Abstract
The search for novel tumour antigens that are either uniquely expressed or over-expressed in a wide variety of tumours is still ongoing. Because of their expression in a broad spectrum of cancers and limited expression in normal tissues, cancer/testis antigens are considered to be potentially reliable targets for immunotherapy of cancer in general. The helicase antigen HAGE has been identified as a cancer/testis antigen. However, little is known about its expression in normal and cancer tissues. Using a newly developed antibody against HAGE, specific staining of its expression by immunohistochemistry was validated and optimised on murine tumours transfected to express the HAGE protein. The antibody was subsequently used to determine HAGE expression in normal human and cancer tissue microarrays. HAGE protein expression was confirmed in 75% (12/16) of carcinomas as compared to normal tissues, which either did not express HAGE at all or expressed HAGE at very low levels with the exception of testis. Interestingly, discrepancies were also found between mRNA analysis by real time quantitative PCR (RT-qPCR) and protein analysis by immunohistochemistry, emphasising the need to validate the expression of cancer/testis antigens at the protein level prior to the development of new vaccine strategies. HAGE is therefore proposed to be a valid candidate for designing a broad spectrum vaccine against cancer.
Keywords: human, cancer, HAGE, RT-PCR, immunohistochemistry
Introduction
There is an ever increasing need for new targets to treat cancer considering the limitations of some of the current therapies. It is therefore still very important to identify new immunogenic tumour-associated antigens (TAAs) that could be potentially targeted by immunotherapy in late stage diseases or in combination therapy with more traditional treatments at early stages to prevent tumour cell proliferation and invasion. The helicase antigen, HAGE, might represent such an antigen. It was first identified together with sarcoma antigen (SAGE) using representational difference analysis in a sarcoma cell line. cDNA clones were identified as tumour-specific with a pattern of expression very similar to the genes from the MAGE family (1). The HAGE gene was mapped to chromosome 6 (6q12-q13) by radiation hybrid analysis and encodes a putative 73 kDa protein. Analysis of the protein sequence of HAGE revealed that it has a DEAD box characteristic of the family of ATP-dependent RNA helicases. The sequence also displayed 55% homology with DDX5 (p68), another member of this family. RNA metabolism, control of cell cycle, spermatogenesis and embryogenesis are among the possible processes that HAGE may be involved in. However, little is known about its localisation or its precise function (1, 2).
Northern blot analysis showed that HAGE transcripts were present at a level that was 100-fold higher in a number of tumours of varying histological types compared to normal tissues, except testis (1). HAGE was later found to be over-expressed at the mRNA level in a small number of normal salivary glands but in a higher proportion in benign and malignant salivary gland neoplasms (3). HAGE is also over-expressed in more than 50% of chronic myeloid leukaemias (CMLs), in 20% of acute myeloid leukaemias (AMLs) (4) and more than 40% of multiple myelomas (5) and, although HAGE is present in a small numbers of lung cancers (1), there is no correlation between HAGE gene expression and clinicopathological factors, indicating that the detection of HAGE in this type of cancer has limited clinical utility (6). More recently, we showed that the frequency of HAGE-positive individual melanoma tissues reached 50% at the mRNA level, although only 20% of samples tested demonstrated a significant HAGE expression twice above testis level (7), a result in agreement with a previous publication (17%) by Scanlan et al. (8). We also showed for the first time HAGE expression at the protein level in several melanoma cell lines (7). Interestingly, a recent study has described the methylation status of the HAGE gene in CML patients and cell lines and showed that, like most cancer/testis antigens, hypomethylation of the HAGE gene promoter correlated with increased HAGE expression and that its expression was strongly associated with advanced disease and poor prognosis, suggesting a potential role of HAGE in increased cellular proliferation and/or survival and as a marker of disease progression (9).
Here, we have optimised and validated the specific immunohistochemical detection of HAGE at the protein level on in vitro and in vivo derived materials using murine tumour cells stably transfected to express the HAGE protein. Following optimisation, the techniques were applied to multiple normal and cancer tissue microarrays. For the first time, HAGE protein was detected at different levels in a variety of tumour tissues including bladder, brain, breast, colon, esophagus, kidney, liver, lung, stomach and small intestine among others, but not in normal tissues or at very low levels with the exception of testis. Considering the expression pattern and the diversity of HAGE expression in different tumours, HAGE may represent a suitable target for immunotherapy.
