Abstract
Intraluminal occlusion of the middle cerebral artery (MCA) is used extensively in cerebral ischemia research. We tested a modified nylon suture in a rat model of middle cerebral artery occlusion (MCAO) under two anesthesia regimens. Sprague-Dawley rats were divided into six groups (Group 1, Poly-L-lysine-coated suture under ketamine/xylazine anesthesia; Group 2, modified suture under ketamine/xylazine anesthesia; Group 3, Poly-L-lysine-coated suture under ketamine/xylazine anesthesia with mechanical ventilation; Group 4, modified suture under ketamine/xylazine anesthesia with mechanical ventilation; Group 5, Poly-L-lysine-coated suture under isoflurane anesthesia; Group 6, modified suture under isoflurane anesthesia) and subjected to 2 hours MCAO. Regional cerebral blood flow (rCBF) was monitored by Laser-Doppler flowmetry. Neurological evaluation and ischemic lesion (TTC stain) were assessed at 24 hr of reperfusion. The total ischemic lesion (sum of areas with lacking and intermediate TTC staining) was similar among all six groups. Compared with a Poly-L-lysine-coated suture technique, the modified suture technique produced a lower rCBF, larger infarct size, smaller variance of infarct size, and greater neurological deficit. In addition, isoflurane significantly reduced infarct size. We conclude that use of this modified suture technique with ketamine/xylazine anesthesia and mechanical ventilation produces a more consistent change in cerebral ischemic damage following MCAO in rats.
INTRODUCTION
Ischemic stroke is a leading cause of mortality and long-term disability. Recently, recanalization with drugs during acute ischemic stroke have been shown to be clinically effective for treating ischemic stroke [24]. Thus, a reliable animal model of transient focal ischemia is becoming more important in order to study the pathophysiology of ischemic stroke and evaluate new therapeutic approaches. Intraluminal occlusion of the middle cerebral artery (MCA) in rats using a suture methodology was first described by Koizumi et al., and has been extensively applied and studied due to its minimal invasiveness [13]. Due to the variability in the final infarct size this method has been modified by many investigators [2,14,17,23,28,29]. Previous studies have demonstrated that rat strain, animal age, blood pressure, brain temperature, blood glucose, suture type, and site of filament insertion are factors contributing to the variability in the final infarct size [2]. However, we suggest that anatomical variations of the origin of the MCA might be a primary factor resulting in a difference in blood flow interdiction of the MCA supplying area during middle cerebral artery occlusion (MCAO), and finally a difference in ischemic brain damage [8,25]. In this study, we used a modified suture technique to produce a more complete and consistent blood flow interdiction of the MCA supplying area for any variation of MCA origination. Thus, our first goal was to compare the effect of a Poly-L-lysine-coated suture technique and our modified suture technique on transient focal ischemia-induced brain damage and cerebral blood flow.
Anesthesia also may be an important factor affecting infarct size in a MCAO model. Several anesthetics including ketamine, urethane, chloral hydrate, ether, halothane, and isoflurane have being used in a MCAO rat model [4,14,16,19,27,31]. However, anesthetics, especially volatile anesthetics, have been shown to temporally reduce ischemic cerebral injury during the early stage of reperfusion [9,10]. In addition, isoflurane was recently reported to provide protection against transient focal ischemia [21]. Thus, our second goal was to compare the effect of ketamine/xylazine and isoflurane on transient focal ischemia-induced brain damage.
RESULTS
Physiological parameter
The physiological parameters measured immediately before MCAO are summarized in Table 1. There was no significant difference in mean arterial blood pressure (MABP), heart rate (HR), or arterial blood gas (pH, PCO2, and PO2) between the Poly-L-lysine-coated suture group and the modified suture group regardless of the anesthesia method. In addition, no significant difference was observed in MABP among all six groups. Anesthesia with ketamine/xylazine induced a respiratory acidosis in groups 1 and 2, which was rectified by mechanical ventilation in groups 3 and 4.
Table 1.
Physiological Parameters
| Group | MABP (mmHg) | HR | pH | PCO2 (mmHg) | PO2 (mmHg) | |
|---|---|---|---|---|---|---|
| Poly-L-lysine-coated | ||||||
| | ||||||
| Ketamine/xylazine | Suture (Group 1) | 94±2 | 246±8 | 7.36±0.01 | 50±1 | 75±1 |
| Modified Suture (Group 2) | 90±3 | 254±13 | 7.37±0.01 | 48±1 | 78±2 | |
| Ketamine/xylazine with mechanical ventilation | Poly-L-lysine-coated Suture (Group 3) | 97±3 | 258±10 | 7.40±0.01*# | 39±2*# | 120±5*# |
| Modified Suture (Group 4) | 94±3 | 251±8 | 7.39±0.004*# | 41±1*# | 126±8*# | |
| | ||||||
| Poly-L-lysine-coated | ||||||
| Suture (Group 5) | 92±1 | 342±8*#? | 7.41±0.01*# | 43±1*# | 133±5*# | |
| Isoflurane | Modified Suture (Group 6) | 94±3 | 333±8*#? | 7.41±0.01*# | 40±1*# | 136±2*# |
P < 0.05 vs. Poly-L-lysine-coated suture under ketamine/xylazine
P < 0.05 vs. modified suture under ketamine/xylazine
P < 0.05 vs. ketamine/xylazine.
