Abstract
Defects of complex I of the mitochondrial respiratory chain are important causes of neurological disease. We report studies that demonstrate a severe deficiency of complex I activity with less severe abnormalities of complexes III and IV (less than 5, 63, and 30% of control values, respectively) in a skeletal muscle mitochondrial fraction from a 22-yr-old female with weakness, lactic acidemia, and the deposition of intramuscular neutral lipid. The observation that lipid accumulates in this and other patients with complex I deficiency suggests impaired mitochondrial fatty acid oxidation. To investigate this mechanism we have shown impaired flux through beta-oxidation [( U-14C]hexadecanoate oxidation was 66% of control rate) and accumulation of specific acyl-CoA ester intermediates. The changes in fatty acid metabolism in complex I deficiency are secondary to the reduced state within the mitochondrial matrix with low NAD+/NADH ratios.
Full text
PDF

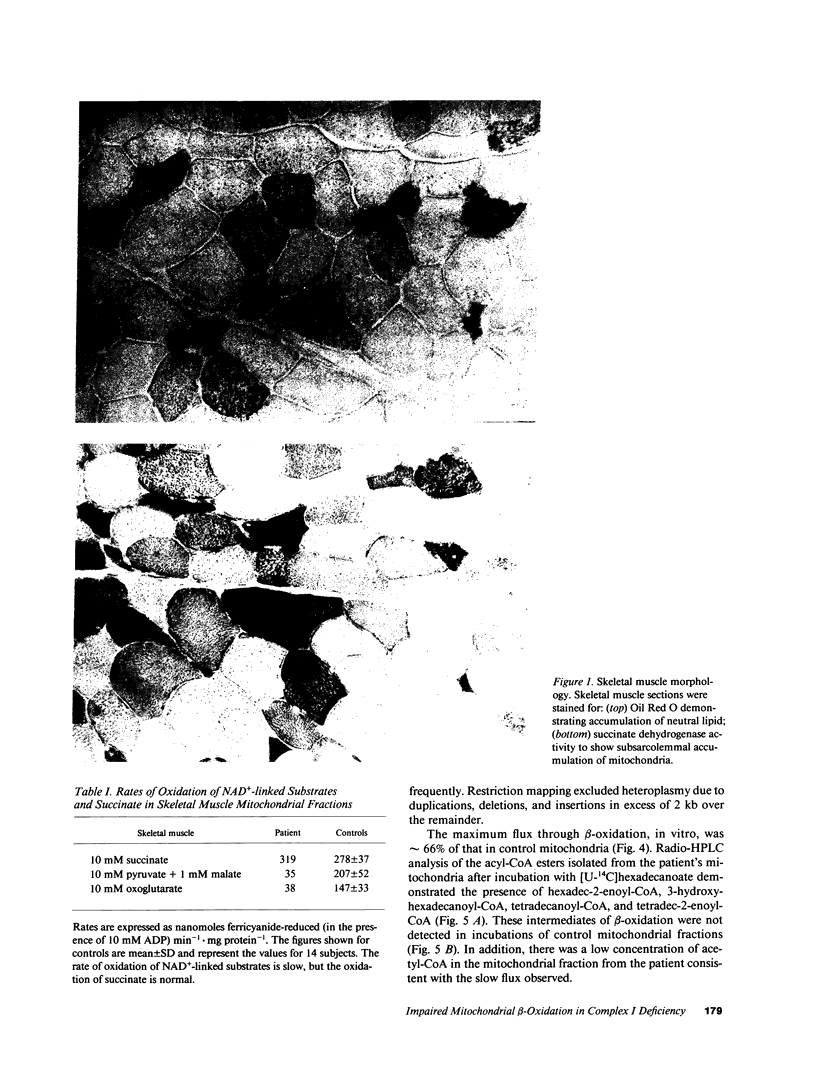
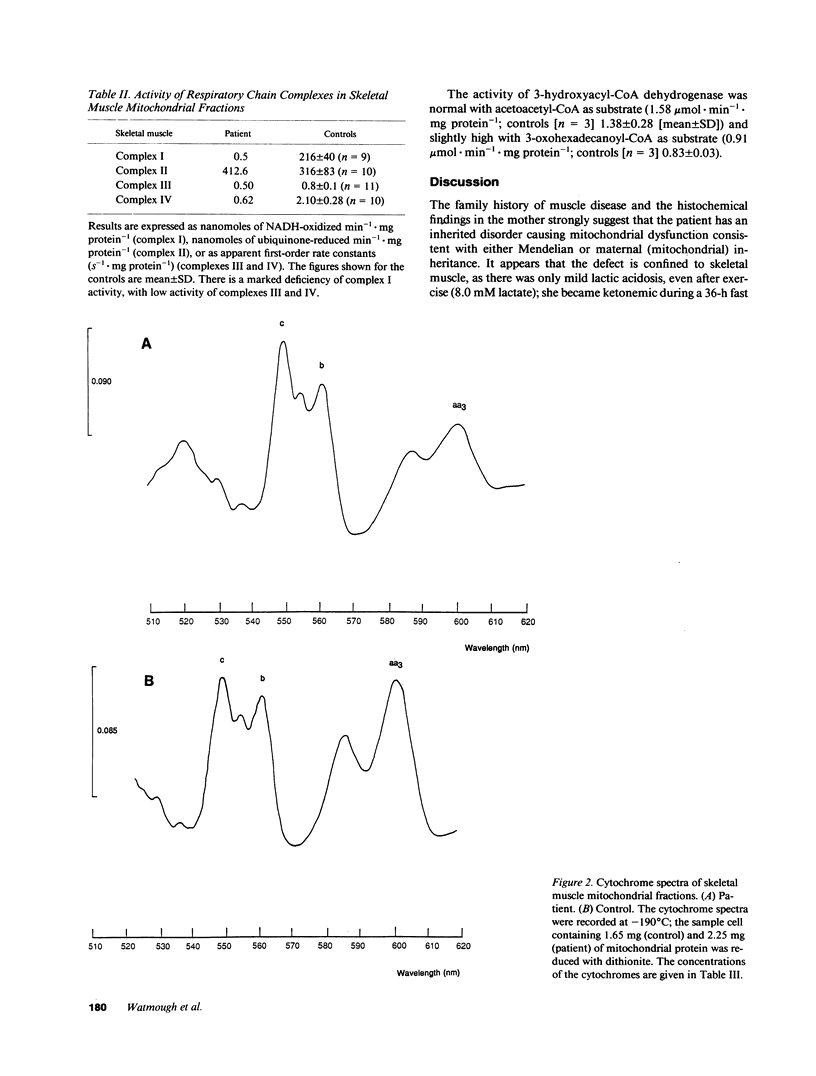
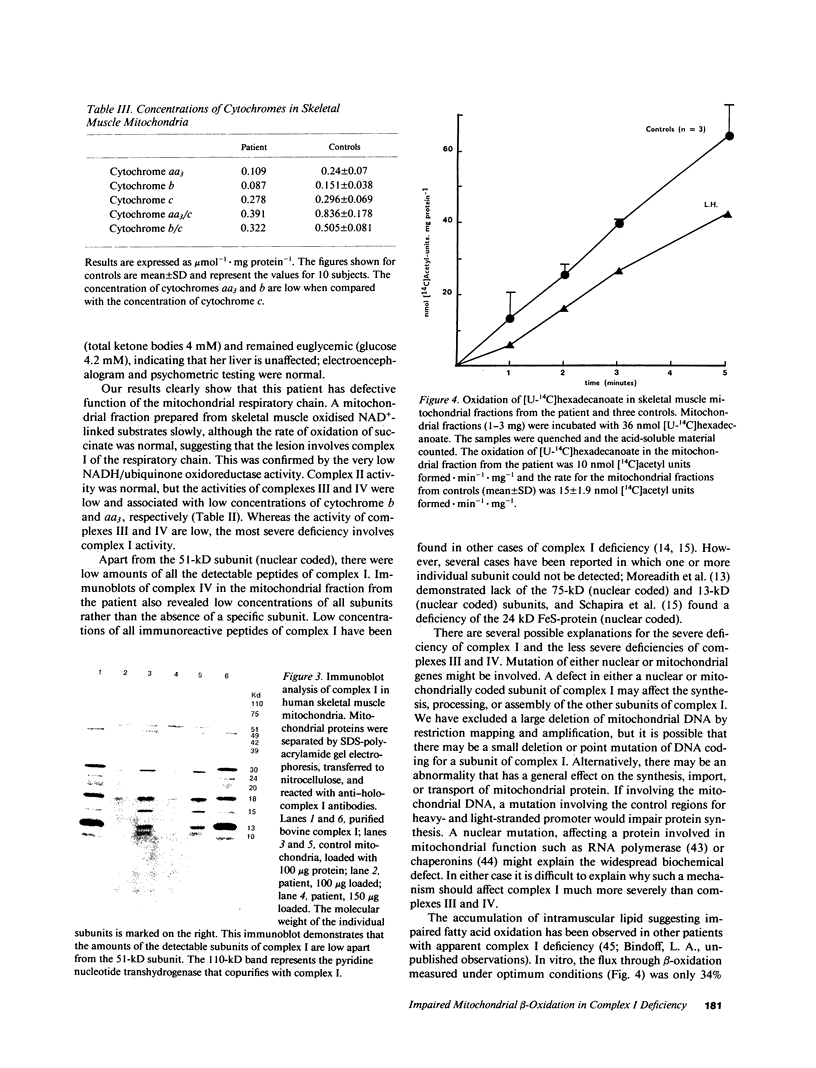


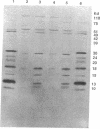
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckmann J. D., Frerman F. E., McKean M. C. Inhibition of general acyl CoA dehydrogenase by electron transfer flavoprotein semiquinone. Biochem Biophys Res Commun. 1981 Oct 30;102(4):1290–1294. doi: 10.1016/s0006-291x(81)80151-9. [DOI] [PubMed] [Google Scholar]
- Birch-Machin M. A., Shepherd I. M., Watmough N. J., Sherratt H. S., Bartlett K., Darley-Usmar V. M., Milligan D. W., Welch R. J., Aynsley-Green A., Turnbull D. M. Fatal lactic acidosis in infancy with a defect of complex III of the respiratory chain. Pediatr Res. 1989 May;25(5):553–559. doi: 10.1203/00006450-198905000-00025. [DOI] [PubMed] [Google Scholar]
- Bradley W. G., Hudgson P., Gardner-Medwin D., Walton J. N. Myopathy associated with abnormal lipid metabolism in skeletal muscle. Lancet. 1969 Mar 8;1(7593):495–498. doi: 10.1016/s0140-6736(69)91593-1. [DOI] [PubMed] [Google Scholar]