Results
Analysis of HAGE expression in murine lymphoma and human melanoma cell lines
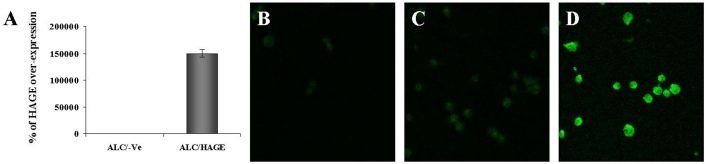
Full length HAGE cDNA was cloned into the pBudCE4.1 mammalian expression vector. HLA-A2-positive murine lymphoma cells (ALC) were grown in a 24-well plate and transfected with this plasmid using Lipofectamine 2000 reagent as per the manufacturer's instructions. Two days after transfection, cells were lysed for RNA extraction and HAGE cDNA amplification, or fixed, permeabilised and stained to determine HAGE expression by confocal analysis using a polyclonal antibody directed against a HAGE-derived peptide predicted to be antigenic in silico and produced in rabbits. RT-qPCR confirmed the expression of HAGE at the mRNA level in cells transfected with pBudCE4.1/HAGE but not in cells transfected with the empty vector pBudCE4.1/-Ve (Figure 1A). Secondary antibody tagged with fluorescein highlights the expression and localisation of HAGE within the cells. As can be seen from Figure 1, no HAGE protein expression was detected in ALC cells transiently transfected with pBudCE4.1/-Ve only, whereas cells transfected with pBudCE4.1/HAGE showed a significant level of HAGE protein expression ubiquitously found in the nucleus of the cells (Figure 1, panels B and D). However, ALC/HAGE cells incubated with rabbit control antibody and FITC-conjugated goat anti-rabbit antibody showed no staining above background (Figure 1C).
Figure 1.
HAGE expression in ALC cells determined by RT-qPCR and immunofluorescence. Efficiency of the transfection with the pBudCE4.1/HAGE plasmid was confirmed by RT-qPCR (A). Immunofluorescence was observed under a confocal microscope in ALC cells stably transfected with HAGE cDNA (D) but not in ALC cells stably transfected with the empty vector (B). No non-specific staining was obtained when ALC/HAGE cells were incubated with rabbit control and secondary antibodies (C). HAGE protein expression is mainly localised in the nucleus. Objective magnification: 50x.
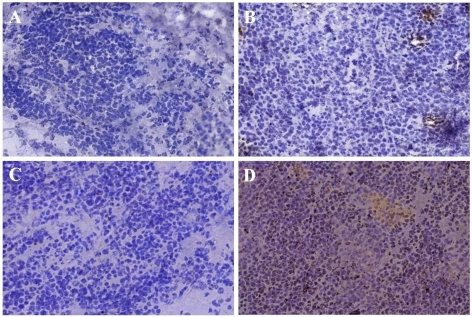
Murine lymphoma ALC cells were thereafter stably transfected with pBudCE4.1/-Ve or pBudCE4.1/HAGE using Lipofectamine 2000 and, following multiple passages in media containing the selective antibiotic zeocin, the resulting ALC/-Ve and ALC/HAGE cells were used in tumour transplantation experiments in HHDII-DR1 double transgenic mice in view of developing a tumour model for the design of cancer vaccines activating both cytotoxic and helper T cells. After determination of the tumorigenic dose rate 50 (TD50; 6 x 104 cells), ten times the TD50 was used in the in vivo tumour models. The in vivo expression of HAGE was then confirmed by performing immunohistochemistry on excised tumours. Whilst no significant staining in ALC/-Ve tumours was obtained (Figure 2C), ALC/HAGE tumours showed positive staining with the HAGE antibody in a vast majority of cells present in the tumour sections (Figure 2D). Sections incubated with isotype control did not demonstrate any staining above background for both ALC/-Ve and ALC/HAGE tumours (Figure 2, panels A and B).
Figure 2.
HAGE protein expression in ALC/-Ve and ALC/HAGE tumours determined by immunohistochemical staining. Immunohistochemistry demonstrated the in vivo expression of HAGE at the protein level in ALC/HAGE tumours (D), but not in ALC/-Ve tumours (C) excised from DR1/HHDII mice. No non-specific secondary staining was seen in both ALC/-Ve (A) and ALC/HAGE (B) tumours. HAGE expression was found in the cytoplasm and in the nucleus and appeared to be granular. Objective magnification: 20x.
