Abstract
Medullary carcinoma (MC) of the breast is a high grade carcinoma that has a relatively favourable prognosis compared to atypical medullary carcinoma (AMC) and other more common breast carcinomas. In a retrospective study in Brunei Darussalam of all available biopsy samples, we compared the nature of the tumour-infiltrating lymphocytes (TILs) in MC and AMC in relation to recorded tumour characteristics. CD4, CD8, CD20, CD25, CD45RO, and CD56 and common tumour biomarkers were detected immunohistochemically. The 11 cases of MC had no nodal metastases and survived without relapse, suggesting good tumour control. In contrast, 7 cases of nodal metastases and 1 relapse were observed in 12 AMCs. Although not statistically significant, there was a tendency for a greater proportion of AMCs to express the Her2/neu oncogene. Higher proportions of CD45RO+ and CD8+ cells, and lower levels of CD20+ cells, were characteristic of TILs in MC compared to AMC. The ratio of CTL to B-lineage cells in TILs in both tumours considered together was inversely related to the expression of HER2/neu and the presence of nodal metastases. The findings suggest that CTLs, rather than antibodies, may give better tumour control in MC relative to AMC. We propose that a comparison of the cellular, molecular and immunological characteristics of MC and AMC, as a paired model system, in a multi-centre investigation with a much larger number of samples will be valuable for better understanding mechanisms of tumour immunity.
Keywords: human, breast, medullary carcinoma, atypical medullary carcinoma, TILs, immunohistochemistry
Introduction
Breast carcinoma is the most common malignancy in women in many countries. Data from the Brunei Darussalam National Cancer Registry for the period 2001-2007 show that typical medullary carcinoma (MC) of the breast accounts for about 4.5% of all breast carcinomas in the country. Typical MC has defined morphological characteristics including predominantly syncytial growth, macroscopically and microscopically circumscribed tumour margins, marked or moderate lymphoplasmocytic infiltration of the stroma, pleomorphic nuclei with a high mitotic index, and the absence of tubule formation and an in situ component (1, 2). Atypical medullary carcinomas (AMCs) are a class of related breast carcinomas that also have a predominantly syncytial growth pattern but lack up to two of the other features of MCs. AMCs may therefore show up to two of the following features: focal areas of tumour infiltration at the margins, absence of or mild lymphoplasmocytic infiltration of the tumour or lymphoplasmocytic infiltration only at the tumour margins, uniform nuclei with infrequent mitosis, focal tubule formation and the presence of an in situ component (1, 2). MC is of particular interest because, despite being a high grade carcinoma, it has a more favourable prognosis after surgical resection than AMC and the other more common breast carcinomas, such as invasive ductal and lobular carcinomas (1-6).
Previous studies on immunophenotyping TILs in medullary-type carcinomas of the breast showed the presence of CD8+ T cells (7-9) and B-lineage cells (1, 8, 10, 11), but these studies have generally not differentiated between MC and AMC. The TILs have been considered to reflect an immune response controlling tumour growth, resulting in a better prognosis for MC (1, 7-11). We hypothesised that MC and AMC may constitute a useful paired model system for investigating mechanisms of tumour immunity. Therefore, in a retrospective study, we characterised the TILs by immunohistochemistry and examined their relationship to nodal metastases and relapses in MC and AMC specimens, as well as routinely determined tumour biomarkers.
Results
The average age of the 11 MC and 12 AMC patients whose tumour tissues were analysed were similar, being 48.9 yr (range 41 yr - 76 yr) and 43.1 yr (range 22 yr - 55 yr) respectively. All of the 11 MC patients survived without relapse to date, while 1 of the 12 AMC patients had a relapse since surgery and commencement of treatment. None of the 11 MC patients had nodal metastases, while 7 of 12 AMC patients had nodal metastases detected on biopsy, a difference which was statistically significant (Fisher's exact two-tail test, P = 0.005).