Anatomical variation of MCA origin
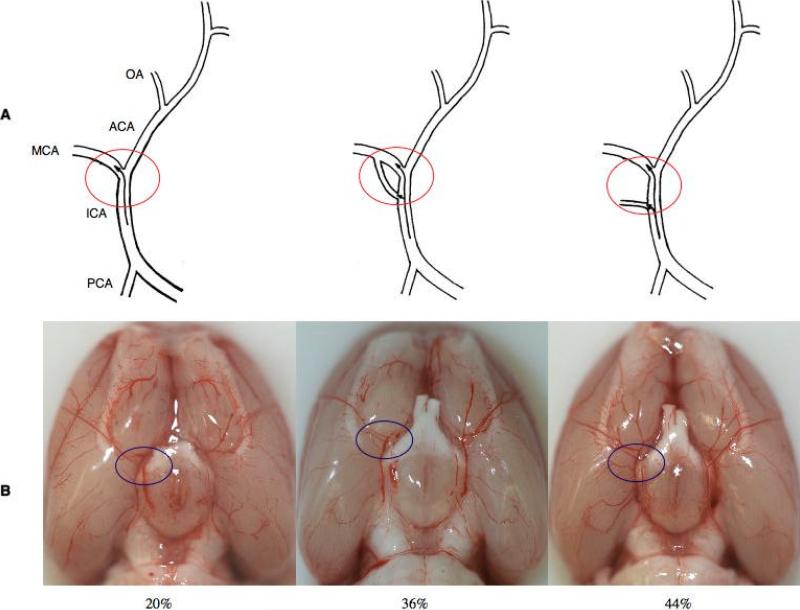
Anatomical variations of the origin of the MCA were observed in the rats (Figure 2). 36% (n=18) of the rats had two branches originating from the ICA to combine into one MCA. 44% (n=22) of the rats had an additional small branch originating from the ICA to supply MCA area. In 20% (n=10) of the rats, the ICA simply divided into MCA and ACA (Figure 2).
Figure 2.
(A) Three anatomical variations of MCA origin. (B) Representative pictures of anatomical variations in MCA origin.
Subarachnoid hemorrhage
Subarachnoid hemorrhage (SAH) resulted from arterial rupture was found in 5 rats, all from the Poly-L-lysine-coated suture groups (Group 1 (3), Group 3 (1), Group 5 (1)). Those rats with SAH were excluded from further measurement of infarct size and neurological evaluation. In contrast, no SAH was found in the modified suture groups.
Infarct size
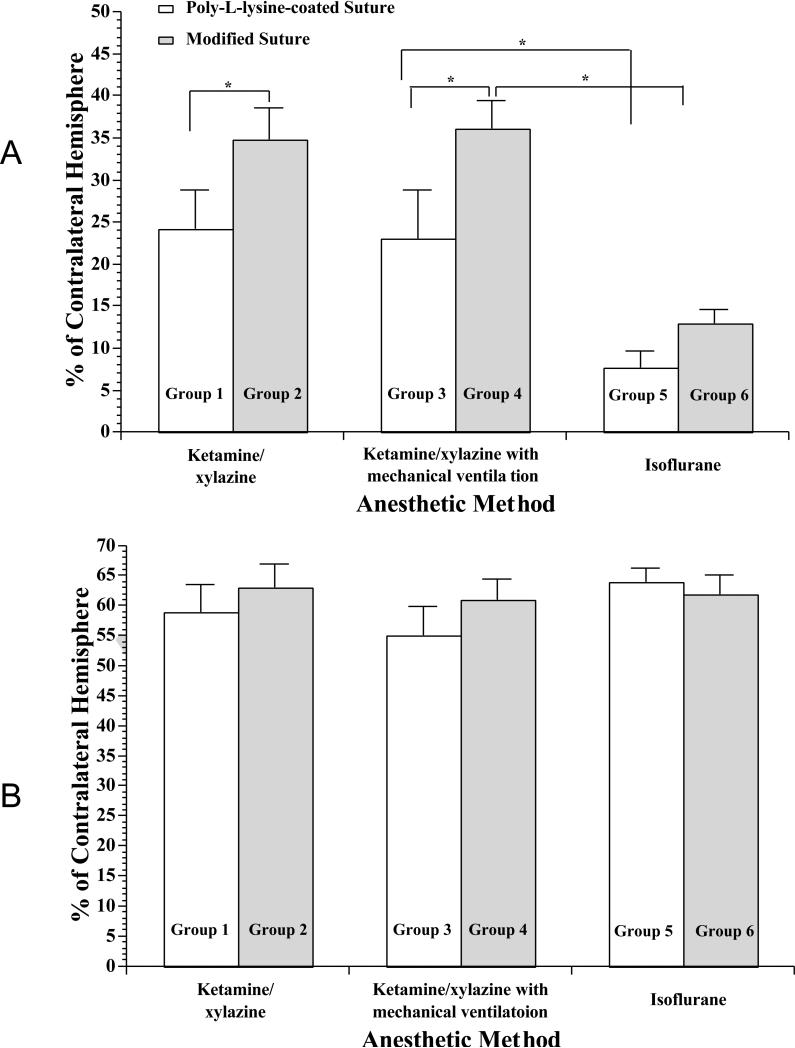
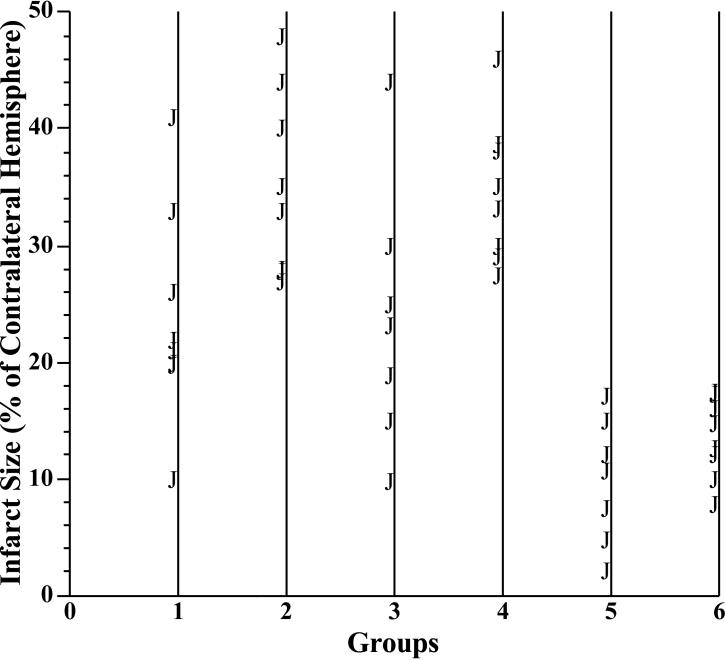
As shown in Figures 3 and 4A, the infarct size was significantly larger in rats using the modified suture technique than those using the Poly-L-lysine-coated suture technique under ketamine/xylazine anesthesia and ketamine/xylazine anesthesia with mechanical ventilation. However, mechanical ventilation did not alter the infarct size in rats anesthetized with ketamine/xylazine (Figures 3 and 4A). Compared with rats anesthetized with ketamine/xylazine, the