Abstract
Recent progress in the biochemical classification and structural determination of allergens and allergen–antibody complexes has enhanced our understanding of the molecular determinants of allergenicity. Databases of allergens and their epitopes have facilitated the clustering of allergens according to their sequences and, more recently, their structures. Groups of similar sequences are identified for allergenic proteins from diverse sources, and all allergens are classified into a limited number of protein structural families. A gallery of experimental structures selected from the protein classes with the largest number of allergens demonstrate the structural diversity of the allergen universe. Further comparison of these structures and identification of areas that are different from innocuous proteins within the same protein family can be used to identify features specific to known allergens. Experimental and computational results related to the determination of IgE binding surfaces and methods to define allergen-specific motifs are highlighted.
Keywords: allergen PFAM classification, cross-reactiviy, allergen specific motifs, IgE epitopes, structural database of allergenic proteins, specific immunotherapy
Introduction
Our current view of allergy as a treatable illness began with our ability to first, define and control the allergic reaction, and second, to better identify the allergens involved. The results of this study, as we will discuss in more detail below, have indicated that while allergens do not have one common structure, most of the major allergens can be grouped according to common features and, in some cases, enzymatic activities. Identifying similarities in allergens can be used clinically, to alert patients who are sensitive to substances known to trigger extreme allergic reactions, such as peanuts, shellfish, or latex, to the presence of similar proteins in different sources. Defining characteristic features of allergenicity is also needed for regulatory purposes, to avoid introducing novel allergenic foods, drugs or genetically modified organisms that contain proteins similar to known allergens1 and to stipulate manufacturing precautions and labeling requirements. Future advances in these areas depend on our ability to reliably discriminate the properties of allergens that distinguish them from structurally related innocuous proteins. Here, we discuss some of the physicochemical and structural features of allergens, and motifs that can be defined for allergens in the same group that differentiate them from non-allergenic structural relatives.
Not All Proteins are Allergenic
Fortunately, only a relatively small fraction of proteins in our environment are allergenic. The first efforts to define common properties of allergenic proteins in the 1970s were limited to characteristics that were measureable at that time, such as molecular size, the abundance of the protein in airborne sensitizing particles or, for food allergens, acid or heat stability.2 We now have gene and protein sequence information for more than 800 characterized allergens. Nuclear Magnetic Resonance (NMR) and X-ray crystal three dimensional (3D) structures are available in the Protein Data Bank (PDB)3 for over 60 allergens, including such clinically important allergens as birch pollen Bet v 1,4 cockroach allergen Bla g 2,5 olive pollen Ole e 6,6 maize pollen Zea m 1,7 and cedar pollen Jun a 1.8 In addition, a large percentage of all allergens without an experimentally determined 3D structure can be reliably modelled with current 3D prediction tools.9 This increasing knowledge of the structural and biochemical properties of allergens makes it feasible to classify allergenic proteins by their 3D structures and to identify shared substructures that might be important for allergic sensitization and reactions. While the idea that a single structural feature common to all allergenic proteins responsible for IgE binding has yielded to results showing a plethora of different structures, we do know that many of the most allergenic proteins closely resemble one another and can be grouped into discrete families.
Allergen Nomenclature and Databases
In response to the large amount of new information about individual allergens, the International Union of Immunological Societies (IUIS) established in 1986 nomenclature rules and criteria to evaluate the clinical data for substantiating that a protein is allergenic,10 which were revised in 1994.11 The list of proteins that met these criteria grew rapidly, leading to a website (www.allergen.org) dedicated to proteins that are generally recognized as causing allergic symptoms in humans. The approved allergens were named systematically according to the Latin species name of their natural source and the order in which they were identified. By 2001, it was clear that bioinformatics tools were required to define important similarities in the amino acid sequences and structural features of these allergens, since molecular similarities of allergen from different sources are not reflected in the nomenclature described above. For example, Ara h 1, the first allergen to be identified in peanut (Arachis hypogaea), is similar to the vicilin allergen Jug r 2, from English walnut (Juglans regia), with a sequence identity of 36% and E-value of 1.8 × 10−22. The E-values, provided routinely as an indication of the extent of sequence similarity identified by FASTA12 or BLAST searches,13 is a measure of how many matches with the same sequence would be expected to occur randomly in the database. A very low E-value (generally <0.001) generally indicates a highly significant sequence match.
