Abstract
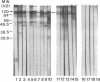
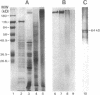
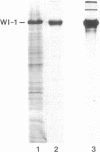
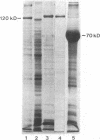
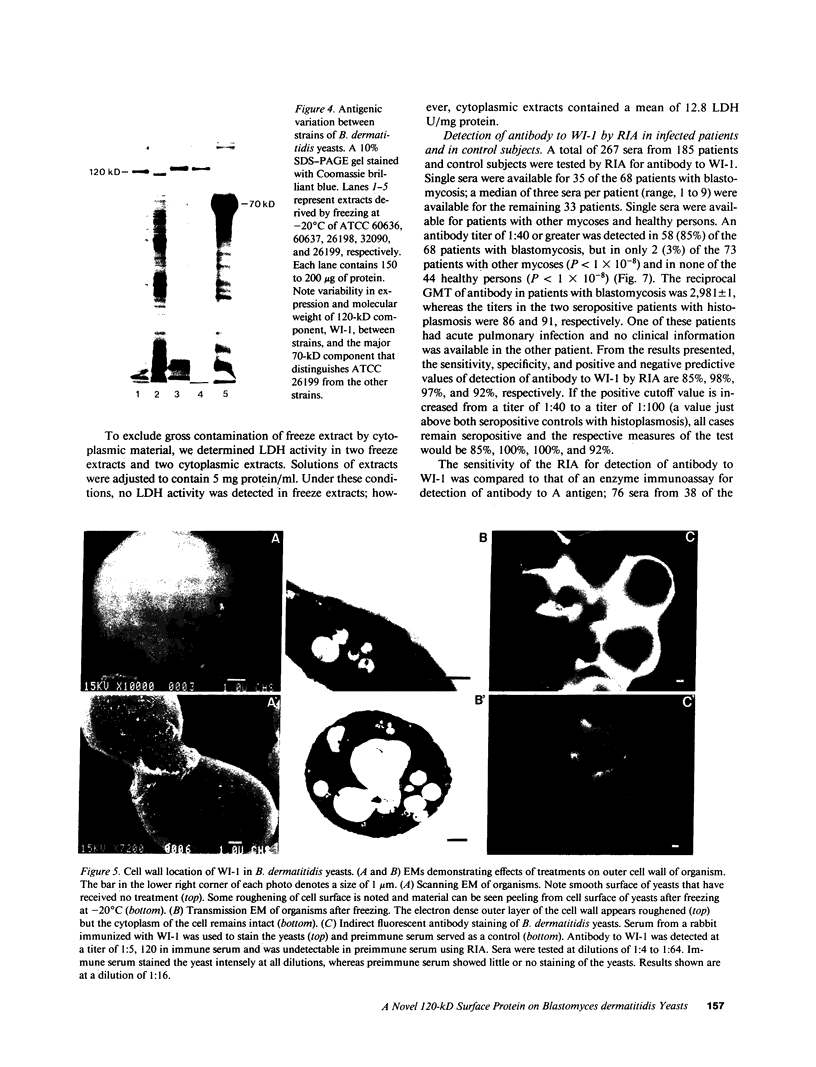
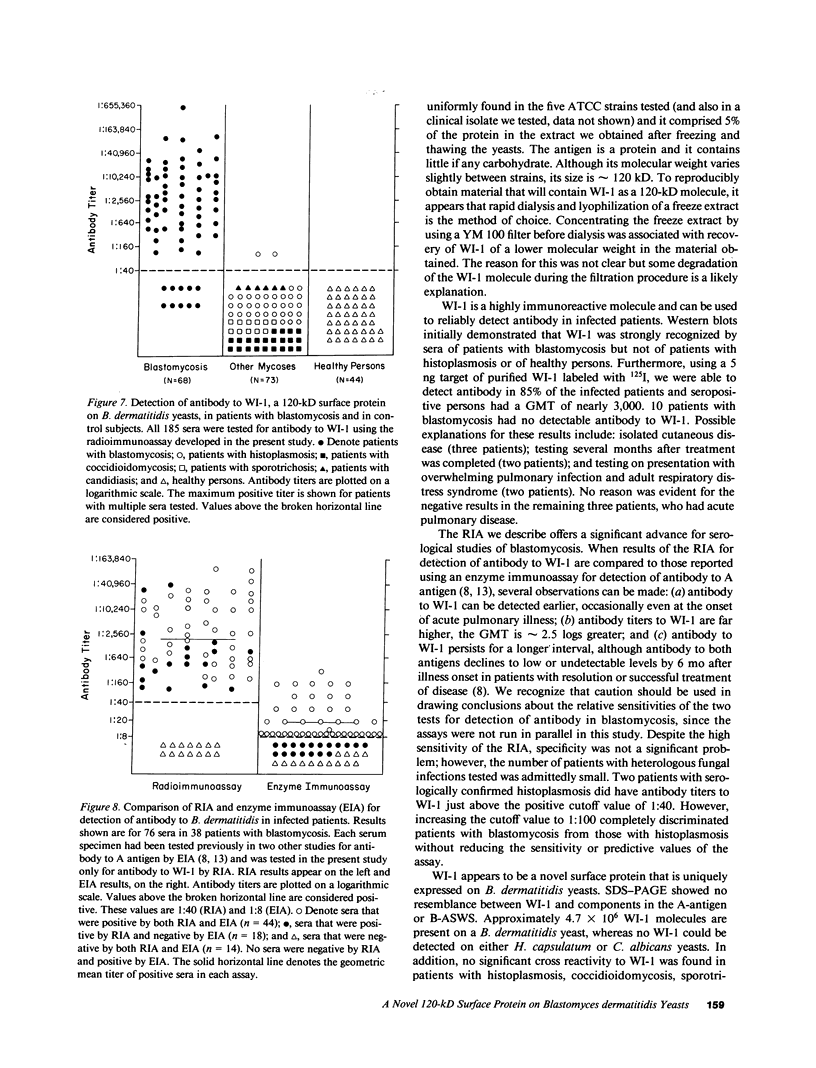
No well-defined Blastomyces-specific antigens are currently available. We used sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting to identify immunologically active molecules in the cell wall of B. dermatitidis. A major immunoreactive 120-kD protein (WI-1) was present in all five strains studied and comprised 5% of the protein in the cell wall extract obtained after freezing and thawing yeast cells. WI-1 was recognized by serum from all 10 patients with blastomycosis but by none of those from 5 patients with histoplasmosis. It was purified by electroelution, radiolabeled with 125I, and incorporated into a radioimmunoassay (RIA) for serodiagnosis of blastomycosis. Antibody to WI-1 was detected in 58 (85%) of 68 patients with blastomycosis (geometric mean titer, 1:2,981), in two (3%) of 73 patients with histoplasmosis, coccidioidomycosis, sporotrichosis, or candidiasis (titers, 1:86 and 1:91) and in none of 44 healthy persons. WI-1 was shown to be a surface molecule abundant on B. dermatitidis yeasts that were indirectly stained with serum from a rabbit immunized with WI-1. Approximately 0.93 pg of WI-1 or 4.7 x 10(6) WI-1 molecules were found on the surface of an individual yeast using an antigen-inhibition RIA; none was found on Histoplasma capsulatum or Candida albicans yeasts. We conclude that WI-1 is a novel, immunologically active surface molecule on the invasive form of B. dermatitidis and that WI-1 can be used to reliably detect antibody and study the immunopathogenesis of blastomycosis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azuma I., Kanetsuna F., Tanaka Y., Yamamura Y., Carbonell L. M. Chemical and immunological properties of galactomannans obtained from Histoplasma duboisii, Histoplasma capsulatum, Paracoccidioides brasiliensis and Blasomyces dermatitidis. Mycopathol Mycol Appl. 1974 Oct 15;54(1):111–125. doi: 10.1007/BF02055979. [DOI] [PubMed] [Google Scholar]
- Cox R. A., Larsh H. W. Isolation of skin test-active preparations from yeast-phase cells of Blastomyces dermatitidis. Infect Immun. 1974 Jul;10(1):42–47. doi: 10.1128/iai.10.1.42-47.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. A., Larsh H. W. Yeast- and mycelial-phase antigens of Blastomyces dermatitidis: comparison using disc gel electrophoresis. Infect Immun. 1974 Jul;10(1):48–53. doi: 10.1128/iai.10.1.48-53.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DENTON J. F., McDONOUGH E. S., AJELLO L., AUSHERMAN R. J. Isolation of Blastomyces dermatitidis from soil. Science. 1961 Apr 14;133(3459):1126–1127. doi: 10.1126/science.133.3459.1126. [DOI] [PubMed] [Google Scholar]