- Bremer J., Wojtczak A. B. Factors controlling the rate of fatty acid -oxidation in rat liver mitochondria. Biochim Biophys Acta. 1972 Dec 8;280(4):515–530. doi: 10.1016/0005-2760(72)90131-2. [DOI] [PubMed] [Google Scholar]
- Cheng M. Y., Hartl F. U., Martin J., Pollock R. A., Kalousek F., Neupert W., Hallberg E. M., Hallberg R. L., Horwich A. L. Mitochondrial heat-shock protein hsp60 is essential for assembly of proteins imported into yeast mitochondria. Nature. 1989 Feb 16;337(6208):620–625. doi: 10.1038/337620a0. [DOI] [PubMed] [Google Scholar]
- Chomyn A., Cleeter M. W., Ragan C. I., Riley M., Doolittle R. F., Attardi G. URF6, last unidentified reading frame of human mtDNA, codes for an NADH dehydrogenase subunit. Science. 1986 Oct 31;234(4776):614–618. doi: 10.1126/science.3764430. [DOI] [PubMed] [Google Scholar]
- Chomyn A., Mariottini P., Cleeter M. W., Ragan C. I., Matsuno-Yagi A., Hatefi Y., Doolittle R. F., Attardi G. Six unidentified reading frames of human mitochondrial DNA encode components of the respiratory-chain NADH dehydrogenase. Nature. 1985 Apr 18;314(6012):592–597. doi: 10.1038/314592a0. [DOI] [PubMed] [Google Scholar]
- Clark J. B., Hayes D. J., Morgan-Hughes J. A., Byrne E. Mitochondrial myopathies: disorders of the respiratory chain and oxidative phosphorylation. J Inherit Metab Dis. 1984;7 (Suppl 1):62–68. doi: 10.1007/BF03047377. [DOI] [PubMed] [Google Scholar]
- Di Donato S., Frerman F. E., Rimoldi M., Rinaldo P., Taroni F., Wiesmann U. N. Systemic carnitine deficiency due to lack of electron transfer flavoprotein:ubiquinone oxidoreductase. Neurology. 1986 Jul;36(7):957–963. doi: 10.1212/wnl.36.7.957. [DOI] [PubMed] [Google Scholar]
- DiMauro S., Bonilla E., Zeviani M., Servidei S., DeVivo D. C., Schon E. A. Mitochondrial myopathies. J Inherit Metab Dis. 1987;10 (Suppl 1):113–128. doi: 10.1007/BF01812852. [DOI] [PubMed] [Google Scholar]