infarct size was significantly smaller in rats anesthetized with isoflurane (Figures 3 and 4A). The range of infarct size was 10.0-41.0% in Group 1, 26.8-48.0% in Group 2, 9.7-44% in Group 3, 27.4-46% in Group 4, 2.0-15.0% in Group 5, 7.8-17.5% in Group 6. The standard deviation of infarct size was 10.06 in Group 1, 8.56 in Group 2, 10.69 in Group 3, 6.17 in Group 4, 5.44 in Group 5, 3.64 in Group 6. Thus, it appears that the variance of infarct size is smaller in the modified suture groups regardless of the anesthesia. Figure 5 is the scatter plot showing the variance of infarct size in each group. In contrast, there was no significant difference in total lesion among all six groups (Figure 4B).
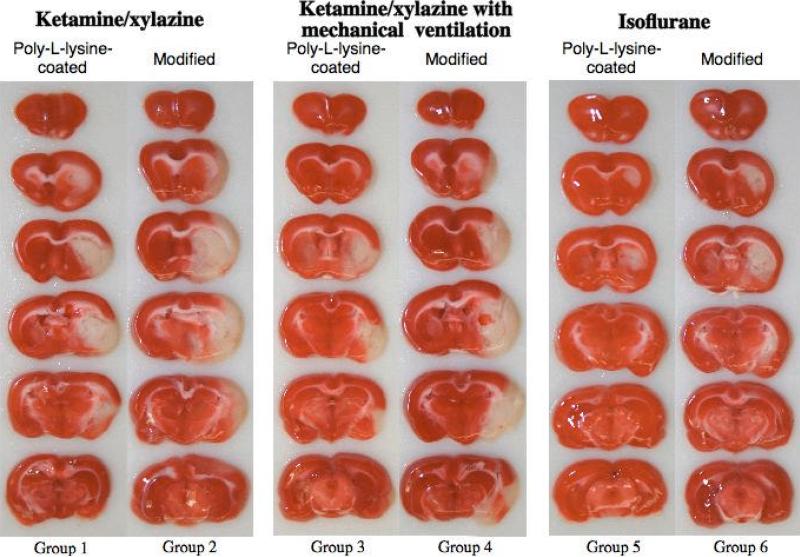
Figure 3.
Representative 2 mm-thick, TTC-stained coronal sections of the brain from groups 1-6 subjected to a 2-hr MCAO/24-hr reperfusion. Dark stain indicates viable tissue, and complete lack of stain is defined as infarct lesion.
Figure 4.
Infarct size (A) and total lesion (B) at 24 hr in Poly-L-lysine-coated or modified silicon-coated suture-produced 2-hr right MCAO under ketamine/xylazine, ketamine/xylazine with mechanical ventilation, or isoflurane anesthesia. Complete lack of TTC staining is defined as infarct lesion. Total lesion is the sum of intermediate staining and infarct lesion. Values are means ± SE. *P < 0.05.
Figure 5.
Scatter plot showing the variance of infarct size in each group.
