Abstract
Background:
Acute paraplegia is a true emergency. It is often the result of trauma but is rarely reported in association with cervical disk herniation in patients without antecedent injury.
Methods:
Case report.
Findings:
This 75-year-old man presented with acute paraplegia due to severe compression of the spinal cord by herniation of the C4-C5 cervical disk. He underwent emergency diskectomy and anterior fusion. Postoperatively, his neurologic functions improved gradually.
Conclusions:
Cervical disk herniation should be considered in the differential diagnosis of nontraumatic acute paraplegia. Pre-existing narrowed canal is an important predisposing factor and excessive neck movements are believed to be triggering factors. Immediate early decompressive surgery is recommended to avoid irreversible progression of neurologic deficit.
Keywords: Paraplegia, acute, nontraumatic; Cervical vertebrae; Intervertebral disk displacement; Spinal cord compression; Rehabilitation, physical
INTRODUCTION
Acute paraplegia is a catastrophic event that is medically and personally devastating. It often results from motor vehicle accidents, falls, violence, and sports. Although uncommon, acute paraplegia has been reported in patients without obvious antecedent injury (1–3), making the diagnosis much more difficult. However, nontraumatic acute paraplegia caused by disk herniation is rare, especially in the cervical spine. Since the first case reported in 1973, only a few have been described in detail (4–9). We present a case of C4-C5 cervical disk herniation causing severe compression of the spinal cord resulting in acute paraplegia. We also review and discuss the etiology, clinical findings, and surgical outcome for reported cases in the literature.
CASE REPORT
This 75-year-old man was admitted to the emergency room of our hospital with neck pain and motor weakness in his lower limbs. His past medical history was significant for hypertension controlled by medication and a diagnosis of cervical spondylosis 10 years prior. He presented with a 2-month history of numbness in all limbs, worse on the left than the right side, first noted in his fingers followed by gradual progression.
On the day of admission, he felt a sharp pain in the posterior neck as he bent forward to pick up a newspaper. The pain soon became electric-like, radiating to his back and buttocks. Two hours later he noticed hypoesthesia and motor weakness of both distal lower limbs. His hypoesthesia and weakness progressed upward and developed rapidly within the next few hours; over the course of 4 hours he became unable to move his lower limbs.