- Domer J. E. Monosaccharide and chitin content of cell walls of Histoplasma capsulatum and Blastomyces dermatitidis. J Bacteriol. 1971 Sep;107(3):870–877. doi: 10.1128/jb.107.3.870-877.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield R. A., Stephens J. L., Bussey M. J., Jones J. M. Quantitation of antibody to Candida mannan by enzyme-linked immunosorbent assay. J Lab Clin Med. 1983 May;101(5):758–771. [PubMed] [Google Scholar]
- HEINER D. C. Diagnosis of histoplasmosis using precipitin reactions in agargel. Pediatrics. 1958 Oct;22(4 Pt 1):616–627. [PubMed] [Google Scholar]
- Jones J. M. Quantitation of antibody against cell wall mannan and a major cytoplasmic antigen of Candida in rabbits, mice, and humans. Infect Immun. 1980 Oct;30(1):78–89. doi: 10.1128/iai.30.1.78-89.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanetsuna F., Carbonell L. M. Cell wall composition of the yeastlike and mycelial forms of Blastomyces dermatitidis. J Bacteriol. 1971 Jun;106(3):946–948. doi: 10.1128/jb.106.3.946-948.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanetsuna F., Carbonell L. M., Gil F., Azuma I. Chemical and ultrastructural studies on the cell walls of the yeastlike and mycelial forms of Histoplasma capsulatum. Mycopathol Mycol Appl. 1974 Oct 15;54(1):1–13. doi: 10.1007/BF02055967. [DOI] [PubMed] [Google Scholar]
- Kanetsuna F., Carbonell L. M., Moreno R. E., Rodriguez J. Cell wall composition of the yeast and mycelial forms of Paracoccidioides brasiliensis. J Bacteriol. 1969 Mar;97(3):1036–1041. doi: 10.1128/jb.97.3.1036-1041.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapitany R. A., Zebrowski E. J. A high resolution PAS stain for polyacrylamide gel electrophoresis. Anal Biochem. 1973 Dec;56(2):361–369. doi: 10.1016/0003-2697(73)90202-9. [DOI] [PubMed] [Google Scholar]
- Kaufman L., Blumer S. Occurrence of serotypes among Histoplasma capsulatum strains. J Bacteriol. 1966 Apr;91(4):1434–1439. doi: 10.1128/jb.91.4.1434-1439.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein B. S., Kuritsky J. N., Chappell W. A., Kaufman L., Green J., Davies S. F., Williams J. E., Sarosi G. A. Comparison of the enzyme immunoassay, immunodiffusion, and complement fixation tests in detecting antibody in human serum to the A antigen of Blastomyces dermatitidis. Am Rev Respir Dis. 1986 Jan;133(1):144–148. doi: 10.1164/arrd.1986.133.1.144. [DOI] [PubMed] [Google Scholar]
- Klein B. S., Vergeront J. M., DiSalvo A. F., Kaufman L., Davis J. P. Two outbreaks of blastomycosis along rivers in Wisconsin. Isolation of Blastomyces dermatitidis from riverbank soil and evidence of its transmission along waterways. Am Rev Respir Dis. 1987 Dec;136(6):1333–1338. doi: 10.1164/ajrccm/136.6.1333. [DOI] [PubMed] [Google Scholar]
