Abstract
Background:
Oxidative stress is a mediator of secondary injury to the spinal cord following trauma.
Objective:
To investigate the putative neuroprotective effect of α-lipoic acid (LA), a powerful antioxidant, in a rat model of spinal cord injury (SCI).
Methods:
Wistar albino rats were divided as control, vehicle-treated SCI, and LA-treated SCI groups. To induce SCI, a standard weight-drop method that induced a moderately severe injury (100 g/cm force) at T10 was used. Injured animals were given either 50 mg/kg LA or saline at 30 minutes postinjury by intraperitoneal injection. At 7 days postinjury, neurologic examination was performed, and rats were decapitated. Spinal cord samples were taken for histologic examination or determination of malondialdehyde (MDA) and glutathione (GSH) levels, myeloperoxidase (MPO) activity, and DNA fragmentation. Formation of reactive oxygen species in spinal cord tissue samples was monitored by using a chemiluminescence (CL) technique.
Results:
SCI caused a significant decrease in spinal cord GSH content, which was accompanied with significant increases in luminol CL and MDA levels, MPO activity, and DNA damage. Furthermore, LA treatment reversed all these biochemical parameters as well as SCI-induced histopathologic alterations. Conversely, impairment of the neurologic function caused by SCI remained unchanged.
Conclusion:
The present study suggests that LA reduces SCI-induced oxidative stress and exerts neuroprotection by inhibiting lipid peroxidation, glutathione depletion, and DNA fragmentation.
Keywords: Alpha-lipoic acid; Antioxidants; Spinal cord injuries; Trauma; Neuroprotection; Lipid Peroxidation; Glutathione, Myeloperoxidase; DNA damage
INTRODUCTION
The pathophysiology of spinal cord injury (SCI) is characterized by the initial, primary injury followed by secondary injury processes involving cascades of biochemical, molecular, and cellular changes, which can produce even more extensive damage (1,2). Although mechanical disruption of the nerve axons in the spinal cord is not amenable to neuroprotective therapy, changes in secondary injury are susceptible to therapeutic intervention. These secondary events include microvascular ischemia, oxidative stress, excitotoxicity, ion dysregulation, and inflammation. A growing body of biochemical, physiologic, and pharmacologic evidence has suggested that oxygen-free-radical–induced lipid peroxidation, working in concert with aberrant calcium fluxes and eicosanoid generation in particular, plays a key role in progressive posttraumatic spinal cord degeneration (2,3).
The brain and nervous system are highly vulnerable to free-radical-mediated insult because of their high lipid content. Free radicals damage various cellular components, including proteins, lipids, and DNA. Lipid peroxidation has been linked to microvascular damage and hypoperfusion, which, if severe enough, can lead to a secondary ischemic insult to the tissue following SCI. Moreover, inflammation is closely related to the overproduction of the reactive oxygen species (ROS) and plays an important role in various neuropathologies. Thus, agents with antioxidant and anti-inflammatory properties are proposed to be useful in the clinical setting of neuronal damage (4).
Alpha-lipoic acid (LA) and its reduced form, dihydrolipoic acid (DHLA), have gained considerable attention because of their roles as biologic thiol antioxidants, which are central to antioxidant defense in the brain and other tissues. Several features have been described for LA, which make it an outstanding antioxidant (5,6). LA readily crosses the blood–brain barrier and is a “metabolic antioxidant”; that is, it is accepted by human cells as substrate and is reduced to DHLA. Therefore, unlike ascorbic acid, DHLA is not destroyed by quenching free radicals, but rather can be recycled from LA. Moreover, LA and DHLA are amphipathic molecules and may act as antioxidants in hydrophilic and lipophilic environments. It shows beneficial effects in oxidative stress conditions because of its synergistic action with other antioxidants (7).
Kagan et al have shown that LA and DHLA may act as a strong direct chain-breaking antioxidant and may enhance the antioxidant potency of other antioxidants (ascorbate and vitamin E) in the aqueous and the hydrophobic membranous phases (8). Furthermore, reports emphasize that administration of lipoic acid has remarkable effects on tissue thiol status, increasing glutathione levels probably by reducing extracellular cystine to cysteine, which bypasses the cystine transporter (9). Because of its antioxidant activity, LA has been shown to be beneficial as a therapeutic agent in ischemia and reperfusion injury and diabetic complications (10–14) as well as various neurologic disorders related to oxidative stress (15–17). Also, it was previously shown that LA has neuroprotective effects in experimental brain injury caused by trauma and subarachnoid hemorrhage (18,19).
Accordingly, this study was designed to determine the possible protective effect of LA against oxidative stress following spinal cord injury in rats by determining biochemical parameters and histologic examination. We also evaluated the neurologic impairments caused by SCI.
