Abstract
Objective:
To examine position-dependent (semireclined to standing) and walking speed–dependent soleus H-reflex modulation after motor incomplete spinal cord injury (SCI).
Participants:
Twenty-six patients with motor incomplete SCI (mean: 45 ± 15 years) and 16 noninjured people (mean: 38 ± 14 years).
Methods:
Soleus H-reflexes were evoked by tibial nerve stimulation. Patients were tested in semireclined and standing positions (experiment 1) and in midstance and midswing positions (experiment 2).
Results:
H-reflexes were significantly greater after SCI in all positions compared with noninjured people (P < 0.05). Position-dependent modulation from semireclined to standing (normally observed in noninjured people) was absent after SCI. In SCI patients, H-reflex modulation was not significantly different at 1.2 m/s compared with 0.6 m/s treadmill walking speed; in noninjured people, H-reflex modulation was significantly greater at 1.2 m/s compared with 0.6 m/s treadmill walking speed. There was a significant positive correlation between modified Ashworth scores, a clinical measure of spasticity and soleus H-reflex amplitudes tested in all positions. A significant negative correlation was also found between H-reflexes in standing and midstance positions and the amount of assistance patients required to walk.
Conclusions:
An improvement in position-dependent and walking speed–dependent reflex modulation after SCI may indicate functional recovery. Future studies will use H-reflex testing to track changes as a result of therapeutic interventions.
Keywords: Spinal cord injuries, incomplete; Ambulation; Spasticity; Locomotor training; Standing; Walking speed; Assistive devices; Treadmill; Rehabilitation; Soleus H-reflex
INTRODUCTION
Spinal cord injury (SCI) leads to functional impairment, such as leg weakness and slow walking speed (1), and neurophysiologic impairment, such as a generalized upregulation of H-reflexes (2–5). H-reflex testing is a reliable (6–10) technique that provides a window into the spinal cord to observe the neuromodulatory processes (11). H-reflexes are task specific, and their amplitude either increases or decreases depending on the body position. In noninjured people, soleus H-reflexes are typically modulated in a position-dependent manner such that they are significantly smaller during the static standing vs semireclined position and, in addition, are modulated in a task-dependent manner as observed during the swing phase of walking vs the stance phase of walking (12–15). In addition to upregulation of H-reflexes, SCI may lead to an inability to modulate reflexes in a position-dependent as well as task-dependent manner.
The position- and task-specific nature of H-reflexes reflects the capacity of the nervous system to modulate reflexes that are essential for postural control and locomotion (12,13). Spinal cord injury leads to disruption of neural pathways that control position and phase-specific neuromodulatory processes (3,14,15). As a result, soleus reflex upregulation occurs after SCI and is manifested as an increase in reflex amplitudes (2–4,16). Previous findings demonstrate the increase in H-reflex amplitude in non–weight-bearing positions, such as sitting, supine, and prone, and weight-bearing positions, such as standing (active and passive). However, it is not known whether the reflex amplitude modulates after SCI based on testing position, as normally seen in noninjured people. An ability to modulate the reflexes in a position-dependent manner may be an essential background for successful execution of functional tasks.
In addition to position-dependent reflex modulation, noninjured people also demonstrate an ability to modulate reflexes based on walking speed. Previous reports suggest that the soleus H-reflex amplitude decreases with increasing walking speeds (17–20). However, it is not known if the soleus H-reflexes decrease with an increase in walking speed in patients after SCI. Soleus H-reflexes tested during walking are significantly greater in amplitude after SCI compared with noninjured controls (2,4,5), and we have previously reported that walking on a treadmill with body weight support and manual assistance can decrease the soleus H-reflex amplitudes in patients after SCI walking at their self-selected speed (2). Thus, the changes in H-reflex amplitude related to walking speed may provide critical insights regarding optimal walking speed used for retraining walking in patients after SCI. Currently, there is no consensus on the optimal training speed for locomotor training studies, and several variations in treadmill training speeds exist (21). Gait patterns at velocities less than 0.6 m/s are susceptible to variability in gait parameters, reduced electromyogram (EMG) amplitude, and abnormal EMG activation patterns due to the slow walking speed, as well as deviations as a consequence of the pathology (22,23). Thus, training patients with SCI at faster, near-normal walking speeds (5,24) has been suggested.