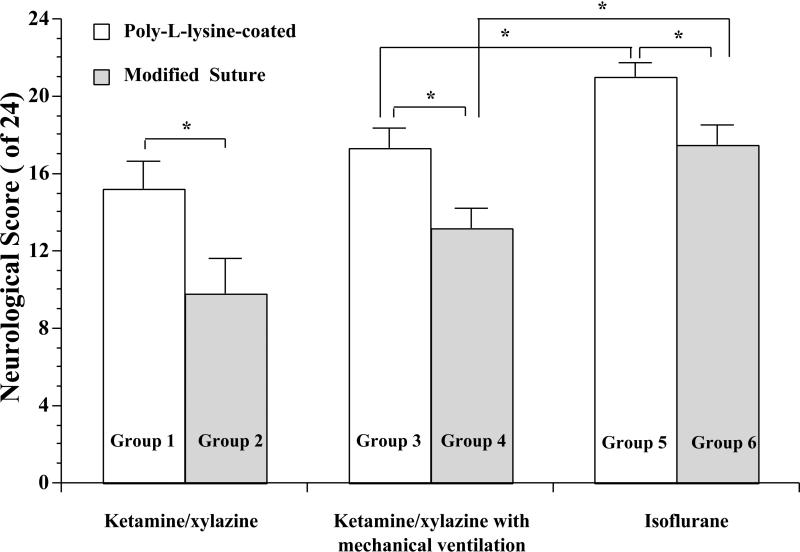
Neurological deficits
Compared with the Poly-L-lysine-coated suture groups, a worse neurological outcome was observed in the modified suture groups regardless of the anesthesia (Figure 6). Compared with rats anesthetized with ketamine/xylazine, a better neurological outcome was found in rats anesthetized with isoflurane (Figure 6).
Figure 6.
Neurological deficit scores at 24 hr in Poly-L-lysine-coated or modified silicon-coated suture-produced 2-hr right MCAO under ketamine/xylazine, ketamine/xylazine with mechanical ventilation, or isoflurane anesthesia. Values are means ± SE. *P < 0.05.
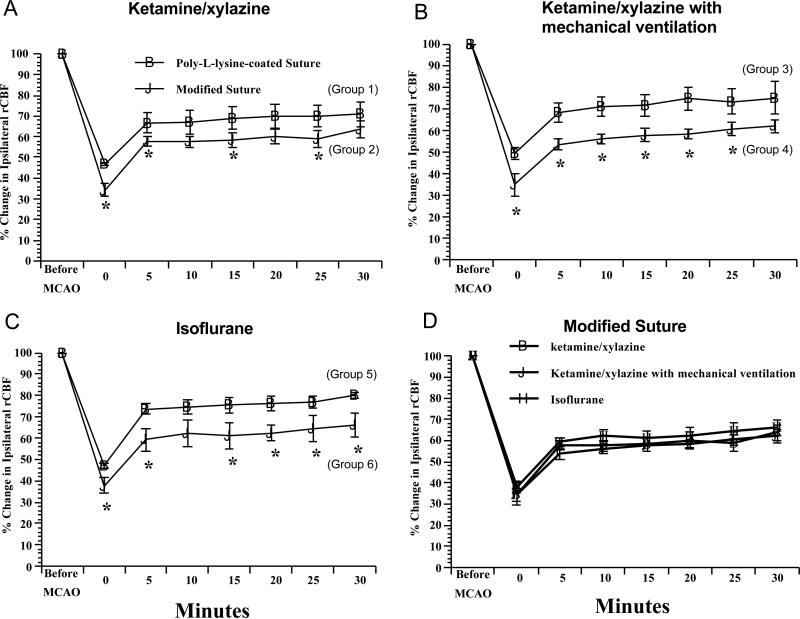
rCBF
Compared with the Poly-L-lysine-coated suture groups, rCBF was significantly lower at MCAO onset and during the first 30 minutes of MCAO in the modified suture groups regardless of the anesthesia (Figures 7A, 7B, and 7C). Compared with rats anesthetized with ketamine/xylazine, rCBF was not altered in rats anesthetized with isoflurane (Figure 7D).
Figure 7.
Ipsilateral parietal cerebral blood flow during first 30 minutes in Poly-L-lysine-coated or modified silicon-coated suture-produced 2-hr right MCAO under ketamine/xylazine, ketamine/xylazine with mechanical ventilation, or isoflurane anesthesia. Values are means ± SE. *P < 0.05 vs. Poly-L-lysine-coated suture.
DISCUSSION
There are several new findings in the present study. First, our modified silicon-coated suture produced a larger infarct size, smaller variance of infarct size, and greater neurological deficit than the Poly-L-lysine-coated suture method in a rat transient MCAO model. In addition, a lower rCBF at MCAO onset and during MCAO was found in the modified suture groups. Second, mechanical ventilation prevented ketamine/xylazine-induced respiratory acidosis, but did not affect the final infarct size and neurological deficit at 24 hr of reperfusion. Third, isoflurane significantly improved neurological deficit and reduced infarct size without altering rCBF at MCAO onset and during MCAO at 24 hr of reperfusion. Our findings suggest that our modified suture technique may provide a more consistent MCAO than the Poly-L-lysine-coated suture methodology. In addition, neuroprotection by isoflurane may be not related to an improvement of rCBF during ischemia.
In the Poly-L-lysine-coated suture-produced MCAO models, the rounded head of 3-0 nylon suture is large enough in diameter to obstruct blood flow through the MCA. However, the lack of suitable thickness in the trunk of the suture may allow the MCA area to be supplied by the ICA and the PCA via two types of branches originating from the ICA, which exist in about 80% rats in the present study [8,25]. Thus, a small and variable ischemic infarct size has been observed in the plain suture-produced rat MCAO model [2,23,29]. A recent study reported that intraluminal occlusion of MCA with ligation of the distal branch of ICA around the intraluminal suture produced a larger infarct size than intraluminal occlusion of MCA without ligation did [5]. Ligation of the distal branch of ICA may prevent the MCA area to be supplied by the ICA via two types of branches. In our modified silicon-coated suture-produced MCAO model, the rounded head of 4-0 nylon suture is advanced into the ACA and its thickened trunk remains in the ICA. This modified suture can obstruct all sources of blood flow from the ICA, the ACA and posterior communicating artery (PCA) into the MCA area, and thus provides a more consistent ischemia in the MCA area than the Poly-L-lysine-coated suture. The difference between the two types of sutures is reflected by similar total lesion and different rCBF, infarct size, variance of infarct size, and neurological deficit between the Poly-L-lysine-coated suture group and the modified suture group regardless of the anesthesia method. In addition to a more consistent occlusion, removal of the silicon layer at the tip of the suture makes its tip more easily inserted into the ACA.