MATERIALS AND METHODS
Animals
Three-month-old male Wistar albino rats (300–350 g) were housed in an air-conditioned room with 12-hour light-and-dark cycles, where the temperature (22 ± 2°C) and relative humidity (65–70%) were kept constant. All experimental protocols were approved by the Marmara University School of Medicine Animal Care and Use Committee.
Rats were divided into 4 groups of 24 rats in each group: (a) control group that underwent sham surgery and received saline intraperitoneally (ip); (b) LA (50 mg/kg/d ip); (c) SCI group that underwent surgery for SCI induction and was given saline; (d) SCI-induced and LA (50 mg/kg, ip) administered group. Three series of each group were used in our studies. The first serial was used for malondialdehyde, glutathione levels, and myeloperoxidase activity. The second serial was used for chemiluminescence and DNA fragmentation, while histopathologic evaluation was performed in the third serial. The tissue samples taken for each test was standardized, that is, it was taken from the same part in all groups.
Induction of SCI
Anesthetized (ip ketamine and chlorpromazine; 75 mg/kg and 1 mg/kg, respectively) rats were positioned in a prone position. Under sterile conditions, following T5–T12 midline skin incision and paravertebral muscle dissection, spinous processes and laminar arcs of T7–T10 were removed. The dura was left intact. Modified weight-drop model was performed for SCI (20). The animals were subjected to an impact of 100 g/cm (10-g weight from 10-cm height) to the dorsal surface of the spinal cord. The force was applied via a stainless steel rod (3-mm diameter tip) that was rounded at the surface. The rod was dropped vertically through a 10-cm guide tube that was positioned perpendicular to the center of the spinal cord. Afterwards, the muscles and the incision were sutured.
A week after SCI induction, neurologic examinations were performed in all groups. Then, rats were decapitated to obtain spinal cord tissue samples (the epicenter to caudal parts of the injury) for the biochemical and histologic analysis. The tissues were stored in −80°C for biochemical analysis.
Neurologic Examination
The neurologic examination scores were assessed according to motor function score of Gale et al (21). The evaluation was as follows: No movement of hind limbs, 0; Perceptible movement, 1; Visible joint movements, 2; Hind limb movement but cannot support body weight, 3; Hind limb movement and can support body weight, 4; Walking with mild deficit, 5; Normal walking, 6. All behavioral tests were conducted by a blinded investigator. The sequence of testing animals by a given task was randomized for the animals.
Chemiluminescence Assay
To assess the role of reactive oxygen species in SCI-induced tissue damage, luminol and lucigenin chemiluminescences were measured as indicators of radical formation. Lucigenin (bis-N-methyl-acridinium nitrate) and luminol (5-amino-2,3-dihydro-1,4-phthalazinedione) were obtained from Sigma (St Louis, MO). Measurements were made at room temperature using Junior LB 9509 luminometer (EG&G Berthold, Germany). Specimens were put into vials containing PBS-HEPES buffer (0.5 M PBS containing 20 mM HEPES, pH 7.2). Reactive oxygen metabolites were quantitated after the addition of the enhancers, lucigenin or luminol, for a final concentration of 0.2 mM. Luminol detects a group of reactive species, such as hydroxyl radical, hydrogen peroxide, and hypochlorous acid, while lucigenin is selective for superoxide anion radical (22). Counts were obtained at 1-minute intervals, and the results were given as the area under curve for a counting period of 5 minutes. Counts was corrected for wet tissue weight and expressed as relative light units (rlu) per milligram of tissue (23).
Measurement of Myeloperoxidase Activity
Myeloperoxidase (MPO) activity in tissues was measured by a procedure similar to that described by Hillegass et al (24). Spinal cord tissue samples were homogenized in 50 mM potassium phosphate buffer with a pH of 6.0 and centrifuged at 41,400g for 10 minutes. The pellets were then suspended in 50 mM PB containing 0.5% hexadecyltrimethylammonium bromide. After 3 freeze-and-thaw cycles, with sonication between cycles, the samples were centrifuged at 41,400g for 10 minutes. Aliquots (0.3 mL) were added to 2.3 mL of reaction mixture containing 50 mM PB, o-di-anisidine, and 20-mM H2O2 solution. One unit of enzyme activity was defined as the amount of MPO present that caused a change in absorbance, measured at 460 nm for 3 minutes. MPO activity was expressed as U/g tissue.
Malondialdehyde and Glutathione Assays
Spinal cord samples were homogenized with ice-cold 150-mM KCl for the determination of malondialdehyde (MDA) and glutathione (GSH) levels. The MDA levels were assayed for the products of lipid peroxidation, and results are expressed as nmol MDA/g tissue (25). GSH was determined by a spectrophotometric method based on the use of Elman's reagent, and results are expressed as µmol GSH/g tissue (26).
