6 μg/L; red blood cell count = 98,541/mm3; transferrin receptor concentration = 14.26 mg/L). No digestive tract carriage of hematophagous helminths was observed. Also, the absence of signs of hemolysis ruled out the possibility of serious thalassemia, which is, in any case, very rare in the Maroni populations. Blood lead levels were normal in the patients (0.22 μmol/L). Aluminum concentrations were significantly higher (P < 0.0001) in the geophagous anemic women than in the controls, with OR of 6.83 (95% CI = 2.72–19.31) for plasma concentrations and 5.44 (95% CI = 2.17–14.8) for urinary concentrations. Considerable variability in these concentrations was observed in the geophagous group. There was no significant correlation between hemoglobin concentration and aluminemia in either the geophagous (Spearman's ρ = −0.06; P = 0.5) or the control group (Spearman's ρ = 0.16; P = 0.17).
Table 4.
Biological characteristics of the two groups
| Geophagy | Control | Statistics* | |
|---|---|---|---|
| Hemoglobin (g/L) | 67 ± 9.9 | 112 ± 8.8 | P < 0.001 |
| Mean globule volume (μm3) | 66 ± 8.8 | 82.7 ± 5.7 | P < 0.001 |
| Hematocrit (%) | 24.7 ± 5.3 | 33 ± 3.2 | P < 0.001 |
| Corpuscle Hb concentration (%) | 27.9 ± 2.4 | 33.2 ± 2 | P < 0.001 |
| Ferritinemia (μg/L) | 6.6 ± 4.8 | 17.6 ± 23 | P < 0.001 |
| Vitamin B12 (pmol/L) | 172.7 ± 73.1 | 186.6 ± 85.5 | P = 0.25 |
| Erythrocyte folate concentration (nmol/L) | 698.11 ± 360.3 | 689.1 ± 566.5 | P = 0.89 |
| Plasma aluminum (μg/L) | 13.92 | 4.95 | P < 0.0001 (OR = 6.83; 95% CI = 2.72–19.31) |
| ≤ 10 μg/L | 56 | 67 | |
| > 10 μg/L | 40 | 7 | |
| Urinary aluminum (μg/L) | 92.83 | 12.11 | P < 0.0001 (OR = 5.44; 95% CI = 2.17–14.8) |
| ≤ 20 μg/L | 45 | 56 | |
| > 20 μg/L | 35 | 8 |
Student t test. OR = odds ratio; 95% CI = 95% confidence interval.
Figure 1.
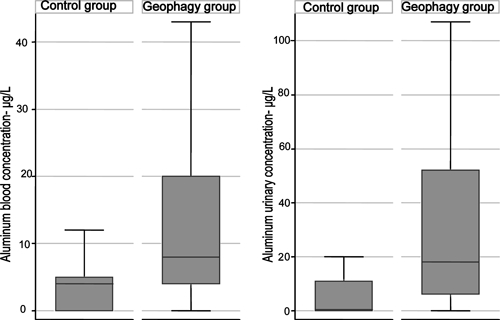
Box plot showing differences in aluminemia (left graph) and aluminuria (right graph) between the control and geophagy groups. The horizontal line within the boxes, the limits of the boxes, and the whiskers extending beyond the boxes represent the median, quartiles, and values below the first quartile and above the fourth quartile, respectively, excluding outliers.
Figure 2.
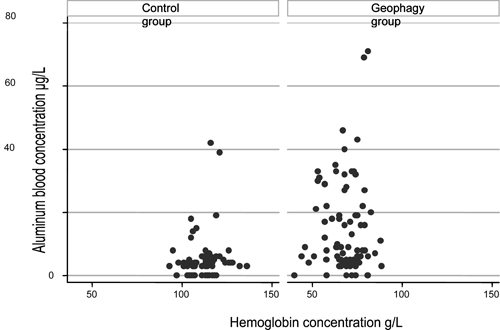
Distribution of plasma aluminum concentration as a function of hemoglobin concentration in the control (left) and geophagy (right) groups
Pemba consumption data recorded in the patients' medical history.