Upon admission, he had reduced neck mobility and his Glasgow Coma Score was 14/15 (E3V5M6). Neurologic examinations revealed a spastic paraplegia and a high-dose methylprednisolone injection was prescribed (10). He was able to move his upper limbs (grade 4–5/5 in left; grade 5/5 in right), but the muscle power in the lower limbs was grade 1/5. Muscle tone also increased with hyperreflexia, more significant on the left side. Babinski reflex and ankle clonus were positive. The last normal sensory level was T1 dermatome. Both light touch and pinprick sensations were present but altered below this level. On rectal examination, the bulbocavernosus and anal reflexes were absent. There was no sensation of bladder distention or of insertion of an indwelling catheter.
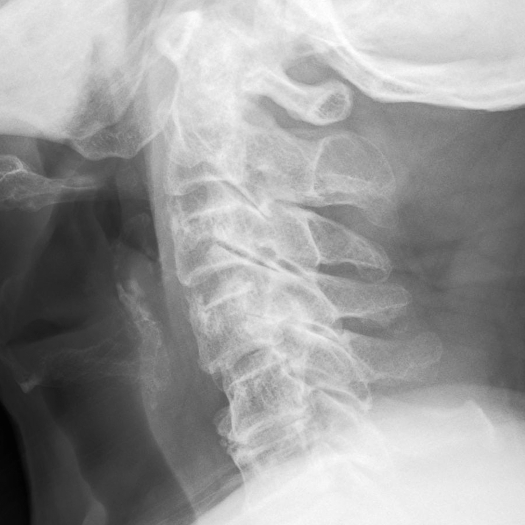
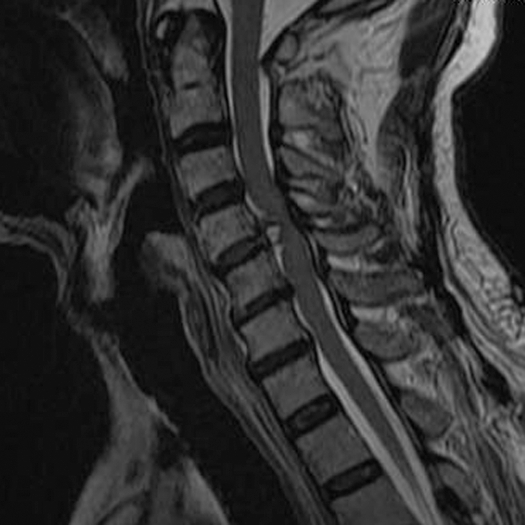
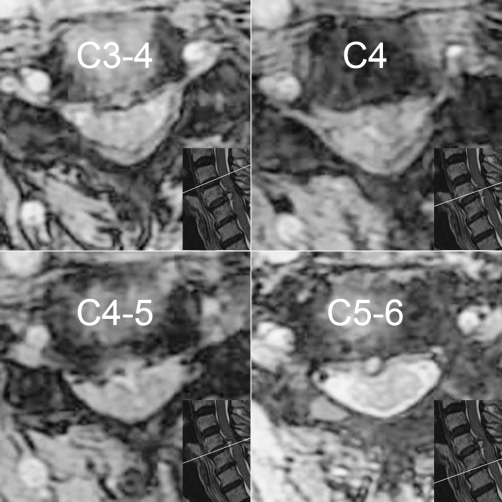
A plain lateral radiograph showed osteophytes at multiple levels in the cervical spine, loss of the lordotic curve, and slight spondyloptosis at C5 vertebra (Figure 1). Emergency magnetic resonance imaging (MRI) revealed localized compression of the cervical spinal cord at C4-C5 level by a sizable mass with high-intensity signal (Figure 2). This lesion was also associated with mild herniation and degeneration of C3-C4 and C5-C6 disks. According to T1-weighted axial images, the spinal cord was normal at C3-C4 and C5-C6 levels; however, the spinal canal at C4-C5 level was occupied by a heterogeneous mass and the spinal cord was compressed mainly from the anterior site (Figure 3). Acute idiopathic epidural hematoma or ruptured disk was highly suspected.
Figure 1.
A lateral radiograph showed osteophytes at multiple levels, loss of lordotic curve, and slight spondyloptosis at C5 vertebra.
Figure 2.
Sagittal T2-weighted magnetic resonance imaging revealed a sizable herniated mass at C4-C5 with high-intensity signal and mild herniation and degeneration at C3-C4 and C5-C6.
Figure 3.
Axial T1-weighted magnetic resonance of the C3-C4, C4, C4-C5, and C5-C6 levels. At C4-C5, a heterogeneous mass occupied the spinal canal anterolaterally, causing the spinal cord compression.
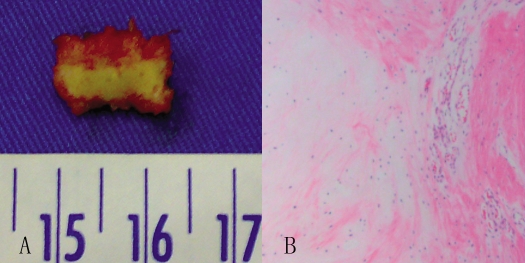
A standard anterior microsurgical approach to the C4-C5 interspace was performed, followed by a diskectomy and interbody fusion with iliac crest bone graft. Intraoperatively, a sequestered disk fragment was found to have compressed the dural sac posteriorly. It was rectangular with dimensions of 1.3 × 1.0 × 0.2 cm and excised successfully without any damage or adhesion. Histologic evaluation demonstrated fiber alignment irregularity accompanied with vascularized granulation tissue (Figure 4).
Figure 4.
(A) The excised sequestrated disk was rectangular and measured 1.3 × 1.0 × 0.2 cm. (B) Photomicrograph of this tissue specimen (stained with hematoxylin-eosin), showing fiber alignment irregularity accompanied by vascularized granulation tissue.