DNA Fragmentation Assay
Samples from spinal cord tissues were homogenized in 9× volumes of a lysis buffer (5 mM Tris–HCl, 20 mM ethylene diamine tetra-acetic acid [EDTA] and 0.5% [v/v] t-octylphenoxy polyethoxyethanol [Triton-X 100]; pH 8.0). Two separate samples of 1 mL each were taken from the sample and centrifuged at 25,000g for 30 minutes to separate the intact chromatin in the pellet from the fragmented DNA in the supernatant.
The supernatant was taken out to be saved, and the pellet was resuspended in 1 mL Tris-EDTA buffer (pH 8.0) (10 mM∶1 mM). Both the supernatant and the resuspended pellet were then assayed for DNA content determination by the diphenylamine reaction described by Burton (27).
Histologic Analysis
Anesthetized (ip ketamine 75 mg/kg and chlorpromazine 1mg/kg) rats were perfused through the heart with a solution of 4% paraformaldehyde in 0.1 M PBS (pH 7.4). For light microscopic analysis, perfused specimens were fixed in 10% formaldehyde, dehydrated in alcohol series, cleared in toluene, and embedded in paraffin. Paraffin sections (5 µm) were stained with hematoxylin and eosin (HE) and examined with an Olympus BX51 (Tokyo, Japan) photomicroscope.
In addition to the HE staining, Luxol fast-blue (LFB) stain was used in this study. The presence of myelin damage was assessed by staining transverse sections of the spinal cord with the LFB stain. LFB stains myelinated axons a bright blue color; areas with pale staining have myelin damage or loss (28). A semiquantitative evaluation was done according to the criteria defined by Lee et al (28); the degree of myelin damage is classified as following: 0: presence of normal intensity of LFB stain. 1 (Mild damage): Presence of decreased intensity of LFB stain compared with that of the control group. 2 (Moderate damage): Presence of decreased intensity of LFB stain and vacuole formation.
Statistical Analysis
Statistical analysis was done using a GraphPad Prism 3.0 (GraphPad Software, San Diego, CA). All data are expressed as means ± SEM. Groups of data were compared with ANOVA followed by Tukey's multiple comparison tests. The neurologic examination scores were evaluated by Kruskal-Wallis test followed by Dunn's multiple comparison test. Values of P < 0.05 were considered significant.
RESULTS
The number of deaths in 1-week posttrauma was 7/24 29%) in the trauma group, while it was 6/24 (25%) in LA-treated group.
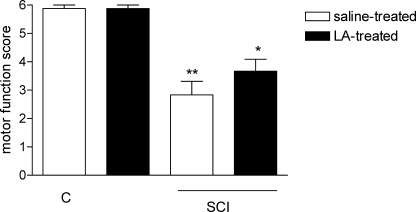
When compared with the control group (5.8 ± 0.1), the neurologic examination score was significantly lower in the vehicle-treated SCI group (2.6 ± 0.4; P < 0.001). Although the score was increased in the LA-treated SCI group (3.7 ± 0.4; Figure 1), it was not statistically significant.
Figure 1.
Motor function scores at 7 days following SCI. Each group consists of 17 to 18 rats. *P < 0.05, ***P < 0.001 vs control group. Values are represented as mean ± SEM.
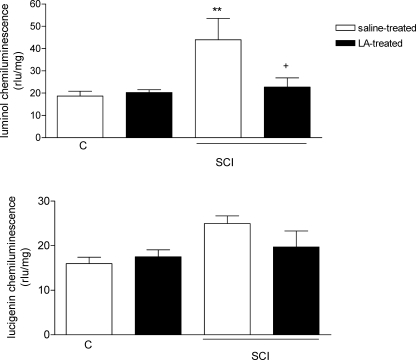
As an indicator of reactive oxygen species in SCI-induced tissue damage, chemiluminescence levels were detected in the spinal cord samples by both luminol and lucigenin probes. Luminol levels were showed significant increases in the vehicle-treated SCI group (43.9 ± 9.6 rlu/mg, P < 0.01; Figure 2a) as compared with the luminol CL levels of the control group (18.6 ± 2.1 rlu/mg). However, treating the SCI-induced rats with LA abolished the elevations in luminol-detected CL values (22.7 ± 4.1 rlu/mg, P < 0.05). Conversely, the lucigenin CL levels of spinal tissue samples were not statistically significant among the groups (Figure 2b).
Figure 2.
(a) Luminol and (b) lucigenin chemiluminescence values in the spinal cord tissues of control, saline, and LA-treated SCI groups. For each group, n = 6. **P < 0.01, +:P < 0.05 vs vehicle-treated SCI group. Values are represented as mean ± SEM.