In total, 71 of 98 women in the geophagous group agreed to provide details of their geophagy during informal interviews (Table 2). Most stated that they regularly consumed earth, with only 15 (21%) considering their consumption to be occasional. The frequency of their purchases of balls of pemba (each weighing about 500 g) provided a rough estimate of the amounts of earth ingested. There was considerable diversity between individuals, with the largest consumers (17% of those questioned) buying two or more balls of pemba per week and 27% having bought only one ball of pemba during the pregnancy. Most women ingested the pemba with water (30%) or by itself (47%), the saliva transforming the powder into a mouthful of paste that was swallowed directly. Some women also reported ingesting pemba with beer (11%), milk (6%), or in a small minority of cases (3%), soda or citrus juice. One woman added the pemba to cooked dishes. Another consumed pemba only with plants as part of a traditional remedy for stomach ache. Most of the women complained of dependence on pemba. Those expressing themselves on this subject were aware of the danger to their health of this practice and were worried about its effects on their babies, but they were unable to stop consuming pemba, despite advice to do so from medical professionals or family and friends.
Discussion
In the Bas Maroni region, severely anemic and geophagous pregnant women are more likely than non-geophagous non-anemic women to have plasma and urinary aluminum concentrations above the upper limit of the normal range.
Balls of pemba produced from the clay soils around Moengo, a town in Suriname known for its bauxite mines, are widely available from the markets of St. Laurent du Maroni. The pharmacopeia of this region is very rich, and pemba has traditionally been used among the Djukas in small quantities during pregnancy.13 Pregnant women who are very fond of pemba may eat large quantities of it, but this consumption is often denied or played down. The feelings of guilt associated with geophagy, which has been stigmatized since the times of slavery and subsequently, by doctors, precluded recruitment based solely on declared pemba consumption.14 We, therefore, deliberately decided to use severe iron-deficient anemia of unknown cause as a surrogate for regular consumption of pemba to ensure that we obtained representative information about geophagy. The lack of correlation between hemoglobin and aluminum concentrations (Figure 2) suggests that the link between pemba consumption and aluminemia was not caused by a confounding effect of differences in hemoglobulinemia between groups. It was difficult for the interviewers to obtain spontaneous and reproducible responses concerning the amounts consumed and the chronology of pemba intake. The interview data (Table 2) were too imprecise to show a correlation between the quantity ingested and Hb or aluminum concentration. Constitution of the control group was more difficult than anticipated because of the low socioeconomic status of the population, which resulted in few women having a hemoglobin concentration greater than 105 g/L, the threshold used to define anemia during pregnancy.15 This, together with a widespread fear of blood sampling, accounts for the control group being smaller than the study group.
The difficulties encountered in the recruitment of non-anemic women in this study also reflect the high frequency of geophagy in this population.6 This compulsive consumption of earth has many consequences, and the balance between the advantages and risks remains a matter of heated debate.16,17 Geophagous individuals prefer clays, like those eaten by the Maroni women. Clays are complex materials consisting of negatively charged microcrystalline leaflets delimiting interleaflet spaces in which cations are adsorbed.18 Their mineralogical composition includes primarily aluminum and silicon together with variable quantities of other chemical elements, as shown by the chemical analyses of the pemba consumed by our patients (Table 3). Because of their unusual chemical structure, clays form colloidal suspensions with water, and they are able to absorb all sorts of toxic substances on their surfaces and exchange cations with the surrounding environment.7,19,20 These exchanges are the cause of the iron deficiency of geophagous women through the chelation of iron ions by the clay matrix.3,21 Hooda and Henry,1 who investigated the movements of iron, copper, and zinc in the digestive tract of geophagous individuals, concluded that, despite being rich in metallic micronutrients, clays tend to aggravate various mineral deficiencies by decreasing their intestinal bioavailability; this is particularly true for iron.1,22,23 The characteristics of the severe anemia observed in our patients are entirely consistent with those reported in other studies: microcytosis, very low ferritin concentrations, and high levels of transferrin receptors—all features indicative of profound iron deficiency.5,6,21 Geophagy was the only element in the medical histories of these patients that could account for this iron deficiency. Indeed, socioeconomic status, parity, and time between pregnancies were similar in the anemic and control groups.24