Postoperatively, the patient was treated with a cervical collar. For the first 24 hours, neurologic examination was performed every hour. Pain and numbness disappeared almost completely within 48 hours after surgery, and neurologic function improved gradually. He was able to move his legs at 3 weeks after surgery, but hypoesthesia was still observed below the T10 dermatome. The patient was referred to a rehabilitation unit for a series of intensive rehabilitation therapy. According to his physician's report, there was no residual spinal cord compression. Four months later, although he was still unable to walk, the patient regained good motor power in both lower limbs (grade 3–4/5) but still required intermittent catheterization. At the latest follow-up, 15 months after surgery, he had mild hypoesthesia in the left leg and was provided with a manual wheelchair.
DISCUSSION
Nontraumatic acute paraplegia is a true emergent condition. Without obvious antecedent injury, accurate diagnosis is considerably difficult. When evaluating a patient with such a condition, various etiologies should be kept in mind, including a parasagittal lesion in the brain, a pontine lesion involving the select corticospinal fibers, and spinal cord lesions at any level. Additionally, disorders of muscle or neuromuscular junctions or psychogenia should also be considered (11). In the present case, the constellation of a recent history of numbness of the limbs, severe neck pain radiating to the back and buttocks, motor paresis, sensory deficits, and urinary retention led us to consider a lesion within the spinal cord, and the pattern of hypoesthesia below T1 dermatome implied the likely location to be in the cervical or upper thoracic cord.
The origins of such cord lesions include disk herniation, hematoma, inflammation, and neoplasm. However, the latter two have a more escalating presentation (11,12). In the present case, the acute onset of symptoms and imaging studies suggested the lesion may be a herniated disk or epidural hematoma (Figures 2 and 3); intraoperative and postoperative evaluations confirmed it actually was a sequestered disk fragment (Figure 4).
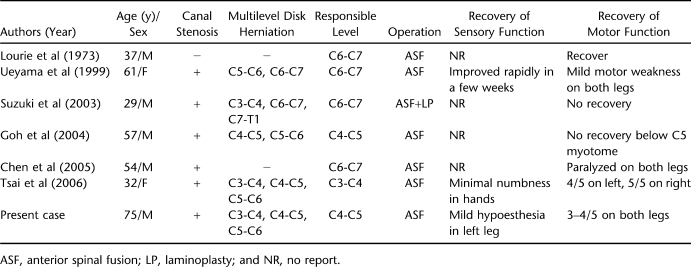
Cervical disk herniation rarely causes nontraumatic acute paraplegia. Since it was first reported in 1973, only 6 cases have been described in detail (4–9); the other 2 cases in Japanese were cited by Suzuki et al (6). Therefore, with our own case, until now only 9 cases have been published in the international literature.
In considering the 7 cases described in detail (Table 1), the age at presentation ranged from 29 to 75 years (mean, 49.3 years) with a male predominance. Pre-existing spinal stenosis was present in the majority. The sagittal diameter of the spinal canal has been established as an important predisposing factor for developing cord compression and myelopathy (13–15). We also noticed that most cases (including 2 cases in Japanese cited by Suzuki et al) were from Asia. It is consistent with the constitutionally narrower canal in Asians, making them more vulnerable to compressive disturbances.
Table 1.
Summary of Reported Cases of Nontraumatic Acute Paraplegia Associated With Cervical Disk Herniation
In the literature, the disk herniation involved one interspace in 2 cases (4,8), 2 contiguous interspaces in 2 cases (5,7), and 3 in the other 3 cases (including our own case) (6,9). The herniated disk responsible for clinical symptoms occurred at the level of C6-C7 followed by C4-C5 and C3-C4 (Table 1). It is impossible to tell how long the herniated disk had existed, or whether it occurred just before the onset of paraplegia. However, patients usually had preceding neck pain and/or progressive numbness in the limbs. Some authors demonstrated that local neck pain or cervical spondylosis should be regarded as a prelude to neurologic deterioration (5,16).
Our review of the literature showed that the symptoms could be provoked during physical examination, change of position, general anesthesia, or even labor. Under these circumstances, excessive flexion/extension in the neck was involved, and we speculated it may be a triggering factor. The average value of cervical intradiskal pressure has been proved to be much higher during flexion/extension both in vivo and in vitro (17). In our own case, we surmised that preceding intrinsic disk degeneration due to age (75 years) caused mild disk bulging at C3-C6; then rapid and momentary rise of the intradiskal pressure due to excessive neck movements occurred when he bent forward, followed by disk rupture at C4-C5. In conjunction with a pre-existing narrowed canal, it was logical that the herniated disk then readily caused severe compression of the spinal cord.