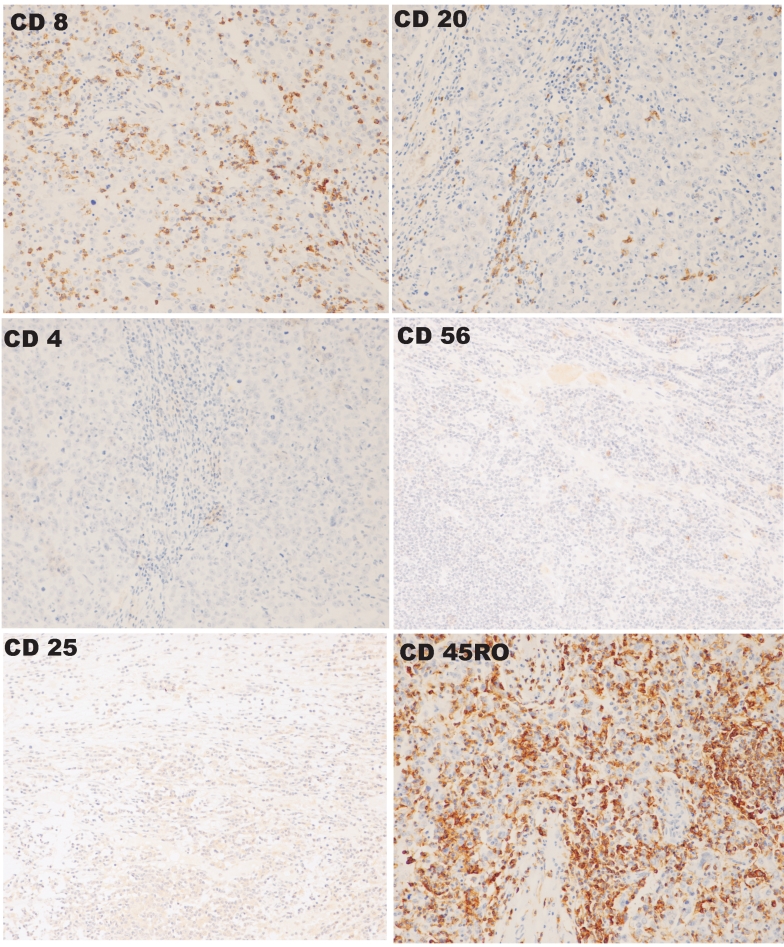
The results of immunohistochemical staining of sections derived from a single MC are shown in Figure 1. The proportion of MCs positive by immunohistochemistry for progesterone receptor (PR), oestrogen receptor (ER), HER2/neu, epidermal growth factor (EGFR) and p53 were 0/11, 0/11, 4/11, 0/11 and 7/11, respectively. The corresponding proportions of AMCs positive for PR, ER, HER2/neu, EGFR and p53 were 3/12, 4/12, 9/12, 3/12 and 6/12, respectively. No statistically significant difference in the expression of any of the examined markers between the two tumour types could be shown, although there was a tendency for more AMC samples to express HER2/neu (Fisher's exact two-tail test, P = 0.1).
Figure 1.
Immunohistochemical staining for the markers CD8, CD20, CD4, CD56, CD25 and CD45RO in a single MC specimen. Different sections are shown at a 200x magnification.
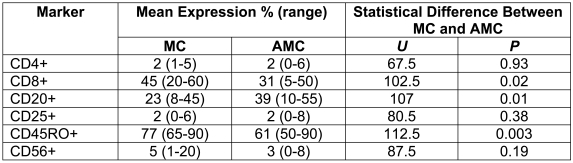
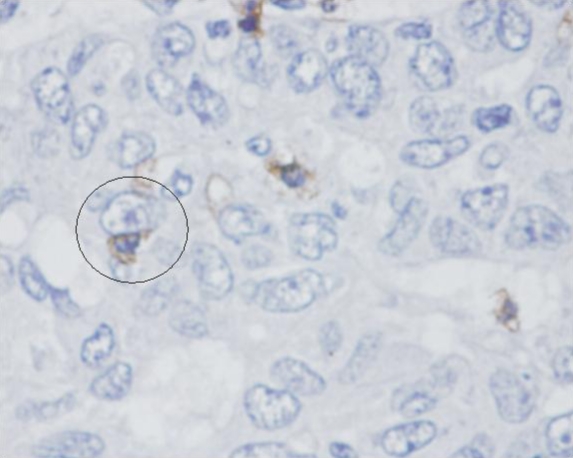
The immunophenotypes of the TILs are shown in Table 1. The proportion of CD4+ cells, as well as that of CD25+ cells, was relatively low in comparison to CD8+ and CD20+ cells in both MCs and AMCs. There was a significantly greater proportion of CD8+ cells and a lower proportion of CD20+ cells in MCs than in AMCs. This was consistent with a higher ratio of CD8+ cells to CD20+ cells in MCs (mean 2.6, range 0.7-6.4) than in AMCs (mean 1.1, range 0.2-1.8), a difference which was statistically significant (Mann-Whitney two-tail U test, U = 113.5, P = 0.002). There were also significantly more CD45RO+ cells in MC TILs compared to AMC. Close contacts between CD8+ cells and tumour cells were frequently observed in MC (Figure 2).