The first experiment was designed to examine whether H-reflexes modulate with a change in position from semireclined to standing in noninjured people and patients with SCI. We hypothesized that position-dependent H-reflex depression would be significantly less in SCI patients vs noninjured people. The second experiment examined the effect of treadmill speed on phase-dependent soleus H-reflex modulation. We hypothesized that an increase in treadmill walking speed will induce greater modulation of H-reflexes in noninjured people vs patients with SCI. Soleus H-reflex testing is a useful noninvasive method to study the underlying neurophysiologic mechanisms after SCI. It is important, however, to fully understand the association between reflex amplitudes and clinical measures of impairment after SCI. In the third part of the study, we tested the correlation between soleus H-reflex amplitude and clinical measures of spasticity and walking assistance. We hypothesized that the H-reflex size would be directly proportional to spasticity and inversely proportional to the level of walking assistance in patients with SCI.
METHODS
Twenty-six adults with incomplete SCI (i-SCI; mean: 45 ± 15 years) and 16 nondisabled control participants (mean: 38 ± 14 years) were recruited for this study (Table 1). All participants reviewed and signed an informed consent form approved by the Institutional Review Board of the University of Florida prior to initiating the study. Criteria included adults with SCI aged 18 to 65 years; a diagnosis of first-time SCI, including etiology from trauma, vascular, or orthopedic pathology at cervical or thoracic levels; ASIA Impairment Scale categories C or D (25); physician's medical status approval; and the ability to walk independently a minimum of 30 feet with or without an assistive device. Healthy noninjured people with no history of neurologic or orthopedic problems that could impair walking function were recruited.
Table 1.
Demographic Data for Patients With Spinal Cord Injury in Experiment 1
Evaluation and Testing Procedures
Soleus H-reflexes were evoked on the more involved side (determined by AISA Impairment Scale motor score) of people with i-SCI and on the dominant side of the noninjured participants. Skin was shaved and cleaned for application of electrodes. Soleus EMG activity was recorded using a bipolar (2-cm interelectrode distance) Ag-AgCl surface electrode (Therapeutics Unlimited, Iowa City, IA [company no longer in business]). The electrodes are embedded in an epoxy mount with preamplifier circuitry. The preamplifier and second stage amplifier provided a total amplification of 1,000× with a frequency band pass of 20 Hz to 4 kHz. A reference electrode was placed on the distal tibia.
To evoke H-reflexes, current pulses were delivered via a dual-channel constant-current stimulator (model S88 with a modified CCU1, Grass Technologies, West Warwick, RI) using a 2-cm half-sphere silver cathode placed in the popliteal fossa and a circular 10-cm-diameter silver dispersive pad (anode) positioned just superior to the patella. The half-sphere shape of the cathode helped in ensuring good contact with the skin; the cathode was held in place using a custom fabricated Velcro strap. Data were acquired at a sampling rate of 10 kHz per channel.
Fifteen H-reflexes with an associated M-wave value between 7% and 13% of maximum were pooled and averaged for each testing position. Stimulation intensity was adjusted throughout the experiment to maintain consistent stimulation level that evoked an M-wave value between 7% and 13% of maximum. The step cycle was divided into midstance and midswing, and the stimuli were presented randomly during a continuous gait cycle (eg, every fourth step at midstance). H-reflexes of the same effective stimulus strength, reflected by M-wave amplitude, were included and compared. Peak-to-peak H-reflex amplitudes for each phase component were pooled and averaged. We did not control for the order effect of the testing positions on H-reflexes. All participants were tested in the order of semireclined, standing, midstance, and midswing.