However, disk herniation usually shows stepwise neurologic deterioration. The progressions in these cases were rapid, mostly within a few minutes or hours. It indicated that a cascade of secondary injury mechanisms must exist. Vascular abnormality has been proven as a key factor in damage secondary to the injury of the spinal cord, including loss of microcirculation, glutamate-mediated excitotoxicity, and failure of autoregulation (18,19). In these cases, an insufficiency of blood supply to the anterior spinal artery and its branches may be a factor. We speculated that herniated disk with pre-existing narrowed canal caused severe spinal cord compression and induced a major reduction in blood flow at the lesion site immediately, whereas vascular abnormalities promoted ischemia and contributed to the acute presentation in these patients. On the other hand, the increased spinal signal could also be detected near or around the lesion site on MRI (5–7,9). It was consistent with histologic findings of ischemia-reperfusion injured cord seen in experimental animals, which indicated the presence of demyelination and cord edema (6,7,20). These findings strongly suggested the necessity of early surgical decompression to avoid progression into permanent neurologic deficit.
All patients were treated surgically with diskectomy and anterior spinal fusion, but the outcomes were varied (Table 1). Also, the persistence of high signal intensity of spinal cord on postoperative MRI did not affect the neurologic recovery. Ueyama et al (5) reported that, although the neurologic function was significantly improved, the signal change in spinal cord remained 4 months later. Suzuki et al (6) performed single-level anterior decompression at first; however, a high signal intensity change in the spinal cord spread postoperatively. Even after an additional laminoplasty, there was still no neurologic recovery. Tsai et al (9) reported a woman who entered rehabilitation programs after surgery; 18 months later her neurologic functions had improved with only minimal residual numbness in the hands. It seems that the various outcomes may be related to patient characteristics, completeness of the lesion, surgical timing, and postoperative rehabilitation therapy (21).
CONCLUSION
In conclusion, a rare clinical picture of acute paraplegia revealing a disk herniation at the level of C4-C5 is reported. This devastating event seems to be underdiagnosed and underrepresented in the international literature. Pre-existing narrowed canal is an important predisposing factor and excessive neck movements are believed to be triggering factors. Appropriate diagnosis is based on accurate neurologic examination and imaging studies. An urgent surgical decompression is recommended to avoid irreversible progression of neurologic deficit.
References
- Kyriakides AE, Lalam RK, El Masry WS. Acute spontaneous spinal subdural hematoma presenting as paraplegia: a rare case. Spine. (Phila Pa 1976) 2007;32(21):E619–E622. doi: 10.1097/BRS.0b013e318154c618. [DOI] [PubMed] [Google Scholar]
- Rathore MF, Gill ZA, Malik AA, Farooq F. Acute flaccid paraplegia: a rare complication of meningococcal meningitis. Spinal Cord. 2008;46(4):314–316. doi: 10.1038/sj.sc.3102120. [DOI] [PubMed] [Google Scholar]
- Huang MK, Chen CC, Wang TL, Lee YK, Su YC. Simultaneous bilateral femoral arterial emboli presenting with acute paraplegia: an uncommon case. Am J Emerg Med. 2009;27(6):753.e3–4. doi: 10.1016/j.ajem.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Lourie H, Shende MC, Stewart DH., Jr The syndrome of central cervical soft disk herniation. JAMA. 1973;226(3):302–305. [PubMed] [Google Scholar]