The spinal cord tissue MDA content in the control group (26.0 ± 2.3 nmol/g) was significantly elevated by the induction of SCI in the vehicle-treated rats (42.6 ± 2.8 nmol/g, P < 0.01); however, LA treatment completely prevented the SCI-induced elevation in tissue MDA level (27.0 ± 3.3 nmol/g; P < 0.01; Figure 3a). In accordance with that, SCI caused a significant decrease in tissue GSH level (0.72 ± 0.05 µmol/g; P < 0.001) as compared with that of the sham-operated control group (1.53 ± 0.13 µmol/g; Figure 3b), while in the LA-treated SCI group, spinal cord GSH content was found to be preserved (1.31 ± 0.12 µmol/g; P < 0.01) and not different from that of the control group.
Figure 3.
(a) MDA and (b) GSH levels in the spinal cord tissues of control, saline, and LA-treated SCI groups. For each group, n = 6. **: P < 0.01, ***: P < 0.001 vs control group; +: P < 0.05, +++: P < 0.001 vs vehicle-treated SCI group. Values are represented as mean ± SEM.
Myeloperoxidase activity, an indicator of neutrophil infiltration, was significantly elevated in the spinal cord tissues of the vehicle-treated SCI group (6.4 ± 0.4 U/g; P < 0.001) as compared with that of the sham-operated control group (3.4 ± 0.1 U/g; Figure 4a). Conversely, when SCI rats were treated with LA, spinal cord MPO level was significantly decreased (4.3 ± 0.3 U/g; P < 0.001) and was not different than that of the control group.
Figure 4.
(a) MPO and (b) DNA fragmentation (%) in the spinal cord tissues of control, saline, and LA-treated SCI groups. For each group, n = 6. *: P < 0.05, ***: P < 0.001 vs control group; +: P < 0.05, +++: P < 0.001 vs vehicle-treated SCI group. Values are represented as mean ± SEM.
DNA fragmentation (%) in the spinal cord tissue was analyzed as an indicator of cell death, including apoptosis. In the SCI group treated with vehicle, DNA fragmentation was elevated significantly (0.16 ± 0.02; P < 0.001) when compared with the control group (0.03 ± 0.01; Figure 4b), while LA treatment significantly prevented the DNA damage of the spinal cord tissue (0.10 ± 0.02; P < 0.05).
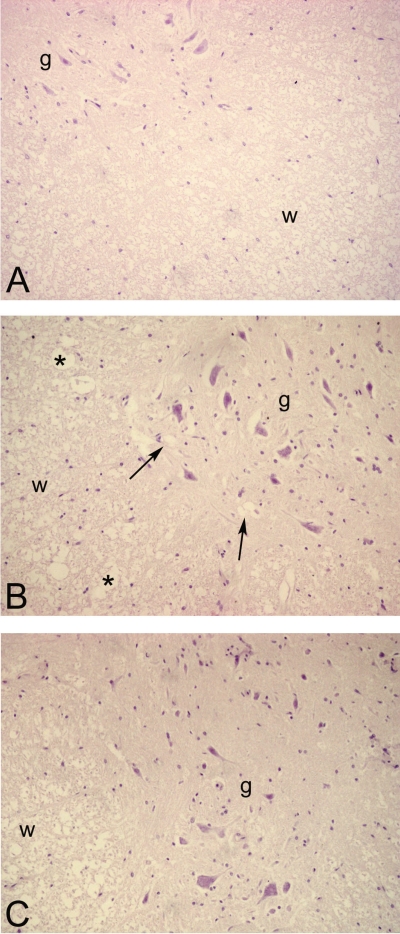
Light microscopic evaluation revealed that SCI resulted in severe degeneration of the white matter besides moderate degeneration of the gray matter (Figure 5B) when compared with control group (Figure 5A). The overall morphology of white matter, which was composed widely of axons, was disturbed. In the gray matter, vacuole formation was observed. LA treatment resulted with a healing morphology with a decreased disruption of axons and preserved general architecture (Figure 5C).
Figure 5.
HE-stained sections of spinal cord demonstrating general morphology of white (w) and gray (g) matter. (A) Control group: Regular morphology of white and gray matter; (B) SCI group: Severe degeneration of spinal cord section when compared with control group. Arrows indicate vacuolizations in the neuropil of gray matter. Note the vacuole formation in the white matter (*); (C) LA-treated spinal cord injury group: near-regular architecture with reduced damage in the gray and white matter is apparent. ×200, original magnification.
LFB staining of spinal cord sections from the control group revealed no evidence of myelin damage. However, in the spinal cords of injured animals, decreased staining intensity was evident. The white matter of spinal cords in this group showed prominent vacuole formation. In the LA-treated SCI group, the spinal cord of the animals revealed nearly normal staining intensity as it is observed in control group, and less vacuole formation was observed compared with spinal cord injury group (Figure 6).
Figure 6.
LFB-stained sections of spinal cords (white matter). The spinal cords of the control group showed normal staining intensity (A). Spinal cord of the SCI group showed myelin sheaths with decreased staining intensity compared with that of the control group. Vacuole formation (arrow; “*” in inset) was apparent (B). Spinal cord of ALA-treated SCI group showed normal staining intensity and less vacuole formation (arrow; “*” in inset) (C). LFB ×400; insets ×1,000, original magnification.