Experiment 1
Soleus H-reflexes were elicited in 26 patients after SCI and 16 noninjured people in 2 positions: semireclined (foot fixed against a footplate at an angle of 100°) and static standing (SCI patients were allowed to use the assistive device of their choice for minimal balance if needed). Fifteen H-reflexes each were evoked in these positions. In addition, the position-dependent modulation was defined as the change in H-reflex amplitude from the semireclined to static standing position. Position-dependent modulation was calculated using the following formula (modified from the formula in Yang et al (26)):
 |
Experiment 2
Eight patients with SCI and 5 noninjured participants (a subset of the experiment 1 participants) walked at 2 different treadmill walking speeds: 0.6 m/s and 1.2 m/s. As described above, 15 H-reflexes were evoked in midstance and midswing phases of walking at both speeds (4,13). Electrical pulses were delivered manually by visually identifying the midstance phase. The testing phase was confirmed using a footswitch marker (insole with contacts in heel, first and fifth metatarsal, and great toe, B & L Engineering, Santa Ana, CA). To compare the reflex modulation between the 2 treadmill speeds, a walking modulation index (26) was calculated using the formula below:
Spatial and Temporal Gait Parameters
Subjects walked the length of a 25′10″ × 4′8′ walkway that included an embedded 11′9″ × 2′8″ computerized gait mat (GAIT MAT II: E.Q., Inc, Chalfont, PA) recording footfall patterns. Participants were instructed to walk at their “natural comfortable walking speed” using an assistive device of their choice.
Walking Environment (Locomotor Training)
Participants were fitted with a trunk and pelvic harness (Robertson Harness, Henderson, NV) specifically designed to comfortably assist in support of their body weight during locomotor training on a treadmill. The Vigor Body Weight support system (Vigor Therapy Solutions, Stevensville, MI) was used to pneumatically adjust the support to 40% of the person's body weight (10,24). A force transducer placed in line with the overhead harness system was used to record body weight support simultaneously with EMG and H-reflex stimuli during testing. A Biodex Rehabilitation treadmill (Biodex Medical Systems, Shirley, NY) with 0.1-mph speed increments was used for the training. No assistive devices were used by the patients with i-SCI during testing on the treadmill. Manual assistance for stepping on the treadmill was provided by skilled trainers specifically trained to assist subjects to initiate or maintain good stepping kinematics (defined as coordinated, rhythmic steps with knee, hip, and ankle motions that visually appeared to be most efficient as well as consistent with normal stepping spatial-temporal characteristics).
Walking Index for SCI
Walking Index for SCI (WISCI) has been reported to be a valid and reliable measure for measuring walking ability after SCI (27). WISCI scores were calculated based on the assistive device and level of assistance needed. The WISCI was used to categorize the level of physical assistance and use of assistive devices required for walking. The WISCI is a 20-item scale with a score of 0 (patient unable to walk) to 20 (patient can walk with no assistive device, no braces, and no assistance for at least 10 m). Spasticity of the calf muscles was tested in a supine position using a modified Ashworth score (MAS; a popular clinical test) (28). This score measures the degree of muscle hypertonia on a 5-point scale, ranging from 0 to 4 (0: no resistance to passive movement; 4: affected part is rigid).
Data Analysis
Peak-to-peak amplitude of all H-reflexes and M waves was calculated. All the H-reflexes were normalized to the maximum M wave. Paired t tests were calculated to examine differences in H-reflexes in semireclined and standing in the noninjured and SCI groups. Paired t tests were also used to compare soleus H-reflex Modulation Index at 2 treadmill speeds (0.6 m/s and 1.2 m/s) in both groups. Pearson's correlation coefficients were calculated. Background soleus EMG activity for all H-reflex trials was calculated by measuring the root mean square of the soleus EMG activity 100 ms before tibial nerve stimulation.
All statistical tests were performed using SPSS software (SPSS Inc, Chicago, IL), and significance for all tests was set at the alpha level of 0.05.
H-reflex Outcome Parameters
H-reflex upregulation and loss of H-reflex inhibition/depression seen after SCI refers to greater reflex amplitude compared with noninjured people. H-reflex depression as a result of change in posture or task refers to the ability of the spinal cord to inhibit the reflex, resulting in smaller reflex amplitudes. Soleus H-reflex modulation refers to the ability to increase or decrease the H-reflex amplitude in a task-specific manner. Thus, an impaired or decreased postural reflex modulation would refer to the inability to change (modulate) reflex amplitude based on the changing posture or task. Modulation index value reflects level of reflex modulation. Increase in the modulation index from one task to another would refer to greater reflex modulation. No change in modulation index would reflect a lack of or impaired reflex modulation.
RESULTS
Table 1 lists the demographic data and scores from clinical tests.