Several systemic factors may affect ischemic brain damage in the rat MCAO model. These factors include blood pressure, blood pH, and blood gas. Browning et al recently reported that hypercapnia increases injury volume in a feline model of focal cerebral ischemia, suggesting that PCO2 should be controlled in experimental focal cerebral ischemia models [3]. In addition, Pignataro et al found that attenuating brain acidosis is protective in a mouse model of transient focal ischemia [18]. In the present study, four experimental groups anesthetized with ketamine/xylazine had a similar blood pressure and heart rate, but two groups not ventilated mechanically produced a respiratory acidosis before introducing MCAO. However, compared with two groups anesthetized with ketamine/zylazine and mechanically ventilated, which kept the arterial blood gas in a normal range before and during MCAO, the total lesion and infarct size were not significantly altered in rats anesthetized with ketamine/xylazine without mechanical ventilation at 24-hr of reperfusion. Although the duration of respiratory acidosis is not known and if such a respiratory acidosis would indeed increase the infarct size at later time points, we suggest that it is prudent to control the physiological parameters within a normal range in experimental focal cerebral ischemia.
There is a general agreement that volatile anesthetics applied before and during cerebral ischemia confer neuroprotection [9,10]. Recently, isoflurane has been shown to provide short-term neuroprotection in a variety of experimental models of ischemia including transient focal ischemia [9,10]. However, it is controversial if the neuroprotection of isoflurane is sustained. Sakai et al reported that isoflurane provided long-term protection against transient focal cerebral ischemia when the common carotid artery is preserved in a rat MCAO model [7,11,21]. However, others found that the neuroprotective efficacy of isoflurane was not sustained when the recovery period was extended to 2 weeks [7,11]. In the present study, we found that infarct size was significantly smaller at 24-hr of reperfusion in rats anesthetized with isoflurane compared with rats anesthetized with ketamine/xylazine. It is possible that the difference in infarct size between two anesthetics was resulted from increased infarct size in ketamine/xylazine-anesthetized groups and/or reduced infarct size in isoflurane-anesthatized groups. Kawai et al reported that katamine/xylazine increases infarct size via a hyperglycemia [12]. However, one recent study found that isoflurane also produces acute hyperglycermia similar to ketamine/xylazine [20]. Thus, it appears that isoflurane has at least short-term neuroprotection in this type ischemic model. Mechanisms that account for neuroprotection with isoflurane include reducing glutamate release, antagonizing postsynaptic glutamate receptors, and/or enhancing GABA receptor A-mediated hyperpolarization [9]. More recent data have indicated that isoflurane can produce a preconditioning in brain tissue [30]. In addition, isoflurane is a potent cerebral vasodilator [15], and thus may reduce ischemic brain damage through an increase in blood flow from surround-unoccluded arteries into the ischemic area. In the present study, percent change of CBF in the border area during ischemia was not altered in rats anesthetized with isoflurane compared with the rats anesthetized with ketamine/xylazine. The blood flow in border area is directly supplied by the MCA, and potentially supplied by the ACA through ACA-MCA anastomoses [6]. Thus, it appears that neuroprotective mechanisms of isoflurane may be related to a higher absolute basal CBF, but not to an improvement in CBF during ischemia.
In summary, our modified suture provides a more consistent occlusion of the MCA in rats. Due to neuroprotection, isoflurane should be cautiously used as anesthetic agent at least for early evaluation of cerebral ischemic damage, and it appears that ketamine/xylazine anesthesia may be a more appropriate choice for ischemia-related studies. We suggest that our modified suture method may aid investigators in selecting the most appropriate technique to assess cerebral ischemic damage following occlusion of the MCA.
MATERIALS AND METHODS
Experimental groups
All procedures were in accordance with the “Principle of Laboratory Animal Care” (NIH publication No. 86-23, revised 1985) and were approved by the Institutional Animal Care and Use Committee. Male Sprague-Dawley rats (body weight 380-420g) were purchased from Harlan and assigned to one of six groups. Group 1 (n=10): Poly-L-lysine-coated suture under ketamine/xylazine (80 mg/kg IP, 40 mg/kg IP for supplement as needed) anesthesia. Group 2 (n=8): Modified suture under ketamine/xylazine (80 mg/kg IP, 40 mg/kg IP for supplement as needed) anesthesia. Group 3 (n=8): Poly-L-lysine-coated suture under ketamine/xylazine (60 mg/kg IP for induction and 20 mg/kg/hr IV for maintenance) anesthesia with mechanical ventilation (orally intubated and ventilated mechanically with room air and supplemental oxygen). Group 4 (n=8): Modified suture under ketamine/xylazine (60 mg/kg IP for induction and 20 mg/kg/hr IV for maintenance) anesthesia with mechanical ventilation (orally intubated and ventilated mechanically with room air and supplemental oxygen). Group 5 (n=8): Poly-L-lysine-coated suture under isoflurane (5% for induction and 2% for maintenance in 30% oxygen-balance nitrogen) anesthesia. Group 6 (n=8): Modified suture under isoflurane (5% for induction and 2% for maintenance in 30% oxygen-balance nitrogen) anesthesia.