- Klein B. S., Vergeront J. M., Kaufman L., Bradsher R. W., Kumar U. N., Mathai G., Varkey B., Davis J. P. Serological tests for blastomycosis: assessments during a large point-source outbreak in Wisconsin. J Infect Dis. 1987 Feb;155(2):262–268. doi: 10.1093/infdis/155.2.262. [DOI] [PubMed] [Google Scholar]
- Klein B. S., Vergeront J. M., Weeks R. J., Kumar U. N., Mathai G., Varkey B., Kaufman L., Bradsher R. W., Stoebig J. F., Davis J. P. Isolation of Blastomyces dermatitidis in soil associated with a large outbreak of blastomycosis in Wisconsin. N Engl J Med. 1986 Feb 27;314(9):529–534. doi: 10.1056/NEJM198602273140901. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lancaster M. V., Sprouse R. F. Isolation of a purified skin test antigen from Blastomyces dermatitidis yeast-phase cell wall. Infect Immun. 1976 Sep;14(3):623–625. doi: 10.1128/iai.14.3.623-625.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. L., Buckley H. R., Campbell C. C. An amino acid liquid synthetic medium for the development of mycelial and yeast forms of Candida Albicans. Sabouraudia. 1975 Jul;13(2):148–153. doi: 10.1080/00362177585190271. [DOI] [PubMed] [Google Scholar]
- Reiss E., Miller S. E., Kaplan W., Kaufman L. Antigenic, chemical, and structural properties of cell walls of Histoplasma capsulatum yeast-form chemotypes 1 and 2 after serial enzymatic hydrolysis. Infect Immun. 1977 May;16(2):690–700. doi: 10.1128/iai.16.2.690-700.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarosi G. A., Hammerman K. J., Tosh F. E., Kronenberg R. S. Clinical features of acute pulmonary blastomycosis. N Engl J Med. 1974 Mar 7;290(10):540–543. doi: 10.1056/NEJM197403072901004. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner S., Kaufman L., Jalbert M. Diagnostic assessment of an enzyme-linked immunosorbent assay for human and canine blastomycosis. J Clin Microbiol. 1986 Feb;23(2):294–297. doi: 10.1128/jcm.23.2.294-297.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WROBLEWSKI F., LADUE J. S. Lactic dehydrogenase activity in blood. Proc Soc Exp Biol Med. 1955 Oct;90(1):210–213. doi: 10.3181/00379727-90-21985. [DOI] [PubMed] [Google Scholar]
- Yokogawa K., Kawata S., Nishimura S., Ikeda Y., Yoshimura Y. Mutanolysin, bacteriolytic agent for cariogenic Streptococci: partial purification and properties. Antimicrob Agents Chemother. 1974 Aug;6(2):156–165. doi: 10.1128/aac.6.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young K. D., Larsh H. W. Identification of the active precipitin components in a purified preparation of the A antigen of Blastomyces dermatitidis. Infect Immun. 1981 Jul;33(1):171–177. doi: 10.1128/iai.33.1.171-177.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]