Abstract
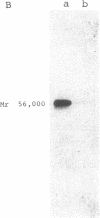
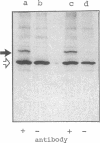
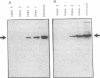
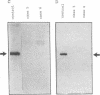
Cultured skin fibroblasts or lymphoblastoid cells from eight patients with clinical symptoms of prolidase deficiency were analyzed in terms of enzyme activity, presence of material crossreacting with specific antibodies, biosynthesis of the polypeptide, and mRNA corresponding to the enzyme. There are at least two enzymes that hydrolyze imidodipeptides in these cells and these two enzymes could be separated by an immunochemical procedure. The specific assay for prolidase showed that the enzyme activity was virtually absent in six cell strains and was markedly reduced in two (less than 3% of controls). The activities of the labile enzyme that did not immunoprecipitate with the anti-prolidase antibody were decreased in the cells (30-60% of controls). Cell strains with residual activities of prolidase had immunological polypeptides crossreacting with a Mr 56,000, similar to findings in the normal enzyme. The polypeptide biosynthesis in these cells and the controls was similar. Northern blot analyses revealed the presence of mRNA in the polypeptide-positive cells, yet it was absent in the polypeptide-negative cells. The substrate specificities analyzed in the partially purified enzymes from the polypeptide-positive cell strains differed, presumably due to different mutations. Thus, there seems to be a molecular heterogeneity in prolidase deficiency. There was no apparent relation between the clinical symptoms and the biochemical phenotypes, except that mental retardation was present in the polypeptide-negative patients. The activities of the labile enzyme may not be a major factor in modifying the clinical symptoms.
Full text
PDF

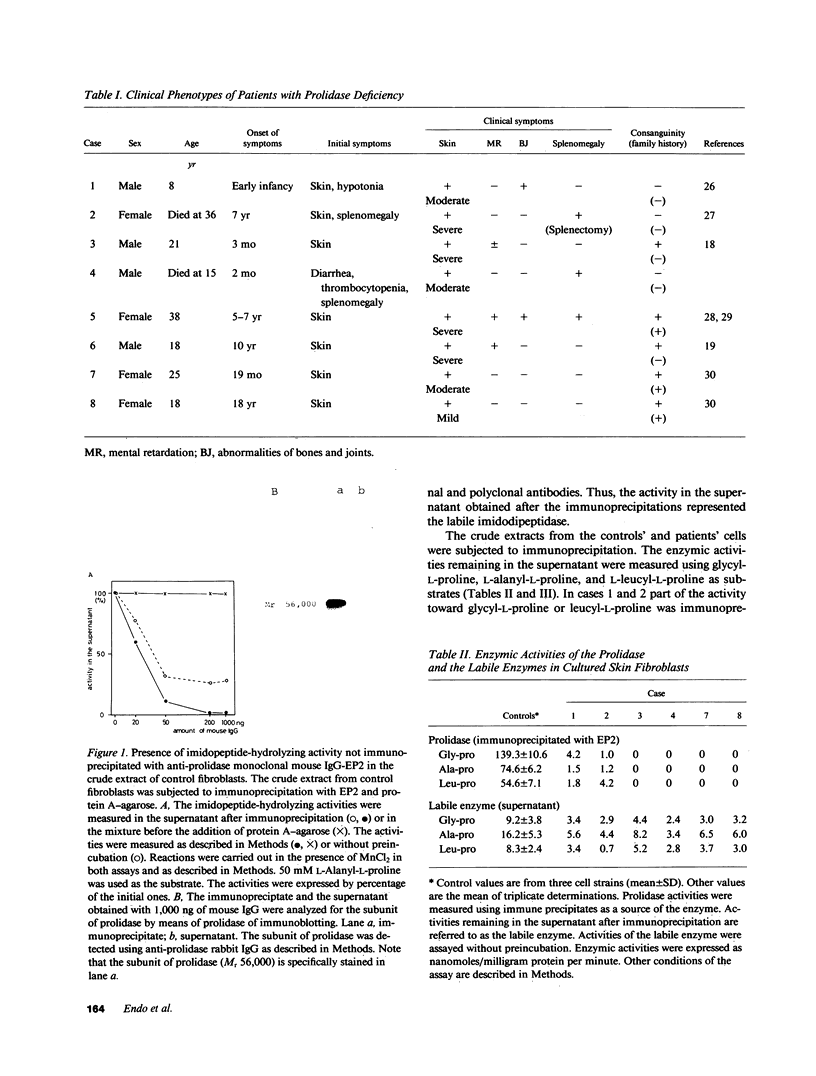

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arata J., Umemura S., Yamamoto Y., Hagiyama M., Nohara N. Prolidase deficiency: its dermatological manifestations and some additional biochemical studies. Arch Dermatol. 1979 Jan;115(1):62–67. doi: 10.1001/archderm.115.1.62. [DOI] [PubMed] [Google Scholar]
- Boright A. P., Scriver C. R., Lancaster G. A., Choy F. Prolidase deficiency: biochemical classification of alleles. Am J Hum Genet. 1989 May;44(5):731–740. [PMC free article] [PubMed] [Google Scholar]
- Butterworth J., Priestman D. A. Presence in human cells and tissues of two prolidases and their alteration in prolidase deficiency. J Inherit Metab Dis. 1985;8(4):193–197. doi: 10.1007/BF01805434. [DOI] [PubMed] [Google Scholar]
- Butterworth J., Priestman D. Substrate specificity of manganese-activated prolidase in control and prolidase-deficient cultured skin fibroblasts. J Inherit Metab Dis. 1984;7(1):32–34. doi: 10.1007/BF01805618. [DOI] [PubMed] [Google Scholar]
- Endo F., Hata A., Indo Y., Motohara K., Matsuda I. Immunochemical analysis of prolidase deficiency and molecular cloning of cDNA for prolidase of human liver. J Inherit Metab Dis. 1987;10(3):305–307. doi: 10.1007/BF01800088. [DOI] [PubMed] [Google Scholar]
- Endo F., Matsuda I., Ogata A., Tanaka S. Human erythrocyte prolidase and prolidase deficiency. Pediatr Res. 1982 Mar;16(3):227–231. doi: 10.1203/00006450-198203000-00013. [DOI] [PubMed] [Google Scholar]
- Endo F., Matsuda I. Screening method for prolidase deficiency. Hum Genet. 1981;56(3):349–351. doi: 10.1007/BF00274691. [DOI] [PubMed] [Google Scholar]
- Endo F., Motohara K., Indo Y., Matsuda I. Immunochemical studies of human prolidase with monoclonal and polyclonal antibodies: absence of the subunit of prolidase in erythrocytes from a patient with prolidase deficiency. Pediatr Res. 1987 Dec;22(6):627–633. doi: 10.1203/00006450-198712000-00002. [DOI] [PubMed] [Google Scholar]
