Abstract
Melanoma patients treated with anti-CTLA-4 have shown a range of anti-tumor responses. In this report, we describe the response of a single patient to anti-CTLA-4, with individual lesions disappearing, others stabilizing, and others progressing. These responses can be viewed as a clear manifestation of cancer immunoediting and its three phases of elimination, equilibrium and escape, with each tumor in this patient being at a discrete stage in the process. The patient's course and associated immunological monitoring and other laboratory data are presented in an immunogram, a way to visualize temporal associations between the multiple clinical and laboratory parameters.
Keywords: human, melanoma, ipilimumab, case report, immunogram, immunoediting
Introduction
Cytotoxic T lymphocyte antigen 4 (CTLA-4) is a co-inhibitory molecule expressed by activated T cells and a subset of regulatory T cells (1-3). CTLA-4 is of primary importance in maintaining immune homeostasis by down-regulating T cell signaling to inhibit the CD28-B7 costimulatory pathway, limiting T cell responses and contributing to tolerance to self-antigens (4, 5). Therefore, blockade of CTLA-4 is thought to prevent down-regulation of T cells and can potentiate immune responses against antigens expressed on tumor cells (6-9).
A CTLA-4 blocking antibody has shown highly promising clinical results in patients with melanoma (10), and large scale clinical trials of anti-CTLA-4 are underway in patients with other tumor types. The challenge now is to understand the basis for the anti-tumor response to anti-CTLA-4 and to identify correlates, both immunological and non-immunological, that are associated with, and predictive of, responses. Given the multiple parameters and data points coming from such an intense analysis, there is a need to present the information in a way that facilitates visual integration of the data. For this purpose, we have developed a display we call an immunogram, and use it to detail the response of an anti-CTLA-4 treated patient that clearly illustrates the process of cancer immunoediting in a single patient.
Results
Treatment history
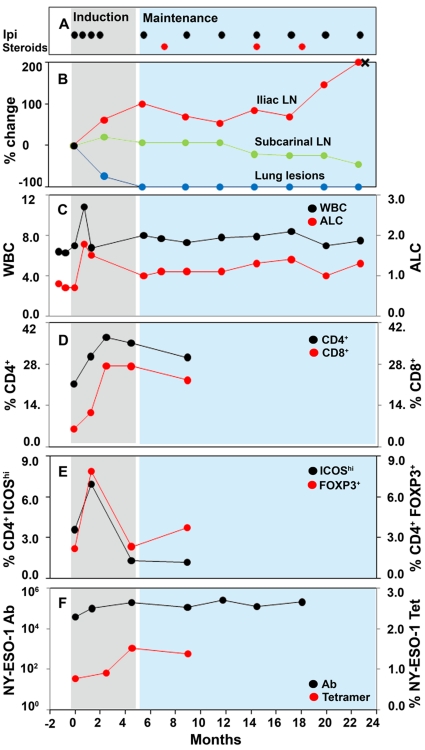
Patient IMF-16 is a 44 year-old woman with melanoma metastatic to bone, lungs and lymph nodes, previously treated with primary resection, temozolomide and resection of bone metastasis. Ipilimumab therapy was initiated in December 2006 as part of protocol CA184-008 using four induction doses of ipilimumab given at 10 mg/kg every 3 weeks x 4. At the first tumor assessment at week 12, the patient experienced a mixed response in evaluable lesions with overall stable disease according to modified World Health Organization (mWHO) criteria. She then continued to receive maintenance ipilimumab and, at her last follow-up 28 months after initiating therapy, demonstrated control of her disease (Figure 1, panel A). Ipilimumab therapy was complicated by grade 2 rash and pruritus, requiring intermittent low-dose oral corticosteroids which did not alter the durability of the clinical response.
Figure 1.
Immunogram of patient IMF-16. WBC and ALC are graphed in ×103/mm3 units; NY-ESO-1 titers are graphed in log10 as an inverse titer.