Intraluminal sutures
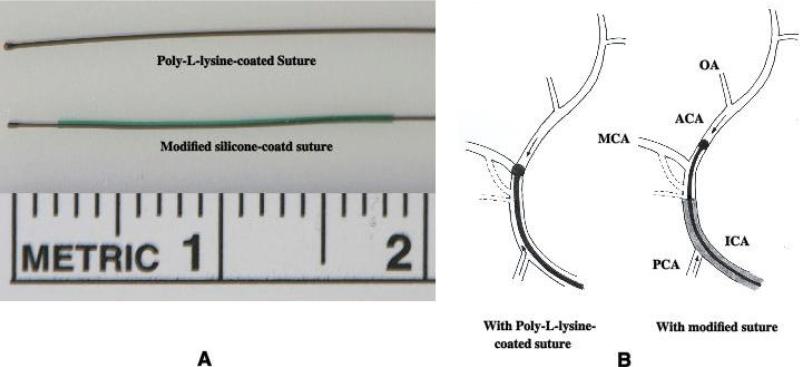
Two types of sutures, Poly-L-lysine-coated and modified silicon-coated, were prepared for performing intraluminal occlusion of the MCA. Poly-L-lysine-coated suture was prepared from a 4-5 cm length 3-0 monofilament nylon suture by heating the tip over a flame into a rounded shape. Suture 25 mm in length from the tip was coated with Poly-L-lysine solution (Sigma) and dried in a 60 °C oven for 1 hour [1]. The diameter of the rounded tip was approximately 400-420μm (Figure 1). A modified silicon-coated suture was prepared from a 4-5 cm length 4-0 monofilament nylon suture as described by Schmid-Elsaesser et al and Bouley et al. with modification [2,22]. First, the tip of the suture was heated into a rounded shape as the Poly-L-lysine-coated suture. The diameter of the rounded tip was approximately 290-310μm (Figure 1). Second, the nylon suture was inserted into CO-EX™ tubing (inner diameter = 430 μm) for 20 mm. The tubing was filled with silicon (Silicone Elastomer, World Precision Instruments, Sarasota FL, USA), and then the tubing was removed 10 minutes after the silicon coagulated. Third, a 2.5 mm silicon layer from the tip was removed to expose the inner nylon suture. Thus, the nylon suture was covered by a silicon layer for 17.5 mm (Figure 1). Fourth, to prevent the sliding of the silicon layer along the nylon suture during the surgical procedures both ends of silicon layer were adhered to the nylon suture by superglue.
Figure 1.
(A) Poly-L-lysine-coated and modified silicone-coated sutures. (B) Schematic representation of MCAO by the Poly-L-lysine-coated or modified silicone-coated sutures. After inserting a Poly-L-lysine-coated suture a reduced blood flow from ICA and/or PCA might enter into MCA through a bypass or supply MCA area via a small branch. The modified silicone-coated suture can prevent such an incomplete occlusion. PCA, posterior communciating artery; OA, olfactory artery.
Surgical procedures
To avoid anesthetic accidents during the surgical procedures, rats were fasted for at least 2 hours before the experiments. The right femoral artery was cannulated for continuous monitoring of mean arterial blood pressure (MABP) and for obtaining a blood sample to measure pH, PaCO2 and PaO2. The right femoral vein was cannulated for the administration of anesthetics. Rectal temperature was maintained at 37 °C using a temperature controlled heating pad. To measure regional cerebral blood flow (rCBF), a laser Doppler flow probe (PeriFlux System 5000, Perimed) was attached to the right side of the dorsal surface of the skull 1-2 mm caudal and 5-6 mm lateral to bregma. Blood flow to this area is directly supplied by the MCA, and potentially supplied by the anterior cerebral artery (ACA) through ACA-MCA anastomoses [6]. MCAO onset will induce a rapid drop in rCBF. However, dilation of those arterial anastomoses in response to hypoxia may produce a rebound increase in rCBF.
MCAO was induced using the intraluminal suture occlusion technique. The right common and external carotid arteries were exposed and ligated. The MCA was occluded by inserting a suture from the basal part of the external carotid artery and advanced cranially into the internal carotid artery (ICA). The Poly-L-lysine-coated suture was inserted into the ICA about 17-18 mm, and the modified silicon-coated suture was inserted into the ICA about 19-20 mm. The onset of MCAO was determined by a rapid drop in rCBF, which was assessed for 30 min following placement of the suture. The suture was carefully withdrawn 2 hours after MCAO onset. Neurological evaluation was performed 24 hours after reperfusion, and then the rats were euthanized for the measurement of infarct size.
Neurological evaluation
We measured neurological deficits at 24 h of reperfusion. Scoring was done blindly with a 24-point scale modified from Garcia's neurological scoring system [26].
