Abstract
Ipilimumab is a fully human, monoclonal antibody that blocks cytotoxic T-lymphocyte antigen-4 to potentiate an antitumor T-cell response. This agent improved overall survival in a phase III trial in previously treated patients with advanced melanoma. Because the mechanism of action for ipilimumab is thought to be HLA independent, most trials enrolled patients without regard to HLA subtype. However, enrollment in the phase III trial was restricted to class-I HLA-A*0201-positive patients because two of the three arms contained an HLA-A*0201-restricted gp100 vaccine. HLA typing was also performed prospectively in several phase II trials and was available for 93.5% of patients. In this retrospective analysis, pooled efficacy and safety data are presented according to HLA-A*0201 status and dose from pretreated patients randomized to 0.3, 3, or 10 mg/kg ipilimumab in four phase II trials. Median overall survival (OS) was similar for the 187 HLA-A*0201-positive [9.3 months, 95% CI (confidence interval) 7.4-11.5] and 266 HLA-A*0201-negative patients [11.4 months, 95% CI 9.3-15.1] randomized to ipilimumab at all doses across the four phase II trials. These data are comparable to the OS for the 137 HLA-A*0201-positive patients randomized to ipilimumab in the phase III study [10.1 months, 95% CI 8.0-13.8]. Ipilimumab-induced adverse events and immune-related adverse events (skin, gastrointestinal, hepatic, other) also occurred at similar frequencies among patients in the phase II and III trials, regardless of HLA-A*0201 status. These findings support the hypothesis that ipilimumab-treated patients with advanced melanoma have similar outcomes regardless of their HLA-A*0201 status.
Keywords: clinical trial, melanoma, ipilimumab, HLA, treatment outcome
Introduction
Ipilimumab is a fully human, monoclonal antibody that blocks cytotoxic T-lymphocyte antigen-4 to potentiate an antitumor T-cell response. The mechanism of action of ipilimumab (i.e., cytotoxic T-lymphocyte antigen-4 receptor-ligand interaction) is thought to be HLA independent. Ipilimumab improved OS, either as monotherapy or combined with a gp100 vaccine, compared with a vaccine-only control group, in a phase III, randomized, controlled trial in previously treated patients with advanced melanoma (MDX010-020) (1). In the MDX010-020 phase III trial, patients randomized to receive ipilimumab monotherapy at 3 mg/kg had a median OS of 10.1 months (95% CI 8.0-13.8) compared with 6.4 months (95% CI 5.5-8.7) in patients randomized to vaccine alone. The 1- and 2-year OS rates with ipilimumab monotherapy were 45.6% and 23.5%, respectively, compared with 25.3% and 13.7% with vaccine alone. Addition of the gp100 vaccine did not influence the survival outcome (hazard ratio 1.04).
Entry criteria for the phase III trial restricted enrollment to HLA-A*0201-positive patients because an HLA-A*0201-restricted gp100 vaccine was used as an active control and in combination with ipilimumab. To elicit an immune response, peptide vaccines like gp100 must be presented to the immune system in the HLA context to which they are restricted. Since the gp100 peptide vaccine antigens were originally identified as binding to HLA-A*0201, trial enrollment was restricted to HLA-A*0201-positive patients. However, this is the only ipilimumab trial with a vaccine arm requiring an HLA enrollment restriction.
The overall ipilimumab clinical development program is being conducted largely without consideration of HLA subtype because the mechanism of action of ipilimumab is not related to HLA and is therefore likely to be HLA independent. Prospective HLA typing was, however, performed in several phase II trials, as part of an investigation of potential biomarkers of response or safety (1-5). Earlier findings from a pooled analysis of data from 139 patients with metastatic melanoma in two trials suggested that HLA-A*0201 status was not predictive for objective response rate to ipilimumab (6). Fifty-four patients received ipilimumab with peptide vaccination and 85 received intra-patient, dose-escalated ipilimumab and were randomized to receive peptides in accordance with HLA status. The objective response rate was 17% in all patients, 18% in HLA-A*0201-positive patients and 13% in HLA-A*0201-negative patients (6).