When the tissues were evaluated semiquantitatively, SCI caused a significant (P < 0 < 01) degeneration, whereas the LA-treated group was not significantly different than the controls. The scores were as follows: Control: 0 ± 0.01, SCI: 1.833 ± 0.17, SCI + LA: 0.833 ± 0.31.
DISCUSSION
Spinal cord injury leads to cascades of noxious pathologic mechanisms that substantially exacerbate the primary injury, then trigger secondary injury and result in permanent functional deficits. Several pathophysiologic events have been proposed to contribute to secondary neuronal dysfunction and death, including events such as ischemia, edema, ionic imbalances, compromised energy metabolism, and biochemical changes resulting in neurotoxicity (1,3). Glutamate-mediated excitotoxicity, the formation of reactive oxygen species (ROS), and lipid peroxidation are prominent events thought to contribute to neuronal dysfunction and cell loss following traumatic damage and ischemic injury to the central nervous system (CNS) (1). There is direct evidence that activation of glutamate receptors and the influx of Ca2+ result in the formation of ROS, especially superoxide anion and hydrogen peroxide (29,30).
In the present study, the results of chemiluminescence analysis demonstrated that luminol CL levels, sensitive to hydrogen peroxide, hypochlorite, peroxynitrite, and hydroxyl and lipid peroxyl radicals, were elevated in the spinal cord tissues of injured rats, implicating enhanced generation of toxic oxygen metabolites. In accordance with the increases in toxic oxygen metabolites, the spinal cord MDA level was also significantly increased, indicating the presence of enhanced lipid peroxidation caused by SCI. Several studies have demonstrated that trauma to the spinal cord tissue is associated with lipid peroxidation, which is an autocatalytic mechanism leading to oxidative destruction of cellular membranes (3,31). It is known that cells are able to defend themselves from the damaging effects of oxygen radicals by way of their own antioxidant mechanisms; however, depletion of the antioxidant defense system caused by oxidative stress enhanced the susceptibility of the tissues to oxidative injury. In this sense, GSH and other antioxidants play a critical role in limiting the propagation of free-radical reactions, which would otherwise result in extensive lipid peroxidation. An increased tissue level of GSH is an important protective mechanism against overproduction of ROM and free-radical reactions (32). In this study, SCI caused significant decrease in spinal cord GSH levels, while LA treatment effectively reversed this effect, reducing both luminol- and lucigenin-enhanced CL levels and lipid peroxidation.
Both in vitro and in vivo studies have demonstrated the wide-ranging ability of LA to influence biologic functions. LA and DHLA act as antioxidants to directly scavenge ROS and reactive nitrogen species, chelate transition and heavy metal ions, and mediate recycling of other endogenous antioxidants such as glutathione. LA also modulates various signaling cascades either by receptor-mediated or nonreceptor-mediated processes (33,34). Thus, LA may be effective in a variety of pathologic conditions, especially those that are associated with oxidative stress (16,33,34). In accordance with these studies, we have previously demonstrated that LA improves stomach, kidney, and liver damage in rat models of ulcer, ischemia/reperfusion, cerebral trauma, and subarachnoid hemorrhage (12,18,19,35,36).
Traumatic injury to the spinal cord also leads to a strong inflammatory response, with the recruitment of peripherally derived inflammatory cells, including macrophages. Following SCI, neutrophils are activated, and they then migrate into visceral organs, a phenomenon occurring in parallel with their well-known entry into the cord injury site (37). Bernards and Akers (38) have demonstrated that spinal cord injury significantly increased the amount of myeloperoxidase, an index of neutrophil infiltration, in the cerebrospinal fluid dialysates. Taoka et al (39), who studied the leukocyte depletion and anti-P-selectin monoclonal antibody treatment in the SCI rats, showed that both of them reduced the accumulation of neutrophils in the damaged spinal cord segment where myeloperoxidase activity was found to be decreased. Thus, the systemic inflammatory response to SCI should be targeted in the development of new therapeutic strategies to treat SCI. In this sense, LA may be suggested to be neuroprotective agent since in the present study, treatment with LA in the SCI groups reduced the MPO activity in addition to inhibiting effects on lipid peroxidation. Zhang et al (40) demonstrated that LA exerts anti-inflammatory effects by inhibiting TNF-α and lipopolysaccharide-induced endothelial and monocyte activation in vitro and lipopolysaccharide-induced acute inflammatory responses in vivo. They also showed that dietary LA supplementation, inhibiting expression of adhesion molecules and proinflammatory cytokines, reduces atherosclerotic lesion formation and also supports the anti-inflammatory effects of LA. Kolgazi et al (41) demonstrated that LA exerts anti-inflammatory and antioxidant effects in the TNBS-induced gastric mucosal inflammation via suppression of neutrophil accumulation, preservation of endogenous glutathione, and inhibition of reactive oxidant generation.