Experiment I
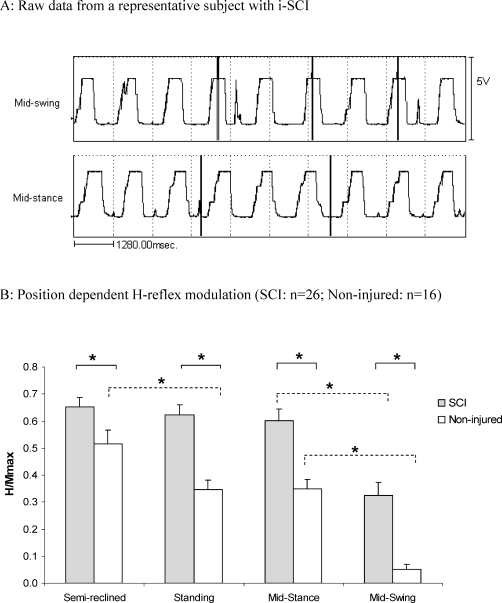
The noninjured participants showed a significant soleus H-reflex depression from semireclined to standing (P < 0.05; Figure 1). However, patients with SCI showed no change in soleus H-reflex amplitude from semireclined to standing. H-reflex was significantly greater in SCI patients compared with noninjured participants in all positions (See Figure 1 for semireclined, standing, and midstance and midswing phases of walking; P < 0.05). Position-dependent H-reflex modulation was significantly greater in noninjured participants (0.22) compared with SCI patients (−0.06).
Figure 1.
(A) Footswitch data showing accuracy and consistency of visual determination of the point of stimulation in midstance and midswing phases of walking in a representative spinal cord injury patient. Footswitches were turned off (flatline) upon weight bearing in the stance phase and turned on (positive deflection) as soon as weight was taken off the leg in the swing phase. The duration of the flatline indicates the entire stance phase, and the duration of the positive deflection indicates the entire swing phase. The vertical lines indicate the point of electrical stimulation to evoke H-reflexes. Note that stimulation was accurately and consistently delivered in the midstance and midswing phases of the walking cycle. (B) * = P < 0.05; vertical bars represent standard error of mean.
Experiment II
In noninjured participants, the soleus H-reflex modulation index of walking was significantly greater (P < 0.05) at 1.2 m/s vs 0.6 m/s (Figure 2). However, the walking modulation index in SCI patients remained the same at both fast and slow speeds.
Figure 2.
The soleus H-reflex modulation index of walking at 1.2 m/s compared with 0.6 m/s. * = P < 0.05; vertical bars represent standard error of the mean.
Correlation Between H-reflexes and Clinical Tests
We found statistically significant (P < 0.05) positive correlations between MAS and H-reflexes tested in semireclined (0.65), static standing (0.60), midstance (0.70), and midswing (0.44) positions. We also found statistically significant (P < 0.05) negative correlations between the WISCI scores and H-reflexes in standing (−0.47) and midstance (−0.50). A significant (P < 0.05) negative correlation was also found between WISCI and MAS (−0.52).
Based on our data and previous data on position-dependent reflex modulation in noninjured people (∼15 ± 5%) (29), we divided the SCI patients into 2 subgroups based on the cut-off value of 10% position-dependent reflex modulation. The 2 groups were normal position-dependent modulation (>10%; normal group, n = 8) and impaired position-dependent modulation group (<10%; impaired group, n = 18). The normal modulation group showed a significantly greater modulation and greater WISCI scores compared with the impaired modulation group (P < 0.0001). The mean modulation and WISCI scores of the control group were 23% and 19% and in the impaired modulation group were −4% and 13%, respectively.
Background EMG activity in the soleus muscle did not change systematically along with soleus H-reflex amplitude (Table 2).
Table 2.
Mean Background Soleus Electromyogram Activity 100 ms Before H-reflex
DISCUSSION
The soleus H-reflexes were highly modulated in a position-dependent and walking speed-dependent manner in participants with an intact spinal cord. In contrast, in SCI patients, we found that the position-dependent soleus H-reflex modulation from semireclined to standing and walking speed-dependent modulation from 0.6 m/s to 1.2 m/s treadmill speed were both not modulated.