As part of the deregulation process for genetically modified (GM) foods, regulatory agencies around the world required a more systematic overview of allergen sequences to detect proteins that were potentially allergenic based on their sequence similarities. The first guidelines were based only on protein sequence comparisons. A committee organized by the World Health Organization (WHO)/Food and Agriculture Organization (FAO) and the European Food Safety Authority (EFSA) proposed that a novel protein was likely to be an allergen if its amino acid sequence was >35% identical to any known allergen, over a window of 80 amino acids with a suitable gap penalty, or it contained 6 (or more recently, 8) contiguous amino acids that are identical to a known allergen.14–16 Several cross-referenced databases created for the comparison of the sequences and properties of allergens (Table 1)17–23 can be used to objectively test the utility of these guidelines. While a recent statistical analysis of the WHO guidelines demonstrates that a 35% sequence identity is a realistic cut-off value to achieve a good balance between sensitivity and specificity,24 the simple identity search for 6 or 8 contiguous residue matches is not a reliable criterion, as such analysis provides too many false positives.1,25 In one case, cross-reactivity between the Cry1F protein and the Der p 7 allergen of dust mite could not be confirmed experimentally, even though they share a stretch of 6 contiguous amino acids.26
Table 1.
Allergen databases and servers.
| Web site and URL | Features |
|---|---|
| IUIS International Union of Immunological Societies http://www.allergen.org |
lists official names links to GenBank, UniProt, PDB |
| SDAP Structural Database of Allergenic Proteins http://fermi.utmb.edu/SDAP |
allergen sequences, structures, IgE epitopes links to GenBank, UniProt, PDB FASTA search in SDAP LAST search in GenBank, SwisProt, PIR Pfam classification FAO/WHO allergenicity rules tools for sequence and epitope comparison high-quality allergen models |
| FARRP Food Allergy Research and Resource Program http://www.allergenonline.org |
allergen list FASTA search in FARRP FAO/WHO allergenicity rules links to GenBank |
| Allergome http://www.allergome.org |
allergen list links to PubMed, Uniprot, PDB links to sequence databases |
| AllFam http://www.meduniwien.ac.at/allergens/allfam |
Pfam classification |
| CSL (Central Science Laboratory, UK) http://allergen.csl.gov.uk//index.htm |
allergen list links to GenBank IgE epitopes |
| InformAll http://foodallergens.ifr.ac.uk |
clinical data |
| ADFS (Allergen Database for Food Safety) http://allergen.nihs.go.jp/ADFS/ |
allergen list sequences IgE epitopes 3D structures epitope search FAO/WHO allergenicity rules motif-based allergenicity prediction |
| All Allergy http://allallergy.net |
allergen list species description |
| IEDB Immune Epitope Database and Analysis Resource http://immuneepitope.org |
T-cell and B-cell epitopes |
| Allermatch http://www.allermatch.org |
FAO/WHO allergenicity rules |
Bioinformatics tools to implement the WHO/FAO criteria are widely available, and include the FARRP database (www.allergenonline.com), the Structural Database of Allergenic Proteins (SDAP; http://fermi.utmb.edu/SDAP/)27 and Allermatch (http://allermatch.org/),28 where a user can rapidly compare molecular features of individual or groups of known allergens, such as protein sequence, 3D structure, IgE epitopes and literature references. Bioinformatics tools incorporated in some of these databases allow the user to find additional information by cross reference to other data bases, such as the protein family of an allergen in Pfam,29 the experimentally determined 3D structure in the Protein Data Bank,3 gene sequences in GenBank,30 or publications in PubMed. Bioinformatics analysis of the properties of allergens has progressed greatly in the last few years, and the established databases now provide a solid scientific foundation beyond the WHO guidelines for assessing the potential risk of allergenicity in GM foods.24,31 Additional information on B-cell and T-cell epitopes is collected in the Immune Epitope Database (http://www.immuneepitope.org/).32 This site also lists peptides from the sequences of allergens that have been tested and shown not to bind IgE. This information can be useful for those designing hypoallergenic proteins designed to induce T-cell tolerance.