- Domin B. A., Serabjit-Singh C. J., Philpot R. M. Quantitation of rabbit cytochrome P-450, form 2, in microsomal preparations bound directly to nitrocellulose paper using a modified peroxidase-immunostaining procedure. Anal Biochem. 1984 Feb;136(2):390–396. doi: 10.1016/0003-2697(84)90234-3. [DOI] [PubMed] [Google Scholar]
- El-Fakhri M., Middleton B. The existence of an inner-membrane-bound, long acyl-chain-specific 3-hydroxyacyl-CoA dehydrogenase in mammalian mitochondria. Biochim Biophys Acta. 1982 Nov 12;713(2):270–279. doi: 10.1016/0005-2760(82)90244-2. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Frerman F. E. Acyl-CoA dehydrogenases, electron transfer flavoprotein and electron transfer flavoprotein dehydrogenase. Biochem Soc Trans. 1988 Jun;16(3):416–418. doi: 10.1042/bst0160416. [DOI] [PubMed] [Google Scholar]
- Hatefi Y. The mitochondrial electron transport and oxidative phosphorylation system. Annu Rev Biochem. 1985;54:1015–1069. doi: 10.1146/annurev.bi.54.070185.005055. [DOI] [PubMed] [Google Scholar]
- Hay R., Böhni P., Gasser S. How mitochondria import proteins. Biochim Biophys Acta. 1984 Jan 27;779(1):65–87. doi: 10.1016/0304-4157(84)90004-2. [DOI] [PubMed] [Google Scholar]
- Hoppel C. L., Kerr D. S., Dahms B., Roessmann U. Deficiency of the reduced nicotinamide adenine dinucleotide dehydrogenase component of complex I of mitochondrial electron transport. Fatal infantile lactic acidosis and hypermetabolism with skeletal-cardiac myopathy and encephalopathy. J Clin Invest. 1987 Jul;80(1):71–77. doi: 10.1172/JCI113066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiki T., Tanaka M., Nishikimi M., Suzuki H., Ozawa T., Kobayashi M., Wada Y. Deficiency of subunits of Complex I and mitochondrial encephalomyopathy. Ann Neurol. 1988 Mar;23(3):287–294. doi: 10.1002/ana.410230312. [DOI] [PubMed] [Google Scholar]
- Kacser H., Burns J. A. The control of flux. Symp Soc Exp Biol. 1973;27:65–104. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Latipä P. M., Kärki T. T., Hiltunen J. K., Hassinen I. E. Regulation of palmitoylcarnitine oxidation in isolated rat liver mitochondria. Role of the redox state of NAD(H). Biochim Biophys Acta. 1986 Feb 12;875(2):293–300. doi: 10.1016/0005-2760(86)90179-7. [DOI] [PubMed] [Google Scholar]