In the results from the first experiment, the impairment of neuromodulatory processes after SCI was evident in the finding of significantly greater H-reflexes compared with noninjured participants in all tested positions (semireclined, standing, midstance, and midswing). These results are in agreement with past reports showing the significant loss of H-reflex depression in SCI patients compared with noninjured people (2,4,30). In addition to greater reflex amplitudes in all positions for SCI patients compared with noninjured controls, we also found that position-dependent modulation typically seen in noninjured people was severely impaired after SCI. People with an intact spinal cord show the ability to modulate the H-reflexes with a change in body position, such as from prone to standing (29,31,32), supine to standing (33), and sitting to standing (33–35). The neurophysiologic changes underlying body position changes are based on the specific demands of the task. Switching from a sitting, supine, or prone position to static standing position imposes additional demands of posture as the base of support decreases. Depression of reflex amplitude in standing in noninjured people may be the result of a lower base of support that induces a high amplitude reflex response, which can potentially destabilize the posture.
Position-dependent H-reflex modulation from prone to standing has been previously reported to reverse in elderly (mean age 71 years) compared with young (mean age 23 years) noninjured people (29). In our study, we did not see such a reversal in reflex amplitude, but, on the contrary, the mean H-reflex amplitude significantly decreased from ∼50% to ∼35% in the noninjured participants (P < 0.05; Figure 1). However, the mean age of our SCI patients (45 years) was not significantly different from the noninjured participants (38 years; P > 0.05). Thus, the lack of position-dependent H-reflex modulation seen in our patients with SCI was not an age-related phenomenon but instead was related to SCI. Our results of loss of position-dependent reciprocal inhibition of soleus H-reflexes after SCI are also in agreement with previous reports documenting impaired reflex inhibition after SCI (14,15).
In comparison, position-dependent H-reflex modulation may not be impaired in persons with complete SCI. Kawashima et al reported that position-dependent soleus H-reflex modulation from sitting to standing was intact in patients with motor complete SCI (35). In addition to SCI severity, different testing conditions may also explain the lack of modulation seen in our study. We used active independent standing and semireclined testing positions compared with the passive standing and sitting positions used in the Kawashima et al study. The results of our current study, however, are in agreement with the study in patients with i-SCI by Perez and Field-Fote, who reported loss of position-dependent modulation of reciprocal inhibition from sitting to active standing (14).
In the second experiment, our results further elucidate the specificity of impairments in H-reflex modulation after SCI. The increase in soleus H-reflex modulation at faster treadmill speed seen in noninjured participants was absent in patients with SCI. Thus, it may be that speed-dependent gating necessary to facilitate walking at faster speed was absent, and thus the reflex modulation was identical at 2 speeds (0.6 m/s and 1.2 m/s) after SCI. Previous studies have reported that locomotor EMG pattern after SCI can be modulated within a range of treadmill walking speeds (36–38); however, in the current study, we did not test H-reflexes at speeds from 0.6 m/s (slow walking speed) to 1.2 m/s (normal walking speed).
Most patients demonstrating an impaired reflex modulation during walking present with gait impairments that limit the velocity and increase the energy requirement of walking (23,39–41). As suggested in previous literature, reduced presynaptic inhibition, associated with spasticity, may hinder heel contact and forward progression over the stance limb, and impaired gating of incoming Ia afferent discharge associated with soleus stretch may account for difficulty in forward progression (4,42).
Although soleus H-reflexes provide us with a window into the spinal cord to study spinal excitability, the nature of correlation with clinical tests is not clear. In this study, we report significant moderate to strong positive and negative correlations with clinical tests, such as MAS and WISCI. We found a significant positive correlation between H-reflex amplitudes and MAS scores, suggesting that the bedside clinical assessment of muscle tone (of both neural and musculo-tendinous origin) can give us an estimate of spinal excitability of the lower limb motoneurons after SCI. We also found a significant negative correlation between H-reflexes in standing and midstance positions and WISCI scores. Thus, a lower H-reflex amplitude was related to a greater WISCI score (greater independence during walking). We also found that the subgroup of SCI patients with normal position-dependent modulation had significantly greater WISCI scores, thereby suggesting that reflex amplitude modulation in standing and midstance positions may be an important factor underlying static and dynamic balance (29).