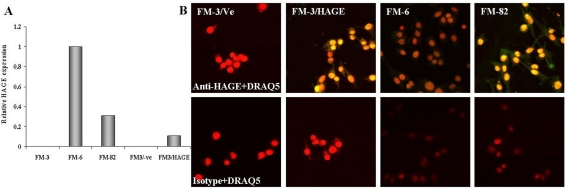
The human melanoma cell line, FM-3, was grown in a 24-well plate and stably transfected with either pcDNA3/-Ve or pcDNA3/HAGE using Lipofectamine 2000. Stable transfectants and two other HAGE-positive melanoma cell lines, FM-6 and FM-82, were lysed for RNA extraction and HAGE cDNA amplification, or fixed, permeabilised and stained for HAGE expression analysis by confocal microscopy using a polyclonal anti-HAGE antibody. RT-qPCR confirmed the expression of HAGE at the mRNA level in FM-3/HAGE, FM-6 and FM-82 cells but not in FM-3 cells transfected with the empty vector pcDNA3/-Ve (Figure 3A). Secondary antibody tagged with fluorescein highlights the expression and localisation of HAGE within the cells. As can be seen from Figure 3, no HAGE protein expression was detected in FM-3 cells transiently transfected with pcDNA3/-Ve only, whereas cells transfected with pcDNA3/HAGE showed a significant level of HAGE protein expression in the nucleus of the cells (Figure 3B). This result was confirmed in both HAGE-positive FM-6 and FM-82 cells (Figure 3B). FM-3/-Ve, FM-3/HAGE, FM-6 and FM-82 cells incubated with rabbit control antibody and FITC-conjugated goat anti-rabbit antibody showed no staining above background (Figure 3B).
Figure 3.
HAGE protein expression in human melanoma cell lines determined by immunofluorescence. Immunofluorescence was observed under a confocal microscope in the nucleus of a HAGE-negative FM-3 cell line transfected with HAGE cDNA (FM-3/HAGE) but not in a HAGE-negative FM-3 cell line transfected with an empty plasmid (FM-3/Ve). Immunofluorescence was also detected under a confocal microscope predominantly in the nucleus of two HAGE-positive melanoma cell lines, FM-6 and FM-82. Cells stained with a rabbit isotype control only showed staining from the DRAQ5 compound specific to the nucleus. Objective magnification: 50x.
Analysis of HAGE expression in normal tissues
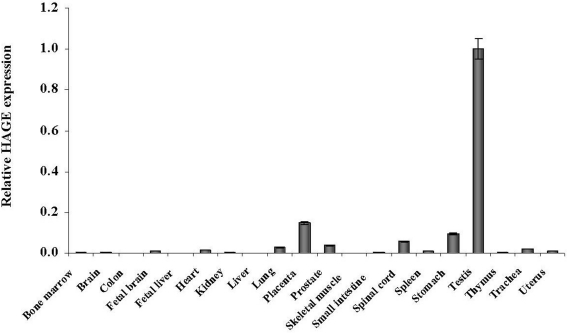
HAGE expression was then assessed in normal human tissues using real time quantitative RT-PCR. Primers for HAGE give a product size of 220 bp. 2 µg of RNA was used to reverse transcribe and generate cDNA using oligo(dT) primers. Real time PCR was performed for 40 cycles and relative gene expression was derived by calculating the 2δCt values using hypoxanthine guanine phosphoribosyltransferase 1 (HPRT1) as the housekeeping gene. As seen in Figure 4, relative HAGE expression proved to be undetectable or extremely low in all the normal tissues tested (0/19) when compared to placenta and testis.
Figure 4.
HAGE mRNA expression in human normal tissues determined by RT-qPCR. Real time quantitative PCR analysis was carried out on 20 human normal tissues. Data are expressed relative to the mRNA level of normal testis which was arbitrarily set as 1.
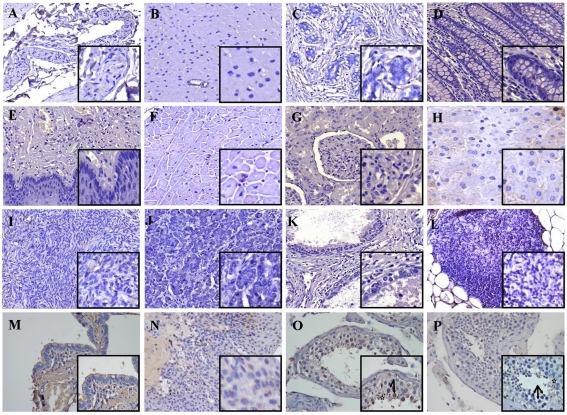
Similarly to the analysis performed on murine cells in vitro and ex vivo, the anti-human HAGE polyclonal antibody was then applied to multiple normal tissue microarrays (TMAs), which were stained for HAGE expression following antigen retrieval, antiserum incubation and diaminobenzidine tetrahydrochloride (DAB) staining. Sixteen healthy tissues coming from two donors were examined. Figure 4 demonstrates that whilst no significant (Figure 4, panels A-L) or weak (Figure 4, panels M and N) staining of normal tissues was observed (2/15), normal testis tissues showed positive staining with the antibody in the nucleus of spermatogonia and primary spermatocytes of both healthy donors (Figure 5O). No non-specific staining was obtained when multiple normal TMAs were incubated with isotype control and secondary antibody (Figure 5P). Therefore, protein expression was shown to correlate with the mRNA levels observed earlier by RT-qPCR.