- Endo F., Tanoue A., Nakai H., Hata A., Indo Y., Titani K., Matsuda I. Primary structure and gene localization of human prolidase. J Biol Chem. 1989 Mar 15;264(8):4476–4481. [PubMed] [Google Scholar]
- Endo F., Tanoue A., Ogata T., Motohara K., Matsuda I. Immunoaffinity purification of human erythrocyte prolidase. Clin Chim Acta. 1988 Aug 31;176(2):143–149. doi: 10.1016/0009-8981(88)90201-x. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Indo Y., Kitano A., Endo F., Akaboshi I., Matsuda I. Altered kinetic properties of the branched-chain alpha-keto acid dehydrogenase complex due to mutation of the beta-subunit of the branched-chain alpha-keto acid decarboxylase (E1) component in lymphoblastoid cells derived from patients with maple syrup urine disease. J Clin Invest. 1987 Jul;80(1):63–70. doi: 10.1172/JCI113064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isemura M., Hanyu T., Gejyo F., Nakazawa R., Igarashi R., Matsuo S., Ikeda K., Sato Y. Prolidase deficiency with imidodipeptiduria. A familial case with and without clinical symptoms. Clin Chim Acta. 1979 May 2;93(3):401–407. doi: 10.1016/0009-8981(79)90291-2. [DOI] [PubMed] [Google Scholar]
- Jackson S. H., Dennis A. W., Greenberg M. Iminodipeptiduria: a genetic defect in recycling collagen; a method for determining prolidase in erythrocytes. Can Med Assoc J. 1975 Oct 18;113(8):759, 762-3. [PMC free article] [PubMed] [Google Scholar]
- Kitano A., Endo F., Matsuda I., Miyabayashi S., Dahl H. H. Mutation of the E1 alpha subunit of the pyruvate dehydrogenase complex, in relation to heterogeneity. J Inherit Metab Dis. 1989;12(2):97–107. doi: 10.1007/BF01800710. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lapiere C. M., Nusgens B. Plaies cutanees torpides et trouble du metabolisme du collagene. Arch Belg Dermatol Syphiligr. 1969;25(3):353–356. [PubMed] [Google Scholar]
- Lombeck I., Wendel U., Versieck J., van Ballenberghe L., Bremer H. J., Duran R., Wadman S. Increased manganese content and reduced arginase activity in erythrocytes of a patient with prolidase deficiency (iminodipeptiduria). Eur J Pediatr. 1986 Apr;144(6):571–573. doi: 10.1007/BF00496038. [DOI] [PubMed] [Google Scholar]
- Miech G., Myara I., Mangeot M., Voigtlander V., Lemonnier A. Prolinase activity in prolidase-deficient fibroblasts. J Inherit Metab Dis. 1988;11(3):266–269. doi: 10.1007/BF01800368. [DOI] [PubMed] [Google Scholar]
- Ogata A., Tanaka S., Tomoda T., Murayama E., Endo F., Kikuchi I. Autosomal recessive prolidase deficiency. Three patients with recalcitrant ulcers. Arch Dermatol. 1981 Nov;117(11):689–697. [PubMed] [Google Scholar]
- Ohhashi T., Ohno T., Arata J., Kodama H. Biochemical studies on prolidase in sera from control, patients with prolidase deficiency and their mother. J Inherit Metab Dis. 1988;11(2):166–173. doi: 10.1007/BF01799867. [DOI] [PubMed] [Google Scholar]
- Powell G. F., Kurosky A., Maniscalco R. M. Prolidase deficiency: report of a second case with quantitation of the excessively excreted amino acids. J Pediatr. 1977 Aug;91(2):242–246. doi: 10.1016/s0022-3476(77)80820-2. [DOI] [PubMed] [Google Scholar]
- Powell G. F., Rasco M. A., Maniscalco R. M. A prolidase deficiency in man with iminopeptiduria. Metabolism. 1974 Jun;23(6):505–513. doi: 10.1016/0026-0495(74)90078-x. [DOI] [PubMed] [Google Scholar]
- Sheffield L. J., Schlesinger P., Faull K., Halpern B. J., Schier G. M., Cotton R. G., Hammond J., Danks D. M. Iminopeptiduria, skin ulcerations, and edema in a boy with prolidase deficiency. J Pediatr. 1977 Oct;91(4):578–583. doi: 10.1016/s0022-3476(77)80506-4. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemura S. Studies on a patient with iminodipeptiduria. II. Lack of prolidase activity in blood cells. Physiol Chem Phys. 1978;10(3):279–283. [PubMed] [Google Scholar]