Table 1.
Immunophenotype of TILs in MC and AMC specimens analyzed.
Figure 2.
Close contact between a CD8+ lymphocyte and a tumour cell in an MC specimen (circled). The MC specimen was first stained with anti-CD8 antibodies, followed by peroxidase-labelled secondary antibodies. Magnification 400x.
There was a trend for CD56+ cells, although fewer in number compared to CD8+ cells, to be relatively increased in MC TILs, although the difference was not statistically significant (Table 1).
There was a significantly higher ratio of CD8+ to CD20+ cells in the TILs of patients without nodal metastases when both tumour types were considered together (Mann-Whitney two-tail U test, U = 88.5, P = 0.03; the mean CD8/CD20 ratio was 0.8 in patients with nodal metastases and 2.2 in patients without metastases). The ratio of CD8+ to CD20+ cells in TILs was also greater in tumours of both types lacking HER2/neu (mean ratio of 2.6) than in those expressing detectable HER2/neu (mean ratio of 1.2), a statistically significant difference (Mann-Whitney two-tail U test, U = 105.5, P = 0.01).
Discussion
The innate and adaptive immune systems are able to control the growth of many cancers through a process termed immunosurveillance (12), until variant cancer cells arise that are either poorly immunogenic (immunoselection) or able to subvert effector immune mechanisms (immunosubversion) (12). The ability of cancers to overcome immunosurveillance through various methods, a process referred to as immunoediting, is likely to be a characteristic of many cancers (13).
Previous studies on TILs in MC and AMC have mostly grouped them together for comparison with the more common invasive ductal carcinomas despite the very different prognosis for MC and AMC. Our data from Brunei are consistent with the prior observations (1, 2) that MC has a better prognosis, in terms of relapse-free survival and nodal metastases, than AMC. Importantly, our findings suggest that there are significant differences in the predominant types of TILs present in MC and AMC. CD8+ CTLs are more predominant in MC while CD20+ B-lineage cells are more prevalent in AMC, although the two cell types were present in relatively high proportions in the TILs of both types of tumours. Breast cancer antigens recognised by B cells in medullary-type tumour infiltrates reportedly include actin (14) and ganglioside GD3 (10), but our findings suggest that the possible protective role of antibodies in anti-tumour immunity needs to be further evaluated in these carcinomas. CD45RO is expressed on activated and memory T helper and cytotoxic cells, as well some B cells, NK cells, monocytes/macrophages, granulocytes and dendritic cells in humans and therefore its greater prevalence is consistent with a preponderance of activated CTLs in MC. Close contact observed between tumour cells and CD8+ cells are consistent with CTL-mediated killing being a major effector mechanism limiting tumour growth and invasiveness in MC. The significant relationship between a higher proportion of CD8+ CTLs to B-lineage cell in the TILs of both types of tumours and the absence of nodal metastases is also consistent with such a role. The small number of CD4+ and CD25+ cells in both MC and AMC may indicate significant absence of helper and regulatory/suppressor T cell activity in the tumour infiltrates. A possible increase in CD56+ NK cells in the TILs of MC, and hence their potential contribution to killing tumour cells, needs to be further tested in studies with larger numbers of samples than are available to us in Brunei.
HER2/neu is one of a family of epidermal growth factor receptors which is amplified in 15%-20% of human carcinomas and whose over-expression is associated with poor prognosis in breast cancers (15, 16), as is the absence of ER and PR (16). HER2/neu and ER are however the targets for treatment of breast cancers with the humanised monoclonal antibody, Herceptin, and ER antagonists, such as Tamoxifen, respectively. In Brunei Darussalam, as in other Southeast Asian countries, breast cancer arises in a slightly younger age group than in the USA, and of all types of breast cancers, including the more common ductal carcinomas, about 64% express immunohistochemically detectable HER2/neu at a level of 1+ or more (17). Our findings show that there was a tendency for more AMC cases to express HER2/neu than MC (although the result was not statistically significant) and that a proportion of the AMCs examined express ER and PR, although these hormone receptors were not detected in the MCs examined. However, similar levels of expression of HER2/neu were observed in MCs and AMCs in an Italian study (18). The same report showed that MCs had more CD3+ T cells than AMCs, a finding consistent with our own results. Our finding that a detectable level of expression of HER2/neu is inversely related to the ratio of CTLs to B-lineage cells in the TILs is consistent with a poorer prognosis for tumours expressing HER2/neu (15, 16), if CTLs are indeed the main effectors of anti-tumour immunity. However the underlying mechanisms for this relationship are unclear at present and merit further investigation.
