Abstract
Exogenous antigens enter antigen-presenting cells through non-specific mechanisms and are presented by the MHC II molecules. We show here that antigens chaperoned by the heat shock protein gp96 enter dendritic cells and B cells through a specific, CD91- and LOX-1-mediated mechanism, and are presented by MHC II molecules, in addition to MHC I molecules as previously demonstrated. Receptor utilization results in high efficiency uptake such that antigen concentrations as low as 10-9 M, if chaperoned by gp96, lead to productive antigen presentation. Chaperoning by gp96 increases the efficiency of uptake over un-chaperoned peptides by up to two orders of magnitude. Consistent with these studies in vitro, immunization of mice with gp96-peptide complexes (containing 5 ng peptide) results in generation of a peptide-specific CD4+ T cell response. The high efficiency suggests a mechanism in which dendritic cells, exposed in vivo to heat shock protein-chaperoned peptides liberated by virus-infected host cells or by the lysis of infecting bacteria, may prime and expand specific CD4+ responses.
Keywords: mice, immunization, heat shock proteins, peptides, cellular immunity
Introduction
Selected heat shock proteins (HSPs) have been shown previously to interact with antigen-presenting cells (APCs) through the HSP receptor CD91 (1-4). Peptides chaperoned by these HSPs enter the APCs through this pathway and get presented by the MHC I molecules of the APCs in spite of their exogenous administration, as demonstrated in the murine (5-7) as well as in the human system (8, 9). We show here that the peptides chaperoned by the HSP gp96 also enter the exogenous antigen presentation pathway and get presented by the MHC II molecules of the APCs. Re-presentation of exogenous antigens through the exogenous pathway is not novel in and of itself (10). The novelty of the study derives from the demonstration that re-presentation of gp96-chaperoned peptides by MHC II molecules of the APCs is largely dependent upon the interaction of HSPs with the HSP receptors CD91 and LOX-1 on the APCs, and further, that the receptor-mediated re-presentation is considerably more antigen-efficient than non-receptor-mediated re-presentation of un-chaperoned peptides. CD91- and LOX-1-mediated uptake of gp96-chaperoned antigens through the exogenous pathway thus has the characteristics of surface immunoglobulin- (sIg) (11), Fc receptor- (12), mannose receptor- (13) and DEC205-mediated (14) uptake of exogenous antigens. Our present results also bear on the ability of immunization with HSP-peptide complexes to elicit T cell help, CD4+ T cell-mediated suppression and the elicitation of memory T cell and antibody responses.
Results
Re-presentation of gp96-chaperoned peptides by the MHC II molecules of APCs
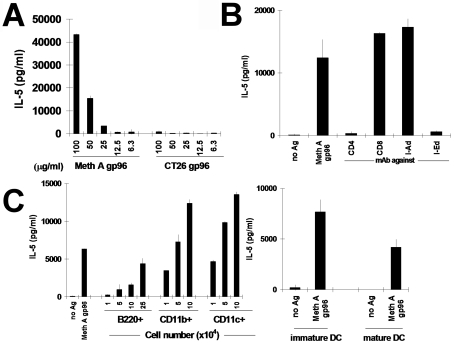
We have generated a number of CD4+ T lymphocyte clones from spleens of BALB/c mice immunized with Meth A sarcoma. The clones 24D3 and 12D1 are typical examples of these. They proliferate and secrete IL-5 specifically in response to APCs pulsed with lysates of Meth A but not CMS5, P815 or other tumors (15). These clones also secrete IL-5 specifically in response to APCs pulsed with gp96 derived from Meth A, but not gp96 derived from an antigenically distinct tumor line CT26 (H-2d) (Figure 1A). The activation of 24D3, elicited by Meth A gp96, is blocked identically by anti-CD4 or anti-IEd antibody, but not by anti-CD8 or anti-IAd antibody (Figure 1B).
Figure 1.
APCs process and present naturally-derived gp96-peptide complexes to CD4+ T cells in a source-specific manner. Data for clone 24D3 are shown; clone 12D1 behaves similarly. (A) 24D3 cells (2 x 104 cells) and irradiated splenic APCs (5 x 105 cells) were cultured with gp96 derived from either Meth A or an antigenically distinct tumor (CT26) at the concentrations indicated. Secretion of IL-5 by T cells was measured. (B) Stimulation of 24D3 by Meth A gp96 (100 µg/ml) is CD4-restricted, I-Ed restricted, but not CD8-restricred or I-Ad-restricted. Assays were done as in A and monoclonal antibodies against CD4, CD8, I-Ad, or I-Ed (2.5 µg/ml) were added. (C) Identity of splenic APCs. Splenic APCs were tested for MHC II-mediated antigen presentation of Meth A-derived gp96. CD4+ T cell clone 24D3 cells (2 x 104) were cultured with either 5 x 105 irradiated syngeneic splenic APCs or 104, 5 x 104 or 105 CD11b+, CD11c+, or B220+ cells purified from splenic APCs by MACS™ column, in the presence or absence of gp96 derived from Meth A or CT26 (100 µg/ml), as a negative control. For B220+ APCs, an additional titration of 2.5 x 105 cells was tested because of the relative inefficiency of these APCs in re-presentation. Bone-marrow derived immature DCs or mature DCs (1 x 105) were also tested in a similar manner as APCs.