Classification of Allergens
Grouping allergens into protein families based upon their amino acid sequences and biochemical functions, as exemplified by the Pfam database,29 has shown that there are only a limited number of allergen structural classes. The Pfam database is a comprehensive collection of families of proteins with similar biochemical functions and sequence similarities. As proteins are generally composed of one or more domains, each domain is separately represented by multiple sequence alignments and hidden Markov models (HMMs) and annotated with its biochemical function. There are two components of Pfam: Pfam-A, a set of high quality, manually curated families and Pfam-B, a set of automatically generated domain usually of lower quality. Pfam assignments can be obtained from precomputed domains using UniProt, TrEMBL or SwissProt accession numbers, or individual BLAST or HMM profile searches of individual allergen sequences.
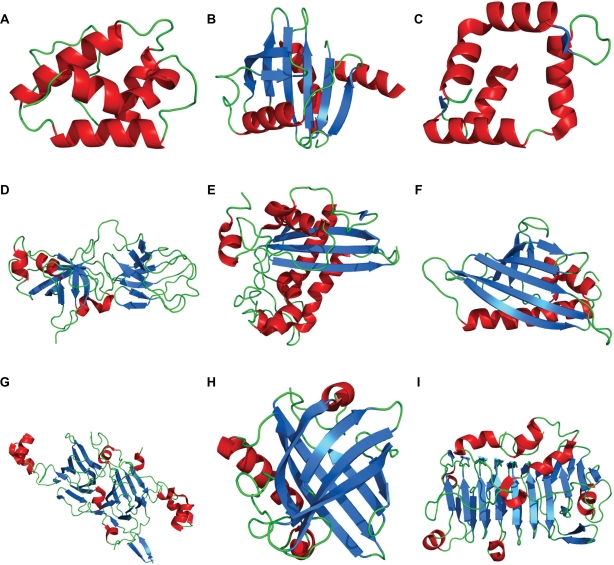
Two independent studies of the classification of all known allergens within two different databases came to similar conclusions. All the allergens in the Allfam database (http://www.meduniwien.ac.at/allergens/allfam) could be grouped into 143 of the 9,318 distinct families in the Pfam database,33 whereas the allergens listed in SDAP database can be grouped into 130 Pfam families.34 Representative 3D folds of nine major protein families that encompass the majority of known allergens illustrate the variety of different folds of these families (Fig. 1). Members of the same protein family in general have very similar 3D protein folds, so given a sufficient degree of sequence identity (above about 35%),35 one can model the structure of other family members using the known structures as templates. Reliable 3D models, which can be used to map experimentally determined linear epitopes and predict conformational epitopes, have been prepared for most of the allergens in SDAP.9
Figure 1.
Representative experimentally determined X-ray crystal and NMR structures of allergens from the nine most abundant Pfam database protein families. A) Protease inhibitor/seed storage/LTP family (Pfam ID = PF00234); representative allergen LTP from maize (Zea m 14) B) Profilin family (PF00235); birch pollen profilin (Bet v 2) C) EF hand (PF00036); pollen allergen from Timothy grass (Phl p 7) D) Expansin family (2 domains PF01357 and PF03330); beta-expansin from maize (Zea m 1) E) Cysteine-rich secretory protein family (PF00188); venom allergen III from fire ant (Sol i 3) F) pathogenesis related protein family PR10 (PF00407); cherry allergen (Pru av 1) G) Cupins (PF00190); peanut allergen (Ara h 3) H) lipocalin (PF00061); alpha-2U-globulin from mouse (Mus m 1) I) Pectate lyase family (PF00544); major cedar pollen allergen (Jun a 1).
Molecular and Structural Features of Cross-reactive Allergens
Clinical cross-reactivity is frequently observed between allergens from different sources
Clinical cross-reactivity between two allergens refers to the situation in which a patient who has been sensitized to and has IgE antibodies specific for one allergen, also reacts to a second allergen. Patients need not have been exposed to the related allergen; for example, IgE antibodies in sera of patients sensitized to Texas mountain cedar also recognize allergens from Japanese red cedar (“Sugi”) pollen.36 Such cross-sensitivity can be identified from clinical history, in vitro quantification of allergen-specific IgE, skin test reactivity and provocation challenges with purified allergens. Competition assays can be used to quantify and verify the findings: preincubation of sera with the suspected cross-reactive antigen should reduce the binding of the IgE to the sensitizing antigen.
