Abstract
Cryptosporidium is a leading cause of childhood diarrhea in developing countries. We investigated symptomatic and asymptomatic cryptosporidiosis in 20 children less than two years of age in a semi-urban slum in southern India. All surveillance (conducted every two weeks) and diarrheal samples from 20 children (n = 1,036) with cryptosporidial diarrhea previously identified by stool microscopy were tested by polymerase chain reaction–restriction fragment length polymorphism for species and subgenotype determination. Thirty-five episodes of cryptosporidiosis were identified in 20 children, of which 25 were diarrheal. Fifteen episodes were associated with prolonged oocyst shedding. Multiple episodes of cryptosporidiosis occurred in 40% of the children. Most infections were with C. hominis, subtype Ia. Children with multiple infections had significantly lower weight-for-age and height-for-age Z scores at 24 months but had scores comparable with children with a single episode by 36 months. Multiple symptomatic Cryptosporidium infections associated with prolonged oocyst shedding occur frequently in this disease-endemic area and may contribute to the long-term effects of cryptosporidiosis on physical growth in these children.
Introduction
Cryptosporidium spp. is a leading cause of diarrhea in children in developing countries where it primarily affects those less than five years of age. Diarrhea caused by these parasites in early childhood has been associated with subsequent cognitive function deficits and growth faltering and stunting, and the risk of stunting increases with the number of episodes per year.1–6 A study from Peru found that symptomatic and asymptomatic cryptosporidiosis in children were associated with growth faltering after an infection but recovery was slower in children with symptomatic infection.7 In India, several studies have reported Cryptosporidium spp. in children with diarrhea (ranging from 1.3% to 18.9%) and in asymptomatic children (0–3%),8–12 and one study reported up to 9.8%13 positivity in asymptomatic children (with 13.1% positivity in symptomatic children).
In a previous study on a well-defined birth cohort of 452 children followed-up for 3 years in a semi-urban slum community in Vellore in southern India, 53 children with diarrhea were identified to have Cryptosporidium by microscopic examination of stool samples (cryptosporidial diarrhea). Most (41 of 53) children had cryptosporidial diarrhea at ≤ 2 years of age. The most common species associated with diarrhea was C. hominis, and subgenotyping by polymerase chain reaction–restriction fragment length polymorphism (PCR-RFLP) at the polymorphic Cpgp40/15 locus identified Ia as the most common subgenotype.14 In another study on children hospitalized with diarrhea, we found that PCR detected more than three times the number of cryptosporidial infections detected by microcopy.15,16 On the basis of these findings, we applied PCR detection to a longitudinal investigation of 20 randomly selected children previously positive for cryptosporidiosis by microscopy in the semi-urban slum community cohort. The goal of the study was to identify the frequency of symptomatic and asymptomatic cryptosporidial infections by using PCR to detect Cryptosporidium spp. in all surveillance and diarrheal stool samples.
Methods
Study participants and samples.
Twenty of 41 children who had at least one episode of cryptosporidial diarrhea identified by microscopy at ≤ 2 years of age were selected for this longitudinal study by using a random numbers list.14 All children belonged to a birth cohort residing in the semi-urban slum areas of Vellore in southern India. Recruitment and follow-up of the cohort have been described.17 Surveillance stool samples collected every two weeks, and diarrheal stool samples collected each time the child had a diarrheal episode were stored at –70°C. All available surveillance and diarrheal stool samples for the 20 children collected from birth to two years of age (n = 1,036 samples, 196 diarrheal samples, and 840 surveillance samples) were examined for Cryptosporidium spp. by PCR using the methods described below.
An episode of diarrhea was defined as at least one day of diarrhea (with ≥ 3 watery stools in a 24-hour period) preceded and followed by ≥ 2 days without diarrhea. An episode of cryptosporidiosis was defined as a period during which stool samples were positive for Cryptosporidium by PCR. The episode was considered to have ended when at least two consecutive samples showed negative results. An episode was classified as symptomatic or associated with diarrhea if at least one sample in that episode was a diarrheal sample. Co-infection was defined as identification of Cryptosporidium and at least one other enteric pathogen in a diarrheal stool sample.
Assessment of diarrhea severity.