The stimulatory activity of gp96 is titratable (Figure 1A). Significant release of T cells is detected by incubation of APCs with up to 50 µg/ml gp96, or 10 µg gp96 in absolute quantity (taking into account the assay volume). This quantity of gp96 exists within approximately 107 cells. The T cell cultures contain 5 x 105 APCs; thus, assuming that all APCs are equal recipients of gp96, each APC receives gp96 from approximately 20 Meth A cells in order to present antigen productively.
The identity of the APCs responsible for presentation was tested. The spleen was fractionated into CD11b+, CD11c+, or B220+ cells and each cell type in titrated numbers (104-105 cells for each APC population, except for an additional titration of 2.5 x 105 that was included for B220+ cells) was pulsed with either medium alone, Meth A-derived gp96, or gp96 derived from CT26 (negative control). The ability of the pulsed APCs to stimulate CD4+ lymphocytes was tested. It was observed (Figure 1C) that, on a per cell basis, the splenic CD11c+ sub-set was the most efficient in presentation, followed in quick succession by the CD11b+ sub-set (that contains CD11c+ cells within it). B220+ cells could also present detectably but poorly. Considering the absolute numbers of these cell types, the B220+ cells (approximately 50% of total spleen, as opposed to approximately 1% CD11c+ cells) may be considered to make the major contribution to MHC II-mediated presentation of gp96-chaperoned peptides by splenic APCs in vitro. Only macrophages and DCs have been shown to be capable of re-presenting HSP-chaperoned peptides thus far (2, 4). The demonstration that B cells may do it as well is consistent with our results that a proportion of B cells express CD91 (R. Binder and P.K. Srivastava, unpublished). The ability of immature and mature bone marrow-derived DCs to re-present gp96-chaperoned peptides through the exogenous pathway was investigated. It was observed that both immature and endotoxin-matured DCs could mediate the process effectively and more efficiently than B cells; however, immature DCs were superior to mature DCs in this regard (Figure 1C).
High efficiency re-presentation of gp96-chaperoned peptides by MHC II molecules
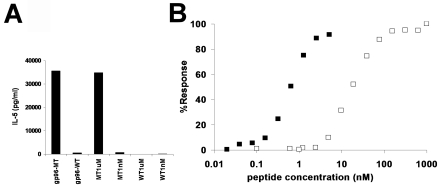
Gp96 (and other HSP) molecules are associated in vivo with a wide array of peptides including antigenic peptides (16-18). HSPs can also be complexed in vitro with antigenic peptides and the chaperoned peptides can be re-presented by the MHC I molecules of the APCs (6, 19). Re-presentability of in vitro reconstituted gp96-chaperoned peptides by the MHC II molecules of APCs was tested. Gp96 from normal liver was complexed to the antigenic peptide recognized by the 24D3 CD4+ cells and the antigen-specific activity of the complex tested. The antigen recognized by the Meth A-specific CD4+ cells is derived from a Meth A-specific mutant allele of the L11 ribosomal protein (15). The clone 24D3 can be stimulated with the mutant (KVREYELRKHNFSDTGNFG) but not with the wild type (KVREYELRKNNFSDTGNFG) allele at a free peptide concentration of 1 µM (Figure 2A). The wild type or mutant peptide was complexed non-covalently with liver-derived gp96 (13) and both gp96-peptide complexes were tested for their ability to stimulate CD4+ cells. The quantity of peptide which could be complexed to the gp96 preparations was in the nM range [see (19) and the Materials and methods section to see how this was determined]. At this low peptide concentration, the free wild type or mutant peptides could not stimulate the CD4+ T cells. However, the mutant peptide complexed with gp96 was effective in stimulating the CD4+ cells, whereas the wild type allele complexed with gp96 at the same concentration as the mutant peptide could not stimulate them (Figure 2A). In addition to demonstrating that antigenic specificity derives not from gp96 itself but from the associated peptides, this observation hinted that the efficiency of uptake and presentation of the mutant allele was enhanced by association with gp96. This was formally tested.
Figure 2.
High efficiency re-presentation of gp96-chaperoned peptides by MHC II molecules. (A) APCs present in vitro the reconstituted complex of gp96 with the antigenic mutant but not wild type peptide through MHC II molecules to CD4+ T cells. CD4+ T cell clone 24D3 (2 x 104) cells were cultured with 1 µM or 1 nM of either free, wild type L11 peptide (WT), the CD4 epitope, mutated L11 peptide (MT) or gp96 complexed with these peptides, in the presence of irradiated syngeneic splenic APCs. Activity was measured by IL-5 release as in Figure 1. The quantity of peptide in gp96-peptide complexes was determined to be in the nM range. (B) Comparison of efficiency of antigen presentation through MHC II, by free peptides and gp96-chaperoned peptides. Radiolabeled peptides of known specific radioactivity (cpm/µg peptide) were complexed with known quantities of gp96. Defined quantities of gp96 were counted after removal of unbound peptides and the absolute quantity of bound peptide per µg gp96 was thus determined. CD4+ T cell clone 24D3 (2 x 104) cells were cultured with free mutant L11 peptide (open square) or gp96 complexed with mutant L11 peptide (filled square) at the concentrations indicated for 2 days and stimulation of 24D3 was measured by IL-5 release in the supernatant by ELISA. The % response was determined by comparison of the activity of gp96-peptide complexes or that of free MT peptide with the activity of 1 µM free MT peptide (set as 100%). The average of 3 experiments is shown for each point.