An increase in the bioavailability of calcium and lead has occasionally been reported in geophagous individuals.17,19,25 We were able to rule out induced saturnism, which might have worsened anemia, in our patients (mean blood lead level = 0.22 ± 0.13 μmol/L). We did not determine calcium concentrations. Paradoxically, aluminum fluxes have not been studied in detail by specialists in the field of geophagy, despite the abundance of this metal in clays. It has been assumed that aluminum is stably trapped in the microcrystalline matrix in the form of aluminum silicate complexes, which have a low bioavailability. Nevertheless, Callahan11 did raise the question of the possible release of aluminum during clay ingestion and of the immunological adjuvant properties of this metal stimulating immunoglobulin A (IgA) production in the intestinal walls of earth eaters. Kawai and others6 suggested that the presence of aluminum, which is hemotoxic, might aggravate the anemia of his patients. Studies of clay soils in natural environments have shown that, in certain conditions of salinity and pH resembling those in the digestive system, aluminum release may occur.26
Aluminum, the most abundant metallic element on Earth, is ubiquitous in our environment. It is found in kitchen utensils, packaging, tap water flocculants, food additives, antiperspirants, and antacid medication, for example.26 In a report on the studies of the United States Food and Drug Administration, Priest27 estimated that daily aluminum intake is of the order of 10 mg in populations with a Western-style diet. Antiacid drugs, produced from clays very similar to those preferred by geophagous individuals, may supply up to 1 g of aluminum per day, about ten times as much as a normal diet.27 Aluminum ingestion, whether dietary or medical, is followed by very low levels of absorption in the digestive tract of the order of 1%. This absorption is greatly increased by the concomitant intake of citrate, which is present in citrus juices, soda, and some beers that are often taken with pemba.27,28 No significant difference was found between our anemic patients and controls in terms of easily identifiable sources of aluminum, such as drinking water (the aluminum content of drinking water in West French Guiana regularly exceeds recommended limits) and the consumption of drinks from metal cans. If we also consider plasma folate and vitamin B12 concentrations, we can assume that the women in the two groups had largely similar dietary habits. We were unable to investigate dietary aspects further. The care staff found no evidence of abusive antacid consumption. We, therefore, conclude that the marked difference in plasma and urinary concentrations of aluminum between the two groups was probably directly linked to geophagy.
After absorbed, aluminum has a half-life of several hours in the blood; it is almost completely eliminated in urine over the course of several days. A small amount of aluminum (about 1%) is stored in the bones and brain. The plasma aluminum concentrations of our patients were between 0 and 71 μg/L, and the urinary aluminum concentrations were between 0 and 1,550 μg/L, with the upper limits of the normal range being 10 μg/L and 20 μg/L, respectively. The short half-life of aluminum in the blood and the random timing of sampling with respect to pemba ingestion probably account for the considerable variability of the concentrations observed (Figure 1). Few data are available concerning the acute oral toxicity of aluminum. Signs of lethal encephalopathies do not seem to occur until plasma concentrations are of the order of 500 μg/L.26 By contrast, chronic hematological effects are observed at plasma aluminum concentrations of about 100 μg/L along with the onset of reversible hypochromic anemia in dialysis patients caused by the direct effects of aluminum on erythrocyte progenitors and possibly, an additional chelation effect; this effect would account for the difficulties encountered when trying to correct the anemia of geophagous individuals by iron supplementation.29–31 The lack of correlation between hemoglobin and aluminum concentrations suggests that aluminum played only a minor role in the anemia of our patients, at least for concentrations of 10–100 μg/L. Neurocognitive problems and subtle electroencephalographic changes have been observed in aluminum workers with urinary aluminum concentrations of the order of 41 μg/L, a level reached in one-third of our patients.32,33 The prospect of fetal damage is even more worrying, because aluminum crosses the placenta and may become fixed in the fetal brain, disturbing its development.12,34 Reports of psychomotor abnormalities in young rats born to dams fed clay during the gestation period seem to confirm this hypothesis.35 Geophagy, therefore, seems to provide a potentially large dietary supply of aluminum.