The severity of the diarrheal episode was assessed by using the Vesikari scoring system, which is commonly used to assess severity of rotavirus diarrhea in children. The 20-point score is determined by the total duration of diarrhea, the maximum number of stools passed in 24 hours, the duration of vomiting (if present), the maximum number of vomiting episodes in 24 hours, fever (in °C), and the degree of dehydration.18 Although the score is designed for acute watery diarrhea caused by rotavirus, total and component assessment have previously yielded useful correlation with severity in a previously published report of cryptosporidiosis in the cohort, of which the 20 study children formed a subset.14
Baseline sociodemographic data.
Baseline sociodemographic data including sex, socioeconomic status, religion, family size, number of siblings, maternal education, education, and occupation of the head of the household were available. In addition, a hygiene assessment carried out at least every six months included information elicited by enquiry on hand-washing before feeding the child and after defecation, and by observation on covering drinking water, use of dedicated dippers to obtain drinking water stored in large containers, and washing of vegetables, fruit, and cooking vessels.
Anthropometric measurements.
All children had birth weights and lengths recorded, and heights and weights were measured each month for the 36 months of follow-up. Weight-for-age (WAZ) and height-for age (HAZ) z-scores were calculated by using the 2006 World Health Organization child growth standards as the reference population.19
DNA extraction and PCR.
DNA extraction using a QIAamp stool DNA extraction kit (Qiagen, Valencia, CA) and nested PCR of the Cryptosporidium small subunit ribosomal RNA (SSU rRNA) locus20,21 were performed on all diarrheal and surveillance stool samples. Initially, three sequentially collected surveillance samples from each child were pooled and resulted in 343 pools derived from 1,036 samples, DNA was extracted from the pool and analyzed by PCR. From all positive pools, DNA was re-extracted from individual stool samples, and the infecting species and subgenotype were identified by PCR-RFLP at the SSU rRNA locus20 and the Cpgp40/15 locus,22 respectively, by using previously described protocols.
Diarrheal stool samples were also screened for parasite ova and cysts by microscopy, for bacterial pathogens by culture, and for rotavirus by enzyme-linked immunosorbent assay (Rota IDEIA; Dako, Ely, United Kingdom). A subset were also screened for Campylobacter, diarrheagenic Escherichia coli,23 norovirus genogroups I and II, sapovirus,24 and adenovirus25 by PCR.
Statistical analysis.
Comparison of diarrheal severity and duration among children with single and multiple episodes of cryptosporidiosis was performed by using the Mann-Whitney U test. Nutritional status among children with single and multiple cryptosporidial infections were compared at the time of weaning (median age = 3 months, interquartile range [IQR] = 1.9–4 months26), 24 months, and 36 months of age by using the Mann-Whitney U test. Baseline sociodemographic parameters, hygiene, and breastfeeding history were compared by using Fisher's exact test for categorical variables and two-tailed t-test or the Mann-Whitney U test for continuous variables.
The study was reviewed and approved by the Institutional Review Board of Christian Medical College, Vellore, and informed consent was obtained from the parents.
Results
Episodes of cryptosporidiosis.
A mean (SD) of 42 (9.6) surveillance stool samples and 10 (5.4) diarrheal stool samples per child were examined, and 1,036 samples were tested in 343 pools of 3 consecutive samples each by SSU rRNA PCR. A total of 28 (8.2%) pools were positive, of which 5 (17.9%) were consecutive pools. Twelve (60%) children had more than one positive pool. When the individual stool samples in the positive pools were tested, 87 (8.4%) of 1,036 samples (both diarrheal and asymptomatic) were positive (Figure 1).
Figure 1.
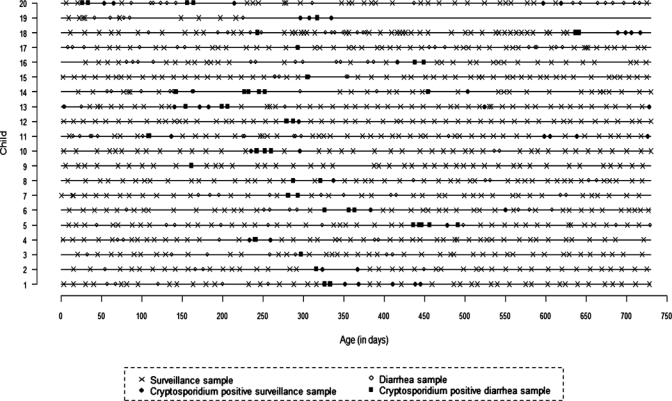
Cryptosporidium detection by polymerase chain reaction in diarrheal and surveillance stool samples of children in southern India.