Titrated quantities of free or gp96-associated mutant peptide were used to pulse APCs and to stimulate CD4+ cells. The quantity of peptide bound to the gp96 molecules was determined as described (19). Briefly, a known quantity of peptide was radiolabeled and the cpm/mole of the peptide determined. Defined titrated quantities of this labeled peptide were complexed in vitro with a defined quantity of gp96 and un-complexed peptides washed off. The quantity of labeled peptide associated with gp96 could be ascertained by counting the label in the gp96-peptide complex. It was observed (Figure 2B) that at their highest concentrations, free or gp96-chaperoned antigens stimulated CD4+ cells to a similar degree. However, the quantity of peptide required for maximal or half-maximal stimulation was strikingly different, depending on whether it was free or gp96-chaperoned. The gp96-chaperoned mutant peptide was approximately 20- to 50-fold more efficient than free mutant peptide for half-maximal stimulation of the CD4+ T lymphocytes, and over 100-fold more efficient for maximal stimulation. Albumin, which also binds peptides effectively and comparably, did not confer this high efficiency (data not shown). These data are reminiscent of a similar high efficiency of endogenous presentation conferred on the peptide upon association with gp96 or hsp70 (6).
CD91 and LOX-1 dependence of antigen presentation
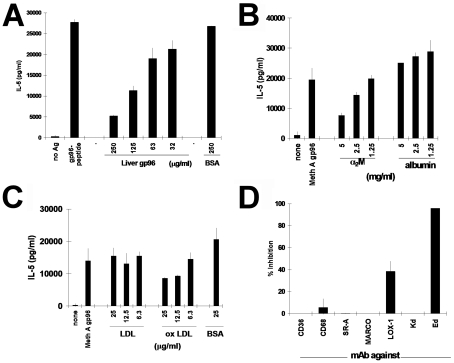
Soluble exogenous antigens have been routinely demonstrated to be taken up by the APCs through non-specific mechanisms and presented by the MHC II molecules. Receptors are not necessary for antigen-APC interaction in this pathway. However, the remarkably high antigenic efficiency of presentation of gp96-chaperoned versus free peptides led us to explore a role for a receptor in this process. Liver-derived gp96, but not albumin, inhibited the re-presentation of gp96-chaperoned peptides to 24D3 CD4+ cells, titratably (Figure 3A). Near complete inhibition was observed at the highest concentration of gp96 tested. Gp96 has been shown to interact with APCs through CD91 (1-3). The ability of a previously known CD91 ligand, α2-macroglobulin (α2M), to inhibit the presentation of gp96-chaperoned peptides by MHC II molecules to CD4+ lymphocytes was tested. CD4+ clone 24D3 cells (2 x 104) were cultured (as in Figure 1A) with Meth A-derived gp96 and 5 x 105 irradiated syngeneic APCs in the presence of titrated quantities of α2M or albumin (as a negative control) at inhibitor concentrations of 0.15, 0.3, 0.6 and 1.2 µM. Figure 3B shows the antigen presentation activity as evidenced by the secretion of IL5 by CD4+ T cells in the presence of inhibitors expressed as the % of the same activity in the absence of inhibitors. α2M inhibited re-presentation of gp96-chaperoned L11-derived antigenic peptide titratably, and by approximately 80% at the highest concentration. Higher concentrations of α2M could not be tested due to difficulties in dissolving α2M at concentrations >1.2 µM. Albumin, even at the highest concentrations tested, did not inhibit re-presentation.
Figure 3.
CD91- and LOX-1-dependence of presentation of gp96-chaperoned peptides by MHC II. Presentation of gp96-chaperoned peptides by immature bone marrow-derived DCs can be inhibited by CD91 ligands α2-macroglobulin or antigen-negative gp96, but not by serum albumin. Re-presentation assays were performed as in Figure 1, in the presence of titrated concentrations of antigen-negative liver gp96 (A), α2M (B) or albumin (A, B) at the concentrations indicated. (C) Re-presentation assays were carried out in the presence of titrated concentrations of LDL, oxidized LDL or BSA, as indicated. Re-presentation was quantitated as in A and B. (D) Antibodies to scavenger receptors CD36, CD68, SR-A, MARCO or LOX-1 (10 µg/ml each) were included in the re-presentation assays as in C. In addition, antibodies to I-Ed (as a positive control) or Kd (as negative control) were included. Percent inhibition (as compared to activity in the absence of inhibiting antibody) is plotted.
LOX-1, a member of the scavenger receptor family, has been implicated in hsp70-DC interaction (4). As CD91 interacts with gp96 and hsp70 (2), and as scavenger receptors bind a broad spectrum of ligands, the role of LOX-1 in re-presentation of gp96-chaperoned peptides was tested. Oxidized low density lipoprotein (LDL), a known ligand of LOX-1, was tested for its ability to inhibit re-presentation of gp96-chaperoned peptides. An oxidized LDL preparation (in which the oxidized LDL/native LDL ratio was 2.20 ± 0.11) inhibited re-presentation, while control ligands such as LDL or BSA failed to do so at similar or higher concentrations (Figure 3C). An antibody to LOX-1 also inhibited re-presentation to the same degree as oxidized LDL, while antibodies to the scavenger receptors CD36, SR-A, CD68 and MARCO did not. As positive and negative controls, an antibody to I-Ed also inhibited re-presentation while an antibody to Kd did not (Figure 3D).