CTLs have previously been proposed as the main effectors limiting tumour growth in MCs (7, 9) and many other tumours (19). Our findings are consistent with a supporting hypothesis that a higher quality CTL response, with possible adjunct NK cell-mediated cytotoxicity, may give rise to the better tumour control in MCs compared to AMCs, that effectively reduces nodal metastases and, after surgery and treatment, relapses in MCs. Immunophenotyping more cellular markers in larger numbers of MCs and AMCs could help extend these findings. Our results suggest that a detailed examination of the nature of antigens recognised by CTLs on MCs and the antigen receptors used by the CTLs may open new avenues for immunotherapy of breast carcinomas. It would be useful in this context to also determine the molecular and cellular differences between MCs and AMCs that elicit the different types of immune responses and to examine the role of the possible differential expression of HER2/neu in the biology of the two types of tumours. The data presented here support our hypothesis that MCs and AMCs constitute a good paired model system for detailed investigations of tumour immunity. Because the numbers of samples of MC and AMC available to us in Brunei were relatively small, the present findings provide valuable initial data that need to be extended in studies with larger numbers of tumour samples. This would only be possible in a large multi-national investigation that can also be designed to include ethnically different patient populations.
Abbreviations
- AMC
atypical medullary carcinoma
- MC
medullary carcinoma
References
- 1.Ridolfi RL, Rosen PP, Port A, Kinne D, Mike V. Medullary carcinoma of the breast: a clinicopathologic study with a 10-year follow up. Cancer. 1977;40:1365–1385. doi: 10.1002/1097-0142(197710)40:4<1365::aid-cncr2820400402>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 2.Sternberg SS, Antonioli DA, Carter D, Mills SE, Oberman HA, editors. Philadelphia (PA): Lippincott Williams & Wilkins; 1999. Diagnostic Surgical Pathology. (Eds.) 3rd edition. [Google Scholar]
- 3.Jensen ML, Kiaer H, Andersen J, Jensen V, Melsen F. Prognostic comparison of three classifications for medullary carcinomas of the breast. Histopathology. 1997;30:523–532. doi: 10.1046/j.1365-2559.1997.5720795.x. [DOI] [PubMed] [Google Scholar]
- 4.Pedersen L, Zedeler K, Holck S, Schiødt T, Mouridsen HT. Medullary carcinoma of the breast. Prevalence and prognostic importance of classical risk factors in breast cancer. Eur J Cancer. 1995;31:2289–2295. doi: 10.1016/0959-8049(95)00408-4. [DOI] [PubMed] [Google Scholar]
- 5.Wargotz ES, Silverberg SG. Medullary carcinoma of the breast: a clinicopathologic study with appraisal of current diagnostic criteria. Human Pathol. 1988;19:1340–1346. doi: 10.1016/s0046-8177(88)80290-9. [DOI] [PubMed] [Google Scholar]
- 6.Rapin V, Contesso G, Mouriesse H, Bertin F, Lacombe MJ, Piekarski JD, Travagli JP, Gadenne C, Friedman S. Medullary breast carcinoma. A re-evaluation of 95 cases of breast cancer with inflammatory stroma. Cancer. 1988;61:2503–2510. doi: 10.1002/1097-0142(19880615)61:12<2503::aid-cncr2820611219>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 7.Kuroda H, Tamaru J, Sakamoto G, Ohnisi K, Itoyama S. Immunophenotype of lymphocytic infiltration in medullary carcinoma of the breast. Virchows Arch. 2005;446:10–14. doi: 10.1007/s00428-004-1143-9. [DOI] [PubMed] [Google Scholar]
- 8.Tamiolakis D, Simopoulos C, Cheva A, Lambropoulou M, Kotini A, Jivannakis T, Papadopoulos N. Immunophenotypic profile of tumor infiltrating lymphocytes in medullary carcinoma of the breast. Eur J Gynaecol Oncol. 2002;23:433–436. [PubMed] [Google Scholar]
- 9.Yakirevich E, Izhak OB, Rennert G, Kovacs ZG, Resnick MB. Cytotoxic phenotype of tumor infiltrating lymphocytes in medullary carcinoma of the breast. Mod Pathol. 1999;12:1050–1056. [PubMed] [Google Scholar]
- 10.Kotlan B, Simsa P, Teillaud JL, Fridman WH, Toth J, McKnight M, Glassy MC. Novel ganglioside antigen identified by B cells in human medullary breast carcinomas: the proof of principle concerning the tumor-infiltrating B lymphocytes. J Immunol. 2005;175:2278–2285. doi: 10.4049/jimmunol.175.4.2278. [DOI] [PubMed] [Google Scholar]
- 11.Coronella JA, Telleman P, Kingsbury GA, Truong TD, Hays S, Junghans RP. Evidence for an antigen-driven humoral immune response in medullary ductal breast cancer. Cancer Res. 2001;61:7889–7899. [PubMed] [Google Scholar]