This is a retrospective analysis of pooled efficacy and safety data stratified by HLA-A*0201 status from 453 pretreated patients randomized to 0.3, 3, or 10 mg/kg ipilimumab in four phase II trials (CA184-004, -007, -008, and -022). Findings were compared with efficacy and safety outcomes from 137 HLA-A*0201-positive patients on the phase III trial (1).
Results
Patient population
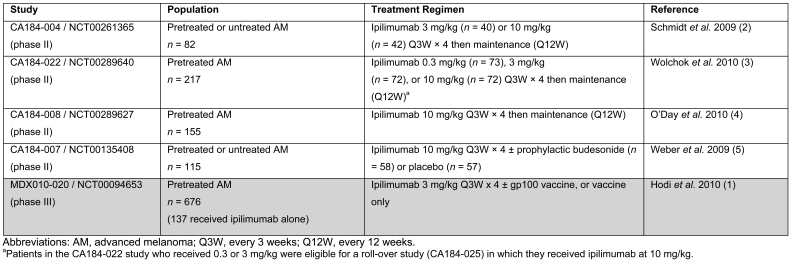
Demographics, disease characteristics, and prior therapies were generally similar across all trials. Details of the four phase II trials used as data sources for the pooled analysis and of the phase III study included for data comparison are shown in Table 1 (1-5). The HLA status (HLA-A*0201 positive or negative) was evaluated prospectively for the majority of patients in MDX010-020 and all four phase II trials (99.7% and 93.5%, respectively). In the phase II trials, 453 previously treated patients were included in the efficacy analyses, of whom 187 (41.3%) were HLA-A*0201 positive and 266 (58.7%) were HLA-A*0201 negative. For safety analyses, 450 previously treated patients were included, of whom 185 (41.1%) were HLA-A*0201 positive and 265 (58.9%) were HLA-A*0201 negative. Patients were randomized to 0.3, 3, or 10 mg/kg ipilimumab depending on the study they participated. Since the 0.3 mg/kg dose appears to be below the minimal threshold for an objective response to ipilimumab (3) the data were also analyzed in patients randomized to either 3 or 10 mg/kg. Patients who initially received 0.3 or 3 mg/kg ipilimumab in the phase II CA184-022 trial were eligible for a roll-over study (CA184-025) in which they received ipilimumab at 10 mg/kg.
Table 1.
Summary of phase II advanced melanoma trials used as data sources for pooled analysis and the comparator phase III study.
Efficacy by HLA-A*0201 status
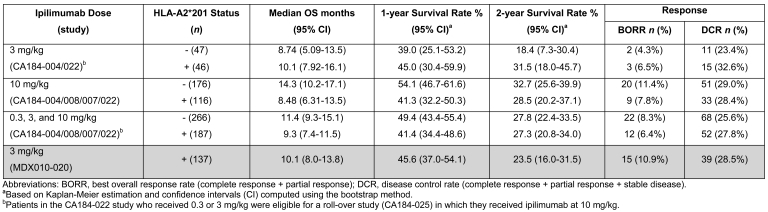
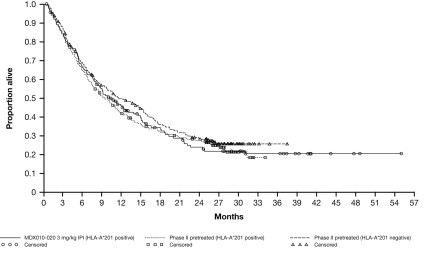
Efficacy data are shown in Table 2. Patients randomized to receive ipilimumab at any dose across the phase II trials had similar median OS regardless of HLA-A*0201 status (Figure 1). When data from patients randomized to a 10 mg/kg ipilimumab dose in the four phase II trials were analyzed, median OS was slightly higher in the HLA-A*0201-negative than in the HLA-A*0201-positive group for that dose. However, this trend is reversed at the 3 mg/kg dose. The conflicting OS observed in the 3 and 10 mg/kg subsets suggests there is no correlation with HLA status. HLA-A*0201-positive patients randomized to ipilimumab 3 mg/kg in two phase II trials (CA184-004/CA184-022) had a median OS similar to that in patients randomized to the same dose in the phase III study (MDX010-020). HLA-A*0201-negative patients randomized to 3 mg/kg ipilimumab in the phase II trials had slightly lower median OS than the HLA-A*0201 positive patients in the phase III study. These data show an expected moderate variability within the populations, and the differences are unlikely to be the result of a true biological effect.