Figure 5.
HAGE protein expression in multiple normal tissue microarrays determined by immunohistochemistry. Immunohistochemical staining demonstrated the in vivo expression of HAGE protein at a high level in testis (O), but not in bladder (A), brain (B), breast (C), colon (D), esophagus (E), heart (F), kidney (G), liver (H), ovaries (I), pancreas (J), prostate (K), thymus (L), lung (M) or uterus (N). No non-specific staining with a rabbit isotype control was obtained in normal testis tissues (P). Visible are spermatogonia (arrow) and mature sperm cells (star). Objective magnification: 40x (inset 100x).
Analysis of HAGE expression in tumour tissues
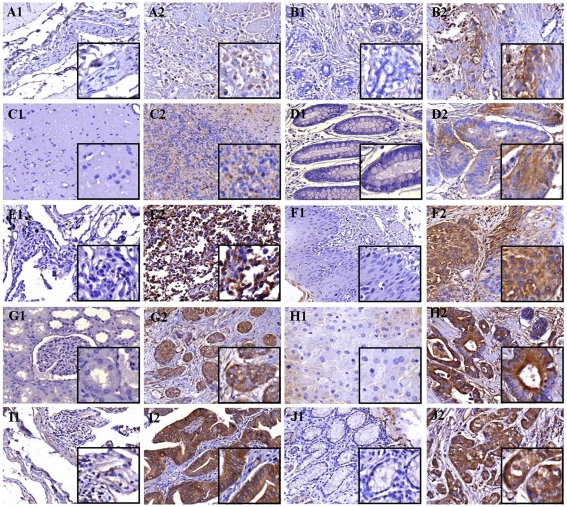
Previous studies have not only focused on the analysis of HAGE gene expression in haematological malignancies but also in solid tumours. Indeed, HAGE expression has been demonstrated to a lower proportion in brain, colon, lung and prostate cancers, among others, at the mRNA level (8). However, to the best of our knowledge, the presence of HAGE at the protein level in these cancers has never been confirmed. Here, immunohistochemistry was performed on multiple cancer TMAs in order to determine whether HAGE is also expressed at the protein level in a large variety of solid tumours. Of the 16 different cancer tissues examined, 10 of them demonstrated HAGE-positive staining (75%) in at least one patient out of four for each of these cancers. Figure 6 shows the in situ expression of HAGE in several cancers from different tissues including bladder (transitional cell carcinoma) and breast (invasive ductal carcinoma) at low protein level; brain (astrocytoma, stage II), colon (adenocarcinoma) and lung (small cell carcinoma) at intermediate protein level; esophagus (squamous cell carcinoma), kidney (clear cell carcinoma), liver (hepatocellular carcinoma), small intestine (papillary adenocarcinoma) and stomach (adenocarcinoma) at high protein level but not in their respective patient-matched normal tissues. In those tissues with a heterogeneous structure, HAGE expression was predominantly located in the glandular epithelium. Also, within the same malignant tissue, HAGE protein expression was quite heterogeneous and not all malignant acini were positive for HAGE. This was true within the same core of tissue and also between tissue cores (data not shown). No non-specific staining was obtained when multiple cancer TMAs were incubated with isotype control and secondary antibody (data not shown). Thus, HAGE appears to be expressed at higher levels in tumour cells than in normal cells and, in the majority of cancers, HAGE protein seems to be located in the cytoplasm when sections were examined at higher magnification.
Figure 6.
HAGE protein expression in multiple cancer tissue microarrays and patient-matched normal tissues determined by immunohistochemistry. Immunohistochemical staining demonstrated the in vivo expression of HAGE protein at a low level in bladder transitional cell carcinoma (A2) and breast invasive ductal carcinoma (B2); at an intermediate level in astrocytoma (C2), colon adenocarcinoma (D2) and lung squamous cell carcinoma (E2); at a high level in esophagus small cell carcinoma (F2), kidney clear cell carcinoma (G2), hepatocellular carcinoma (H2), small intestine papillary adenocarcinoma (I2) and stomach adenocarcinoma (J2) but not in their respective matched normal tissues (A1, B1, C1, D1, E1, F1, G1, H1, I1 and J1). Objective magnification: 40x (inset 100x).