Thirty-five episodes of cryptosporidiosis were identified among the 20 children. Two children (child 18 and child 20) had a gap of more than one month between two available positive samples. Intervening samples were not collected because the children were out of the study area at that time, and these were considered to belong to a single episode. One child dropped out of the study at 10 months (child 19). Twenty-five (71.4%) of 35 episodes were associated with diarrhea and the remaining 10 (28.6%) were asymptomatic. One episode of asymptomatic cryptosporidiosis was preceded by symptomatic cryptosporidiosis less than a month earlier (child 6, Table 1).
Table 1.
Type of episode and duration between episodes in eight children with multiple episodes of cryptosporidiosis, southern India*
| Child no. | First episode | Species, subtype | Interval (days) | Second episode | Species, subtype | Interval (days) | Third episode | Species, subtype | Interval (days) | Fourth episode | Species, subtype |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | S | Ch, Ie | 42 | A | Ch, ND | 28 | A | Ch, ND | |||
| 6 | S | Ch, Ia | 167 | A | ND | ||||||
| 8 | S | Ch, Id | 34 | S | Ch, Id | ||||||
| 11 | S | Ch, Ib | 462 | A | Ch, Ia | 88 | A | Ch, ND | |||
| 13 | S | Ch, Ia | 318 | A | Ch, ND | 204 | A | Ch, ND | |||
| 14 | S | ND | 85 | S | Ch, Ie | 202 | S | Ch, ND | 49 | A | Ch, ND |
| 18 | S | Ch, Ia | 394 | A | Ch, Ia | ||||||
| 20 | S | Ch, ND | 91 | S | Ch, ND | 383 | A | Ch, ND | 22 | A | Cm, ND |
S = symptomatic (diarrhea); Ch = Cryptosporidium hominis; A = asymptomatic; ND = not determined; Cm = C. meleagridis.
Most (31 of 35) symptomatic and asymptomatic infections were caused by C. hominis; there was one episode each caused by C. parvum and C. meleagridis. In two episodes (second episode in child 6 and the first of four episodes in child 14), only a faint band was obtained in the SSU rRNA PCR, and the species could not be identified by RFLP analysis. Twenty-two (71%) of 31 C. hominis could be subgenotyped by PCR-RFLP at the Cpgp40/15 locus, and most (12 of 22, 54.5%) were subgenotype Ia as previously documented in this birth cohort,14 followed by Id (6 of 22) and Ie (2 of 22). The C. parvum belonged to the anthroponotic IIc subgenotype.
Thirteen episodes of cryptosporidial diarrhea in 12 children were followed by asymptomatic oocyst shedding for 7–65 days. Six episodes of cryptosporidial diarrhea in six children were preceded by asymptomatic oocyst shedding that ranged from 7 to 22 days before the onset of diarrhea (Figure 1). In four of six children with asymptomatic oocyst shedding before diarrhea, the diarrheal stool samples were positive for Cryptosporidium and other enteric pathogens, including rotavirus (child 4), enterotoxigenic E. coli (child 16), and adenovirus (child 13 and child 19). Two other children with intermittent diarrhea during a cryptosporidial episode were found to be co-infected with adenovirus and Giardia (child 5) and enteroaggregative E. coli (child 6).
Multiple episodes.
Multiple episodes of cryptosporidial infection occurred in 40% (8 of 20) of the children less than two years of age; three children were infected three times and two children had four episodes each (Table 1). In all the eight children with multiple infections, the first infection was symptomatic, and three of eight second infections, one of five third infections, and zero of two fourth infections were associated with diarrhea. In all but two children with multiple episodes of cryptosporidiosis, subsequent episodes were asymptomatic or associated with a decrease in duration and severity of diarrhea (Table 1). Both children with greater severity during the second episode of cryptosporidial diarrhea were co-infected with other enteric pathogens (adenovirus and enterotoxigenic E. coli in child 14 and adenovirus and rotavirus in child 20).
Almost all recurrent episodes of cryptosporidiosis occurred more than a month after the previous episode (median = 91 days, IQR = 22–462 days). The median duration between a diarrheal episode and an asymptomatic episode of cryptosporidiosis was 242.5 days (IQR = 42–462 days).