- Moreadith R. W., Batshaw M. L., Ohnishi T., Kerr D., Knox B., Jackson D., Hruban R., Olson J., Reynafarje B., Lehninger A. L. Deficiency of the iron-sulfur clusters of mitochondrial reduced nicotinamide-adenine dinucleotide-ubiquinone oxidoreductase (complex I) in an infant with congenital lactic acidosis. J Clin Invest. 1984 Sep;74(3):685–697. doi: 10.1172/JCI111484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreadith R. W., Cleeter M. W., Ragan C. I., Batshaw M. L., Lehninger A. L. Congenital deficiency of two polypeptide subunits of the iron-protein fragment of mitochondrial complex I. J Clin Invest. 1987 Feb;79(2):463–467. doi: 10.1172/JCI112834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan-Hughes J. A., Schapira A. H., Cooper J. M., Clark J. B. Molecular defects of NADH-ubiquinone oxidoreductase (complex I) in mitochondrial diseases. J Bioenerg Biomembr. 1988 Jun;20(3):365–382. doi: 10.1007/BF00769638. [DOI] [PubMed] [Google Scholar]
- Noer A. S., Marzuki S., Trounce I., Byrne E. Mitochondrial DNA deletion in encephalomyopathy. Lancet. 1988 Nov 26;2(8622):1253–1254. doi: 10.1016/s0140-6736(88)90847-1. [DOI] [PubMed] [Google Scholar]
- Petty R. K., Harding A. E., Morgan-Hughes J. A. The clinical features of mitochondrial myopathy. Brain. 1986 Oct;109(Pt 5):915–938. doi: 10.1093/brain/109.5.915. [DOI] [PubMed] [Google Scholar]
- Poulton J., Deadman M. E., Gardiner R. M. Duplications of mitochondrial DNA in mitochondrial myopathy. Lancet. 1989 Feb 4;1(8632):236–240. doi: 10.1016/s0140-6736(89)91256-7. [DOI] [PubMed] [Google Scholar]
