Abstract
Study Design:
Prospective clinical study.
Background:
Pressure ulcers interfere with the rehabilitation process in patients with spinal cord injury (SCI) and are a significant deterrent to participation in activities that contribute to independent, productive, and satisfying life.
Objective:
To evaluate the effect of surgery for pressure ulcers on general health and quality of life in patients with SCI.
Setting:
Tertiary care center in northern India.
Methods:
Various types of flap surgery were performed on 30 patients with SCI and 32 pressure ulcers (stages III and IV). Outcome was evaluated using general improvement in health (hemoglobin, serum proteins, and general well-being), patient satisfaction, and global quality of life scores (according to the visual analog scale).
Results:
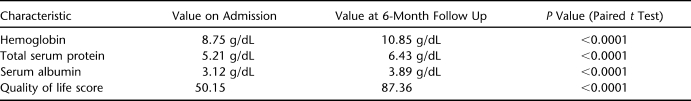
At admission, the mean values of global quality of life, hemoglobin, serum albumin, and total serum proteins were 50.15 (range, 30–65), 8.75 g/dL (range, 6–12 g/dL), 3.12 g/dL (range, 2.9–4.3 g/dL), and 5.21 (range, 5–6.2 g/dL), respectively. At 6-month follow up, mean values of global quality of life score, hemoglobin, serum albumin, and total serum proteins were 87.36 (range, 44–96), 10.85 g/dL (range, 8.2–13.5 g/dL), 3.89 g/dL (range, 3.2–4.5 g/dL), and 6.43 g/dL (range, 5.85–6.70 g/dL), respectively. The overall rise in quality of life scores, hemoglobin, serum albumin, and total serum proteins was statistically significant. Most of the patients (76.7%) reported improvement in subjective well-being, and 83.3% were satisfied with the ultimate outcome of the surgery.
Conclusion:
Results suggest that surgery for stages III and IV pressure ulcers offers the greatest benefit to the patients in terms of improvement in general health (anemia, hypoproteinemia, and general well-being) and quality of life.
Keywords: Spinal cord injuries; Pressure ulcers; Rehabilitation; Quality of life; Surgery, flap
INTRODUCTION
Because life expectancy is steadily improving through modern care, the increased survival in patients with spinal cord injury (SCI) is associated with secondary complications that continue to pose management challenges and impair quality of life (QoL) (1,2). Secondary complications prolong the length of hospital stay, make rehabilitation more difficult, and increase the cost of care (2). Despite advances in medical care, prevention and cure of pressure ulcers (PrUs) remains a significant problem (3). Pressure ulcers were reported as the most frequent secondary medical complications in all years in an analysis of long-term medical complications after traumatic SCI (4). The primary factors include pressure, shear, moisture secondary to perspiration or incontinence, anemia and nutritional deficiencies, and aged skin. Patients with PrUs often suffer from anemia and serum protein deficiencies (total hypoproteinemia and hypoalbuminemia) (5,6). Pressure ulcers have a profound impact, including negative effects on physical, social, and financial status; change of body image; and/or loss of independence and control (7). Pressure ulcers are a major obstacle to long-term rehabilitation and are associated with low QoL scores (1).
In general, superficial PrUs (stages I and II) are likely to respond to conservative treatment, whereas deep PrUs (stages III and IV) often require surgical intervention (8). Conservative treatment is associated with prolonged immobilization and is accompanied by a higher incidence of recurrence. Different operative procedures have been described to repair PrUs, including direct closure, skin grafting, skin flaps, musculocutaneous flaps, fasciocutaneous flaps, and free flaps (8). The present study aimed to evaluate the effect of surgery for PrUs on general health and QoL in SCI patients.
MATERIALS AND METHODS
The study included 30 patients with PrUs (stages III and IV) who presented to a tertiary level referral center from April 2005 through March 2008. All 30 patients met the following eligibility criteria: (a) occurrence of a traumatic event resulting in SCI with PrU, (b) failure of conservative treatment to heal the PrU, (c) minimum regular follow up of 6 months, (d) signed informed consent, (e) age older than 18 years, (f) injury below C4. Patients were excluded if they had chronic medical illness prior to injury that could affect rehabilitation outcome appreciably, such as head injury, neuropsychologic disorder, and brain tumor.