In children with multiple episodes of cryptosporidiosis, almost all (13 of 15) episodes after the first one were caused by C. hominis, and only one child (child 20) had C. meleagridis infection after three C. hominis infections (Table 1). Subgenotypes could be determined for 6 of 8 primary infections but only for 4 of 15 later infections. Thus, subgenotypes were identified for initial and subsequent infections in only three of eight children with multiple infections. Two children (child 8 and child 18) were re-infected with the same subtype (Id and Ia, respectively), and the third child (child 11) had an initial subtype Ib infection and was re-infected with subtype Ia.
When clinical, sociodemographic, and environmental parameters were compared between children with single and multiple episodes of cryptosporidiosis (Table 2), children with multiple episodes were exclusively breastfed for a longer duration than children with a single episode (P < 0.001).
Table 2.
Comparison of clinical, sociodemographic, and environmental parameters in children with single and multiple infections with Cryptosporidium, southern India
| Variable | Single infection (n = 12) | Multiple infections (n = 8) | P |
|---|---|---|---|
| Male sex | 4 | 4 | 0.648* |
| Low socioeconomic status | 7 | 4 | 1.000* |
| No formal education for head of family | 3 | 3 | 0.642* |
| No formal education for mother | 4 | 2 | 1.000* |
| Mean (SD) maternal age at birth (years) | 22.75 (4.33) | 24.88 (2.42) | 0.225† |
| Mean (SD) family size | 6.08 (1.62) | 6.25 (1.28) | 0.810 |
| Mean (SD) no. of siblings | 2 (1.28) | 3 (0.89) | 0.094‡ |
| Mean (SD) birth weight (kg)§ | 2.97 (0.49) | 2.96 (0.24) | 0.931† |
| Mean (SD) duration of exclusive breastfeeding (months) | 1.59 (0.82) | 3.89 (1.26) | 0.001‡ |
| Mean (SD) no. of diarrhea episodes | 5.42 (3.61) | 7.5 (3.16) | 0.173‡ |
| Animals in the household | 1 | 2 | 0.537* |
| Covered drinking water container | 9 | 4 | 0.356* |
| Mother washed hands before feeding the child | 4 | 5 | 0.362* |
By Fisher's exact test.
By two-tailed t-test.
By Mann-Whitney U test.
Data missing for one child.
When the 11 children with a single episode (1 of the 12 dropped out of the study at 10 months of age) and 8 children with multiple episodes of cryptosporidiosis were compared, there were no significant difference in birth weight or in WAZ and HAZ scores at the time of weaning (median = 2 months, IQR = 1–3 months). However, by 24 months of age, children with multiple episodes of cryptosporidiosis had significantly lower Z scores for WAZ and HAZ than those with single infections (P = 0.013 and P = 0.021, respectively) (Figure 2). By 36 months of age, there were no significant differences in WAZ or HAZ scores between children with single and multiple infections, which indicated recovery from malnutrition.
Figure 2.
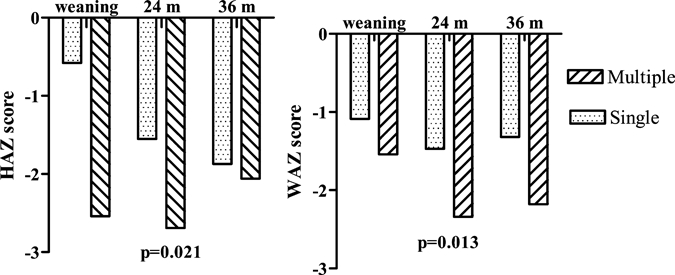
Weight-for-age (WAZ) and height-for-age (HAZ) scores in children with single and multiple episodes of cryptosporidiosis at weaning, 24, and 36 months of age in southern India.
Discussion
Using molecular tools and a longitudinal study design, we found high rates of multiple cryptosporidial infections, prolonged oocyst shedding before and after an episode of cryptosporidiosis, and a greater association of growth faltering with multiple infections in children in this semi-urban slum community in southern India. Other studies of children in developing countries have reported multiple infections in 14–30% of cases.27–29 Longitudinal studies from Brazil30 and Israel28 and cross-sectional studies on children with diarrhea from Uganda and Mexico2,31 have reported a greater prevalence of symptomatic infections than asymptomatic infections. In contrast, a longitudinal study from Peru consistently identified more asymptomatic infections than symptomatic infections.4,27,29 Both longitudinal studies from Brazil and Israel and those from Guinea-Bissau32 and Uganda2 identified more persistent (diarrhea lasting ≥ 14 days) diarrhea than acute diarrhea (diarrhea lasting less than 4 days). However, no persistent diarrhea was found in this study.