Requirement of intracellular processing, including transit through an acidic compartment
The effect of two physiological inhibitors that block distinct steps in the intracellular traffic on the presentation of gp96-chaperoned peptides by APCs (20) was tested. Brefeldin A (BFA) blocks vesicular traffic (21), while chloroquine (CHQ) neutralizes the intracellular acidic compartments (22). The same experimental system as in Figure 1 was used and the inhibitors were incorporated in the assay. Both inhibitors abrogated the presentation of gp96-chaperoned peptides (Figure 4A), thus indicating that the gp96-peptide complex must traverse an acidic compartment and utilize the classical secretory pathway. As a control, the effect of these inhibitors on the endogenous MHC I-mediated presentation of gp96-chaperoned peptides was tested in parallel. Liver gp96 was complexed in vitro to AH1-19, a 19-mer peptide which bears within it the Ld-restricted AH1 epitope of the CT26 colon carcinoma (RVTYHSPSYVYHQFERRAK) (23). The AH1-19 peptide cannot charge the surface Ld molecules but has to be processed internally after being taken up with gp96. Presentation of AH1 by the MHC I molecules was inhibited by BFA but not by CHQ (Figure 4B). This is consistent with previous observations in a different antigenic system (4). Thus, while the introduction of gp96-chaperoned peptides into the endogenous pathway of presentation is insensitive to treatment with CHQ, their introduction into the exogenous pathway is sensitive to the same treatment. BFA inhibited both pathways equally.
Figure 4.
Inhibition of re-presentation of gp96-chaperoned peptides through MHC I or MHC II molecules. (A) CD4+ T cell clone 24D3 cells (2 x 104) were cultured with Meth A-derived gp96 (50 µg/ml) in the presence of 5 x 105 irradiated syngeneic APCs which had or had not been pre-treated with BFA or CHQ. Inhibitors were used as described in the text. Stimulation of CD4+ T cells was measured by IL-5 release. (B) CD8+ cells (104) specific for Ld/AH1 epitope of CT26 were cultured with 104 RAW264.7 cells in the presence of gp96 (50 µg/ml) or gp96-AH1 19-mer peptide complexes (50 µg/ml). The APCs were either untreated or treated previously with BFA or CHQ. Stimulation of CD8+ T cells was measured by interferon-γ release.
Immunization with gp96-peptide complex elicits peptide-specific CD4+ T lymphocytes
BALB/c mice were immunized with in vitro reconstituted non-covalent complexes of gp96 with the wild type or mutant L11 peptide (15). The gp96-peptide complexes contained 25 µg gp96 and 2.5 ng peptide per immunization. Buffer-immunized mice were tested as controls. Splenocytes of all mice were cultured in vitro in the presence of either no peptide, wild type (WT) peptide, or mutant (MT) peptide for 4 days and were tested for proliferation by thymidine incorporation. Mice immunized with the gp96 MT peptide generated a strong primary CD4+ T cell response, while mice immunized with buffer or the wild type peptide did not (Figure 5A). Depletion of CD8+ T cells from the splenocytes did not affect the proliferation activity (data not shown). Further, CD4+ T cell activity was measurable only in cultures of splenocytes derived from mice immunized with the gp96-chaperoned MT peptide and stimulated in vitro with the MT peptide. A long term CD4+ T cell line, TM/JB007, was derived from cultures of such splenocytes. This CD4+ line showed exquisite specificity and proliferated in response to APCs pulsed with the mutant, but not the wild type, L11 peptide and its activity was inhibited in vitro by antibodies to CD4 and I-Ed but not CD8 or I-Ad (Figure 5B).
Figure 5.
Immunization of mice with gp96-peptide complexes elicits antigen-specific CD4+ T cells. (A) BALB/c mice were immunized with either buffer, liver-derived gp96 complexed with WT L11 peptide (25 µg gp96 complexed with approximately 2.5 ng peptide) or liver-derived gp96 complexed with MT L11 (25 µg gp96 complexed with approximately 2.5 ng peptide) twice intradermally, 1 week apart. One week after the last immunization, spleen cells were cultured in vitro either without peptide, with wild type peptide (WT) or with mutated peptide (MT) at 1 µM for 4 days. 3H-thymidine (0.5 µCi/well) was added to the cultures 18 h before harvesting and proliferation was measured. Data from each of two individual mice for each group are shown. (B) The CD4+ T cell line from A was blocked with anti-CD4 and anti-MHC class II mAbs. CD4+ T cell line TM/JB007 cells (2 x 104) from A were cultured with 5 x 105 of irradiated splenic APCs and mutated L11 peptide (0.1 µM) in the presence of the mAb indicated. The response was blocked by anti-CD4 or -I-Ed mAb, but not by anti-CD8 or anti-I-Ad mAb as in Figure 1B.
Discussion
Our observations show that a gp96-chaperoned peptide enters the APCs by a CD91- and LOX-1-mediated mechanism, reaches an acidic compartment, and is presented by the MHC II molecules of the APCs. The processing and presentation are sensitive to BFA and CHQ, indicating that the gp96-chaperoned peptides are not simply charged onto the surface MHC II molecules. The observation that competition with excess α2-macroglobulin, another ligand for CD91, and oxidized LDL, a ligand of LOX-1, inhibits presentation of gp96-chaperoned peptides by MHC II molecules, also argues against surface transfer.