Table 2.
HLA-A2*201 status and efficacy (survival, response, and disease control) across phase II trials versus the phase III study.
Figure 1.
Kaplan-Meier plot of overall survival by HLA status. Data from all pretreated patients randomized to 0.3, 3, or 10 mg/kg ipilimumab from four phase II trials pooled by dose versus HLA-A2*201-positive patients randomized to 3 mg/kg ipilimumab monotherapy in a phase III trial. Patients in the CA184-022 study who received 0.3 or 3 mg/kg were eligible for a roll-over study (CA184-025) in which they received ipilimumab at 10 mg/kg.
Survival rates at 1 and 2 years broadly mirror median OS values between groups and doses. Overall, rates were similar in HLA-A*0201-positive and -negative patients in phase II and phase III trials. However, in the analysis limited to patients randomized to 3 mg/kg ipilimumab, survival rates were slightly higher in HLA-A*0201-positive patients than in HLA-A*0201-negative patients; this pattern in survival rates was inverted at the 10 mg/kg dose, and in pooled data from patients randomized to any ipilimumab dose.
Data for best overall response rate (BORR) and disease control rate (DCR) are summarized for the four phase II trials and the phase III trial by HLA-A*0201 type and ipilimumab dose in Table 2. The BORR and DCR were generally consistent among all subgroups regardless of HLA-A*0201 status.
Immune-related adverse events by HLA-A*201 status
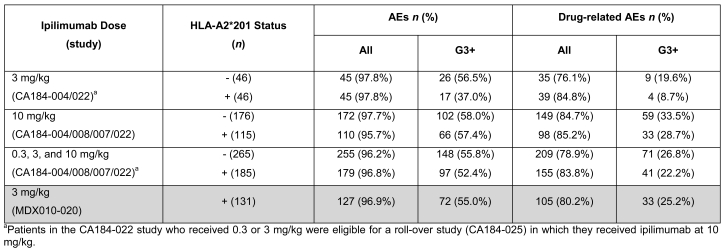
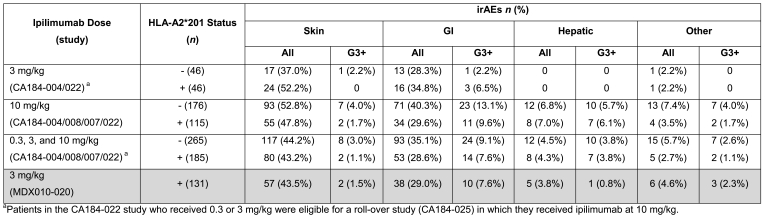
Adverse event (AE) and drug-related AE rates were generally similar across ipilimumab doses of 3 and 10 mg/kg, and when all doses were pooled, regardless of HLA-A*0201 status (Table 3). When pooled data from patients in the four phase II trials across all ipilimumab doses were compared with data from patients in the phase III trial, immune-related adverse events (irAEs) occurred at similar frequencies regardless of HLA-A*0201 status (Table 4). However, patients receiving 3 mg/kg ipilimumab in phase II trials showed a tendency toward a higher frequency of irAEs in HLA-A*201-positive patients compared with HLA-A*0201-negative patients, while this trend was inverted in the 10 mg/kg dose group (Table 4). This is consistent with the observations on survival outcomes in HLA-A*0201-negative or -positive patients in the different dosage groups.
Table 3.
HLA-A2*201 status and safety (all and drug-related AEs) across phase II trials versus the phase III study.
Table 4.
HLA-A2*201 status and immune-related adverse events (irAEs) across phase II trials versus the phase III study.