Occurrence of multiple infections, including repeated episodes of diarrhea, could be caused by infection with different species or subtypes. However, this finding was not supported by our study, where all re-infections, except one, were with C. hominis, and further characterization identified the same subgenotype in two of three children in whom all subgenotyping data were available. In Peru, C. hominis was detected in 6 of 15 children with 2 episodes and 1 of 2 children with 3 infections, and other re-infections were initially with a potentially zoonotic species followed by C. hominis.29 The studies are not directly comparable because of differences in study design and typing methods, but it is interesting to speculate on geographic differences in exposure to different cryptosporidial species and host response to infection.
This study did not evaluate any immunologic parameter. However, the data supports a decrease in frequency of infections and symptoms with age, probably caused by development of immunity. It would have been interesting to have more data on the subgenotypes infecting children with multiple episodes to elucidate whether protection is species or subgenotype specific. We were able to subgenotype 75% of primary infections but only 23% of post-primary infections (Table 1). These data may be partially explained by the fact that most of these were asymptomatic infections in which parasite burden is likely to be lower. The PCR-RFLP for the SSU rRNA gene (which is a multicopy gene33) that is used for species determination is more sensitive than the PCR-RFLP for the gp40/15 gene (which is a single-copy gene34) used for subgenotyping. This finding may explain why it was possible to determine species but not subgenotype in these infections. In two cases, species could not be identified by the SSU rRNA PCR, possibly because of the low oocyst burden.
Our study documented prolonged asymptomatic oocyst shedding before and after cryptosporidial diarrhea in at least 50% of children studied. These findings may have important implications for transmission of disease and long-term effects on growth. The finding of significantly greater growth faltering at 24 months of age in children with multiple infections must be interpreted with caution given the small number of children included in the study. However, this finding is biologically plausible and supports previous studies that have shown deleterious effects on growth and development after symptomatic and asymptomatic infections4 in children who acquired the infection at less than one year of age and with boys rather than girls affected more significantly.4,28,32 In a longitudinal study from Brazil, C. hominis and C. parvum infections were associated with decrease in HAZ scores within three months post-infection but this decrease was found to persist at 3–6 months after C. hominis infections, but not C. parvum infections.35
Low birth weight, malnutrition, stunting and lack of breastfeeding have been reported to predispose children to cryptosporidiosis.2,4,30 In this birth cohort, the median age of weaning was three months (IQR = 1.8–4 months). In our study, multiple infections were significantly associated with a longer duration of exclusive breastfeeding. This is difficult to explain except as an artifact produced by the small sample size, but it was interesting that when we examined the birth month of the children we found that 5 (41.7%) of 12 children with single infections and 2 (25%) of 8 children with multiple infections were born between April and July, the hottest months of the year, when mothers tend give other fluids in addition to breast milk. The finding of a difference in duration of exclusive breastfeeding did not appear to be related to nutritional status because there were no significant differences in WAZ or HAZ scores between the two groups at the time of weaning. A more detailed study will be required to assess nutritional and environmental factors and identify whether the multiple episodes of cryptosporidiosis result in growth deficits, or whether more episodes of cryptosporidiosis occurred in malnourished or more highly exposed children.
There were a number of limitations of this study, the greatest being the small sample size. Another limitation is that although we examined samples collected over a two-year period, the samples were only from children identified by microscopy to have previous symptomatic infections. There were also some occasions when the study child was unavailable for sample collection. Thus, the duration of every episode of cryptosporidiosis could not be estimated. Finally, although the infecting species could be identified in most episodes of cryptosporidiosis, it was not possible to identify the subgenotype in most asymptomatic infections, possibly because of a low parasite load.
Our current efforts are focused on a longitudinal investigation of cryptosporidiosis in a large birth cohort of children followed from birth to three years of age by using molecular techniques to detect, characterize, and quantify single and multiple asymptomatic and symptomatic Cryptosporidium spp. infections in this area. These data can then be correlated with clinical, nutritional, genetic, environmental, and immunologic parameters to determine risk factors for acquiring asymptomatic and symptomatic infections and re-infections with Cryptosporidium spp.
Acknowledgments
These data were presented, in part, at the 13th International Congress on Infectious Diseases at the Kuala Lumpur, Malaysia in June 2008.