Response pattern
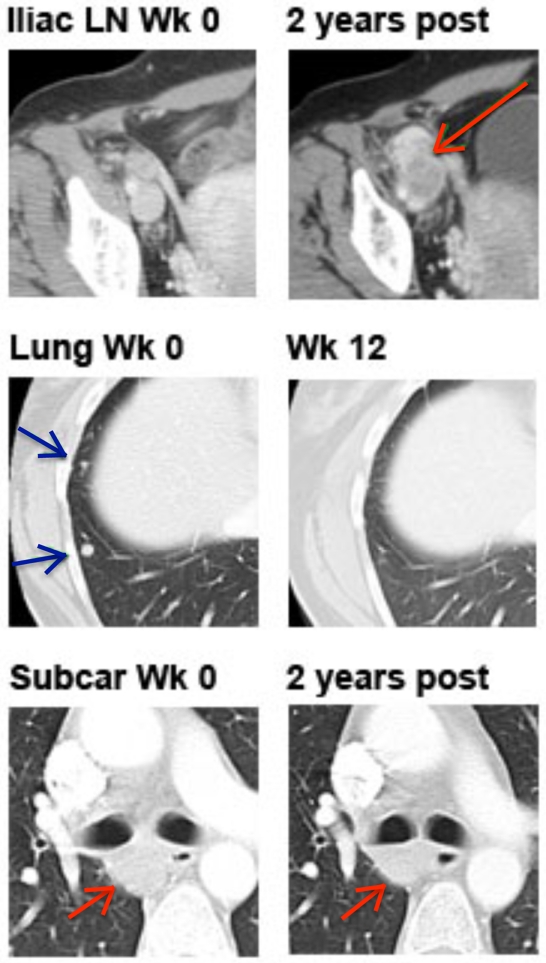
By classical response criteria such as mWHO, IMF-16 would be classified as having stable disease at weeks 12 and 24. However, analysis of individual lesions by computer tomography (CT) demonstrates a heterogeneous response, with complete resolution of lung lesions, stable subcarinal lymphadenopathy and disappearance of bone metastases, but progressive disease in the right pelvic lymph nodes (Figure 2). The patient underwent resection of the progressive iliac nodes 21 months after starting ipilimumab, which confirmed metastatic melanoma (represented by an X in Figure 1, panel B).
Figure 2.
Radiographic images of target lesions of patient IMF-16. Representative computer tomography (CT) images of IMF-16 demonstrating complete response in right lung lesions, progressive disease in right external iliac lymph nodes, and essentially stable subcarinal lymph nodes.
Changes in absolute lymphocyte counts (ALC) and white blood cell counts (WBC)
There are emerging data suggesting that changes in the ALC that occur during ipilimumab therapy may be predictive of clinical outcome. Data presented in abstract form suggest that higher rates of change in the ALC with ipilimumab therapy may be associated with a greater rate of clinical benefit (defined as the sum of complete responses, partial responses and stable disease), while patients whose ALC declines during ipilimumab therapy derive no clinical benefit (11). Similarly, our group has observed that patients with an ALC of 1,000/mm3 or more after two ipilimumab treatments (at week 7) have a significantly higher clinical benefit rate and longer median overall survival than patients with an ALC <1,000/mm3 (12). Consistent with these observations, IMF-16 experienced a rapid and sustained increase in ALC above 1,000/mm3. Of note, the patient's baseline ALC was <1,000/mm3 despite the fact that she had not received chemotherapy in many months, possibly a reflection of underlying immunosuppression from her metastatic melanoma. Further characterization of these lymphocytes revealed that the number of both CD4+ and CD8+ cells increased with ipilimumab therapy (Figure 1, panel D), highlighting the effect of CTLA-4 blockade in both lineages. However, intratumoral CD4+ and CD8+ T cell distribution may not be correlated with peripheral levels and needs to be assessed directly (13).