- Ueyama T, Tamaki N, Kondoh T, Miyamoto H, Akiyama H, Nagashima T. Non-traumatic acute paraplegia associated with cervical disk herniation: a case report. Surg Neurol. 1999;52(2):204–206; discussion 206–207. doi: 10.1016/s0090-3019(97)00422-9. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Abe E, Murai H, Kobayashi T. Nontraumatic acute complete paraplegia resulting from cervical disk herniation: a case report. Spine (Phila Pa 1976) 2003;28(6):E125–E128. doi: 10.1097/01.BRS.0000050404.11654.9F. [DOI] [PubMed] [Google Scholar]
- Goh HK, Li YH. Non-traumatic acute paraplegia caused by cervical disk herniation in a patient with sleep apnoea. Singapore Med J. 2004;45(5):235–238. [PubMed] [Google Scholar]
- Chen SH, Hui YL, Yu CM, Niu CC, Lui PW. Paraplegia by acute cervical disk protrusion after lumbar spine surgery. Chang Gung Med J. 2005;28(4):254–257. [PubMed] [Google Scholar]
- Tsai HH, Li TY, Chang ST. Nontraumatic acute myelopathy associated with cervical disk herniation during labor. J Back Musculoskelet Rehabil. 2006;19(2–3):97–100. [Google Scholar]
- Bracken MB, Shepard MJ, Holford TR, et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury: results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA. 1997;277(20):1597–1604. [PubMed] [Google Scholar]
- Prasad S, Price RS, Kranick SM, Woo JH, Hurst RW, Galetta S. Clinical reasoning: a 59-year-old woman with acute paraplegia. Neurology. 2007;69(24):E41–E47. doi: 10.1212/01.wnl.0000291014.07901.59. [DOI] [PubMed] [Google Scholar]
- Boockvar JA, Black K, Malik S, Stanek A, Tracey KJ. Subacute paraparesis induced by venous thrombosis of a spinal angiolipoma: a case report. Spine (Phila Pa 1976) 1997;22(19):2304–2308. doi: 10.1097/00007632-199710010-00022. [DOI] [PubMed] [Google Scholar]
- Torg JS, Naranja RJ, Jr, Pavlov H, Galinat BJ, Warren R, Stine RA. The relationship of developmental narrowing of the cervical spinal canal to reversible and irreversible injury of the cervical spinal cord in football players. J Bone Joint Surg Am. 1996;78(9):1308–1314. doi: 10.2106/00004623-199609000-00003. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Kadoya S, Iizuka H. Roentgenological study of the sagittal diameter of the cervical spinal canal in normal adult Japanese. Neurol Med Chir (Tokyo) 1998;38(2):83–88; discussion 88–89. doi: 10.2176/nmc.38.83. [DOI] [PubMed] [Google Scholar]
- Sani S, Boco T, Deutsch H. Cervical stenosis presenting with acute Brown-Sequard syndrome: case report. Spine (Phila Pa 1976) 2005;30(16):E481–E483. doi: 10.1097/01.brs.0000174284.08440.1e. [DOI] [PubMed] [Google Scholar]
- Tator CH, Duncan EG, Edmonds VE, Lapczak LI, Andrews DF. Changes in epidemiology of acute spinal cord injury from 1947 to 1981. Surg Neurol. 1993;40(3):207–215. doi: 10.1016/0090-3019(93)90069-d. [DOI] [PubMed] [Google Scholar]
- Pospiech J, Stolke D, Wilke HJ, Claes LE. Intradiskal pressure recordings in the cervical spine. Neurosurgery. 1999;44(2):379–384; discussion 384–385. doi: 10.1097/00006123-199902000-00078. [DOI] [PubMed] [Google Scholar]
- Sekhon LH, Fehlings MG. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine (Phila Pa 1976) 2001;26(24 suppl):S2–S12. doi: 10.1097/00007632-200112151-00002. [DOI] [PubMed] [Google Scholar]
- Kwon BK, Tetzlaff W, Grauer JN, Beiner J, Vaccaro AR. Pathophysiology and pharmacologic treatment of acute spinal cord injury. Spine J. 2004;4(4):451–464. doi: 10.1016/j.spinee.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Wisselink W, Money SR, Crockett DE, et al. Ischemia-reperfusion injury of the spinal cord: protective effect of the hydroxyl radical scavenger dimethylthiourea. J Vasc Surg. 1994;20(3):444–491; discussion 449–450. doi: 10.1016/0741-5214(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Wuermser LA, Ho CH, Chiodo AE, Priebe MM, Kirshblum SC, Scelza WM. Spinal cord injury medicine: 2. Acute care management of traumatic and nontraumatic injury. Arch Phys Med Rehabil. 2007;88(3 suppl 1):S55–S61. doi: 10.1016/j.apmr.2006.12.002. [DOI] [PubMed] [Google Scholar]