Footnotes
Financial support: This study was supported by Fogarty International Research Cooperative Agreement R03TW2711 and Global Infectious Disease Training Grant D43TW007392, National Institutes of Health. Birth cohort studies were supported by Wellcome Trust Grant 063144.
Authors' addresses: Sitara S. R. Ajjampur, Rajiv Sarkar, Premi Sankaran, Arun Kannan, Vipin K. Menon, and Gagandeep Kang, Department of Gastrointestinal Sciences, Christian Medical College, Vellore, India, E-mails: sitararao@cmcvellore.ac.in, rsarkar@cmcvellore.ac.in, arunkannan@cmcvellore.ac.in, vipinmenon@cmcvellore.ac.in, and gkang@cmcvellore.ac.in. Jayaprakash Muliyil, Department of Community Health, Christian Medical College, Vellore, India, E-mail: jayaprakash@cmcvellore.ac. Honorine Ward, Division of Geographic Medicine and Infectious Diseases, Tufts Medical Center, Boston, MA, E-mail: hward@tuftsmedicalcenter.org.
References
- 1.Guerrant DI, Moore SR, Lima AA, Patrick PD, Schorling JB, Guerrant RL. Association of early childhood diarrhea and cryptosporidiosis with impaired physical fitness and cognitive function four-seven years later in a poor urban community in northeast Brazil. Am J Trop Med Hyg. 1999;61:707–713. doi: 10.4269/ajtmh.1999.61.707. [DOI] [PubMed] [Google Scholar]
- 2.Tumwine JK, Kekitiinwa A, Nabukeera N, Akiyoshi DE, Rich SM, Widmer G, Feng X, Tzipori S. Cryptosporidium parvum in children with diarrhea in Mulago Hospital, Kampala, Uganda. Am J Trop Med Hyg. 2003;68:710–715. [PubMed] [Google Scholar]
- 3.Kirkpatrick BD, Daniels MM, Jean SS, Pape JW, Karp C, Littenberg B, Fitzgerald DW, Lederman HM, Nataro JP, Sears CL. Cryptosporidiosis stimulates an inflammatory intestinal response in malnourished Haitian children. J Infect Dis. 2002;186:94–101. doi: 10.1086/341296. [DOI] [PubMed] [Google Scholar]
- 4.Checkley W, Gilman RH, Epstein LD, Suarez M, Diaz JF, Cabrera L, Black RE, Sterling CR. Asymptomatic and symptomatic cryptosporidiosis: their acute effect on weight gain in Peruvian children. Am J Epidemiol. 1997;145:156–163. doi: 10.1093/oxfordjournals.aje.a009086. [DOI] [PubMed] [Google Scholar]
- 5.Checkley W, Epstein LD, Gilman RH, Black RE, Cabrera L, Sterling CR. Effects of Cryptosporidium parvum infection in Peruvian children: growth faltering and subsequent catch-up growth. Am J Epidemiol. 1998;148:497–506. doi: 10.1093/oxfordjournals.aje.a009675. [DOI] [PubMed] [Google Scholar]
- 6.Agnew DG, Lima AA, Newman RD, Wuhib T, Moore RD, Guerrant RL, Sears CL. Cryptosporidiosis in northeastern Brazilian children: association with increased diarrhea morbidity. J Infect Dis. 1998;177:754–760. doi: 10.1086/514247. [DOI] [PubMed] [Google Scholar]
- 7.Berkman DS, Lescano AG, Gilman RH, Lopez SL, Black MM. Effects of stunting, diarrhoeal disease, and parasitic infection during infancy on cognition in late childhood: a follow-up study. Lancet. 2002;359:564–571. doi: 10.1016/S0140-6736(02)07744-9. [DOI] [PubMed] [Google Scholar]
- 8.Kaur R, Rawat D, Kakkar M, Uppal B, Sharma VK. Intestinal parasites in children with diarrhea in Delhi, India. Southeast Asian J Trop Med Public Health. 2002;33:725–729. [PubMed] [Google Scholar]
- 9.Ballal M, Shivananda PG. Rotavirus and enteric pathogens in infantile diarrhoea in Manipal, south India. Indian J Pediatr. 2002;69:393–396. doi: 10.1007/BF02722628. [DOI] [PubMed] [Google Scholar]
- 10.Shetty M, Brown TA, Kotian M, Shivananda PG. Viral diarrhoea in a rural coastal region of Karnataka India. J Trop Pediatr. 1995;41:301–303. doi: 10.1093/tropej/41.5.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jindal N, Arora R, Bhushan B, Arora S. A study of infective aetiology of chronic diarrhoea in children in Amritsar. J Indian Med Assoc. 1995;93:169–170. [PubMed] [Google Scholar]