Discussion
In the search for new TAAs which could be used as targets for anti-cancer therapies, it is important to assess their expression at both the mRNA and protein level, in both normal and cancer tissues. HAGE was initially identified by Martelange et al. (1), who applied the technique of cDNA subtraction to identify novel genes with tumour-specific expression. HAGE mRNA was found to be expressed by a wide range of tumour tissues at levels at least 100-fold that of normal tissues, with the exception of testis where this gene was shown to be highly expressed constitutively. Furthermore, expression of this gene could be induced with the demethylating agent, 5'-AZAC, a classical feature of cancer/testis genes (8). Therefore, the authors concluded that this gene is a member of the cancer/testis family and, based on its tumour-specific expression, may represent a potential target for immunotherapeutic vaccination against cancer. However, in previous reports, the expression of HAGE was not studied in a variety of normal tissues at the mRNA level using a more sensitive approach such as quantitative real time RT-PCR. This technique detected HAGE expression at very low levels in a wide array of 20 normal tissues including lung, prostate, spinal cord, stomach and trachea. Furthermore, HAGE expression was demonstrated to be at least 2 to 1,000-fold higher in placenta and testis than in any other normal tissue tested, hence validating previous studies using Northern blot or conventional RT-PCR analyses (1, 4).
However, these data are all restricted to the detection of HAGE at the messenger RNA level and discrepancies between mRNA and protein levels are commonly found due to factors such as the varying stability of different mRNA molecules, the regulatory process of translation, post-translational modifications and proteasomal degradation (10, 11). Therefore, to truly evaluate the expression of HAGE in normal and malignant tissues, analysis at the protein level was required. Using a monospecific and polyclonal antibody generated against a HAGE-derived peptide predicted in silico to be antigenic and exposed on the surface of the protein to the binding of an antibody, immunofluorescence and immunohistochemistry were respectively carried out on in vitro and in vivo material, as well as multiple normal and cancer TMAs. These results also provide further details regarding the protein's cellular and subcellular localisation.
We first set out to optimise anti-HAGE antibody concentration on HLA-A2-positive murine lymphoma ALC cells, which were transfected with HAGE cDNA for the development of an in vivo tumour model in HHDII-DR1 mice, and on human melanoma cell lines. ALC cells were first transiently and then stably transfected with pBudCE4.1/HAGE to express the HAGE protein. The in vitro and in vivo expression of HAGE was confirmed by immunofluorescence of transiently transfected in vitro cultured ALC cells and immunohistochemistry of stably transfected ALC/-Ve and ALC/HAGE cells grown in HHDII-DR1 mice to a size of 1 cm2 before excision. Transfection of ALC cells led to a predominant nuclear expression of HAGE in vitro and in vivo, with a characteristic and weak granular staining in the cytoplasm as shown by immunohistochemistry. Interestingly, both ALC/-Ve and ALC/HAGE cells were injected into HHDII-DR1 mice and the rate of growth of ALC/HAGE tumours (mean area of 0.76 cm2 ± 0.14 cm2) was significantly (P < 0.05) higher than ALC/-Ve tumours (mean area of 0.46 cm2 ± 0.14 cm2) 15 days after tumour transplantation. This preliminary result suggests that the transfected HAGE gene may be implicated in promoting tumour cell proliferation/survival in vivo, a finding that requires to be confirmed in a NOD/SCID mouse model. Furthermore, HAGE was also detected at both mRNA and protein level in HAGE-positive FM-3/HAGE, FM-6 and FM-82 cells but not in FM-3/-Ve cells. It is also noteworthy to mention that FM-6 cells showed the lowest expression at the protein level, i.e. the lowest fluorescence signal amongst all HAGE-positive cell lines in spite of expressing HAGE mRNA at a higher level than FM-3/HAGE or FM-82 cells, indicating that HAGE protein turnover might be shorter in FM-6 cells.
Upon antigen retrieval and application of the same protocol used for ALC tumours on paraffin-embedded multiple normal human TMAs, antibody staining allowed confirmation of the relative non-expression of the HAGE protein in all normal tissues tested with the exception of testis. Interestingly, immunohistochemistry did not detect HAGE at the protein level in normal stomach tissues from patients with gastric adenocarcinoma (Figure 6J) despite RT-qPCR detecting HAGE mRNA in normal stomach tissues at levels comparable to levels in placenta. Although the RNA was from a different source than the tissue sections, we believe these results emphasise the need to perform protein analysis rather than mRNA analysis in order to confirm the actual translation of a TAA mRNA into a protein before envisaging the use of such a TAA as a target for immunotherapy, as RT-qPCR results alone can be misleading (10, 11).
HAGE was previously shown to be over-expressed at the mRNA level at varying frequencies in various solid tumours such as bladder, brain, breast, colon, esophagus, liver, lung and skin (8). Using paraffin-embedded multiple cancer TMAs including patient-matched adjacent normal tissues, we extended these results to the protein expression of HAGE in various solid tumours. Bladder and breast cancer tissues demonstrated low staining of HAGE protein. Brain, colon, and lung cancer tissues had an intermediate level of staining for HAGE protein. Finally, esophagus, kidney, liver, small intestine and stomach cancer tissues showed a high level of staining for HAGE protein. Expression of HAGE could not be detected in patient-matched adjacent normal tissues. It is also worth considering that high protein expression is not necessarily a prerequisite for an effective anti-tumour CTL-mediated response. p53-specific CTLs were previously shown to be able to recognise tumour cells expressing moderate or low levels of p53, suggesting that in this case the protein turnover is more important for CTL-mediated killing than the actual steady-state level of expression (12).