A potentially promising area of neuroprotective drug discovery for treating acute SCI is the development of agents targeting apoptotic cell death. Free radicals lead to neuronal damage by promoting lipid peroxidation, protein breakdown, and DNA damage, which in turn lead to cellular apoptosis. Previously it has been shown that LA pretreatment reduced radiation-induced apoptotic and necrotic cell death of granule cells and Purkinje cells (15,42). In the present study, DNA fragmentation of spinal cord tissue was found to be increased in the SCI group, while LA treatments reduced this effect of SCI through its antioxidant and anti-inflammatory properties. Apoptosis has long been considered as an essential mechanism for eliminating redundant cells during CNS development. However, a number of recent studies have documented that apoptosis is emerging as a critical factor contributing to ongoing cell loss following traumatic CNS injury, especially in SCI (3). Apoptotic cell death can be detected hours to several weeks following SCI and occurs in numerous cell types, including neurons, oligodendroglia, and inflammatory cells such as neutrophils, microglia, and macrophages (3). The occurrence of programmed apoptotic cell death has been suggested to play a role in the ultimate neurologic insult. Indeed, in the current study, LA treatment caused a significant deterioration in the DNA fragmentation besides reduction of inflammation, lipid peroxidation, and preservation of endogenous antioxidant levels.
In agreement with our biochemical data, light microscopic examination was also consistent. However, during routine histologic processing, the myeline structure was lost, so we could not observe the myeline structure of the axons in the light microscope; we could, however, observe its traces as axonal sites. Thus, light microscopic evaluation revealed that SCI resulted in severe degeneration of the white matter besides moderate degeneration of the gray matter. The overall morphology of white matter, which was composed widely of axons was disturbed, presumably because of intracellular edema and the enlarged and disrupted diameters of axons. In the gray matter, interstitial and intracellular edema were observed. LA treatment resulted in a healing morphology, with a decreased disruption of axons and interstitial edema.
Antioxidant drugs can prevent the onset of pathologies; they also delay pathologic processes or may even take part in repair. Conversely, research on antioxidant drugs and research related to oxidative disease processes have not converged into a therapeutic intervention in which the mode of action is fully comprehended. In the present study, we have initiated LA treatment immediately on the day of the injury and continued for 1 week. Thus, our results imply the benefit of LA in the early phase of the injury. However, it would be interesting to evaluate the effects of LA in the late phase. Therefore, further studies should be conducted by continuing the treatment for a longer period; thus, the time course for LA treatment would be elucidated.
CONCLUSION
In conclusion, current data clearly demonstrate that treatment with LA significantly inhibited SCI-induced generation of free radicals, lipid peroxidation, neutrophil infiltration, and DNA damage in the spinal cord tissue, while the depleted antioxidant GSH levels were increased back to control levels. In addition, LA treatment also improved histologic and neurologic deterioration seen following SCI. Thus, LA needs to be taken into consideration, for it may have a therapeutic value in SCI as an adjuvant therapy.
References
- Azbill RD, Mu X, Bruce-Keller AJ, Mattson MP, Springer JE. Impaired mitochondrial function, oxidative stress and altered antioxidant enzyme activities following traumatic spinal cord injury. Brain Res. 1997;765(2):283–290. doi: 10.1016/s0006-8993(97)00573-8. [DOI] [PubMed] [Google Scholar]
- Park E, Velumian AA, Fehlings MG. The role of excitotoxicity in secondary mechanisms of spinal cord injury: a review with an emphasis on the implications for white matter degeneration. J Neurotrauma. 2004;21(6):754–774. doi: 10.1089/0897715041269641. [DOI] [PubMed] [Google Scholar]