- Poulton J., Turnbull D. M., Mehta A. B., Wilson J., Gardiner R. M. Restriction enzyme analysis of the mitochondrial genome in mitochondrial myopathy. J Med Genet. 1988 Sep;25(9):600–605. doi: 10.1136/jmg.25.9.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson B. H., Ward J., Goodyer P., Baudet A. Respiratory chain defects in the mitochondria of cultured skin fibroblasts from three patients with lacticacidemia. J Clin Invest. 1986 May;77(5):1422–1427. doi: 10.1172/JCI112453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Bugawan T. L., Horn G. T., Mullis K. B., Erlich H. A. Analysis of enzymatically amplified beta-globin and HLA-DQ alpha DNA with allele-specific oligonucleotide probes. Nature. 1986 Nov 13;324(6093):163–166. doi: 10.1038/324163a0. [DOI] [PubMed] [Google Scholar]
- Schapira A. H., Cooper J. M., Morgan-Hughes J. A., Patel S. D., Cleeter M. J., Ragan C. I., Clark J. B. Molecular basis of mitochondrial myopathies: polypeptide analysis in complex-I deficiency. Lancet. 1988 Mar 5;1(8584):500–503. doi: 10.1016/s0140-6736(88)91296-2. [DOI] [PubMed] [Google Scholar]
- Sherratt H. S., Watmough N. J., Johnson M. A., Turnbull D. M. Methods for study of normal and abnormal skeletal muscle mitochondria. Methods Biochem Anal. 1988;33:243–335. doi: 10.1002/9780470110546.ch6. [DOI] [PubMed] [Google Scholar]
- Shuey D. J., Attardi G. Characterization of an RNA polymerase activity from HeLa cell mitochondria, which initiates transcription at the heavy strand rRNA promoter and the light strand promoter in human mitochondrial DNA. J Biol Chem. 1985 Feb 10;260(3):1952–1958. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tervoort M. J., Schilder L. T., Van Gelder B. F. The absorbance coefficient of beef heart cytochrome c1. Biochim Biophys Acta. 1981 Sep 14;637(2):245–251. doi: 10.1016/0005-2728(81)90163-8. [DOI] [PubMed] [Google Scholar]
- Thorpe C. A method for the preparation of 3-ketoacyl-CoA derivatives. Anal Biochem. 1986 Jun;155(2):391–394. doi: 10.1016/0003-2697(86)90452-5. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull D. M., Bartlett K., Stevens D. L., Alberti K. G., Gibson G. J., Johnson M. A., McCulloch A. J., Sherratt H. S. Short-chain acyl-CoA dehydrogenase deficiency associated with a lipid-storage myopathy and secondary carnitine deficiency. N Engl J Med. 1984 Nov 8;311(19):1232–1236. doi: 10.1056/NEJM198411083111906. [DOI] [PubMed] [Google Scholar]