Cancer/testis antigens represent one of the most promising groups of tumour-associated antigens identified to date because of their almost unique and specific expression in tumours, and are rightly investigated for the development of novel T cell-based immunotherapeutic strategies. The results of this study suggest that HAGE could be exploited as a target for T cell-based immunotherapy where the immune response would be predominantly directed against tumour cells with no serious risk of autoimmune reactions against adjacent healthy cells within the same organ. Importantly, the cytoplasmic expression of HAGE does not preclude its expression as a potential immunogenic HLA-linked peptide on the cell surface (13).
Not all tumour antigens are capable of eliciting specific immune responses and the immunogenicity of an antigen would be the deciding factor in validating HAGE as a potential immunotherapeutic target. As immunogenic MHC class I/II derived immunogenic peptides were previously identified (7), we would like to propose HAGE as a valid target for immunotherapy.
Abbreviations
- RT-qPCR
real time quantitative PCR
- TMA
tissue microarray
Acknowledgements
This work was supported by the John and Lucille van Geest Foundation. We also thank Prof. Bharat Jasani (Cardiff University, UK) for critical reading of the manuscript.
References
- 1.Martelange V, De Smet C, De Plaen E, Lurquin C, Boon T. Identification on a human sarcoma of two new genes with tumor-specific expression. Cancer Res. 2000;60:3848–3855. [PubMed] [Google Scholar]
- 2.Rocak S, Linder P. DEAD-box proteins: the driving forces behind RNA metabolism. Nat Rev Mol Cell Biol. 2004;5:232–241. doi: 10.1038/nrm1335. [DOI] [PubMed] [Google Scholar]
- 3.Nagel H, Laskawi R, Eiffert H, Schlott T. Analysis of the tumour suppressor genes, FHIT and WT-1, and the tumour rejection genes, BAGE, GAGE-1/2, HAGE, MAGE-1, and MAGE-3, in benign and malignant neoplasms of the salivary glands. Mol Pathol. 2003;56:226–231. doi: 10.1136/mp.56.4.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adams SP, Sahota SS, Mijovic A, Czepulkowski B, Padua RA, Mufti GJ, Guinn BA. Frequent expression of HAGE in chronic myeloid leukaemias. Leukemia. 2002;16:2238–2242. doi: 10.1038/sj.leu.2402732. [DOI] [PubMed] [Google Scholar]
- 5.Condomines M, Hose D, Raynaud P, Hundemer M, De Vos J, Baudard M, Moehler T, Pantesco V, Moos M, Schved JF, Rossi JF, Rème T, Goldschmidt H, Klein B. Cancer/testis genes in multiple myeloma: expression patterns and prognosis value determined by microarray analysis. J Immunol. 2007;178:3307–3315. doi: 10.4049/jimmunol.178.5.3307. [DOI] [PubMed] [Google Scholar]
- 6.Sasaki H, Moriyama S, Mizuno K, Yukiue H, Yano M, Fukai I, Yamakawa Y, Fujii Y. SAGE mRNA expression in advanced-stage lung cancers. Eur J Surg Oncol. 2003;29:900–903. doi: 10.1016/j.ejso.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Mathieu MG, Knights AJ, Pawelec G, Riley CL, Wernet D, Lemonnier FA, Straten PT, Mueller L, Rees RC, McArdle SE. HAGE, a cancer/testis antigen with potential for melanoma immunotherapy: identification of several MHC class I/II HAGE-derived immunogenic peptides. Cancer Immunol Immunother. 2007;56:1885–1895. doi: 10.1007/s00262-007-0331-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scanlan MJ, Simpson AJG, Old LJ. The cancer/testis genes: review, standardization, and commentary. Cancer Immun. 2004;4:1. http://www.cancerimmunity.org/v4p1/031220.htm [PubMed] [Google Scholar]
- 9.Roman-Gomez J, Jimenez-Velasco A, Agirre X, Castillejo JA, Navarro G, San Jose-Eneriz E, Garate L, Cordeu L, Cervantes F, Prosper F, Heiniger A, Torres A. Epigenetic regulation of human cancer/testis antigen gene, HAGE, in chronic myeloid leukemia. Haematologica. 2007;92:153–162. doi: 10.3324/haematol.10782. [DOI] [PubMed] [Google Scholar]