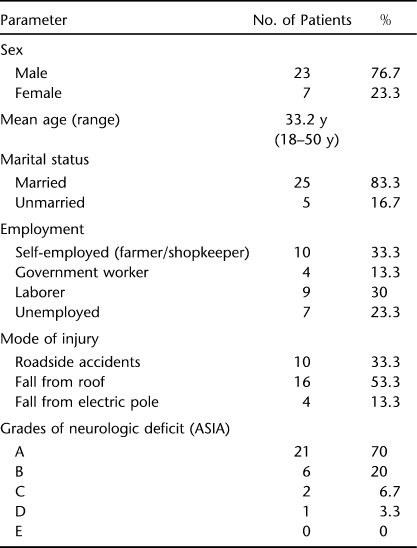
Patients were given detailed information about the purpose of study, and written consent was obtained from all the participants. Evaluation included complete history to rule out occult medical or neuropsychologic problems and complete physical and neurologic examinations. Demographic data are given in Table 1. Most of the patients (53.3%) developed PrUs within 1 month of injury and 33.3% within 1 to 3 months of injury; only 13.3% had chronic SCI. In the present study, PrUs were present from 20 to 270 days, with an average of 91.85 days, before surgery. Neurologic deficits according to the ASIA Impairment Scale were as follows: A in 21, B in 6, C in 2, D in 1, and E in 0. Testing included radiography of the injury site as well as at the site of PrU and routine blood work (hemoglobin, bleeding time, clotting time, blood urea, blood glucose, electrolytes, total serum protein, serum albumin). The eschar was debrided, and PrUs were staged according to the European Pressure Ulcer Advisory Panel (9).
Table 1.
Demographic Profile
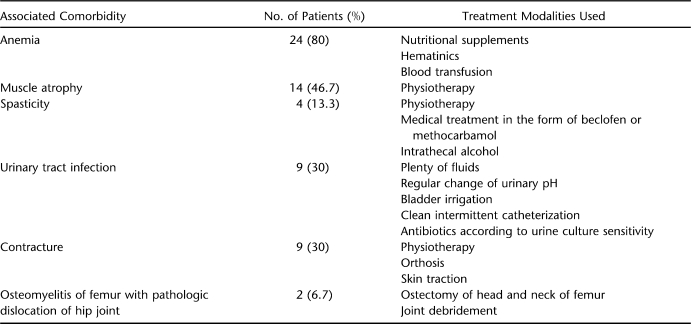
Associated comorbidities were assessed and managed according to the protocol outlined in Table 2. Nutritional status was improved with a diet high in protein and calories and supplemented with iron and vitamins. Severe hypoproteinemia was corrected by transfusing amino acid supplementation or total parenteral nutrition. Severely anemic patients and patients requiring major debridement were given blood transfusions.
Table 2.
Associated Comorbidities and Their Management
Locations of PrUs were 23 on sacrum; 11 on trochanters; 2 on heel; and 1 each on ischial tuberosity, thorax, and lateral malleolus. Systemic antibiotics were used to treat sepsis, advancing cellulitis, or osteomyelitis and were chosen based on the results of blood culture and wound biopsy cultures. Exposed bony prominences were removed with an osteotome. All necrotic tissue was removed, and surgery was performed on a total of 32 stage III and IV PrUs (23 sacral, 7 trochanteric, 1 ischial tuberosity, 1 thorax). Of the surgically treated PrUs, 15 were stage III and 17 were stage IV. The following general measures were taken in all patients: (a) change of posture every 2 hours; (b) use of water or air beds; (c) avoidance of creases in bed sheets of patients; (d) encouragement to use clean intermittent self-catheterization to avoid wetting of the bed and body. Patients with indwelling catheters were also taught intermittent catheterization and encouraged to use this method; (e) good nutritious diet; (f) daily antiseptic dressing or dressing and debridement of the wound preoperatively; (g) patients were taught to lie prone for extended periods, so that they could adopt this posture in postoperative period.
Postoperative Care and Follow Up
In addition, general principles of preoperative care, the following measures were taken: (a) daily inspection of flap by surgeon until the patient was admitted to the hospital and after discharge by patient and/or caretaker; (b) proper positioning to avoid any pressure on the flap and change of posture/turning allowed earliest by 2 weeks of the surgery or later according to the flap-healing situation; (c) indwelling catheter during surgery and at least 2 weeks postoperatively or until patient was allowed to turn; (d) sitting allowed after 6 weeks; (e) proper wheelchair/cushions/orthosis for mobilization and to avoid any pressure on flap; (f) monthly follow up for 3 months and then at 6 months after flap surgery; (g) reinforcement of general care principles to patients and/or caretakers at each follow up to avoid any recurrence.