The CD91- and LOX-1-mediated uptake of antigen leads to a high efficiency of antigen presentation, as demonstrated earlier in the case of antigen uptake through other receptors such as sIg (11), Fc receptor (12), mannose receptor (13) and DEC205 (14). Receptor-mediated antigen uptake thus also suggests a mechanism through which APCs, exposed in vivo to HSP-chaperoned peptides liberated by virus-infected host cells or by the lysis of infecting bacteria, may prime specific CD4+ responses. Consistent with these studies in vitro, immunization of mice with gp96-peptide complexes containing as little as 5 ng peptide results in generation of a peptide-specific CD4+ T response. Utilization of this pathway under conditions of viral infection and tumorigenesis, as well as the relative significance of the pathway, are presently under investigation. Our observation that gp96-peptide complexes from as few as approximately 20 tumor cells is sufficient for productive antigen presentation by a single APC, also lends weight to this premise particularly if one bears in mind the certain possibility that HSPs other than gp96, and far more abundant than gp96, behave in the same way as gp96. The observation that immature DCs are measurably better than endotoxin-matured DCs at taking up and re-presenting gp96-chaperoned peptides is consistent with a physiological role for HSP-peptide complexes in priming CD4+ T responses. The previous demonstration by our group (24) and others (25) that gp96 molecules can mediate maturation of DCs suggests that interaction of immature Langerhans cells or other immature tissue DCs may, on one hand, lead to re-presentation of HSP-chaperoned peptides and, on the other hand, cause their maturation and migration in vivo (26).
An entirely expected difference emerges in the intracellular trafficking pathway of gp96-chaperoned peptides to MHC I or MHC II. The re-presentation of gp96-chaperoned peptides by MHC I molecules has also been shown to require intracellular processing (2, 6, 27). It is however, insensitive to treatment of the APCs with CHQ, indicating the lack of requirement for the access of the peptide to an acidic compartment (6). Those observations, together with the results shown here, suggest that the gp96-peptide complex internalized through CD91 and LOX-1 may enter an acidic or a non-acidic compartment, with consequent introduction of the peptides into the exogenous or endogenous pathway of presentation. As one possible mechanism for the dual outcomes, one can imagine the internalized gp96-peptide containing vesicles stochastically fusing with acidic vesicles (leading to presentation by MHC II) or permitting transport of the gp96-peptide complexes to the cytosol through an as yet unknown mechanism, leading to presentation by MHC I. Re-presentation studies with hybrid peptides containing epitopes for presentation by MHC I and MHC II molecules should shed further light on the intracellular trafficking. The structure of the HSP-chaperoned peptide has been shown to have a defining influence on the intracellular trafficking of the peptide in case of re-presentation of hsp70-chaperoned peptides by MHC I molecules of APCs (7). Similar influences of peptide structure in the exogenous pathway are not unlikely.
The downstream effects of re-presentation of gp96-chaperoned peptides by MHC II molecules include consequences for the generation of CD4+ help, CD4+-mediated suppression, and elicitation of T cell memory and antibody responses. Such re-presentation should lead to the generation of helper T cell responses, in addition to the CD8+ responses traditionally reported as a result of immunization with HSP-peptide complexes. Although priming of CD4+ responses has been shown not to be required for effective immunity in models of prophylaxis against tumors, CD4+ help is essential in the effector phase of the immune response (28). The demonstrated ability of gp96-peptide complexes to elicit such responses adds to the potential immunogenicity of HSP-peptide complexes. Rouse and colleagues have recently shown that immunization of mice with an hsp70-chaperoned MHC I epitope of the herpes simplex virus-2 elicits an excellent CD8+ T cell response and protection from an acute viral challenge; however, the memory response elicited by immunization with the hsp70-peptide complex is less robust than the corresponding response elicited by live attenuated virus (29). In view of the role of help in generation of memory responses, we would predict that immunization with HSP-peptide complexes where the peptides contain MHC I and MHC II epitopes should overcome the memory deficit reported by Rouse and colleagues. Finally, recent studies by at least two groups have demonstrated that immunization with gp96-chaperoned peptides (30) or hsp110-chaperoned proteins (31) elicits antibody responses against the peptides or proteins. These responses are apparently helper-dependent and our demonstration that gp96-chaperoned peptides can be re-presented by the MHC II molecules of the APCs to CD4+ T cells provides a mechanistic basis for the elicitation of antibody responses.
Paradoxically, immunization with high doses of endogenously-derived gp96-peptide complexes has been shown to elicit CD4+ T cells that can down-regulate CD8+ responses in a tumor model (32, 33). The present studies do not shed light on that phenomenon. It is our belief that elicitation of suppressor CD4+ T cells by immunization with high doses of gp96 derives from the conditioning of APCs by high doses of gp96 and the resulting signals derived from that conditioning, that then affect the property of the CD4+ cells primed. This premise requires experimental testing.
HSP-APC interaction has been shown to result in a wide array of immunological events and the results shown here expand that spectrum even further. Thus, HSPs stimulate macrophages to elaborate cytokines (24) and chemokines (34), cause maturation of dendritic cells in vitro (24-26), and their maturation and migration in vivo (26), introduce peptides chaperoned by them into the endogenous and now the exogenous pathway (this study) of antigen presentation. The HSP-peptide and HSP-APC interaction thus leads to a uniquely broad spectrum of antigen-specific and innate immune responses.