CONCLUSIONS
Position-dependent H-reflex modulation from semireclined to standing and from 0.6 m/s to 1.2 m/s, typically seen in noninjured people, is impaired after SCI. Soleus H-reflexes are positively correlated with spasticity (MAS) and negatively correlated with level of walking assistance (WISCI). Impaired position-dependent reflex modulation and strong correlation with clinical tests may assist in demonstrating the underlying functional relevance of neurophysiologic impairment after SCI. Naturally, the restitution of position-dependent H-reflex modulation (tested in different positions) would be considered a favorable change, especially if related to functional improvement. Soleus H-reflexes are strongly correlated with changes in clinical outcomes, such as MAS and WISCI scores, and can be used as a tool to assess the neurophysiologic mechanism underlying the recovery of walking after SCI.
Acknowledgments
This work was supported by Christopher Reeve Paralysis Foundation grant #KA2-000202 (M.H.T., C.G.K., A.L.B.), National Institutes of Health; the National Center for Medical Rehabilitation Research K-O1 Human Development 01348-01 Award (A.L.B.); and Veterans Affairs Rehabilitation Research & Development grant F2182C (A.L.B.). We thank the locomotor training team at the University of Florida and VA Brain Rehabilitation Research Center and all of the study participants. The views expressed do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
References
- Barbeau H, Pepin A, Norman KE, Ladouceur M, Leroux A. Walking after spinal cord injury: control and recovery. Neuroscientist. 1998;4(1):14–24. [Google Scholar]
- Phadke CP, Wu SS, Thompson FJ, Behrman AL. Comparison of soleus H-reflex modulation after incomplete spinal cord injury in 2 walking environments: treadmill with body weight support and overground. Arch Phys Med Rehabil. 2007;88(12):1606–1613. doi: 10.1016/j.apmr.2007.07.031. [DOI] [PubMed] [Google Scholar]
- Phadke CP, Wu SS, Thompson FJ, Behrman AL. Soleus H-reflex modulation in response to change in percentage of leg loading in standing after incomplete spinal cord injury. Neurosci Lett. 2006;403(1,2):6–10. doi: 10.1016/j.neulet.2006.04.058. [DOI] [PubMed] [Google Scholar]
- Yang JF, Fung J, Edamura M, Blunt R, Stein RB, Barbeau H. H-reflex modulation during walking in spastic paretic subjects. Can J Neurol Sci. 1991;18(4):443–452. doi: 10.1017/s0317167100032133. [DOI] [PubMed] [Google Scholar]
- Trimble MH, Behrman AL, Flynn SM, Thigpen MT, Thompson FJ. Acute effects of locomotor training on overground walking speed and H-reflex modulation in individuals with incomplete spinal cord injury. J Spinal Cord Med. 2001;24(2):74–80. doi: 10.1080/10790268.2001.11753558. [DOI] [PubMed] [Google Scholar]
- Mynark RG. Reliability of the soleus H-reflex from supine to standing in young and elderly. Clin Neurophysiol. 2005;116(6):1400–1404. doi: 10.1016/j.clinph.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Palmieri RM, Hoffman MA, Ingersoll CD. Intersession reliability for H-reflex measurements arising from the soleus, peroneal, and tibialis anterior musculature. Int J Neurosci. 2002;112(7):841–850. doi: 10.1080/00207450290025851. [DOI] [PubMed] [Google Scholar]
- Hopkins JT, Ingersoll CD, Cordova ML, Edwards JE. Intrasession and intersession reliability of the soleus H-reflex in supine and standing positions. Electromyogr Clin Neurophysiol. 2000;40(2):89–94. [PubMed] [Google Scholar]
- Handcock PJ, Williams LR, Sullivan SJ. The reliability of H-reflex recordings in standing subjects. Electromyogr Clin Neurophysiol. 2001;41(1):9–15. [PubMed] [Google Scholar]