- 12.Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nature Rev Immunol. 2006;6:715–727. doi: 10.1038/nri1936. [DOI] [PubMed] [Google Scholar]
- 13.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nature Rev Immunol. 2006;6:836–848. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 14.Hansen MH, Nielsen H, Ditzel HJ. The tumour-infiltrating B cell response in medullary breast cancer is oligoclonal and directed against the autoantigen actin exposed on the surface of apoptotic cancer cells. Proc Natl Acad Sci USA. 2001;98:12659–12664. doi: 10.1073/pnas.171460798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ross JS, Fletcher JA. The HER-2/neu oncogene in breast cancer. Prognostic factor, predictive factor and target for therapy. Oncologist. 1998;3:237–252. [PubMed] [Google Scholar]
- 16.Jones C, Lakhani SR. Prognostic and predictive factors in breast cancer. Brunei Int Med J. 2000;2:147–169. [Google Scholar]
- 17.Kang FR, Telisinghe PU, bin Abdallah S, Ramasamy R. Breast cancer in Brunei Darussalam − Differential community distribution and an analysis of common molecular tumour markers. Brunei Darussalam J Health. 2007;2:23–30. [Google Scholar]
- 18.Orlando L, Renner G, Rocca A, Curigliano G, Colleoni M, Severi G, Peruzzotti G, Cinieri S, Viale G, Sanna G, Goldhirsch A. Are high-grade breast cancers with no steroid hormone expression alike? The special case of the medullary phenotype. Ann Oncol. 2005;16:1094–1099. doi: 10.1093/annonc/mdi213. [DOI] [PubMed] [Google Scholar]
- 19.Old LJ. Cancer vaccines: an overview. Cancer Immun. 2008;8(Suppl 1):1. http://www.cancerimmunity.org/v8suppl1p1/080104.htm [PubMed] [Google Scholar]
Materials and methods
Patient data
The standard treatment for breast cancer patients in Brunei Darussalam on diagnosis is surgical resection. Systemic therapy was given as required, particularly for AMC patients. Data on patients with carcinoma of the breast treated in RIPAS Hospital, Brunei Darussalam was available from January 2001 to August 2007. This data was retrieved from the hospital's Histopathology Database and the National Cancer Registry. RIPAS hospital is the reference centre for all cancer patients in the country. Of the 13 cases of MC and 14 cases of AMC diagnosed during this period, tumour tissue derived from mastectomy of 11 MC and 12 AMC patients whose clinical and laboratory data were complete were selected for study. All of the AMCs showed significant lymphoplasmocytic infiltration and were therefore differentiated from MCs by other criteria (1, 2). In the absence of institutional ethical review committees, the study was approved by the Ministry of Health of Brunei Darussalam.
Immunohistochemistry
Formalin-fixed, paraffin-embedded tumour tissue blocks from the hospital's Histopathology Archive were retrieved and manually processed following the department's standard procedures. A 5 µm section from each block was first stained with haematoxylin and eosin to locate the tumour nests. Consecutive 5 µm sections were then cut and tested after de-waxing by a two-step procedure with primary antibodies against CD4, CD8, CD20, CD25, CD45RO, and CD56 followed by peroxidase-conjugated secondary antibody (Dako, Denmark). Each slide was scored according to the standard for grading lymphocyte infiltration in cancer tissues (7). For more quantitative comparisons, stained cells in tumour nests in five different fields, each containing 100 mononuclear cells, were manually counted by the specialist histopathologist (P.U.T) from coded slides without knowing whether the slide corresponded to an MC or an AMC specimen.
Tumour cell markers
The presence (with a reading 1+ or more) of progesterone (PR) and oestrogen (ER) receptors, epidermal growth factor types 1 (EGRF) and 2 (HER2/neu), and p53 were determined in the tumours by routine manual immunohistochemical procedures with antibodies from Dako (Denmark).
Statistical analysis
The significance of differences between the proportions of the different lymphocytes and lymphocyte ratios in MC and AMC samples were determined by the non-parametric Mann-Whitney U test. The significance of differences in proportions of tumour cell markers and metastases were examined using Fisher's exact test. All tests were two-tailed and considered significant for P < 0.05.