Discussion
This analysis shows that HLA-A*0201 status does not have a significant correlation with efficacy or toxicity with ipilimumab monotherapy. This is consistent with findings from a previous analysis of ipilimumab therapy and HLA status in a smaller group of phase II patients (6). The efficacy and safety outcomes in the phase III trial in HLA-A*0201-positive patients were similar to outcomes in the HLA-A*0201-positive and HLA-A*0201-negative patients in the four phase II trials analyzed. These data support that the mechanism of action of ipilimumab is independent of HLA status.
In this analysis, 41.3% of the 453 patients from the phase II trials who were evaluable for efficacy were HLA-A*0201 positive. This is consistent with the proportion of HLA-A*0201-positive individuals in the general population (7-9). OS was similar in HLA-A*0201-positive and HLA-A*0201-negative patients. However, there was an apparent increase in OS in patients who were HLA-A*0201-negative compared with HLA-A*0201-positive patients randomized to the 10 mg/kg dose of ipilimumab and in the pooled data; however this finding was not statistically significant. This was accompanied by a corresponding trend toward an increased frequency of both overall and grade 3 or higher irAEs in HLA-A*0201-negative patients, consistent with previous observations of an association between ipilimumab efficacy and mechanism-related irAEs (5, 6, 10-14). In contrast, in patients randomized to 3 mg/kg ipilimumab, OS and frequency of irAEs tended to be higher in HLA-A*0201-positive than -negative patients (also not statistically significant).
This pooled analysis of data from four phase II trials suggests that there is no apparent correlation between HLA-A2*0201 status and efficacy and safety outcomes in ipilimumab treated patients. However, there may be small trends that are inconsistent in direction by dose level that represent statistical variability rather than a biologic effect. This is particularly relevant to the small survival advantage observed for HLA-A2*0201-negative patients in the 10 mg/kg group and the similarly small disadvantage in the 3 mg/kg group. Confidence intervals for these data were overlapping and the observations seem to be driven by the sample size of the cohorts as the differences reduce as the sample sizes increase. Overall, the retrospective use of data pooled from several studies is a limitation, and imbalances among the populations may account for differences in efficacy and safety. Regardless of the small differences among HLA-A2*0201-positive and -negative patients at the different doses, the 1-year OS rates ranged from 39.0% to 54.1% and these values are similar to those for the ipilimumab-containing arms in the phase III trial (43.6% and 45.6%) (1). These OS rates also compare favorably with that of the vaccine-only control arm in the phase III trial (25.3%) (1) and the overall mean 1-year survival rate of 25% reported from a meta-analysis of 68 phase II trial arms (15).
BORR and DCR were comparable between the MDX010-020 phase III trial and pooled response data from the four phase II trials. Other immunotherapies (with HLA-independent mechanisms of action) also provide benefit regardless of HLA status. An extensive analysis of prospectively collected data from 272 patients with metastatic melanoma receiving interleukin-2-based treatment showed no significant correlation between HLA-A*02 expression and response (16). In another study, there was no association between response and HLA-A*02 expression in 54 patients with metastatic melanoma, including 16 responders and 16 patients with stable disease, who were treated with interferon-alfa and interleukin-2 (17).
Clinical data linking HLA subtype to any melanoma-related risk are limited; observations in the small number of evaluations have not been replicated and none of the putative associations involve the A*0201 allele (18-20). Moreover, HLA characterization does not play any role in the clinical staging and classification of melanoma based on validated prognostic factors (validation based on approximately 50,000 patients) (21). HLA-A*02 was not a gene predictive of outcome in 284 high-risk melanoma patients receiving adjuvant interferon (22).
In summary, this retrospective analysis shows that the survival and toxicity outcomes are generally similar in HLA-A*0201-positive and HLA-A*0201-negative patients who received ipilimumab in the phase II trials, and in the HLA-A*0201-positive patients who received ipilimumab alone in the phase III trial. These data support the hypothesis that HLA-A*0201 status is not a predictive factor for ipilimumab efficacy or safety.
Abbreviations
- CI
confidence interval
- OS
overall survival
Acknowledgements
Trials were supported by Bristol-Myers Squibb. Editorial and writing assistance was provided by StemScientific, funded by Bristol-Myers Squibb.