Changes in ICOS and FOXP3 expression on CD4+ cells
ICOS is a T cell-specific surface molecule that is structurally related to CD28 and CTLA-4 (14, 15). Unlike CD28 which is constitutively expressed, ICOS is upregulated on T cells only after activation. In a small pre-operative trial, six patients with localized bladder cancer received preoperative ipilimumab prior to cystectomy (16). All six patients exhibited an increase in the percentage of CD4+ ICOShi cells in the peripheral blood and tumor tissue after ipilimumab therapy. These CD4+ ICOShi cells had increased production of interferon (IFN)-γ compared to CD4+ ICOShi cells from untreated patients or healthy donors. The ratio of CD4+ ICOShi cells to regulatory T cells (Tregs, CD4+ FOXP3+)(17) after ipilimumab therapy was consistently increased in the peripheral blood and tumor microenvironment. From these findings, it was postulated that CD4+ ICOShi cells may have effector functions. Based on these data, we performed multiparameter flow cytometry on PBMC samples obtained around the period of induction therapy with ipilimumab. Patient IMF-16 exhibited a transient increase in ICOShi and FOXP3+ cells following induction dosing (Figure 1, panel E), which is consistent with the hypothesis that ipilimumab expands both Treg and Teff cells peripherally (18).
Changes in NY-ESO-1 antibody titers and tetramer-reactive CD8+ cells
NY-ESO-1 is a cancer/testis antigen that is expressed in a variety of human malignancies but not in normal tissues except for testis and placenta (19). NY-ESO-1 is highly immunogenic and elicits spontaneous antibody and CD4+ and CD8+ T cell responses in cancer patients; an immune response to NY-ESO-1 is seen at a high frequency in patients with advanced NY-ESO-1-expressing tumors, including melanomas (20). Intriguingly, humoral and cellular immune responses tend to occur in an integrated fashion such that one seems to be dependent on the other (21, 22). Our group previously evaluated NY-ESO-1 antibody and T cell responses in 15 melanoma patients receiving ipilimumab (23). We confirmed the concordance between NY-ESO-1 seropositivity and T cell responses and also noted that NY-ESO-1 T cell responses increased with ipilimumab therapy.
As one of the patients included in the above analysis, patient IMF-16 is illustrative of our findings (Figure 1, panel F). At baseline, she had positive NY-ESO-1 antibody titers (defined as a reciprocal titer of >100), which increased with ipilimumab therapy. Similarly, she also had baseline detectable NY-ESO-1 tetramer-reactive CD8+ cells after 10 days of in vitro culture with NY-ESO-1 overlapping peptides. These tetramer-reactive CD8+ cells increased in frequency with ipilimumab therapy.
In situ tumor monitoring
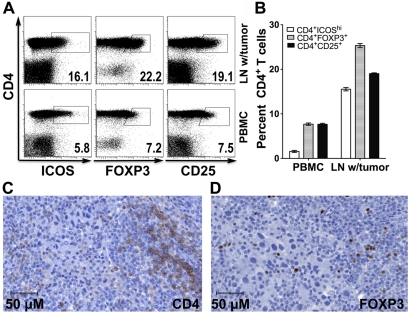
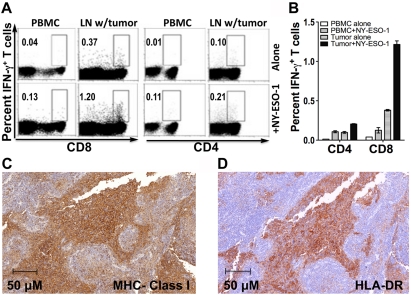
Tissue − consisting of lymph node infiltrated by metastatic melanoma − from the resection of patient IMF-16's right external iliac lymph nodes and fresh PBMCs were obtained for immune analyses. CD4+ cells from the tumor tissue and from PBMCs were analyzed by flow cytometry for CD25 and FOXP3 expression (Figure 3, panels A and B) or by immunohistochemical staining for CD4 and FOXP3 expression (Figure 3, panels C and D). Compared to CD4+ cells from the peripheral blood, CD4+ cells in the tumor tissue had higher expression of FOXP3 and CD25, suggestive of a higher proportion of Tregs. In addition, lymphocytes from the tumor tissue and PBMCs underwent ex vivo stimulation overnight with overlapping NY-ESO-1 peptides before undergoing intracellular cytokine staining (ICS) (Figure 4, panels A and B). This revealed a higher percentage of NY-ESO-1 specific CD8+ IFN-γ+ cells in the tumor tissue than in the peripheral blood, again possibly consistent with the notion of a concentration of NY-ESO-1-specific T cells at the tumor site. Immunohistochemical staining showed that this lymph node metastatic tumor expressed MHC class I and HLA-DR (Figure 4, panels C and D).