Aluminum is found in all the types of earth consumed by geophagous individuals and is undoubtedly a key element in the pathogenic effects of this practice. The findings that we report draw attention to a much neglected aspect of pica: the risk of overexposure to aluminum and its possible consequences for the neurocognitive capacities of geophagous individuals, their hematological status, and the neurological development of the fetuses that they bear. Clays are highly complex chemically, and aluminum bioavailability is highly sensitive to the surrounding factors. Additional studies are, therefore, required to confirm our findings for other types of clays in other social and cultural contexts. The systematic use of IPC-MS for determinations would also greatly improve the quality of studies of this type.36 If the absorption of large quantities of aluminum in geophagous individuals is confirmed, then the clinical consequences of this absorption for pregnant women and their offspring should be explored.
Acknowledgments
We would like to thank all the pregnant women who agreed to participate in this study. The authors thank Aurélie Renaud, Sandrine Petitjean, Audrey Floch, Dominique Gaquière, Sandrine Estrabeaud, Betty Masure, Sandrine Reymond, Nathalie Picolo, Béatrice Fougère, Aristide Odoulami, and the healthcare professionals in the maternity unit of the CHOG who gave up some of their precious time to recruit patients for this study. We would particularly like to thank Professor Alain Verloes from Robert Debré Hospital (Assistance Publique-Hôpitaux de Paris, Paris, France) for his advice during the writing of this manuscript and critical reading.
Footnotes
Authors' addresses: Veronique Lambert, Wael El Guindi, Gabriel Carles, Rachida Bouhari, and Estelle Rouvier, Service de Gynécologie Obstétrique and Service de Biologie Medicale, Centre Hospitalier de l'Ouest Guyanais, Saint Laurent du Maroni, French Guiana, E-mails: v.lambert@ch-ouestguyane.fr, welguindi@yahoo.com, g.carles@ch-ouestguyane.fr, r.bouhari@ch-ouestguyane.fr, and estelle.rouvier@hotmail.fr. Mathieu Nacher, Centre d'Investigations Cliniques, Centre Hospitalier Andre Rosemon, Cayenne, French Guiana, E-mail: mathieu.nacher@ch-cayenne.fr. Jean-Pierre Goulle, Laboratoire de Toxicologie, Groupe Hospitalier du Havre, Le Havre, France, E-mail: jgoulle@ch-havre.fr. Annie Laquerrière, Laboratoire de Pathologie, CHU de Rouen, Hôpital Charles Nicolle, Rouen Cedex, France, E-mail: Annie-Laquerrier@chu-rouen.fr.
References
- 1.Hooda P, Henry J. In: Geophagia and human nutrition. Consuming the Inedible. First edition. Macclancy J, Henry J, Macbeth H, editors. London, UK: Berghahn Books; 2007. pp. 89–111. [Google Scholar]
- 2.Horner RD, Lackey CJ, Kolasa K, Warren K. Pica practices of pregnant women. J Am Diet Assoc. 1991;1:34–38. [PubMed] [Google Scholar]
- 3.Halsted JA. Geophagia in man: its nature and nutritional effects. Am J Clin Nutr. 1968;21:1384–1393. doi: 10.1093/ajcn/21.12.1384. [DOI] [PubMed] [Google Scholar]
- 4.Okcuoglŭ A, Arcasoy A, Minnich V, Tarcon Y, Cin S, Yörükoğlu O, Demirag B, Renda F. Pica in Turkey. 1. The incidence and association with anemia. Am J Clin Nutr. 1966;19:125–131. doi: 10.1093/ajcn/19.2.125. [DOI] [PubMed] [Google Scholar]