- Hall ED, Springer JE. Neuroprotection and acute spinal cord injury: a reappraisal. NeuroRx. Jan 2004;1(1):80–100. doi: 10.1602/neurorx.1.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slemmer JE, Shacka JJ, Sweeney MI, Weber JT. Antioxidants and free radical scavengers for the treatment of stroke, traumatic brain injury and aging. Curr Med Chem. 2008;15(4):404–414. doi: 10.2174/092986708783497337. [DOI] [PubMed] [Google Scholar]
- Packer L, Tritschler HJ, Wessel K. Neuroprotection by the metabolic antioxidant alpha-lipoic acid. Free Radic Biol Med. 1997;22(1–2):359–378. doi: 10.1016/s0891-5849(96)00269-9. [DOI] [PubMed] [Google Scholar]
- Roy S, Packer L. Redox regulation of cell functions by alpha-lipoate: biochemical and molecular aspects. Biofactors. 1998;8(1–2):17–21. doi: 10.1002/biof.5520080104. [DOI] [PubMed] [Google Scholar]
- Suzuki YJ, Tsuchiya M, Packer L. Antioxidant activities of dihydrolipoic acid and its structural homologues. Free Radic Res Commun. 1993;18(2):115–122. doi: 10.3109/10715769309147348. [DOI] [PubMed] [Google Scholar]
- Kagan VE, Shvedova A, Serbinova E, et al. Dihydrolipoic acid—a universal antioxidant both in the membrane and in the aqueous phase. Reduction of peroxyl, ascorbyl and chromanoxyl radicals. Biochem Pharmacol. 1992;44(8):1637–1649. doi: 10.1016/0006-2952(92)90482-x. [DOI] [PubMed] [Google Scholar]
- Han D, Handelman G, Marcocci L, et al. Lipoic acid increases de novo synthesis of cellular glutathione by improving cystine utilization. Biofactors. 1997;6(3):321–338. doi: 10.1002/biof.5520060303. [DOI] [PubMed] [Google Scholar]
- Glantzounis GK, Yang W, Koti RS, Mikhailidis DP, Seifalian AM, Davidson BR. The role of thiols in liver ischemia-reperfusion injury. Curr Pharm Des. 2006;12(23):2891–2901. doi: 10.2174/138161206777947641. [DOI] [PubMed] [Google Scholar]
- Biewenga G, Haenen GR, Bast A. The role of lipoic acid in the treatment of diabetic polyneuropathy. Drug Metab Rev. Nov 1997;29(4):1025–1054. doi: 10.3109/03602539709002242. [DOI] [PubMed] [Google Scholar]
- Sehirli O, Sener E, Cetinel S, Yuksel M, Gedik N, Sener G. Alpha-lipoic acid protects against renal ischaemia-reperfusion injury in rats. Clin Exp Pharmacol Physiol. 2008;35(3):249–255. doi: 10.1111/j.1440-1681.2007.04810.x. [DOI] [PubMed] [Google Scholar]
- Bilska A, Wlodek L. Lipoic acid—the drug of the future. Pharmacol Rep. Sep–Oct 2005;57(5):570–577. [PubMed] [Google Scholar]
- Foster TS. Efficacy and safety of alpha-lipoic acid supplementation in the treatment of symptomatic diabetic neuropathy. Diabetes Educ. 2007;33(1):111–117. doi: 10.1177/0145721706297450. [DOI] [PubMed] [Google Scholar]
- Manda K, Ueno M, Moritake T, Anzai K. Radiation-induced cognitive dysfunction and cerebellar oxidative stress in mice: protective effect of alpha-lipoic acid. Behav Brain Res. 2007;177(1):7–14. doi: 10.1016/j.bbr.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Salinthone S, Yadav V, Bourdette DN, Carr DW. Lipoic acid: a novel therapeutic approach for multiple sclerosis and other chronic inflammatory diseases of the CNS. Endocr Metab Immune Disord Drug Targets. 2008;8(2):132–142. doi: 10.2174/187153008784534303. [DOI] [PubMed] [Google Scholar]
- Maczurek A, Hager K, Kenklies M, Sharman M, Martins R, Engel J, Carlsonn DA, Munch G. Lipoic acid as an anti-inflammatory and neuroprotective treatment for Alzheimer's disease. Adv Drug Deliv Rev. 2008;60(13–14):1463–1470. doi: 10.1016/j.addr.2008.04.015. [DOI] [PubMed] [Google Scholar]
- Toklu HZ, Hakan T, Biber N, Solakoglu S, Ogunc AV, Sener G. The protective effect of alpha lipoic acid against traumatic brain injury in rats. Free Radic Res. 2009;43(7):658–667. doi: 10.1080/10715760902988843. [DOI] [PubMed] [Google Scholar]
- Ersahin M, Toklu HZ, Cetinel S, et al. Alpha lipoic acid alleviates oxidative stress and preserves blood brain permeability in rats with subarachnoid hemorrhage. Neurochem Res. 2010;35(3):418–428. doi: 10.1007/s11064-009-0072-z. [DOI] [PubMed] [Google Scholar]
- Allen AR. Surgery of experimental lesion of spinal cord equivalent to crush injury of fracture dislocation of spinal column. A preliminary report. JAMA. 1911;57:878–880. [Google Scholar]
- Gale K, Kerasidis H, Wrathall JR. Spinal cord contusion in the rat: behavioral analysis of functional neurologic impairment. Exp Neurol. 1985;88(1):123–134. doi: 10.1016/0014-4886(85)90118-9. [DOI] [PubMed] [Google Scholar]