- Turnbull D. M., Sherratt H. S., Davies D. M., Sykes A. G. Tetracyano-2,2-bipyridineiron(iii), an improved electron acceptor for the spectrophotometric assay of beta-oxidation and of succinate dehydrogenase in intact mitochondria. Biochem J. 1982 Sep 15;206(3):511–516. doi: 10.1042/bj2060511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull D. M., Sherratt H. S. Mitochondrial myopathies: defects in beta-oxidation. Biochem Soc Trans. 1985 Aug;13(4):645–647. doi: 10.1042/bst0130645. [DOI] [PubMed] [Google Scholar]
- Watmough N. J., Bhuiyan A. K., Bartlett K., Sherratt H. S., Turnbull D. M. Skeletal muscle mitochondrial beta-oxidation. A study of the products of oxidation of [U-14C]hexadecanoate by h.p.l.c. using continuous on-line radiochemical detection. Biochem J. 1988 Jul 15;253(2):541–547. doi: 10.1042/bj2530541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watmough N. J., Turnbull D. M., Sherratt H. S., Bartlett K. Measurement of the acyl-CoA intermediates of beta-oxidation by h.p.l.c. with on-line radiochemical and photodiode-array detection. Application to the study of [U-14C]hexadecanoate oxidation by intact rat liver mitochondria. Biochem J. 1989 Aug 15;262(1):261–269. doi: 10.1042/bj2620261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. F. Effect of temperature on the spectral properties of some ferrocytochromes. Arch Biochem Biophys. 1967 Sep;121(3):757–768. doi: 10.1016/0003-9861(67)90065-3. [DOI] [PubMed] [Google Scholar]
- Wood R., Lee T. Metabolism of 2-hexadecynoate and inhibition of fatty acid elongation. J Biol Chem. 1981 Dec 10;256(23):12379–12386. [PubMed] [Google Scholar]
- van Erven P. M., Gabreëls F. J., Ruitenbeek W., Renier W. O., Fischer J. C. Mitochondrial encephalomyopathy. Association with an NADH dehydrogenase deficiency. Arch Neurol. 1987 Jul;44(7):775–778. doi: 10.1001/archneur.1987.00520190079019. [DOI] [PubMed] [Google Scholar]