- 10.Chen G, Gharib TG, Huang CC, Taylor JM, Misek DE, Kardia SL, Giordano TJ, Iannettoni MD, Orringer MB, Hanash SM, Beer DG. Discordant protein and mRNA expression in lung adenocarcinomas. Mol Cell Proteomics. 2002;1:304–313. doi: 10.1074/mcp.m200008-mcp200. [DOI] [PubMed] [Google Scholar]
- 11.Rogel A, Popliker M, Webb CG, Oren M. p53 cellular tumor antigen: analysis of mRNA levels in normal adult tissues, embryos, and tumors. Mol Cell Biol. 1985;5:2851–2855. doi: 10.1128/mcb.5.10.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vierboom MP, Zwaveling S, Bos GMJ, Ooms M, Krietemeijer GM, Melief CJ, Offringa R. High steady-state levels of p53 are not a prerequisite for tumor eradication by wild-type p53-specific cytotoxic T lymphocytes. Cancer Res. 2000;60:5508–5513. [PubMed] [Google Scholar]
- 13.Kloetzel PM. The proteasome and MHC class I antigen processing. Biochim Biophys Acta. 2004;1695:225–233. doi: 10.1016/j.bbamcr.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Gritzapis AD, Mahaira LG, Perez SA, Cacoullos NT, Papamichail M, Baxevanis CN. Vaccination with human HER-2/neu (435-443) CTL peptide induces effective antitumor immunity against HER-2/neu-expressing tumor cells in vivo. Cancer Res. 2006;66:5452–5460. doi: 10.1158/0008-5472.CAN-05-4018. [DOI] [PubMed] [Google Scholar]
Materials and methods
Animals
Mouse class I (H-2) and class II (IA/IE) double knockout C57BL/6 animals transgenic for human HLA-A2.1/-DR1 (HHDII-DR1) were received as a generous gift from Dr. F. Lemonnier (Institut Pasteur, Paris). Colonies were bred at Nottingham Trent University animal house in accordance with the UK Home Office Codes of Practice for the housing and care of animals.
Cell lines, transfection assays and injection procedures
The HAGE-positive melanoma cell lines, FM-6 and FM-82, were cultured in RPMI1640 media supplemented with 10% fetal calf serum (FCS) and 5 mM L-glutamine. The pcDNA3 mammalian expression plasmid incorporating the entire HAGE cDNA sequence and an ampicillin/neomycin resistance cassette was kindly provided by Prof. Thierry Boon (Ludwig Institute for Cancer Research, Belgium). The HAGE-negative melanoma cell line, FM-3, was seeded at 6 x 105 cells per well in a 24-well plate (Sarstedt). The cells were then transfected overnight using Lipofectamine 2000 (Invitrogen) (1.2 µg pcDNA3/-Ve or 1.2 µg pcDNA3/HAGE) and then washed with fresh media containing 500 µg/ml G418 (Cambrex). Stable transfectants were made by plating 100 cells per well in 200 µl of selective medium in a 96-well plate. Upon repeated splitting in 96-well plates in selective medium, stable FM-3/-Ve and FM-3/HAGE cells were obtained, bulked up and stored in liquid nitrogen until required.
The murine lymphoma cell line ALC/A2 (H-2b) (a generous gift from Dr. Constantin Baxevanis, Cancer Immunology and Immunotherapy Centre, Athens), which is transgenic for the HLA-A2.1 class I molecule, was cultured with RPMI1640 supplemented with 10% FCS, 5 mM L-glutamine and 500 µg/ml G418 (Cambrex) (14). The entire open reading frame of HAGE was cloned from the pcDNA3 vector into the HindIII and XbaI sites of the pBudCE4.1 mammalian expression vector (Invitrogen) containing a zeocin selection marker. Using a similar protocol as described above, stable transfectants were made with either 1.2 µg pBudCE4.1/-Ve or 1.2 µg pBudCE4.1/HAGE in selective media containing 50 µg/ml zeocin (Invitrogen), bulked up and stored in liquid nitrogen until required.
HHDII/DR1 mice were injected with 6 x 105 ALC/A2/-Ve or ALC/A2/HAGE cells. Animals were monitored three times a week for tumour development and size, and were sacrificed when tumours reached a size of 1 cm2 according to the Home Office guidelines.