The outcomes of the study were evaluated at 6-month follow up using (a) general improvement in health (hemoglobin, serum albumin, total serum proteins, well-being), (b) patient satisfaction, and (c) global quality of life scores (according to the visual analog scale) (10). Patient satisfaction was assessed by the following questions: (a) Had surgery resulted in improvement in subjective well-being? (b) Was the patient satisfied with ultimate rehabilitation outcome after surgery? (c) Had surgery led to improvement in QoL? (d) In what areas of life had QoL improved after surgery?
Student's t test for independent samples was used to compare the data between 2 groups of values, that is, at the time of admission and at 6-month follow up. Bonferroni correction was used to test the statistical significance, and P ≤ 0.01 was considered significant.
Statement of Ethics
“We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of this research.”
RESULTS
At admission, the mean values of global QoL, hemoglobin, serum albumin, and total serum proteins were 50.15 (range, 30–65), 8.75 g/dL (range, 6–12 g/dL), 3.12 g/dL (range, 2.9–4.3 g/dL), and 5.21 (range, 5–6.2 g/dL), respectively. Fasciocutaneous rotation flap surgery was performed on 7 PUs, tensor fascia lata flap surgery on 7, gluteus maximus island flap surgery on 3, unilateral gluteus maximus V-Y advancement flap surgery on 4, and bilateral gluteus maximus V-Y advancement flap surgery on 11. An average of 1.72 units of blood/patient (range 0–4 units) were transfused preoperatively, and 1 unit (range 0–2 units) was transfused perioperatively or postoperatively. Patients were discharged from the hospital 35.84 days (range, 17–92 days) after surgery. Total duration of stay in hospital averaged 93.20 days (range, 25–211 days). At 6-month follow up, mean values of global QoL, hemoglobin, serum albumin, and total serum proteins were 87.36 (range, 44–96), 10.85 g/dL (range, 8.2–13.5 g/dL), 3.89 g/dL (range, 3.2–4.5 g/dL), and 6.43 g/dL (range, 5.85–6.70 g/dL), respectively, indicating a total rise in hemoglobin of 2.1 g/dL, a rise in serum albumin by 0.72 g/dL, and a rise in the total serum protein of 1.22 g/dL from the mean preoperative values. Overall rise in global QoL score, hemoglobin, serum albumin, and total serum protein did reach the level of statistical significance (Table 3). The following areas of QoL improved in patients after surgery: self-esteem in 26 (86.6%), personal hygiene in 28 (93.3%), social participation in 23 (76.6%), mobility in 20 (66.6%), family/partner relations in 21 (70%), and financial status in 24 (80%).
Table 3.
Comparison of Values of Hemoglobin, Serum Protein, Serum Albumin, and Quality of Life on Admission and at 6-Month Follow Up
At 6-month follow up, response to the questions about subjective well-being and patient satisfaction with rehabilitation outcome were as follows: 76.7% patients reported improvement in subjective well-being, and 83.3% were satisfied with the ultimate rehabilitation outcome after the surgery. There was one partial flap necrosis and 3 recurrences of stage I or II PUs at the same site of flap surgery. These were managed conservatively, and none required resurgery.
DISCUSSION
People with SCI are vulnerable across their lifespan to tissue breakdown that can interfere with initial rehabilitation in the acute post-traumatic recovery phase and successful reintegration into the communities, as well as lead to more serious medical complications and have profound psychologic consequences. Pressure ulcers are responsible for physical, social, vocational, and economic costs (11) and impair QoL (1). Operative management of deep PrUs allows rapid healing and reduces the risks of subsequent infection and recurrence (8). In the present study, we evaluated the effect of operative treatment of PrUs on general health and QoL in SCI patients.
The study has some limitations. Results were not compared with a control group in whom the PrUs healed conservatively or with patients living with a PrU 6 months after discharge. Flap surgery led to improvement in anemia, hypoalbuminemia, and hypoproteinemia in the present study, although overall improvement may be due to a summation effect of our comprehensive approach of dietary therapy, blood transfusion, and flap surgery.