- 5.Geissler PW, Shulman CE, Prince RJ, Mutemi W, Mnazi C, Friis H, Lowe B. Geophagy, iron status and anaemia among pregnant women on the coast of Kenya. Trans R Soc Trop Med Hyg. 1998;92:549–553. doi: 10.1016/s0035-9203(98)90910-5. [DOI] [PubMed] [Google Scholar]
- 6.Kawai K, Saathoff E, Antelman G, Msamanga G, Fawzi WW. Geophagy (soil-eating) in relation to anemia and helminth infection among HIV-infected pregnant women in Tanzania. Am J Trop Med Hyg. 2009;80:36–43. [PMC free article] [PubMed] [Google Scholar]
- 7.Tateo F, Summa V. Element mobility in clays for healing use. Appl Clay Sci. 2007;36:64–76. [Google Scholar]
- 8.Nchito M, Geissler PW, Mubila L, Friis H, Olsen A. Effects of iron and multimicronutrient supplementation on geophagy: a two-by-two factorial study among Zambian schoolchildren in Lusaka. Trans R Soc Trop Med Hyg. 2004;98:218–227. doi: 10.1016/s0035-9203(03)00045-2. [DOI] [PubMed] [Google Scholar]
- 9.El Guindi W, Pronost J, Carles G, Largeaud M, El Gareh N, Montoya Y, Arbeille P. Severe maternal anemia and pregnancy outcome. J Gynecol Obstet Biol Reprod (Paris) 2004;33:506–509. doi: 10.1016/s0368-2315(04)96563-5. [DOI] [PubMed] [Google Scholar]
- 10.Ziegler JL. Geophagy: a vestige of palaeonutrition? Trop Med Int Health. 1997;2:609–611. doi: 10.1046/j.1365-3156.1997.d01-359.x. [DOI] [PubMed] [Google Scholar]
- 11.Callahan GN. Eating dirt. Emerg Infect Dis. 2003;9:1016–1021. doi: 10.3201/eid0908.030033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Domingo JL. Reproductive and developmental toxicity of aluminum: a review. Neurotoxicol Teratol. 1995;17:515–521. doi: 10.1016/0892-0362(95)00002-9. [DOI] [PubMed] [Google Scholar]
- 13.Vernon D. Les représentations du corps chez les Noirs Marrons Ndjuka du Surinam et de la Guyane Française. 1992. http://www.documentation.ird.fr/hor/fdi:36991 Available at. Accessed June 1, 2010.
- 14.Higman BW. Slave Populations of the British Caribbean, 1807–1834. 2nd ed. Kingston, Jamaica: The Press University of the West Indies; 1995. pp. 294–298. [Google Scholar]
- 15.Milman N. Prepartum anaemia: prevention and treatment. Ann Hematol. 2008;87:949–959. doi: 10.1007/s00277-008-0518-4. [DOI] [PubMed] [Google Scholar]
- 16.Stokes T. The earth-eaters. Nature. 2006;444:543–544. doi: 10.1038/444543a. [DOI] [PubMed] [Google Scholar]
- 17.Abrahams PW. Soils: their implications to human health. Sci Total Environ. 2002;291:1–32. doi: 10.1016/s0048-9697(01)01102-0. [DOI] [PubMed] [Google Scholar]
- 18.Murray HH. Traditional and new applications for kaolin, smectite, and palygorskite: a general overview. Appl Clay Sci. 2000;17:207–221. [Google Scholar]
- 19.Dominy NJ, Davoust E, Minekus M. Adaptive function of soil consumption: an in vitro study modeling the human stomach and small intestine. J Exp Biol. 2004;207:319–324. doi: 10.1242/jeb.00758. [DOI] [PubMed] [Google Scholar]
- 20.Wilson MJ. Clay mineralogical and related characteristics of geophagic materials. J Chem Ecol. 2003;29:1525–1547. doi: 10.1023/a:1024262411676. [DOI] [PubMed] [Google Scholar]
- 21.Minnich V, Okçuoğlu A, Tarcon Y, Arcasoy A, Cin S, Yörükoğlu O, Renda F, Demirağ B. Pica in Turkey. II. Effect of clay upon iron absorption. Am J Clin Nutr. 1968;21:78–86. doi: 10.1093/ajcn/21.1.78. [DOI] [PubMed] [Google Scholar]