- 12.Das P Roy SS, MitraDhar K, Dutta P, Bhattacharya MK, Sen A, Ganguly S, Bhattacharya SK, Lal AA, Xiao L. Molecular characterization of Cryptosporidium spp. in children in Kolkata, India. J Clin Microbiol. 2006;44:4246–4249. doi: 10.1128/JCM.00091-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mathan MM, Venkatesan S, George R, Mathew M, Mathan VI. Cryptosporidium and diarrhoea in southern Indian children. Lancet. 1985;2:1172–1175. doi: 10.1016/s0140-6736(85)92691-1. [DOI] [PubMed] [Google Scholar]
- 14.Ajjampur SS, Gladstone BP, Selvapandian D, Muliyil JP, Ward H, Kang G. Molecular and spatial epidemiology of cryptosporidiosis in children in a semiurban community in south India. J Clin Microbiol. 2007;45:915–920. doi: 10.1128/JCM.01590-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ajjampur SS, Rajendran P, Ramani S, Banerjee I, Monica B, Sankaran P, Rosario V, Arumugam R, Sarkar R, Ward H, Kang G. Closing the diarrhoea diagnostic gap in Indian children by the application of molecular techniques. J Med Microbiol. 2008;57:1364–1368. doi: 10.1099/jmm.0.2008/003319-0. [DOI] [PubMed] [Google Scholar]
- 16.Ajjampur SS, Liakath FB, Kannan A, Rajendren P, Sarkar R, Moses PD, Simon A, Agarwal I, Mathew A, O'Connor R, Ward H, Kang G. Multisite study of cryptosporidiosis in children with diarrhea in India. J Clin Microbiol. 2010;48:2075–2081. doi: 10.1128/JCM.02509-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Banerjee I, Ramani S, Primrose B, Moses P, Iturriza-Gomara M, Gray JJ, Jaffar S, Monica B, Muliyil JP, Brown DW, Estes MK, Kang G. Comparative study of the epidemiology of rotavirus in children from a community-based birth cohort and a hospital in south India. J Clin Microbiol. 2006;44:2468–2474. doi: 10.1128/JCM.01882-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruuska T, Vesikari T. Rotavirus disease in Finnish children: use of numerical scores for clinical severity of diarrhoeal episodes. Scand J Infect Dis. 1990;22:259–267. doi: 10.3109/00365549009027046. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization HO . WHO Child Growth Standards: Methods and Development: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index for Age. Geneva: World Health Organization; 2006. [Google Scholar]
- 20.Xiao L, Escalante L, Yang C, Sulaiman I, Escalante AA, Montali RJ, Fayer R, Lal AA. Phylogenetic analysis of Cryptosporidium parasites based on the small-subunit rRNA gene locus. Appl Environ Microbiol. 1999;65:1578–1583. doi: 10.1128/aem.65.4.1578-1583.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiao L, Bern C, Limor J, Sulaiman I, Roberts J, Checkley W, Cabrera L, Gilman RH, Lal AA. Identification of 5 types of Cryptosporidium parasites in children in Lima, Peru. J Infect Dis. 2001;183:492–497. doi: 10.1086/318090. [DOI] [PubMed] [Google Scholar]
- 22.Leav BA, Mackay MR, Anyanwu A, O'Connor RM, Cevallos AM, Kindra G, Rollins NC, Bennish ML, Nelson RG, Ward HD. Analysis of sequence diversity at the highly polymorphic Cpgp40/15 locus among Cryptosporidium isolates from human immunodeficiency virus-infected children in South Africa. Infect Immun. 2002;70:3881–3890. doi: 10.1128/IAI.70.7.3881-3890.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vidal M, Kruger E, Duran C, Lagos R, Levine M, Prado V, Toro C, Vidal R. Single multiplex PCR assay to identify simultaneously the six categories of diarrheagenic Escherichia coli associated with enteric infections. J Clin Microbiol. 