Some common allergen cross-reactivities have been explained by sequence/structural similarities between proteins from different sources. For example, shellfish allergies have been linked to reaction to tropomyosins of more distantly related arthropods, such as cockroaches or dust mites, using in vitro and animal models.37,38 The cross-reactivity observed for cedar pollens across a large array of taxonomically related groups,36 can be explained by the fact they all contain forms of the major allergenic proteins (particularly pectate lyases and certain pathogenesis-related (PR) proteins) that are highly similar in sequence. Similar cross-reactivities to plants from different phyla have been related to their nearly identical profilins, lipid transfer proteins, calcium-binding proteins and PR proteins.39,40
The situation is more complex in other important food sources, such as nut proteins, where several major allergens have been identified. About 35% of patients who are allergic to peanuts also react to tree nuts, particularly walnuts.41 The major allergenic proteins in peanuts and walnuts are vicilins, albumins, and pathogenesis related proteins, which have a high structural similarity. While the vicilins are quite similar, the percent identities of the other allergens lie well below the 35% cutoff listed in the WHO rules. Sera from patients with nut allergies detect many proteins and subsequences of known allergens on Western blots and microarrays, and the patterns differ greatly from one patient to another.42 Thus, much more effort will be required to establish which of the protein groups in the two sources is most important for cross-reactivity.
In some cases, the source of allergenic triggers do not appear to be related to each other; for example, in pollen–food allergy syndrome (also known as oral allergy syndrome (OAS)), the sensitizing allergen is often a plant pollen and the trigger is a food protein. Pollen-food allergy syndrome is elicited by a variety of plant proteins cross-reacting with airborne allergens. Symptoms are mostly confined to the oral and pharyngeal region after eating foods that have not been denatured by cooking. It is estimated that OAS affects up to 50%–70% of patients suffering from pollen allergy, especially to birch and ragweed. These patients were sensitized with pollen allergens and symptoms develop when they ingest food which contains homologous allergens.
Allergens that can both sensitize and trigger reactions are known as “complete” allergens; those that can only trigger reactions in previously sensitized individuals are known as “incomplete” allergens. The latter include Group 2 food allergens, which are not sensitizing but cross-react with IgE antibodies that individuals produce in response to aeroallergens, and are implicated in OAS.40 For example, some individuals sensitive to the birch pollen allergen Bet v 1 can experience OAS after eating fruits of the Rosaceae such as apple, cherries, celery root, and carrots, which contain the allergens Mal d l, Pru av 1, Api g 1 and Dau c 1, respectively, all of which share sequence identity of more than 35% with Bet v 1.43–46
Cross-reactive allergens are often from the same protein family
The classification of allergens according to Pfam also provides a framework to explain clinically observed cross-reactivities.27,47–49 For example, similar lipid transfer proteins (LTP) have been implicated in food allergies to cherry (Pru av 3), apricot (Pru ar 3), hazelnut (Cor a 8), peach (Pru p 3) and corn (Zea m 14). The 3D structures of these proteins form a compact four-helix bundle (Fig. 1a) which is stabilized by disulfide bonds. A structural homologue of these allergens in plane tree pollen (Pla a 3) may be the sensitizing allergen for cross reactivity with pollen fruit allergens in the Mediterranean population.50 Several other studies demonstrated cross-reactivities of structural homologues of LTPs in other foods, such as rice, strawberry and cabbage.40
Profilins (Fig. 1b) are pan allergens, considered to be responsible for cross-reactivities between latex, pollen and plant food.51,52 However, not all plant profilins are cross-reactive to the same extent, and ELISA inhibition data with sera from different patients could be correlated in a semi-quantitative analysis with conserved and species-specific epitopes of profilin.53 The extent of cross-reactivity among profilins from Timothy grass, birch, latex and celery was greater than cross-reactivity to mugwort and bell pepper profilins.