Figure 3.
CD4+ CD25+ and CD4+ FOXP3+ Tregs in peripheral blood and progressive right inguinal lymph node in patient IMF-16. CD4+ cells from the tumor tissue and from PBMCs were analyzed by flow cytometry and immunohistological staining for CD25 and FOXP3 expression. (A) Representative dot plots. (B) Compiled data. (C) CD4 immunohistochemical staining (clone BC/1F6). (D) FOXP3 immunohistochemical staining (clone 236A/E7).
Figure 4.
NY-ESO-1 antigen-specific CD8+ IFN-γ+ and CD4+ IFN-γ+ responses. The IFN-γ gate was set based on a negative control (alone) sample and applied across other samples (+NY-ESO-1). (A) Representative dot plots. (B) Compiled data. (C) MHC class I immunohistochemical staining (clone A4). (D) HLA-DR immunohistochemical staining (clone YE2/36HLK).
Cancer/testis antigen expression on a progressive lymph node with tumor
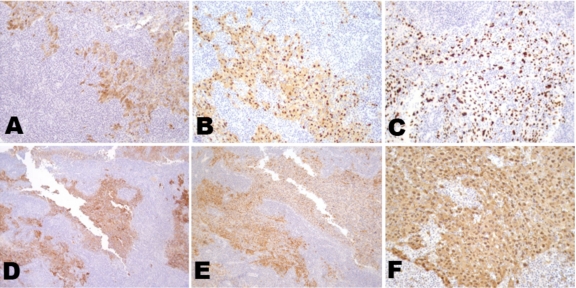
Immunohistochemical staining of a lymph node metastasis was performed for additional cancer/testis antigens. Extensive immunoreactivity was seen with mAb MA454 (to MAGE-A1), mAb M3H67 (to several MAGE-A antigens), mAb CT10-5 (to CT10/MAGE-C2), mAb E978 (to NY-ESO-1), mAb CT7-33 (to CT7/MAGE-C1), and mAb #26 (to GAGE) (Figure 5).
Figure 5.
Cancer/testis antigen expression on a progressive lymph node with tumor. Immunohistochemical staining of a lymph node metastasis for several cancer/testis antigens. Extensive immunoreactivity was seen with monoclonal antibodies (A) MA454 (to MAGE-A1), (B) M3H67 (to several MAGE-A antigens), (C) CT10-5 (to CT10/MAGE-C2), (D) E978 (to NY-ESO-1), (E) CT7-33 (to CT7/MAGE-C1), and (F) #26 (to GAGE).
Discussion
The immunogram that we introduce in this brief report was developed to provide a temporal survey of laboratory and clinical data from an individual patient. The immunogram for patient IMF-16 validates many previously described conclusions coming from the clinical studies of anti-CTLA-4 antibody therapy. For example, the immunogram clearly demonstrates that: responses occur despite corticosteroid treatment (24, 25), responses may be atypical compared to conventional cytotoxic therapy (26), response correlates with early increases in ALC (11, 12), anti-CTLA-4 therapy expands both Teff and Treg cells, and response may be correlated with NY-ESO-1-specific T cell and B cell activity (22, 23).