Abbreviations
- BFA
brefeldin A
- CHQ
chloroquine
- HSP
heat shock protein
- LDL
low density lipoprotein
- MT
mutant
- WT
wild type
Acknowledgements
This work was supported by an NIH grant to PKS (CA84479).
References
- 1.Binder RJ, Han DK, Srivastava PK. CD91: a receptor for heat shock protein gp96. Nat Immunol. 2000;1:151–155. doi: 10.1038/77835. [DOI] [PubMed] [Google Scholar]
- 2.Basu S, Binder RJ, Ramalingam T, Srivastava PK. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity. 2001;14:303–313. doi: 10.1016/s1074-7613(01)00111-x. [DOI] [PubMed] [Google Scholar]
- 3.Binder RJ, Srivastava PK. Essential role of CD91 in re-presentation of gp96-chaperoned peptides. Proc Natl Acad Sci USA. 2004;101:6128–6133. doi: 10.1073/pnas.0308180101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delneste Y, Magistrelli G, Gauchat J, Haeuw J, Aubry J, Nakamura K, Kawakami-Honda N, Goetsch L, Sawamura T, Bonnefoy J, Jeannin P. Involvement of LOX-1 in dendritic cell-mediated antigen cross-presentation. Immunity. 2002;17:353–362. doi: 10.1016/s1074-7613(02)00388-6. [DOI] [PubMed] [Google Scholar]
- 5.Srivastava PK, Udono H, Blachere NE, Li Z. Heat shock proteins transfer peptides during antigen processing and CTL priming. Immunogenetics. 1994;39:93–98. doi: 10.1007/BF00188611. [DOI] [PubMed] [Google Scholar]
- 6.Suto R, Srivastava PK. A mechanism for the specific immunogenicity of heat shock protein-chaperoned peptides. Science. 1995;269:1585–1588. doi: 10.1126/science.7545313. [DOI] [PubMed] [Google Scholar]
- 7.Castellino F, Boucher PE, Eichelberg K, Mayhew M, Rothman JE, Houghton AN, Germain RN. Receptor-mediated uptake of antigen/heat shock protein complexes results in major histocompatibility complex class I antigen presentation via two distinct processing pathways. J Exp Med. 2000;191:1957–1964. doi: 10.1084/jem.191.11.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castelli C, Ciupitu AM, Rini F, Rivoltini L, Mazzocchi A, Kiessling R, Parmiani G. Human heat shock protein 70 peptide complexes specifically activate antimelanoma T cells. Cancer Res. 2001;61:222–227. [PubMed] [Google Scholar]
- 9.Noessner E, Gastpar R, Milani V, Brandl A, Hutzler PJ, Kuppner MC, Roos M, Kremmer E, Asea A, Calderwood SK, Issels RD. Tumor-derived heat shock protein 70 peptide complexes are cross-presented by human dendritic cells. J Immunol. 2002;169:5424–5432. doi: 10.4049/jimmunol.169.10.5424. [DOI] [PubMed] [Google Scholar]
- 10.SenGupta D, Norris PJ, Suscovich TJ, Hassan-Zahraee M, Moffett HF, Trocha A, Draenert R, Goulder PJ, Binder RJ, Levey DL, Walker BD, Srivastava PK, Brander C. Heat shock protein-mediated cross-presentation of exogenous HIV antigen on HLA class I and class II. J Immunol. 2004;173:1987–1993. doi: 10.4049/jimmunol.173.3.1987. [DOI] [PubMed] [Google Scholar]
- 11.Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature. 1985;314:537–539. doi: 10.1038/314537a0. [DOI] [PubMed] [Google Scholar]
- 12.Guermonprez P, Valladeau J, Zitvogel L, Théry C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annu Rev Immunol. 2002;20:621–667. doi: 10.1146/annurev.immunol.20.100301.064828. [DOI] [PubMed] [Google Scholar]
- 13.Mansour MK, Schlesinger LS, Levitz SM. Optimal T cell responses to Cryptococcus neoformans mannoprotein are dependent on recognition of conjugated carbohydrates by mannose receptors. J Immunol. 2002;168:2872–2879. doi: 10.4049/jimmunol.168.6.2872. [DOI] [PubMed] [Google Scholar]
- 14.Jiang W, Swiggard WJ, Heufler C, Peng M, Mirza A, Steinman RM, Nussenzweig MC. The receptor DEC-205 expressed by dendritic cells and thymic epithelial cells is involved in antigen processing. Nature. 1995;375:151–155. doi: 10.1038/375151a0. [DOI] [PubMed] [Google Scholar]
- 15.Matsutake T, Srivastava PK. The immunoprotective MHC II epitope of a chemically induced tumor harbors a unique mutation in a ribosomal protein. Proc Natl Acad Sci USA. 2001;98:3992–3997. doi: 10.1073/pnas.071523398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nieland TJ, Tan MC, Monne-van Muijen M, Koning F, Kruisbeek AM, van Bleek GM. Isolation of an immunodominant viral peptide that is endogenously bound to the stress protein GP96/GRP94. Proc Natl Acad Sci USA. 1996;93:6135–6139. doi: 10.1073/pnas.93.12.6135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Udono H, Srivastava PK. Heat shock protein 70-associated peptides elicit specific cancer immunity. J Exp Med. 1993;178:1391–1396. doi: 10.1084/jem.178.4.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Breloer M, Marti T, Fleischer B, von Bonin A. Isolation of processed, H-2Kb-binding ovalbumin-derived peptides associated with the stress proteins HSP70 and gp96. Eur J Immunol. 1998;28:1016–1021. doi: 10.1002/(SICI)1521-4141(199803)28:03<1016::AID-IMMU1016>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 19.Blachere NE, Li Z, Chandawarkar RY, Suto R, Jaikaria NS, Basu S, Udono H, Srivastava PK. Heat shock protein-peptide complexes, reconstituted in vitro, elicit peptide-specific cytotoxic T lymphocyte response and tumor immunity. J Exp Med. 1997;186:1315–1322. doi: 10.1084/jem.186.8.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moriwaki H, Kume N, Sawamura T, Aoyama T, Hoshikawa H, Ochi H, Nishi E, Masaki T, Kita T. Ligand specificity of LOX-1, a novel endothelial receptor for oxidized low density lipoprotein. Arterioscler Thromb Vasc Biol. 1998;18:1541–1547. doi: 10.1161/01.atv.18.10.1541. [DOI] [PubMed] [Google Scholar]