2005;43:5362–5365. doi: 10.1128/JCM.43.10.5362-5365.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monica B, Ramani S, Banerjee I, Primrose B, Iturriza-Gomara M, Gallimore CI, Brown DW, Moses PD, Gray JJ, Kang G. Human caliciviruses in symptomatic and asymptomatic infections in children in Vellore, south India. J Med Virol. 2007;79:544–551. doi: 10.1002/jmv.20862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phan TG, Nguyen TA, Yan H, Yagyu F, Kozlov V, Kozlov A, Okitsu S, Muller WE, Ushuijma H. Development of a novel protocol for RT-multiplex PCR to detect diarrheal viruses among infants and children with acute gastroenteritis in Eastern Russia. Clin Lab. 2005;51:429–435. [PubMed] [Google Scholar]
- 26.Gladstone BP, Muliyil JP, Jaffar S, Wheeler JG, Le Fevre A, Iturriza-Gomara M, Gray JJ, Bose A, Estes MK, Brown DW, Kang G. Infant morbidity in an Indian slum birth cohort. Arch Dis Child. 2008;93:479–484. doi: 10.1136/adc.2006.114546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bern C, Ortega Y, Checkley W, Roberts JM, Lescano AG, Cabrera L, Verastegui M, Black RE, Sterling C, Gilman RH. Epidemiologic differences between cyclosporiasis and cryptosporidiosis in Peruvian children. Emerg Infect Dis. 2002;8:581–585. doi: 10.3201/eid0806.01-0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fraser D, Dagan R, Naggan L, Greene V, El-On J, Abu-Rbiah Y, Deckelbaum RJ. Natural history of Giardia lamblia and Cryptosporidium infections in a cohort of Israeli Bedouin infants: a study of a population in transition. Am J Trop Med Hyg. 1997;57:544–549. doi: 10.4269/ajtmh.1997.57.544. [DOI] [PubMed] [Google Scholar]
- 29.Cama VA, Bern C, Roberts J, Cabrera L, Sterling CR, Ortega Y, Gilman RH, Xiao L. Cryptosporidium species and subtypes and clinical manifestations in children, Peru. Emerg Infect Dis. 2008;14:1567–1574. doi: 10.3201/eid1410.071273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newman RD, Sears CL, Moore SR, Nataro JP, Wuhib T, Agnew DA, Guerrant RL, Lima AA. Longitudinal study of Cryptosporidium infection in children in northeastern Brazil. J Infect Dis. 1999;180:167–175. doi: 10.1086/314820. [DOI] [PubMed] [Google Scholar]
- 31.Miller K, Duran-Pinales C, Cruz-Lopez A, Morales-Lechuga L, Taren D, Enriquez FJ. Cryptosporidium parvum in children with diarrhea in Mexico. Am J Trop Med Hyg. 1994;51:322–325. doi: 10.4269/ajtmh.1994.51.322. [DOI] [PubMed] [Google Scholar]
- 32.Molbak K, Andersen M, Aaby P, Hojlyng N, Jakobsen M, Sodemann M, da Silva AP. Cryptosporidium infection in infancy as a cause of malnutrition: a community study from Guinea-Bissau, west Africa. Am J Clin Nutr. 1997;65:149–152. doi: 10.1093/ajcn/65.1.149. [DOI] [PubMed] [Google Scholar]
- 33.Le Blancq SMKN, Zamani F, Upton SJ, Wu TW. Ribosomal RNA gene organization in Cryptosporidium parvum. Mol Biochem Parasitol. 1997;90:463–478. doi: 10.1016/s0166-6851(97)00181-3. [DOI] [PubMed] [Google Scholar]
- 34.Cevallos AM, Zhang X, Waldor MK, Jaison S, Zhou X, Tzipori S, Neutra MR, Ward HD. Molecular cloning and expression of a gene encoding Cryptosporidium parvum glycoproteins gp40 and gp15. Infect Immun. 2000;68:4108–4116. doi: 10.1128/iai.68.7.4108-4116.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bushen OY, Kohli A, Pinkerton RC, Dupnik K, Newman RD, Sears CL, Fayer R, Lima AA, Guerrant RL. Heavy cryptosporidial infections in children in northeast Brazil: comparison of Cryptosporidium hominis and Cryptosporidium parvum. Trans R Soc Trop Med Hyg. 2007;101:378–384. doi: 10.1016/j.trstmh.2006.06.005. [DOI] [PubMed] [Google Scholar]