The immunogram of IMF-16 also clearly demonstrates the clinical reality of "immunoediting", advanced by Dunn, Old and Schreiber several years ago (27). This concept suggests a dynamic host-tumor relationship whereby the host immune system recognizes and eliminates incipient cancers, yet exerts a Darwinistic pressure that selects for tumor variants with low or absence of immunogenicity. This dynamic relationship results in tumor elimination, tumor escape, or a meta-stable equilibrium which may persist indefinitely. Three lesion sites in IMF-16 exemplify these three "Es" of immunoediting: the elimination of lung lesions, the equilibrium of a subcarinal lymph node and the escape of right pelvic lymph nodes.
Extending the immunological analysis to tissues specimens e.g. lymph node, tumor, ("in situ immunology") rather than limiting it to the peripheral blood compartment is essential for a comprehensive view of the immune response to cancer. We have obtained a progressive lymph node from patient IMF-16 (Figure 3). Compared to PBMCs, CD4+ cells in the tumor tissue had higher expression of FOXP3, CD25 and ICOS, suggestive of greater proportions of both Teffs and Tregs. ICS analysis of ex vivo NY-ESO-1 overlapping peptide-stimulated PBMCs and tumor-infiltrating lymphocytes revealed more NY-ESO-1-specific CD8+ IFN-γ+ cells in the tumor tissue than in the peripheral blood, implying functional antigen recognition at the tumor site (Figure 4, panels A and B). Immunohistochemical tumor staining demonstrates positivity for NY-ESO-1 antigen, MHC class I and HLA-DR (Figure 4, panels C and D). Taken together, these data suggest that the "escape" mechanism may be related to Tregs or other immunosuppression mechanisms, rather than loss of MHC expression and antigen recognition.
A correlation between NY-ESO-1 immunity and response to anti-CTLA-4 therapy has been observed in metastatic melanoma patients (23) and recently in patients with prostate cancer (28, 29). Is NY-ESO-1 immunity involved in the anti-tumor effects of anti-CTLA-4 or is it simply an associated non-related event? And are responses to other tumor antigens correlated with anti-CTLA-4 therapeutic effects? These associations should be further investigated in large scale trials and other anti-CTLA-4 therapy settings.
The immunogram provides a useful approach to the comparative analysis of laboratory and clinical data and should be helpful in clarifying the role of immunity in the anti-tumor effects of anti-CTLA-4 therapy and other immunotherapeutic approaches to cancer.
Abbreviations
- ALC
absolute lymphocyte counts
Acknowledgements
This work was supported by NIH P01CA33049, Swim Across America, the Experimental Therapeutics Center of MSKCC and Ludwig Trust. JDW was supported by a Damon Runyon-Lilly Clinical Investigator Award and the Melanoma Research Alliance. JY was supported by Memorial Sloan-Kettering Cancer Center Pilot Grant number P50AT002779 from the National Center for Complementary and Alternative Medicine (NCCAM) and the Office of Dietary Supplements (ODS).