- 21.Lippincott-Schwartz J, Yuan L, Tipper C, Amherdt M, Orci L, Klausner RD. Brefeldin A's effects on endosomes, lysosomes, and the TGN suggest a general mechanism for regulating organelle structure and membrane traffic. Cell. 1991;67:601–616. doi: 10.1016/0092-8674(91)90534-6. [DOI] [PubMed] [Google Scholar]
- 22.Harding CV, Collins DS, Slot JW, Geuze HJ, Unanue ER. Liposome-encapsulated antigens are processed in lysosomes, recycled, and presented to T cells. Cell. 1991;64:393–401. doi: 10.1016/0092-8674(91)90647-h. [DOI] [PubMed] [Google Scholar]
- 23.Huang AY, Gulden PH, Woods AS, Thomas MC, Tong CD, Wang W, Engelhard VH, Pasternack G, Cotter R, Hunt D, Pardoll DM, Jaffee EM. The immunodominant major histocompatibility complex class I-restricted antigen of a murine colon tumor derives from an endogenous retroviral gene product. Proc Natl Acad Sci USA. 1996;93:9730–9735. doi: 10.1073/pnas.93.18.9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int Immunol. 2000;12:1539–1546. doi: 10.1093/intimm/12.11.1539. [DOI] [PubMed] [Google Scholar]
- 25.Singh-Jasuja H, Scherer HU, Hilf N, Arnold-Schild D, Rammensee HG, Toes RE, Schild H. The heat shock protein gp96 induces maturation of dendritic cells and down-regulation of its receptor. Eur J Immunol. 2000;30:2211–2215. doi: 10.1002/1521-4141(2000)30:8<2211::AID-IMMU2211>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 26.Binder RJ, Anderson KM, Basu S, Srivastava PK. Cutting edge: heat shock protein gp96 induces maturation and migration of CD11c+ cells in vivo. J Immunol. 2000;165:6029–6035. doi: 10.4049/jimmunol.165.11.6029. [DOI] [PubMed] [Google Scholar]
- 27.Singh-Jasuja H, Toes RE, Spee P, Münz C, Hilf N, Schoenberger SP, Ricciardi-Castagnoli P, Neefjes J, Rammensee HG, Arnold-Schild D, Schild H. Cross-presentation of glycoprotein 96-associated antigens on major histocompatibility complex class I molecules requires receptor-mediated endocytosis. J Exp Med. 2000;191:1965–1974. doi: 10.1084/jem.191.11.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Udono H, Levey DL, Srivastava PK. Cellular requirements for tumor-specific immunity elicited by heat shock proteins: tumor rejection antigen gp96 primes CD8+ T cells in vivo. Proc Natl Acad Sci U S A. 1994;91:3077–3081. doi: 10.1073/pnas.91.8.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumaraguru U, Gierynska M, Norman S, Bruce BD, Rouse BT. Immunization with chaperone-peptide complex induces low-avidity cytotoxic T lymphocytes providing transient protection against herpes simplex virus infection. J Virol. 2002;76:136–141. doi: 10.1128/JVI.76.1.136-141.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Navaratnam M, Deshpande MS, Hariharan MJ, Zatechka DS Jr, Srikumaran S. Heat shock protein-peptide complexes elicit cytotoxic T-lymphocyte and antibody responses specific for bovine herpesvirus 1. Vaccine. 2001;19:1425–1434. doi: 10.1016/s0264-410x(00)00381-9. [DOI] [PubMed] [Google Scholar]
- 31.Manjili MH, Henderson R, Wang XY, Chen X, Li Y, Repasky E, Kazim L, Subjeck JR. Development of a recombinant HSP110-HER-2/neu vaccine using the chaperoning properties of HSP110. Cancer Res. 2002;62:1737–1742. [PubMed] [Google Scholar]
- 32.Chandawarkar RY, Wagh MS, Srivastava PK. The dual nature of specific immunological activity of tumor-derived gp96 preparations. J Exp Med. 1999;189:1437–1442. doi: 10.1084/jem.189.9.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chandawarkar RY, Wagh MS, Kovalchin JT, Srivastava P. Immune modulation with high-dose heat-shock protein gp96: therapy of murine autoimmune diabetes and encephalomyelitis. Int Immunol. 2004;16:615–624. doi: 10.1093/intimm/dxh063. [DOI] [PubMed] [Google Scholar]
- 34.Lehner T, Bergmeier LA, Wang Y, Tao L, Sing M, Spallek R, van der Zee R. Heat shock proteins generate beta-chemokines which function as innate adjuvants enhancing adaptive immunity. Eur J Immunol. 2000;30:594–603. doi: 10.1002/1521-4141(200002)30:2<594::AID-IMMU594>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
Materials and methods
Mice
BALB/c mice were purchased from Jackson Laboratory and maintained in our animal facilities.