Assessment of infarct size
Animals were euthanized after neurological evaluation with thiobutabarbital sodium (inactin) (150 mg/kg body weight) and exsanguinated. The brain was quickly removed and placed in ice-cold sterile saline for 5 min, and cut into six 2-mm coronal sections. Sections were stained with 2% 2,3,5-triphenyltetrazolium chloride (TTC, Sigma). Slice images were digitalized, the ischemic lesion was evaluated using Kodak Molecular Imaging Software. Dark staining with TTC indicates viable tissue, and complete lack of staining is defined as infarct lesion. Total lesion is specified as the sum of intermediate staining and infarct lesion. Total and infarct lesions corrected for cerebral edema were expressed as percentage of the contralateral hemisphere.
Statistical analyses
Data are expressed as mean ± SEM. Statistical differences between the various groups were assessed with one-way ANOVA followed by Tukey's post hoc test. A P value of 0.05 or less was considered to be significant. Range and standard deviation were calculated to express the variance of infarct size.
ACKNOWLEDGMENTS
This study was supported by National Institutes of Health Grants DA 14258, HL79587, AA 11288, a Scientist Development Grant from the American Heart Association (0635052N), and funds from the University of Nebraska Medical Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Belayev L, Alonso OF, Busto R, Zhao W, Ginsberg MD. Middle cerebral artery occlusion in the rat by intraluminal suture. Neurological and pathological evaluation of an improved model. Stroke. 1996;27:1616–22. doi: 10.1161/01.str.27.9.1616. discussion 1623. [DOI] [PubMed] [Google Scholar]
- 2.Bouley J, Fisher M, Henninger N. Comparison between coated vs. uncoated suture middle cerebral artery occlusion in the rat as assessed by perfusion/diffusion weighted imaging. Neurosci Lett. 2007;412:185–90. doi: 10.1016/j.neulet.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Browning JL, Heizer ML, Widmayer MA, Baskin DS. Effects of halothane, alpha-chloralose, and pCO2 on injury volume and CSF beta-endorphin levels in focal cerebral ischemia. Mol Chem Neuropathol. 1997;31:29–42. doi: 10.1007/BF02815158. [DOI] [PubMed] [Google Scholar]
- 4.Butcher KS, Hachinski VC, Wilson JX, Guiraudon C, Cechetto DF. Cardiac and sympathetic effects of middle cerebral artery occlusion in the spontaneously hypertensive rat. Brain Res. 1993;621:79–86. doi: 10.1016/0006-8993(93)90300-c. [DOI] [PubMed] [Google Scholar]
- 5.Chu X, Qi C, Zou L, Fu X. Intraluminal suture occlusion and ligation of the distal branch of internal carotid artery: an improved rat model of focal cerebral ischemia-reperfusion. J Neurosci Methods. 2008;168:1–7. doi: 10.1016/j.jneumeth.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 6.Coyle P, Jokelainen PT. Dorsal cerebral arterial collaterals of the rat. Anat Rec. 1982;203:397–404. doi: 10.1002/ar.1092030309. [DOI] [PubMed] [Google Scholar]
- 7.Du C, Hu R, Csernansky CA, Hsu CY, Choi DW. Very delayed infarction after mild focal cerebral ischemia: a role for apoptosis? J Cereb Blood Flow Metab. 1996;16:195–201. doi: 10.1097/00004647-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Hartkamp MJ, van Der Grond J, van Everdingen KJ, Hillen B, Mali WP. Circle of Willis collateral flow investigated by magnetic resonance angiography. Stroke. 1999;30:2671–8. doi: 10.1161/01.str.30.12.2671. [DOI] [PubMed] [Google Scholar]
- 9.Head BP, Patel P. Anesthetics and brain protection. Curr Opin Anaesthesiol. 2007;20:395–9. doi: 10.1097/ACO.0b013e3282efa69d. [DOI] [PubMed] [Google Scholar]
- 10.Kapinya KJ, Lowl D, Futterer C, Maurer M, Waschke KF, Isaev NK, Dirnagl U. Tolerance against ischemic neuronal injury can be induced by volatile anesthetics and is inducible NO synthase dependent. Stroke. 2002;33:1889–98. doi: 10.1161/01.str.0000020092.41820.58. [DOI] [PubMed] [Google Scholar]
- 11.Kawaguchi M, Kimbro JR, Drummond JC, Cole DJ, Kelly PJ, Patel PM. Isoflurane delays but does not prevent cerebral infarction in rats subjected to focal ischemia. Anesthesiology. 2000;92:1335–42. doi: 10.1097/00000542-200005000-00023. [DOI] [PubMed] [Google Scholar]
- 12.Kawai N, Keep RF, Betz AL. Effects of hyperglycemia on cerebral blood flow and edema formation after carotid artery occlusion in Fischer 344 rats. Acta Neurochir Suppl. 1997;70:34–6. doi: 10.1007/978-3-7091-6837-0_10. [DOI] [PubMed] [Google Scholar]
- 13.Koizumi J, Yoshida Y, Nakazawa T, Oneda G. Experimental studies of ischemic brain edema 1. A new experimental model of cerebral embolism in ratsin which recirculation can be introduced in the ischemia area. Jpn J Stroke. 1986;8:1–8. [Google Scholar]