References
- 1.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbé C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. New Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmidt H, Hamid O, Nissan A, Guida M, Aamdal S, Hansson J, Ridolfi R, Berman D, Chasalow SD. Identification of tumor biopsy markers as potential predictors of ipilimumab clinical activity in patients with advanced melanoma [abstract]. Eur J Cancer Suppl. 2009;7:577. [Google Scholar]
- 3.Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L, Waterfield W, Schadendorf D, Smylie M, Guthrie T Jr, Grob JJ, Chesney J, Chin K, Chen K, Hoos A, O'Day SJ, Lebbé C. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11:155–164. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 4.O'Day SJ, Maio M, Chiarion-Sileni V, Gajewski TF, Pehamberger H, Bondarenko IN, Queirolo P, Lundgren L, Mikhailov S, Roman L, Verschraegen C, Humphrey R, Ibrahim R, de Pril V, Hoos A, Wolchok JD. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm phase II study. Ann Oncol. 2010;21:1712–1717. doi: 10.1093/annonc/mdq013. [DOI] [PubMed] [Google Scholar]
- 5.Weber J, Thompson JA, Hamid O, Minor D, Amin A, Ron I, Ridolfi R, Assi H, Maraveyas A, Berman D, Siegel J, O'Day SJ. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable Stage III or IV melanoma. Clin Cancer Res. 2009;15:5591–5598. doi: 10.1158/1078-0432.CCR-09-1024. [DOI] [PubMed] [Google Scholar]
- 6.Downey SG, Klapper JA, Smith FO, Yang JC, Sherry RM, Royal RE, Kammula US, Hughes MS, Allen TE, Levy CL, Yellin M, Nichol G, White DE, Steinberg SM, Rosenberg SA. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res. 2007;13:6681–6688. doi: 10.1158/1078-0432.CCR-07-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sette A, Sidney J. Nine major HLA class I supertypes account for the vast preponderance of HLA-A and -B polymorphism. Immunogenetics. 1999;50:201–212. doi: 10.1007/s002510050594. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez-Viña MA, Falco M, Sun Y, Stastny P. DNA typing for HLA class I alleles: I. Subsets of HLA-A2 and of -A28. Hum Immunol. 1992;33:163–173. doi: 10.1016/0198-8859(92)90068-x. [DOI] [PubMed] [Google Scholar]
- 9.Krausa P, Brywka M 3rd, Savage D, Hui KM, Bunce M, Ngai JL, Teo DL, Ong YW, Barouch D, Allsop CE, Hill AVS, McMichael AJ, Bodmer JG, Browning MJ. Genetic polymorphism within HLA-A*02: significant allelic variation revealed in different populations. Tissue Antigens. 1995;45:223–231. doi: 10.1111/j.1399-0039.1995.tb02444.x. [DOI] [PubMed] [Google Scholar]
- 10.Attia P, Phan GQ, Maker AV, Robinson MR, Quezado MM, Yang JC, Sherry RM, Topalian SL, Kammula US, Royal RE, Restifo NP, Haworth LR, Levy C, Mavroukakis SA, Nichol G, Yellin MJ, Rosenberg SA. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23:6043–6053. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, Restifo NP, Haworth LR, Seipp CA, Freezer LJ, Morton KE, Mavroukakis SA, Duray PH, Steinberg SM, Allison JP, Davis TA, Rosenberg SA. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2003;100:8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blansfield JA, Beck KE, Tran K, Yang JC, Hughes MS, Kammula US, Royal RE, Topalian SL, Haworth LR, Levy C, Rosenberg SA, Sherry RM. Cytotoxic T-lymphocyte-associated antigen-4 blockage can induce autoimmune hypophysitis in patients with metastatic melanoma and renal cancer. J Immunother. 2005;28:593–598. doi: 10.1097/01.cji.0000178913.41256.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beck KE, Blansfield JA, Tran KQ, Feldman AL, Hughes MS, Royal RE, Kammula US, Topalian SL, Sherry RM, Kleiner D, Quezado M, Lowy I, Yellin M, Rosenberg SA, Yang JC. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006;24:2283–2289. doi: 10.1200/JCO.2005.04.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang JC, Hughes M, Kammula U, Royal R, Sherry RM, Topalian SL, Suri KB, Levy C, Allen T, Mavroukakis S, Lowy I, White DE, Rosenberg SA. Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J Immunother. 2007;30:825–830. doi: 10.1097/CJI.0b013e318156e47e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korn EL, Liu PY, Lee SJ, Chapman JA, Niedzwiecki D, Suman VJ, Moon J, Sondak VK, Atkins MB, Eisenhauer EA, Parulekar W, Markovic SN, Saxman S, Kirkwood JM. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol. 2008;26:527–534. doi: 10.1200/JCO.2007.12.7837. [DOI] [PubMed] [Google Scholar]