References
- 1.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 3.Walunas TL, Bakker CY, Bluestone JA. CTLA-4 ligation blocks CD28-dependent T cell activation. J Exp Med. 1996;183:2541–2550. doi: 10.1084/jem.183.6.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunner MC, Chambers CA, Chan FK, Hanke J, Winoto A, Allison JP. CTLA-4-Mediated inhibition of early events of T cell proliferation. J Immunol. 1999;162:5813–5820. [PubMed] [Google Scholar]
- 5.Karandikar NJ, Vanderlugt CL, Walunas TL, Miller SD, Bluestone JA. CTLA-4: a negative regulator of autoimmune disease. J Exp Med. 1996;184:783–788. doi: 10.1084/jem.184.2.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190:355–366. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maker AV, Attia P, Rosenberg SA. Analysis of the cellular mechanism of antitumor responses and autoimmunity in patients treated with CTLA-4 blockade. J Immunol. 2005;175:7746–7754. doi: 10.4049/jimmunol.175.11.7746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quezada SA, Peggs KS, Curran MA, Allison JP. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest. 2006;116:1935–1945. doi: 10.1172/JCI27745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med. 2009;206:1717–1725. doi: 10.1084/jem.20082492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L, Waterfield W, Schadendorf D, Smylie M, Guthrie T Jr, Grob JJ, Chesney J, Chin K, Chen K, Hoos A, O'Day SJ, Lebbé C. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. doi: 10.1016/S1470-2045(09)70334-1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Berman DM, Wolchok J, Weber J, Hamid O, O'Day S, Chasalow SD. Association of peripheral blood absolute lymphocyte count (ALC) and clinical activity in patients (pts) with advanced melanoma treated with ipilimumab [abstract].; J Clin Oncol (ASCO Annual Meeting); 2009. p. 3020. [Google Scholar]
- 12.Ku GY, Yuan J, Page DB, Schroeder SEA, Panageas KS, Carvajal RD, Chapman PD, Schwartz GK, Allison JP, Wolchok JD. Compassionate-use trial of ipilimumab in patients with advanced melanoma: lymphocyte count after two doses correlates with survival. Cancer. 2009 doi: 10.1002/cncr.24951. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wada H, Sato E, Uenaka A, Isobe M, Kawabata R, Nakamura Y, Iwae S, Yonezawa K, Yamasaki M, Miyata H, Doki Y, Shiku H, Jungbluth AA, Ritter G, Murphy R, Hoffman EW, Old LJ, Monden M, Nakayama E. Analysis of peripheral and local anti-tumor immune response in esophageal cancer patients after NY-ESO-1 protein vaccination. Int J Cancer. 2008;123:2362–2369. doi: 10.1002/ijc.23810. [DOI] [PubMed] [Google Scholar]
- 14.Dong C, Juedes AE, Temann UA, Shresta S, Allison JP, Ruddle NH, Flavell RA. ICOS co-stimulatory receptor is essential for T cell activation and function. Nature. 2001;409:97–101. doi: 10.1038/35051100. [DOI] [PubMed] [Google Scholar]
- 15.Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I, Kroczek RA. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397:263–266. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- 16.Liakou CI, Kamat A, Tang DN, Chen H, Sun J, Troncoso P, Logothetis C, Sharma P. CTLA-4 blockade increases IFNgamma-producing CD4+ICOShi cells to shift the ratio of effector to regulatory T cells in cancer patients. Proc Natl Acad Sci U S A. 2008;105:14987–14992. doi: 10.1073/pnas.0806075105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 18.Kavanagh B, O'Brien S, Lee D, Hou Y, Weinberg V, Rini B, Allison JP, Small EJ, Fong L. CTLA4 blockade expands FoxP3+ regulatory and activated effector CD4+ T cells in a dose-dependent fashion. Blood. 2008;112:1175–1183. doi: 10.1182/blood-2007-11-125435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simpson AJ, Caballero OL, Jungbluth A, Chen YT, Old LJ. Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer. 2005;5:615–625. doi: 10.1038/nrc1669. [DOI] [PubMed] [Google Scholar]
- 20.Gnjatic S, Nishikawa H, Jungbluth AA, Güre AO, Ritter G, Jäger E, Knuth A, Chen YT, Old LJ. NY-ESO-1: review of an immunogenic tumor antigen. Adv Cancer Res. 2006;95:1–30. doi: 10.1016/S0065-230X(06)95001-5. [DOI] [PubMed] [Google Scholar]