Real time quantitative PCR
Samples of mRNA from normal tissues were purchased from Clontech. Reverse transcription using the M-MLV reverse transcriptase kit (Promega) was performed with Oligo(dT)15 according to the manufacturer's protocol. Real-time PCR was carried out using primers specific for HAGE cDNA according to the GenBank entry NM_018665 (HAGE-496 5'-GGAGATCGGCCATTGATAGA-3', HAGE-716 5'-GGATTGGGGATAGGTCGTTT-3'; product size 221 bp), for 40 cycles of 95˚C, 64.5˚C and 72˚C, using a Sybr Green Supermix (BioRad) and a hot start procedure in a Rotagene real time PCR cycler (Corbett). This pair of primers does not hybridise to cDNA encoded by the DDX5 (p68) gene which shares 55% homology with the HAGE gene. The PCR product was sequenced (Eurofins DNA) and determined to be 100% homologous to HAGE cDNA. The HPRT1 gene was used as housekeeping gene to calculate the 2δCt values indicative of the relative expression of our gene of interest, HAGE, in normal human tissues.
Antibodies
Monospecific and polyclonal anti-human HAGE antibody was produced by Pacific Immunology (USA) following sequential immunisation of rabbits in complete Freund's adjuvant (1x) and incomplete Freund's adjuvant (3x) with a HAGE-derived 16-mer coupled to a carrier protein (KLH) via a cysteine that has been added to the N-terminal of the peptide and determined to be immunogenic by in silico analysis (HAGE 517: C-LHGDREQRDREKALEN). The chosen peptide had no homology with highly homologous proteins such as DDX5. Upon serum purification of IgG, this antibody was used in immunofluorescence and immunohistochemistry. A rabbit polyclonal antibody IgG (Affinity BioReagents, USA) was used as an isotype control. Goat anti-rabbit FITC and goat anti-rabbit biotin were obtained from Dako (USA).
Immunofluorescence
Murine lymphoma and human melanoma cells were plated in an eight-chamber slide (BD Biosciences) at a concentration of 1 x 104 cells per well. After overnight incubation at 37˚C, 5% CO2, cells were fixed and permeabilised at room temperature using a solution of 1% (w/v) paraformaldehyde (applied for 10 minutes) and 70% (v/v) ethanol (applied for 10 minutes), respectively. Cells were washed and then stained with 4 µg/ml HAGE-specific rabbit polyclonal anti-serum and 10 µg/ml fluorescein-conjugated goat anti-rabbit Ig with the slides placed on ice. After multiple washes in PBS [0.1% (w/v) BSA, 0.02% (w/v) NaN3], murine lymphoma cells were left to dry at room temperature and prepared with fluorescent mounting media (Dako). Human melanoma cells were washed in PBS [0.1% (w/v) BSA, 0.02% (w/v) NaN3]. Their nuclei were stained with 2.5 mM of the nuclear DNA dye, DRAQ5 (Biostatus Ltd), for 30 minutes at room temperature (RT) and the cells were then left to dry at room temperature and prepared with fluorescent mounting media (Dako). Cells were observed by confocal microscopy using a Leica TCS-NT microscope and captured using Leica TCS-NT software (50x magnification).
Immunohistochemistry
Paraffin-embedded multiple normal and tumour human tissue microarrays (TMAs) were purchased from US Biomax (USA). Tumours (1 cm2) were excised from HHDII/DR1 mice and embedded in OCT media, fixed in a cold solution of 2-butanol (Sigma) and sectioned using a cryostat CM1900 (Leica). Prior to immunohistochemistry, antigen retrieval was carried out on paraffin-embedded tissues. Briefly, sections were de-waxed in xylene and re-hydrated in graded ethanol [100%, 100% and 70% (v/v)]. Slides were consecutively rinsed in tap water and in ddH2O before being heated up in 0.01 M citrate buffer pH 6.0 for 10 minutes in the microwave. Following this stage, both frozen and paraffin-embedded tissues underwent the same protocol. 0.03% (v/v) H202 diluted in 1x PBS was added to the tissue sections for 5 minutes before being washed with PBS. Sections were then blocked for 10 minutes with 10% (v/v) goat serum. Following incubation, the serum was removed and 4 µg/ml of rabbit anti-HAGE or rabbit isotype control were added for overnight incubation at 4˚C. Slides were then washed in 1x PBS and 10 µg/ml of biotin-conjugated goat anti-rabbit IgG was added. Slides were incubated for 30 minutes at RT and washed thoroughly with 1x PBS. The ABC reagent (Vector laboratories) was laid onto the slides according to the manufacturer's protocol, left to react with secondary antibodies for 30 minutes at RT and washed off with PBS. The DAB reagent (Vector laboratories) was added to the sections for 10 minutes at RT following the manufacturer's instructions in order to react with the ABC reagent and was then washed off with ddH2O. Finally, frozen and paraffin-embedded sections were counterstained in Gill's and Harris' staining solutions, respectively, and fixed consecutively in graded ethanol [70%, 100%, 100% (v/v)] and in xylene. Slides were mounted, air-dried and captured using Mirax Scan (Zeiss). Virtual slides were then analysed under (40x) and (100x) magnification.