- 22.Prasad AS. Recognition of zinc-deficiency syndrome. Nutrition. 2001;17:67–69. doi: 10.1016/s0899-9007(00)00469-x. [DOI] [PubMed] [Google Scholar]
- 23.Hooda PS, Henry CJK, Seyoum TA, Armstrong LDM, Fowler MB. The potential impact of soil ingestion on human mineral nutrition. Sci Total Environ. 2004;333:75–87. doi: 10.1016/j.scitotenv.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 24.Adam I, Khamis AH, Elbashir MI. Prevalence and risk factors for anaemia in pregnant women of eastern Sudan. Trans R Soc Trop Med Hyg. 2005;99:739–743. doi: 10.1016/j.trstmh.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 25.Eiley A, Katz S. Geophagy in pregnancy: a test of a hypothesis. Curr Anthropol. 1998;39:532–545. [Google Scholar]
- 26.Agency for Toxic Substances and Disease Registry Toxicological Profile for Aluminum. 2008. http://www.atsdr.cdc.gov/toxprofiles/tp22.pdf Available at. Accessed June 1, 2010. [PubMed]
- 27.Priest ND. The biological behaviour and bioavailability of aluminium in man, with special reference to studies employing aluminium-26 as a tracer: review and study update. J Environ Monit. 2004;6:375–403. doi: 10.1039/b314329p. [DOI] [PubMed] [Google Scholar]
- 28.Sutherland JE, Greger JL. Kinetics of aluminum disposition after ingestion of low to moderate pharmacological doses of aluminum. Toxicology. 1998;126:115–125. doi: 10.1016/s0300-483x(98)00005-5. [DOI] [PubMed] [Google Scholar]
- 29.Touam M, Martinez F, Lacour B, Bourdon R, Zingraff J, Di Giulio S, Drüeke T. Aluminium-induced, reversible microcytic anemia in chronic renal failure: clinical and experimental studies. Clin Nephrol. 1983;19:295–298. [PubMed] [Google Scholar]
- 30.Varma PP, Kumar R, Prasher PK, Roy ND. Hypochromic anaemia in chronic renal failure—role of aluminium. J Assoc Physicians India. 1999;47:690–693. [PubMed] [Google Scholar]
- 31.Vittori D, Nesse A, Pérez G, Garbossa G. Morphologic and functional alterations of erythroid cells induced by long-term ingestion of aluminium. J Inorg Biochem. 1999;76:113–120. doi: 10.1016/s0162-0134(99)00122-1. [DOI] [PubMed] [Google Scholar]
- 32.Sińczuk-Walczak H, Szymczak M, Raźniewska G, Matczak W, Szymczak W. Effects of occupational exposure to aluminum on nervous system: clinical and electroencephalographic findings. Int J Occup Med Environ Health. 2003;16:301–310. [PubMed] [Google Scholar]
- 33.Cutrufo C, Caroli S, Delle Femmine P, Ortolani E, Palazzesi S, Violante N, Zapponi GA, Loizzo A. Experimental aluminium encephalopathy: quantitative EEG analysis of aluminium bioavailability. J Neurol Neurosurg Psychiatry. 1984;47:204–206. doi: 10.1136/jnnp.47.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yumoto S, Nagai H, Matsuzaki H, Matsumura H, Tada W, Nagatsuma E, Kobayashi K. Aluminium incorporation into the brain of rat fetuses and sucklings. Brain Res Bull. 2001;55:229–234. doi: 10.1016/s0361-9230(01)00509-3. [DOI] [PubMed] [Google Scholar]
- 35.Edwards AA, Mathura CB, Edwards CH. Effects of maternal geophagia on infant and juvenile rats. J Natl Med Assoc. 1983;75:895–902. [PMC free article] [PubMed] [Google Scholar]
- 36.Goullé J, Saussereau E, Mahieu L, Bouige D, Guerbet M, Lacroix C. A new medical concept: the metallic profile. Rev Med Interne. 2010;31:128–134. doi: 10.1016/j.revmed.2009.03.360. [DOI] [PubMed] [Google Scholar]