- 21.Gnjatic S, Atanackovic D, Matsuo M, Jäger E, Lee SY, Valmori D, Chen YT, Ritter G, Knuth A, Old LJ. Cross-presentation of HLA class I epitopes from exogenous NY-ESO-1 polypeptides by nonprofessional APCs. J Immunol. 2003;170:1191–1196. doi: 10.4049/jimmunol.170.3.1191. [DOI] [PubMed] [Google Scholar]
- 22.Jäger E, Gnjatic S, Nagata Y, Stockert E, Jäger D, Karbach J, Neumann A, Rieckenberg J, Chen YT, Ritter G, Hoffman E, Arand M, Old LJ, Knuth A. Induction of primary NY-ESO-1 immunity: CD8+ T lymphocyte and antibody responses in peptide-vaccinated patients with NY-ESO-1+ cancers. Proc Natl Acad Sci U S A. 2000;97:12198–12203. doi: 10.1073/pnas.220413497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuan J, Gnjatic S, Li H, Powel S, Gallardo HF, Ritter E, Ku GY, Jungbluth AA, Segal NH, Rasalan TS, Manukian G, Xu Y, Roman RA, Terzulli SL, Heywood M, Pogoriler E, Ritter G, Old LJ, Allison JP, Wolchok JD. CTLA-4 blockade enhances polyfunctional NY-ESO-1 specific T cell responses in metastatic melanoma patients with clinical benefit. Proc Natl Acad Sci U S A. 2008;105:20410–20415. doi: 10.1073/pnas.0810114105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amin A, DePril V, Hamid O, Wolchok J, Maio M, Neyns B, Chin K, Ibrahim R, Hoos A, O'Day S. Evaluation of the effect of systemic corticosteroids for the treatment of immune-related adverse events (irAEs) on the development or maintenance of ipilimumab clinical activity [abstract].; J Clin Oncol (ASCO Annual Meeting); 2009. p. 9037. [Google Scholar]
- 25.Downey SG, Klapper JA, Smith FO, Yang JC, Sherry RM, Royal RE, Kammula US, Hughes MS, Allen TE, Levy CL, Yellin M, Nichol G, White DE, Steinberg SM, Rosenberg SA. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res. 2007;13:6681–6688. doi: 10.1158/1078-0432.CCR-07-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saenger YM, Wolchok JD. The heterogeneity of the kinetics of response to ipilimumab in metastatic melanoma: patient cases. Cancer Immun. 2008;8:1. http://www.cancerimmunity.org/v8p1/080102.htm [PMC free article] [PubMed] [Google Scholar]
- 27.Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–360. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 28.Yuan J, Gnjatic S, Ku G, Jefferson M, Tandon S, Ritter E, Rasalan TS, Orlandi F, Li H, Gallardo HF, Xu YY, Ritter G, Scher H, Lowy I, Old LJ, Wolchok JD, Slovin SF, Allison JP. NY-ESO-1 specific responses in patients with advanced prostate cancer treated with ipilimumab [abstract].; International Society for Biological Therapy of Cancer Annual Meeting; 2009. [Google Scholar]
- 29.Fong L, Kwek SS, O'Brien S, Kavanagh B, McNeel DG, Weinberg V, Lin AM, Rosenberg J, Ryan CJ, Rini BI, Small EJ. Potentiating endogenous antitumor immunity to prostate cancer through combination immunotherapy with CTLA4 blockade and GM-CSF. Cancer Res. 2009;69:609–615. doi: 10.1158/0008-5472.CAN-08-3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barrow C, Browning J, MacGregor D, Davis ID, Sturrock S, Jungbluth AA, Cebon J. Tumor antigen expression in melanoma varies according to antigen and stage. Clin Cancer Res. 2006;12:764–771. doi: 10.1158/1078-0432.CCR-05-1544. [DOI] [PubMed] [Google Scholar]
Materials and methods
Patient
Patient IMF-16 had stage IV melanoma and was enrolled on a clinical trial (NCT00289627) of compassionate-use ipilimumab at the Memorial Sloan-Kettering Cancer Center (MSKCC). She received induction treatment with ipilimumab 10 mg/kg i.v. every three weeks for four doses (on weeks 1, 4, 7 and 10). Because of ongoing clinical benefit at week 24, maintenance ipilimumab was initiated and repeated every 12 weeks. The patient underwent radiographic evaluation at baseline, week 12, week 24 and every 12 weeks thereafter. Responses were classified according to Response Evaluation Criteria in Solid Tumors (RECIST) criteria. Toxicity was assessed using National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0. The protocol had been reviewed by the MSKCC Institutional Review Board and the patient provided informed consent. Tumor tissue and blood samples were obtained under two separate tissue procurement protocols.