Patients with PrUs often suffer from anemia and serum protein deficiencies, which complicate treatment (6). Anemia interferes with rehabilitation. The correct diagnosis is important for proper treatment because the anemia is the result of the inability to use iron stores in the reticuloendothelial system and not iron deficiency (5,6). Factors associated with hypoalbuminemia include losses of protein and albumin in PrU exudates and the presence of a chronic inflammatory state (12). The products of bacterial invasion and tissue breakdown form a foul-smelling, purulent discharge, which itself is destructive of new epithelium. The continuous discharge of proteolysed material leads to protein deficiency, anemia, temperature fluctuation, malaise, and general lowering of the constitutional status (13). Hepatic synthesis of albumin is also inhibited (5,6). Treatment of serum protein alterations should be based on a dietary therapy rich in protein and calories (6).
Altered nutritional status is a contributing factor in the development of PrUs and in delayed healing. Treating malnutrition reduces the risk of developing a PrU or speeds up its healing (14). Rieger et al (15) also emphasized the treatment of concomitant malnutrition. In the present study, almost all the patients were anemic and hypoproteinemic on admission. There was definite improvement in anemia, hypoalbuminemia, and hypoproteinemia after flap surgery, which was statistically significant even after Bonferroni correction. Scivolleto et al (5) reported a significant decrease in the number of red blood cells, decreased hemoglobin and hematocrit, increased numbers of white blood cells and ferritin, and transferrin and transferrin saturation; total hypoproteinemia and hypoalbuminemia with increased alpha-1 and gamma globulins; increased erythrocyte sedimentation rate; and elevated C-reactive protein levels. These alterations returned to normal after surgical intervention. We are of the opinion that the flap surgery leads to improvement in anemia, hypoalbuminemia, and hypoproteinemia, although the overall improvement may be due to a summation effect of a comprehensive approach of dietary therapy, blood transfusion, and flap surgery. Fuoco et al (6) also reported that both anemia and hypoproteinemia disappeared after PrU healing. Healing of PrUs prevents further loss of proteins through the wound (6).
Pressure ulcers contribute to low QoL scores in patients with paraplegia (1,7). Quality of life incorporates such variables as pain and suffering, financial cost of health care, effect on personal resources, and overall impact on one's life and activities of daily living (11,16). Franks et al (17) and Baltzi and Dafogianni (18) reported low self-care and mobility in case groups compared with controls. Social isolation negatively affects the lives of people with PrUs (7,16). Limited positions, odor, bulky dressings, and the need for special beds or a vacuum-assisted device to control exudates can adversely affect image and lifestyle and decrease mobility. Need for the caregiver to change dressings may lead to embarrassment, and relationships may be negatively affected (7). Pressure ulcers cause people with SCI to lose time from work, school, and social activities, leading to many concomitant problems. Losses in income, productivity, progress towards vocational goals, independence, self-esteem, and sense of self worth are some of the ramifications of PrUs (11). In the present study, 76.7% patients reported improvement in subjective well-being and QoL scores after surgical interventions. Improvement in self-esteem, personal hygiene, social participation, family and partner relationships, and finance in the majority of cases shows that the negative effect of PUs can be minimized and QoL can be improved by surgical intervention aimed at healing them.
Surgery allows rapid healing of PrUs and greatly improves QoL in individuals with SCI (19). It does not preclude any possibility of recurrence but has multiple benefits (19). There is evidence that surgical intervention makes it possible to heal existing ulcers more rapidly in patients with SCI, to greatly improve QoL, and to increase survival (20). In the present study, 83.3% patients were satisfied with the ultimate rehabilitational outcome after the surgery. We agree with the findings of Zogovska et al (8) that surgery achieves good long-term results and facilitates rehabilitation.
CONCLUSIONS
Surgery for stages III and IV PrUs benefits patients with SCI in terms of improvement in general health (anemia, hypoproteinemia, and general well-being) and QoL.