- Ali A, Sabbahi MA. Test-retest reliability of the soleus H-reflex in three different positions. Electromyogr Clin Neurophysiol. 2001;41(4):209–214. [PubMed] [Google Scholar]
- Knikou M. The H-reflex as a probe: pathways and pitfalls. J Neurosci Methods. 2008;171(1):1–12. doi: 10.1016/j.jneumeth.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Knikou M, Angeli CA, Ferreira CK, Harkema SJ. Soleus H-reflex modulation during body weight support treadmill walking in spinal cord intact and injured subjects. Exp Brain Res. 2009;193(3):397–407. doi: 10.1007/s00221-008-1636-x. [DOI] [PubMed] [Google Scholar]
- Capaday C, Stein RB. Amplitude modulation of the soleus H-reflex in the humans during walking and standing. J Neurosci. 1986;6(5):1308–1313. doi: 10.1523/JNEUROSCI.06-05-01308.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez MA, Field-Fote EC. Impaired posture-dependent modulation of disynaptic reciprocal Ia inhibition in individuals with incomplete spinal cord injury. Neurosci Lett. 2003;341(3):225–228. doi: 10.1016/s0304-3940(03)00183-6. [DOI] [PubMed] [Google Scholar]
- Field-Fote EC, Brown KM, Lindley SD. Influence of posture and stimulus parameters on post-activation depression of the soleus H-reflex in individuals with chronic spinal cord injury. Neurosci Lett. 2006;410(1):37–41. doi: 10.1016/j.neulet.2006.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phadke CP, Flynn SF, Thompspon FJ, Behrman AL, Trimble MH, Kukulka CG. Comparison of single bout effects of bicycle training versus locomotor training on paired reflex depression of the soleus H-reflex after motor incomplete spinal cord injury. Arch Phys Med Rehabil. 2009;90(7):1218–1228. doi: 10.1016/j.apmr.2009.01.022. [DOI] [PubMed] [Google Scholar]
- Ferris DP, Aagaard P, Simonsen EB, Farley CT, Dyhre-Poulsen P. Soleus H-reflex gain in humans walking and running under simulated reduced gravity. J Physiol. 2001;530(pt 1):167–180. doi: 10.1111/j.1469-7793.2001.0167m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaday C, Stein RB. Difference in the amplitude of the human soleus H reflex during walking and running. J Physiol. 1987;392(Nov):513–522. doi: 10.1113/jphysiol.1987.sp016794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen EB, Dyhre-Poulsen P. Amplitude of the human soleus H reflex during walking and running. J Physiol. 1999;515(pt 3):929–939. doi: 10.1111/j.1469-7793.1999.929ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edamura M, Yang JF, Stein RB. Factors that determine the magnitude and time course of human H-reflexes in locomotion. J Neurosci. 1991;11(2):420–427. doi: 10.1523/JNEUROSCI.11-02-00420.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidler JM. What is next for locomotor-based studies. J Rehabil Res Devel. 2005;42(1):10–16. doi: 10.1682/jrrd.2005.42.0010. [DOI] [PubMed] [Google Scholar]
- Visintin M, Barbeau H. The effects of parallel bars, body weight support and speed on the modulation of the locomotor pattern of spastic paretic gait: a preliminary communication. Paraplegia. 1994;32(8):540–553. doi: 10.1038/sc.1994.86. [DOI] [PubMed] [Google Scholar]
- Knutson E. Studies of Gait Control in Patients With Spastic Paresis. Amsterdam: Elseveir; 1985. [Google Scholar]
- Behrman AL, Harkema SJ. Locomotor training after human spinal cord injury: a series of case studies. Phys Ther. 2000;80(7):688–700. [PubMed] [Google Scholar]
- Maynard FM, Jr, Bracken MB, Creasey G, et al. International Standards for Neurological and Functional Classification of Spinal Cord Injury. American Spinal Injury Association. Spinal Cord. 1997;35(5):266–274. doi: 10.1038/sj.sc.3100432. [DOI] [PubMed] [Google Scholar]
- Yang J, Stein RB, James KB. Contribution of peripheral afferents to the activation of the soleus muscle during walking in humans. Exp Brain Res. 1991;87:679–687. doi: 10.1007/BF00227094. [DOI] [PubMed] [Google Scholar]