ICOS and FOXP3 staining
One million peripheral blood mononuclear cells (PBMCs) were washed with 2 ml FACS buffer (PBS containing 1% bovine serum albumin and 0.05 mM EDTA). The cells were resuspended in 50 µl FACS buffer and stained with 0.375 µl ICOS-biotin antibody (eBioscience, San Diego, CA) for 20 min at 4˚C before being washed again with 2 ml FACS buffer. The following antibodies were then added for 30 min at room temperature: 0.3 µl strepavidin-PE-Cy7 (eBioscience), 3 µl CD3-Pacific Blue (eBioscience), 1 µl CD4-ECD (Beckman Coulter Inc., Fullerton, CA) and 3 µl CD25-APC-Cy (BD Bioscience, San Jose, CA). After re-washing with FACS buffer, the cells were fixed and permeabilized with 250 µl 1× Fixation/Permeabilization solution (eBioscience) for 30 min at 4˚C before being washed with 2 ml 1× Permeabilization buffer (eBioscience). Five µL FOXP3-APC antibody (eBioscience) were then added for 60 min at 4˚C before a final washing with 1× Permeabilization buffer. The cells were then resuspended in 400 µl FACS buffer and acquired on a CyAn flow cytometer with Summit software (DakoCytomation California Inc., Carpinteria, CA). Analysis was performed using FlowJo software (version 8.1; TreeStar, Inc., Ashland, OR). Isotype controls included the appropriate biotin or fluorochrome conjugated mouse IgG1a or IgG2a (Dako).
Tetramer staining and intracellular cytokine staining (ICS)
HLA-A*0201-PE labeled tetramers loaded with NY-ESO-1157-165 (SLLMWITQC) were provided by the Tetramer Core Facility, Ludwig Institute for Cancer Research (Lausanne, Switzerland). Tetramer staining and ICS were performed on cells obtained after cell harvest as previously described (23). A T cell response at a post-vaccination or post-ipilimumab time point was considered positive if it was 3 or more standard deviations greater than the mean value at baseline and had an absolute value >0.1%.
Tumor sample processing
Tissue sections (5 µm) were prepared from formalin-fixed, paraffin-embedded material and collected on Superfrost/plus microscope slides (Fisher Scientific, NJ). After deparaffinization and rehydration, the slides were boiled in 50 mM citrate buffer (pH 6) for 30 minutes to retrieve the antigens. Subsequently, the slides were allowed to cool down at room temperature. Immunohistochemistry (IHC) was performed using the ABC method. Rat anti-human monoclonal antibody HLA-DR (1:200 dilution, clone YE2/36HLK, Abcam) and mouse anti-human monoclonal HLA class I (1:200 dilution, clone A4, eBioscience) were applied as primary antibodies at 4˚C overnight. Before applying primary antibodies, the sections were blocked with 10% rabbit and horse normal serum (Santa Cruz Biotechnology, CA) for HLA-DR and HLA class I respectively. Biotinylated rabbit anti-rat and horse anti-mouse secondary antibodies (Vectastain Elite ABC kit, Vector Labs, Burlingame, CA) were added on the second day, incubated for 30 minutes at room temperature and tertiary reagent was applied according to the manufacturer's instructions. Antigen detection was performed by a color reaction with 3,3'-diaminobenzidine (DAB plus chromogen; DakoCytomation, Germany). The sections were counterstained with hematoxylin and mounted with Permount media (Fisher Scientific, NJ). The slides were scanned with a Mirax Scanner (Carl Zeiss AG, VA) and images were acquired with Mirax Reviewer 1.11 software. IHC detection of MAGE-A1, MAGE-A, CT10/MAGE-C2, NY-ESO-1, CT7/MAGE-C1 and GAGE was performed using monoclonal antibodies MA-454, M3H67, CT10-5, E978, CT7-33 and #26 respectively, as previously described (30).