The 3D structures of the cross-reactive allergens from grass pollen (Phl p 7), tree pollen (Bet v 4) and weed pollen (Che a 3) contain a common domain known as the EF-hand, a structural motif for calcium binding (Fig. 1c).54 Other pollen allergens, grass allergens Phl p 1 and Phl p 2 from timothy grass and Zea m 1 from corn, are expansins that mediate cell wall extension in plants. Expansins contain two domains that form a binding groove for a glycan backbone. The first domain, a double-psi beta barrel,55 resembles the structure of the family-45 glycoside hydrolase (GH45), although Zea m 1 lacks a critical residue in the active site and does not hydrolyze polysaccharides.7 The second domain is an immunoglobulin (Ig)-like β sandwich (Fig. 1d). Common epitopes among corn and grass pollen allergens have been located on the protein portion of these expansins, but these epitopes of group 1 grass allergens are not conserved among all members of the expansin superfamily. Five surface exposed regions in the major latex allergen Hev b 2 are conserved in the 1,3 β-glucanase homologue from banana, and might be responsible for latex-banana cross-reactivity.52
These examples illustrate that clinically observed cross-reactivities of allergens can often be explained by structural similarity of conserved surface patches of allergenic proteins in the same protein family. These observations are also valid for other families, such as the cysteine-rich secretory protein family; PR-10 proteins, cupins, lipocalins, and pectate lyases (Fig. 1e–i). Recent annotations of allergen databases with protein family relations, such as SDAP or Allfam can alert physicians of allergenic patients to sources containing structurally related allergens, and in vitro tests such as Western blotting and ELISA can then be used to determine whether the patient’s serum IgE binds to the allergenic proteins in those sources.27,40,48,49,56 New proteomic microarray technology allow the detection of IgE-related sensitization of large panels of allergens using many sera samples, and can provide a comprehensive basis for the relation between sequence similarity and IgE recognition in the future.57
Linear and Conformational IgE Epitopes
Identifying IgE epitope containing areas as allergen specific motifs
It is possible that small areas of similar sequence might be sufficient to account for cross-reactivity between sources by constituting an IgE epitope. Two different methods have been proposed to define areas of sequence and structural homology that are common and distinctive for homologous allergens,34,58 known as allergen specific motifs (ASMs).
The first approach defines ASMs as MEME motifs 50 amino acids in length occurring repeatedly in protein sequences of currently known allergens.59 MEME motifs are sequence patterns that are represented as probabilities for each amino acid in a position-dependent manner.60 Stadler found that more than 90% of the test allergens shared 52 repeating sequence motifs. A recent test of the MEME methodology showed that a motif-based peptide from tropomyosins had the same reactivity with IgE in patient sera as did full-length tropomyosins from shrimp.58
The second approach uses the Pfam classification of allergens and generates PCPMer motifs, typically 5–20 residues in length. PCPMer motifs are short contiguous sequences with a statistical significant conservation of physicochemical properties of amino acid residues derived from a multiple sequence alignment of proteins.61 PCPMer motifs can be generated online from any protein sequence alignment with the PCPMer suite (http://landau.utmb.edu:8080/pcpmer/index.jsp); these motifs correspond to some extent with known IgE epitopes.34 An algorithm for quantitatively assessing the similarity in physicochemical properties of a sequence amino acids in linear epitopes, known as the property distance (PD) scale, has been experimentally validated and implemented in the SDAP allergen data base.62 The PD scale and PCPMer motifs, available on the SDAP web server, can be used to screen novel proteins for the presence of sequences similar to those found in known allergens to assess the potential risk of allergenicity in recombinant food products.24
Defining conformational epitopes
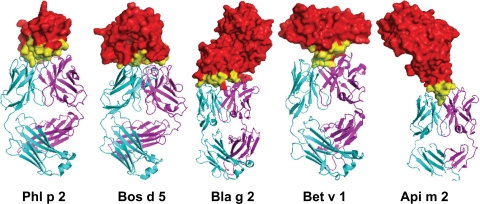
Most of the IgE epitopes that have been elucidated so far are “linear” or “continuous” ASMs as they were defined by probing overlapping synthetic peptides of the allergens for the binding of IgE from the sera of allergic patients. However, the epitopes to which IgE binds most tightly are most commonly formed by residues that become contiguous on the protein surface after folding.5,6,8,63–67 Identifying such “conformational epitopes” is currently a major challenge.17,24,27,48,58,68,69 Experimentally, conformational IgE epitopes can be defined most convincingly by examining the crystal structures of complexes of the allergen and the binding fragments (Fab) of relevant antibodies.66,70–72 However, only five X-ray structures of complexes of allergen–Fab fragments have been determined so far (Fig. 2): the major allergens from Timothy grass pollen Phl p 273 and bovine milk β-lactoglobulin Bos d 571 with recombinant Fab fragments of human IgE; the major allergens from birch pollen Bet v 1,74 bee venom hyaluronidase Api m 2,72 and the major allergen from German cockroach Bla g 2 with monoclonal IgG antibodies66 that were shown in competition assays to bind to or near human IgE epitopes.
Figure 2.