- Ditunno JF, Jr, Ditunno PL, Graziani V, et al. Walking index for spinal cord injury (WISCI): an international multicenter validity and reliability study. Spinal Cord. 2000;38(4):234–243. doi: 10.1038/sj.sc.3100993. [DOI] [PubMed] [Google Scholar]
- Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987;67(2):206–207. doi: 10.1093/ptj/67.2.206. [DOI] [PubMed] [Google Scholar]
- Angulo-Kinzler RM, Mynark RG, Koceja DM. Soleus H-reflex gain in elderly and young adults: modulation due to body position. J Gerontol A Biol Sci Med Sci. 1998;53(2):M120–M125. doi: 10.1093/gerona/53a.2.m120. [DOI] [PubMed] [Google Scholar]
- Trimble MH, Behrman AL, Flynn SM, Thigpen MT, Thompson FJ. Acute effects of locomotor training on overground walking speed and H-reflex modulation in individuals with incomplete spinal cord injury. J Spinal Cord Med. 2001;24(2):74–80. doi: 10.1080/10790268.2001.11753558. [DOI] [PubMed] [Google Scholar]
- Jeon HS, Kukulka CG, Brunt D, Behrman AL, Thompson FJ. Soleus H-reflex modulation and paired reflex depression from prone to standing and from standing to walking. Int J Neurosci. 2007;117(12):1661–1675. doi: 10.1080/00207450601067158. [DOI] [PubMed] [Google Scholar]
- Koceja DM, Markus CA, Trimble MH. Postural modulation of the soleus H reflex in young and old subjects. Electroencephalogr Clin Neurophysiol. 1995;97(6):387–393. doi: 10.1016/0924-980x(95)00163-f. [DOI] [PubMed] [Google Scholar]
- Goulart F, Valls-Sole J, Alvarez R. Posture-related changes of soleus H-reflex excitability. Muscle Nerve. 2000;23(6):925–932. doi: 10.1002/(sici)1097-4598(200006)23:6<925::aid-mus13>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Hayashi R, Tako K, Tokuda T, Yanagisawa N. Comparison of amplitude of human soleus H-reflex during sitting and standing. Neurosci Res. 1992;13(3):227–233. doi: 10.1016/0168-0102(92)90062-h. [DOI] [PubMed] [Google Scholar]
- Kawashima N, Sekiguchi H, Miyoshi T, Nakazawa K, Akai M. Inhibition of the human soleus Hoffman reflex during standing without descending commands. Neurosci Lett. 2003;345(1):41–44. doi: 10.1016/s0304-3940(03)00485-3. [DOI] [PubMed] [Google Scholar]
- Dobkin BH, Harkema S, Requejo P, Edgerton VR. Modulation of locomotor-like EMG activity in subjects with complete and incomplete spinal cord injury. J Neurol Rehabil. 1995;9(4):183–190. [PubMed] [Google Scholar]
- Pepin A, Norman KE, Barbeau H. Treadmill walking in incomplete spinal-cord-injured subjects: 1. Adaptation to changes in speed. Spinal Cord. 2003;41(5):257–270. doi: 10.1038/sj.sc.3101452. [DOI] [PubMed] [Google Scholar]
- Beres-Jones JA, Harkema SJ. The human spinal cord interprets velocity-dependent afferent input during stepping. Brain. Oct 2004;127(pt 10):2232–2246. doi: 10.1093/brain/awh252. [DOI] [PubMed] [Google Scholar]
- Barbeau H, Norman K, Fung J, Visintin M, Ladouceur M. Does neurorehabilitation play a role in the recovery of walking in neurological populations. Ann N Y Acad Sci. 1998;860(Nov):377–392. doi: 10.1111/j.1749-6632.1998.tb09063.x. [DOI] [PubMed] [Google Scholar]
- Conrad B, Benecke R, Meinck HM. Gait Disturbances in Paraspastic Patients. Amsterdam, The Netherlands: Elsevier Science Publishers; 1985. [Google Scholar]
- Perry J, Hoffer MM, Giovan P, Antonelli D, Greenberg R. Gait analysis of the triceps surae in cerebral palsy: a preoperative and postoperative clinical and electromyographic study. J Bone Joint Surg Am. 1974;56(3):511–520. [PubMed] [Google Scholar]
- Crenna P, Frigo C. Excitability of the soleus H-reflex arc during walking and stepping in man. Exp Brain Res. 1987;66(1):49–60. doi: 10.1007/BF00236201. [DOI] [PubMed] [Google Scholar]