- 14.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 15.Matta BF, Heath KJ, Tipping K, Summors AC. Direct cerebral vasodilatory effects of sevoflurane and isoflurane. Anesthesiology. 1999;91:677–80. doi: 10.1097/00000542-199909000-00019. [DOI] [PubMed] [Google Scholar]
- 16.Matucz E, Moricz K, Gigler G, Simo A, Barkoczy J, Levay G, Harsing LG, Jr., Szenasi G. Reduction of cerebral infarct size by non-competitive AMPA antagonists in rats subjected to permanent and transient focal ischemia. Brain Res. 2004;1019:210–6. doi: 10.1016/j.brainres.2004.05.098. [DOI] [PubMed] [Google Scholar]
- 17.Palmer GC, Peeling J, Corbett D, Del Bigio MR, Hudzik TJ. T2-weighted MRI correlates with long-term histopathology, neurology scores, and skilled motor behavior in a rat stroke model. Ann N Y Acad Sci. 2001;939:283–96. doi: 10.1111/j.1749-6632.2001.tb03636.x. [DOI] [PubMed] [Google Scholar]
- 18.Pignataro G, Simon RP, Xiong ZG. Prolonged activation of ASIC1a and the time window for neuroprotection in cerebral ischaemia. Brain. 2007;130:151–8. doi: 10.1093/brain/awl325. [DOI] [PubMed] [Google Scholar]
- 19.Ridenour TR, Warner DS, Todd MM, Baker MT. Effects of ketamine on outcome from temporary middle cerebral artery occlusion in the spontaneously hypertensive rat. Brain Res. 1991;565:116–22. doi: 10.1016/0006-8993(91)91742-j. [DOI] [PubMed] [Google Scholar]
- 20.Saha JK, Xia J, Grondin JM, Engle SK, Jakubowski JA. Acute hyperglycemia induced by ketamine/xylazine anesthesia in rats: mechanisms and implications for preclinical models. Exp Biol Med (Maywood) 2005;230:777–84. doi: 10.1177/153537020523001012. [DOI] [PubMed] [Google Scholar]
- 21.Sakai H, Sheng H, Yates RB, Ishida K, Pearlstein RD, Warner DS. Isoflurane provides long-term protection against focal cerebral ischemia in the rat. Anesthesiology. 2007;106:92–9. doi: 10.1097/00000542-200701000-00017. discussion 8-10. [DOI] [PubMed] [Google Scholar]
- 22.Schmid-Elsaesser R, Zausinger S, Hungerhuber E, Baethmann A, Reulen H. A critical reevaluation of the intraluminal thread model of focal cerebral ischemia: evidence of inadvertent premature perfusion and subarachnoid hemorrhage in rats by laser-Doppler flowmetry. Stroke. 1998;29:2162–2170. doi: 10.1161/01.str.29.10.2162. [DOI] [PubMed] [Google Scholar]
- 23.Shimamura N, Matchett G, Tsubokawa T, Ohkuma H, Zhang J. Comparison of silicon-coated nylon suture to plain nylon suture in the rat middle cerebral artery occlusion model. J Neurosci Methods. 2006;156:161–5. doi: 10.1016/j.jneumeth.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 24.Smith WS. Technology Insight: recanalization with drugs and devices during acute ischemic stroke. Nat Clin Pract Neurol. 2007;3:45–53. doi: 10.1038/ncpneuro0372. [DOI] [PubMed] [Google Scholar]
- 25.Stock KW, Wetzel S, Kirsch E, Bongartz G, Steinbrich W, Radue EW. Anatomic evaluation of the circle of Willis: MR angiography versus intraarterial digital subtraction angiography. AJNR Am J Neuroradiol. 1996;17:1495–9. [PMC free article] [PubMed] [Google Scholar]
- 26.Sun H, Zhao H, Sharpe GM, Arrick DM, Mayhan WG. Effect of chronic alcohol consumption on brain damage following transient focal ischemia. Brain Res. 2008;1194:73–80. doi: 10.1016/j.brainres.2007.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warner DS, Zhou JG, Ramani R, Todd MM. Reversible focal ischemia in the rat: effects of halothane, isoflurane, and methohexital anesthesia. J Cereb Blood Flow Metab. 1991;11:794–802. doi: 10.1038/jcbfm.1991.137. [DOI] [PubMed] [Google Scholar]
- 28.Wegener S, Weber R, Ramos-Cabrer P, Uhlenkueken U, Wiedermann D, Kandal K, Villringer A, Hoehn M. Subcortical lesions after transient thread occlusion in the rat: T2-weighted magnetic resonance imaging findings without corresponding sensorimotor deficits. J Magn Reson Imaging. 2005;21:340–346. doi: 10.1002/jmri.20270. [DOI] [PubMed] [Google Scholar]
- 29.Woitzik J, Schneider UC, Thome C, Schroeck H, Schilling L. Comparison of different intravascular thread occlusion models for experimental stroke in rats. J Neurosci Methods. 2006;151:224–31. doi: 10.1016/j.jneumeth.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 30.Xiong L, Zheng Y, Wu M, Hou L, Zhu Z, Zhang X, Lu Z. Preconditioning with isoflurane produces dose-dependent neuroprotection via activation of adenosine triphosphate-regulated potassium channels after focal cerebral ischemia in rats. Anesth Analg. 2003;96:233–7. doi: 10.1097/00000539-200301000-00047. table of contents. [DOI] [PubMed] [Google Scholar]
- 31.Xu J, Culman J, Blume A, Brecht S, Gohlke P. Chronic treatment with a low dose of lithium protects the brain against ischemic injury by reducing apoptotic death. Stroke. 2003;34:1287–92. doi: 10.1161/01.STR.0000066308.25088.64. [DOI] [PubMed] [Google Scholar]