Allergens in complex with IgE or IgG Fab fragments. Timothy pollen Phl p 2–IgE; bovine milk beta-lactoglobulin Bos d 5–IgE; German cockroach Bla g 2–IgG; birch pollen Bet v 1–IgG; honey bee venom hyaluronidase Api m 2–IgG. Allergens are colored red with epitopes colored yellow, whereas the H and L chains of Fab are colored magenta and cyan, respectively.
Padavattan et al compared the first four of these epitopes defined by X-ray crystallography73 and suggested that the flattened shape (formed by four parallel β strands) of the Bos d 5 and Phl p 2 epitopes recognized by their human IgE antibodies were different from the protruding epitopes of Bet v 1 and Api m 2 (formed from a β hairpin and a helix-loop helix motif) because these epitopes were recognized by IgE vs. IgG antibodies. However, the monoclonal IgG antibodies used in the study could be considered as surrogates for human IgEs, based on their ability to inhibit the binding of human IgG to the target allergen. Subsequently the structure of an epitope of the German cockroach allergen Bla g 2,66 a flat area dominated by a quasi-helical structure, was defined in a co-crystal with a high affinity IgG monoclonal antibody, which effectively competed with patient IgE antibodies for Bla g 2 binding. These observations on structural characteristics of IgE vs. IgG epitopes are based on the rather limited data that are currently available, and additional co-crystal structures are needed to define structural patterns of epitopes that distinguish epitopes for IgE from IgG responses.
The extent of fragmentation of the sequences that form epitopes was also considered as a potential difference between IgG and IgE epitopes. For example, in the IgG epitopes of Bet v 1 and Api m 2, the conformational epitopes consist mainly of one contiguous segment of residues. In contrast, the IgE epitopes from Bos d 5 and Phl p 2 are formed by four fragments of contiguous residues and several isolated residues. The Bla g 2-specific monoclonal antibody 7C11 binds to the same binding site on Bla g 2 as IgE and the epitope consists also of four fragments of three or more contiguous residues, with a long fragment of 11 residues.
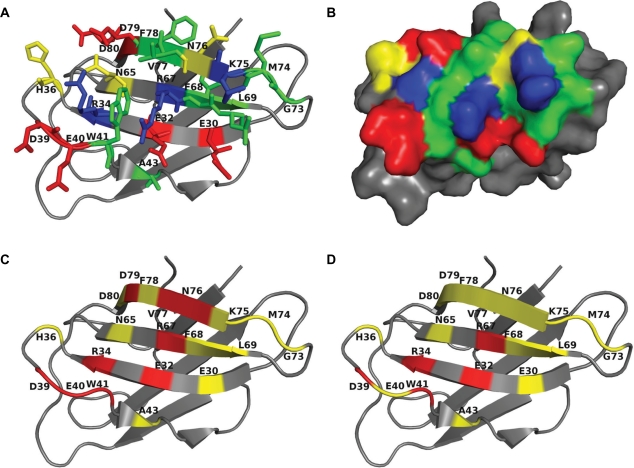
Another use of crystal structures is to determine fine differences that might account for the different reactivities of closely related proteins. For example, grass pollen allergens were grouped in the 1960s based on chemical characteristics, including molecular weight and isoelectric point.75,76 There is extensive cross-reactivity between group 2 and group 3 grass pollen allergens, but none between these and the group 1 allergens. An IgE epitope of the group 2 Timothy grass pollen allergen Phl p 2 is dominated by charged and polar residues that form tight contacts (some of them by hydrogen bonds) to the antibody (Fig. 3a, b).73 Nine residues within hydrogen-bonding distance of IgE are conserved between group 2 and group 3 allergens (red in Fig. 3c) but are different from the five conserved potential hydrogen-bonding residues in group 1 allergens (Fig. 3d). We expect that many clinically observed cross-reactivities between allergens could be elucidated based on the structural features of conformational IgE epitopes.27,40,45,49,54,77
Figure 3.
The Phl p 2 IgE epitope and cross-reactivity between grass pollen from group 2 and group 3 but not group 1. A) and B) Phl p 2 IgE epitope colored by residue type: hydrophobic and aromatic—green; polar—yellow; positive—blue; negative—red; proline—magenta. C and D: sequence conservation of Phl p 2 epitope residues in multiple alignments with homologs from group 2 and group 3 C) and group 1 D) red—conserved or conservatively substituted residues that form hydrogen bonds, yellow—epitope residues that do not form hydrogen bonds.