- 16.Marincola FM, Shamamian P, Rivoltini L, Salgaller M, Cormier J, Restifo NP, Simonis TB, Venzon D, White DE, Parkinson DR. HLA associations in the antitumor response against malignant melanoma. J Immunother Emphasis Tumor Immunol. 1995;18:242–252. doi: 10.1097/00002371-199511000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scheibenbogen C, Keilholz U, Mytilineos J, Suciu S, Manasterski M, Hunstein W. HLA class I alleles and responsiveness of melanoma to immunotherapy with interferon-alpha (IFN-alpha) and interleukin-2 (IL-2). Melanoma Res. 1994;4:191–194. doi: 10.1097/00008390-199406000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Fensterle J, Trefzer U, Berger T, Andersen MH, Ugure S, Becker JC. HLA-B8 association with late-stage melanoma - an immunological lesson? BMC Medicine. 2006;4:5. doi: 10.1186/1741-7015-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JE, Abdalla J, Porter GA, Bradford L, Grimm EA, Reveille JD, Mansfield PF, Gershenwald JE, Ross MI. Presence of the human leukocyte antigen class II gene DRB1*1101 predicts interferon gamma levels and disease recurrence in melanoma patients. Ann Surg Oncol. 2002;9:587–593. doi: 10.1007/BF02573896. [DOI] [PubMed] [Google Scholar]
- 20.Jager MJ, Volker-Dieben HJ, De Wolffrouendaal D, Kakebeeke-Kemme H, D'Amaro J. Possible relation between HLA and ABO type and prognosis of uveal melanoma. Doc Ophthalmol. 1992;82:43–47. doi: 10.1007/BF00156992. [DOI] [PubMed] [Google Scholar]
- 21.Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG, Ding S, Eggermont AM, Flaherty KT, Gimotty PA, Kirkwood JM, McMasters KM, Mihm MC Jr, Morton DL, Ross MI, Sober AJ, Sondak VK. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gogas H, Kirkwood JM, Falk CS, Dafni U, Sondak VK, Tsoutsos D, Stratigos A, Markopoulos C, Pectasides D, Spyropoulou-Vlachou M. Correlation of molecular human leukocyte antigen typing and outcome in high-risk melanoma patients receiving adjuvant interferon. Cancer. 2010;116:4326–4333. doi: 10.1002/cncr.25211. [DOI] [PMC free article] [PubMed] [Google Scholar]
Materials and methods
Data sources
Details of study design, dosing regimens, and patient demographics for the phase II and phase III trials have been reported elsewhere (1-5). Only data from pretreated patients for whom HLA type was available were included for analysis.
Analytical methods
In trials CA184-004, -022, -008, and -007, the limited specificity of the initial HLA-A*02 supergroup identification, which used the Innogenetics INNO-LiPA HLA assay (this assay uses a DNA line-probe methodology and is not FDA approved), meant that a second step to achieve full genomic sequencing of the allelic site (specific allelic identification using the FDA-approved Genovision/Qiagen Olerup SSP TM-Assay) was required in every case.
In the MDX010-020 phase III study, the required study-based HLA testing was performed using the Invitrogen™ SSP UniTray® polymerase chain reaction (PCR)-based assay, which is FDA approved for in vitro diagnostic use, using HLA primers provided by Invitrogen™. Samples from this study that gave ambiguous results using the Invitrogen™ assay kit were referred for full genomic sequencing of the allelic site; this sequenced-based typing was carried out using the HLA-AlleleSEQR kit from Applied BioSystems. In the MDX010-020 dataset, only 9 patients required this second level of testing for HLA classification.