T cell proliferation assay
T cell clones (2 x 104) were cultured with antigens in the presence of 5 x 105 irradiated syngeneic splenic APCs. Activity was measured by 3H-thymidine uptake (0.5 µCi/well of 3H-thymidine added 18 h before harvesting) at day 4 or cytokine release in the supernatant at day 2 by ELISA (Endogen, Woburn, MA). Anti-CD4, -CD8, -I-A/Ed mAbs were purchased from BD Biosciences PharMingen (San Diego, CA). Anti-I-Ed mAb was purchased from Accurate (Westbury, NY). APCs were treated with 0.3 mM chloroquine (CHQ) at 37˚C for 30 min and washed twice and used as APCs. APCs were treated with 5 µg/ml of brefeldin A (BFA) at 37˚C for 3 h and washed 2-3 times and used as APCs. BFA (0.5 µg/ml) was present during culture.
T cell lines and antigens
CD4+ T cell clones were established as described (15). The clones recognize a mutated allele of the ribosomal protein L11 (EYELRKHNFSDTG) generated due to substitution of Asn by His (underlined). Wild type (KVREYELRKNNFSDTGNFG) and mutated (KVREYELRKHNFSDTGNFG) 19-mer peptides were used. The CT26-specific CD8+ T clone recognizes the 9-mer AH1 peptide (SPSYVYHQF) epitope from murine leukemia virus (MuLV) gp70 (23). A 19-mer extended peptide (RVTYHSPSYVYHQFERRAK) was synthesized and used in re-presentation assays.
Purification of antigen-presenting cells
CD11b+, CD11c+, B220+ cells were purified by MACS™ column (Miltenyi Biotec, Auburn, CA). Dendritic cells were prepared from bone marrow culture (treated with anti-CD4, -CD8, -I-A/Ed, -B220, -Gr-1 Abs and incubated on ice for 30 min, followed by incubation with rabbit serum at 37˚C for 45 min, and cultured with 20 ng/ml of GM-CSF). On day 3, medium was removed and 20 ng/ml of GM-CSF was added and the cells cultured for a further 3 days. Day 6 culture was used as immature DCs. For mature DCs, a day 6 culture was treated with 100 ng/ml LPS for 17 h and used. DCs were irradiated (3000 rad) before use.
Purification of gp96 and reconstitution of gp96 and peptides in vitro
Gp96 was purified and complexed with antigenic peptides as described (19). Gp96-bound peptide was quantitated as previously described (19). Radiolabeled peptides of known specific radioactivity (cpm/µg peptide) were complexed with known quantities of gp96 (peptide:gp96 molar ratio of 50:1) by co-incubation at 50˚C for 30 min as described (19). Free, un-complexed peptides were removed through multiple rounds of gel filtration, and the fact of their having been removed was tested by size exclusion chromatography or SDS-PAGE. Defined quantities of gp96-peptide complexes were then resolved on SDS-PAGE and stained with Coomassie blue, and autoradiographed. The gp96 band could be detected by autoradiography due to the bound radiolabeled peptide. The stained gp96 band was cut out, measured on a gamma counter (Model Gamma 5500; Wakefield, MA) and the absolute quantity of bound peptide determined based on the previously determined specific radioactivity of the peptide.
Oxidation of LDL
LDL was purchased from Calbiochem (San Diego, CA). Oxidation was performed as in (20). Briefly, LDL was dialyzed against PBS for 24 h at 4˚C. Oxidation was then performed by incubation with 7.5 µM CuSO4 at 37˚C for 24 h and terminated by incubation with 1 mM EDTA with cooling. After dialyzing against LDL buffer (150 mmol/L NaCl, 0.24 mM EDTA, pH 7.4) for 48 h, oxidized and native LDL were separated according to their relative electrophoretic mobility in a 0.6% agarose gel. The protein concentration of LDL and oxidized LDL was measured using the BCA protein assay (Pierce).
Immunization of mice with gp96-peptide complexes and generation of antigen-specific CD4+ cells
BALB/c mice were immunized intradermally with PBS, 25 µg of liver-derived gp96 conjugated with wild type or mutated L11 peptide (15) twice, 1 week apart. The complexing reaction was carried out as previously described (19). One week after the last immunization, spleen cells were stimulated in vitro either with medium, wild type or mutated L11 peptide at 1 µM for 4 days. 3H-thymidine was added on day 3. Cells were harvested with the cell harvester on day 4 and the 3H-count measured using a beta counter (Model 1450 Microbeta counter; Perkin-Elmer, Waltham, MA). One day before taking out spleen cells, mice were injected with anti-CD8 mAb (YTS 169.4). CD8 depletion was confirmed by FACS analysis. After several rounds of stimulation peptide (approximately 0.1 to 1 µM), T cell lines were obtained and their specificity tested.