- Davies GR, Simmonds NJ, Stevens TR, Grandison A, Blake DR, Rampton DS. Mucosal reactive oxygen metabolite production in duodenal ulcer disease. Gut. 1992;33(11):1467–1472. doi: 10.1136/gut.33.11.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haklar G, Yuksel M, Yalcin AS. Chemiluminescence in the measurement of free radicals: theory and application on a tissue injury model. Marmara Med J. 1998;11:55–60. [Google Scholar]
- Hillegass LM, Griswold DE, Brickson B, Albrightson-Winslow C. Assessment of myeloperoxidase activity in whole rat kidney. J Pharmacol Methods. 1990;24(4):285–295. doi: 10.1016/0160-5402(90)90013-b. [DOI] [PubMed] [Google Scholar]
- Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- Beutler E, Duran O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963;61:882–888. [PubMed] [Google Scholar]
- Burton K. Determination of DNA concentration with diphenylamine. Methods Enzymol. 1968;12:163–166. [Google Scholar]
- Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000;343(13):938–952. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- Emerit J, Edeas M, Bricaire F. Neurodegenerative diseases and oxidative stress. Biomed Pharmacother. 2004;58(1):39–46. doi: 10.1016/j.biopha.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Tilleux S, Hermans E. Neuroinflammation and regulation of glial glutamate uptake in neurological disorders. J Neurosci Res. 2007;85(10):2059–2070. doi: 10.1002/jnr.21325. [DOI] [PubMed] [Google Scholar]
- Adibhatla RM, Hatcher JF. Phospholipase A(2), reactive oxygen species, and lipid peroxidation in CNS pathologies. BMB Rep. 2008;41(8):560–567. doi: 10.5483/bmbrep.2008.41.8.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr. 2004;134(3):489–492. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- Biewenga GP, Haenen GR, Bast A. The pharmacology of the antioxidant lipoic acid. Gen Pharmacol. 1997;29(3):315–331. doi: 10.1016/s0306-3623(96)00474-0. [DOI] [PubMed] [Google Scholar]
- Moini H, Packer L, Saris NE. Antioxidant and prooxidant activities of alpha-lipoic acid and dihydrolipoic acid. Toxicol Appl Pharmacol. 2002;182(1):84–90. doi: 10.1006/taap.2002.9437. [DOI] [PubMed] [Google Scholar]
- Dulundu E, Ozel Y, Topaloglu U, et al. Alpha-lipoic acid protects against hepatic ischemia-reperfusion injury in rats. Pharmacology. 2007;79(3):163–170. doi: 10.1159/000098953. [DOI] [PubMed] [Google Scholar]
- Sehirli O, Tatlidede E, Yuksel M, Sehirli O, Ercan F, Gedik N, Sener G. Antioxidant effect of alpha-lipoic acid against ethanol-induced gastric mucosal erosion in rats. Pharmacology. 2008;81(2):173–180. doi: 10.1159/000111145. [DOI] [PubMed] [Google Scholar]
- Gris D, Hamilton EF, Weaver LC. The systemic inflammatory response after spinal cord injury damages lungs and kidneys. Exp Neurol. 2008;211(1):259–270. doi: 10.1016/j.expneurol.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Bernards CM, Akers T. Effect of postinjury intravenous or intrathecal methylprednisolone on spinal cord excitatory amino-acid release, nitric oxide generation, PGE2 synthesis, and myeloperoxidase content in a pig model of acute spinal cord injury. Spinal Cord. 2006;44(10):594–604. doi: 10.1038/sj.sc.3101891. [DOI] [PubMed] [Google Scholar]
- Taoka Y, Okajima K, Uchiba M, Johno M. Methylprednisolone reduces spinal cord injury in rats without affecting tumor necrosis factor-alpha production. J Neurotrauma. 2001;18(5):533–543. doi: 10.1089/089771501300227332. [DOI] [PubMed] [Google Scholar]
- Zhang WJ, Wei H, Hagen T, Frei B. Alpha-lipoic acid attenuates LPS-induced inflammatory responses by activating the phosphoinositide 3-kinase/Akt signaling pathway. Proc Natl Acad Sci U S A. 2007;104(10):4077–4082. doi: 10.1073/pnas.0700305104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolgazi M, Jahovic N, Yuksel M, Ercan F, Alican I. Alpha-lipoic acid modulates gut inflammation induced by trinitrobenzene sulfonic acid in rats. J Gastroenterol Hepatol. 2007;22(11):1859–1865. doi: 10.1111/j.1440-1746.2006.04504.x. [DOI] [PubMed] [Google Scholar]
- Jia Z, Zhu H, Vitto MJ, Misra BR, Li Y, Misra HP. Alpha-lipoic acid potently inhibits peroxynitrite-mediated DNA strand breakage and hydroxyl radical formation: implications for the neuroprotective effects of alpha-lipoic acid. Mol Cell Biochem. 2009;323(1–2):131–138. doi: 10.1007/s11010-008-9971-6. [DOI] [PubMed] [Google Scholar]