References
- Singh R, Dhankar SS, Rohilla R. Quality of life of people with spinal cord injury in northern India. Int J Rehabil Res. 2008;31(3):247–251. doi: 10.1097/MRR.0b013e3282fb7d25. [DOI] [PubMed] [Google Scholar]
- Drigotaite N, Krisciunas A. Complications after spinal cord injuries and their influence on the effectiveness of rehabilitation. Medicina (Kaunas) 2006;42(11):877–880. [PubMed] [Google Scholar]
- Colquhoun M. Nursing management of pressure sores. Nursing. 1983;11(2):320–322. [PubMed] [Google Scholar]
- McKinley WO, Jackson AB, Cardenas DD, DeVivo MJ. Long-term medical complications after traumatic spinal cord injury: a regional model systems analysis. Arch Phys Med Rehabil. 1999;80(11):1402–1410. doi: 10.1016/s0003-9993(99)90251-4. [DOI] [PubMed] [Google Scholar]
- Scivoletto G, Fuoco U, Morganti B, Cosentino E, Molinari M. Pressure sores and blood and serum dysmetabolism in spinal cord injury patients. Spinal Cord. 2004;42(8):473–476. doi: 10.1038/sj.sc.3101622. [DOI] [PubMed] [Google Scholar]
- Fuoco U, Scivoletto G, Pace A, Vona VU, Castellano V. Anaemia and serum protein alteration in patients with pressure ulcers. Spinal Cord. 1997;35(1):58–60. doi: 10.1038/sj.sc.3100340. [DOI] [PubMed] [Google Scholar]
- Langemo DK, Melland H, Hanson D, Olson B, Hunter S. The lived experience of having a pressure ulcer: a qualitative analysis. Adv Skin Wound Care. 2000;13(5):225–235. [PubMed] [Google Scholar]
- Zogovska E, Novevski L, Agai Li, Mircevski V, Peev I, Dzokic G. Our experience in treatment of pressure ulcers by using local cutaneous flaps. Prilozi. 2008;29(1):199–210. [PubMed] [Google Scholar]
- European Pressure Ulcer Advisory Panel (1999) Pressure ulcer treatment guidelines. EPUAP. Available at: http://www.EPUAP.org. Accessed March 20, 2005.
- Carlsson AM. Assessment of chronic pain. I: aspects of the reliability and validity of the visual analogue scale. Pain. 1983;16(1):87–101. doi: 10.1016/0304-3959(83)90088-X. [DOI] [PubMed] [Google Scholar]
- O'Connor FM, Salcido R. Pressure ulcers and spinal cord injury. In: Kirshblum S, Campagnolo DI, DeLisa JA, editors. Spinal Cord Medicine. Philadelphia, PA: Lippincott Williams & Wilkins; 2002. pp. 207–220. [Google Scholar]
- Perier C, Granouillet R, Chamson A, Gonthier R, Frey J. Nutritional markers, acute phase reactants and tissue inhibitor of matrix metalloproteinase 1 in elderly patients with pressure sores. Gerontology. 2002;48(5):298–301. doi: 10.1159/000065253. [DOI] [PubMed] [Google Scholar]
- Campbell RM, Delgado JP. Pressure sore. In: Converse JM, editor. Reconstructive Plastic Surgery. Philadelphia, PA: WB Saunders; 1977. pp. 3763–3799. [Google Scholar]
- Stratton RJ, Ek AC, Engfer M, et al. Enteral nutritional support in prevention and treatment of pressure ulcers: a systematic review and meta-analysis. Ageing Res Rev. 2005;4(3):422–450. doi: 10.1016/j.arr.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Rieger U, Scheufler O, Schmid D, Zweifel-Schlatter M, Kalbermatten D, Pierer G. Six treatment principles of the basle pressure sore concept. Handchir Mikrochir Plast Chir. 2007;39(3):206–214. doi: 10.1055/s-2007-965311. [DOI] [PubMed] [Google Scholar]
- Fox C. Living with a pressure ulcer: a descriptive study of patients' experiences. Br J Comm Med. 2002;7(6):10–22. doi: 10.12968/bjcn.2002.7.Sup4.12615. [DOI] [PubMed] [Google Scholar]
- Franks PJ, Winterberg H, Moffat CJ. Health-related quality of life and pressure ulceration assessment in patients treated in community. Wound Rep Reg. 2002;10(3):133–140. doi: 10.1046/j.1524-475x.2002.11002.x. [DOI] [PubMed] [Google Scholar]
- Baltzi E, Dafogianni C. Quality of life and bedsores. ICUS Nurs Web J. 2005;24(4):1–10. [Google Scholar]
- Dardour JC, Vilain R, Castro D. Evaluation of 10 years of surgical treatment for decubitus ulcer. Sem Hop. 1984;60(15):1051–1056. [PubMed] [Google Scholar]
- Baskov AV. The surgical treatment of decubitus ulcers in patients with spinal cord injury [in Russian] Zh Vopr Neirokhir Im N N Burdenko. 2000;Jan–Mar(1):7–10. [PubMed] [Google Scholar]