The structural characterization of conformational IgE epitopes by X-ray crystal structures is a difficult and time consuming procedure, since it requires monoclonal antibodies that mimic the reactivity of human IgE antibodies and production of high quality co-crystals. Another approach for defining potential conformational epitopes uses random peptide phage display to identify mimetic peptides (mimotopes). Mimotopes are peptides that are generated by phage display technology and screened using monoclonal antibodies in an iterative selection process.
The selected peptides mimic the binding site of the antigen to the chosen antibody and can be linear or conformational protein, carbohydrates or lipid epitopes. In case of carbohydrates and lipids these mimetic peptides compete in binding to the chosen monoclonal antibody directed against carbohydrate or lipid antigen. Both monoclonal IgG78–80 and purified IgE81,82 from patient sera have been used with some success. Mimotopes can achieve immunogenicity and induce epitope-specific antibody responses upon vaccination when coupled to suitable carriers. Manual or computational methods have been used to locate contiguous regions on the surface of the allergen proteins that most similarly match the physicochemical structure of the peptide mimetics.36,83 The similarity between the phage peptides and areas on the surface of the allergen protein can be assessed and quantified using several web servers.83–85
The relation of binding affinities of specific IgE epitopes on the physiological effect has been directly tested using a panel of recombinant IgE (rIgE) antibodies. For the major dust mite allergen Der p 2 several rIgE antibodies with different binding affinities were tested for their ability to induce degranulation of human blood basophils triggered by different concentrations of rDer p 2.86 Their finding strongly suggests that optimal degranulation of basophils requires two to three epitope-IgE complexes. Equimolar concentrations of the different IgE antibodies were most effective; increasing the concentrations of Der p 2 IgE antibodies relative to unrelated IgE antibodies was important, and certain pairing of their monoclonal antibodies to different epitopes was more effective than others. This observation might be related to the spatial relationships between different IgE epitopes on the Der p 2 protein surfaces. Since many allergens exist in nature as homodimers, which greatly augments their ability to activate cells with IgE receptors, the orientation of the dimerization could also be an important structural determinant of the relative potency of different allergens.
Redesigning Allergenic Proteins
The first efforts to reduce allergenicity used chemical modification, for example polymerization with aldehyde87 or coupling to methoxypolyethylene.88 The polymerized proteins diffuse more slowly and contain more concealed antigenic determinants, which are preferentially processed by phagocytic cells (monocytes, macrophages and dendritic cells),87 are less likely to cross-link IgE antibodies, and more likely to be presented to T cells. Proteins coupled with methoxypolyethylene stimulate antigen-specific, T-supressor cells and down regulate the proliferation of B cell clones engaged in IgE synthesis.88 Another approach is to use fragments of an allergen, obtained proteolytically or by recombinant synthesis; for example, fragments of Bet v 1 cannot bind IgE but can activate T cells.89 However, the fragments might not fold correctly, thus greatly decreasing their ability to generate an IgG response that could reduce subsequent IgE sensitization.90 Treatment with fragments did not significantly prevent side reactions, such as flushing or swelling at the injection site, generalized urticaria, pruritus, and even asthma, dyspnoea, circulatory dysregulation, and gastrointestinal disturbance when compared to the wild type extract.91
The advent of recombinant allergen synthesis allowed the production of allergen isoforms that are not only hypoallergenic, i.e, with reduced IgE binding,92,93 but that specifically stimulate the production of protective antibodies (eg, specific IgG, IgA and IgM) and regulatory T-cells. For grass pollen allergens, IgE epitopes appear different from those that bind other allergen classes.94 One can also generate IgG antibodies (IgG1, IgG4 and IgG2 subclasses) to major birch pollen allergen Bet v 1 epitopes distinct from those recognized by IgE.95–97 There are 10 regions on the five allergenic caseins in milk, αS1-casein, αS2-casein, β-casein, β-lactoglobulin and κ-casein, that are more reactive for IgE than for IgG4.98 At least 44 different allergens, mostly aeroallergens, have been modified in some way to decrease their allergenicity.99 There is also an effort to find ways to modify the immune response in individuals with food allergies, or at least to label all composite foods to alert those with allergies to possible trace contaminants.
Structural studies can also play an important role in the redesigning of proteins to mitigate allergenicity. Consistent with the structural observations, patients allergic to dust mite produced IgG with binding affinity only to conformational epitopes of the major allergen Der p 1, whereas IgG from non-symptomatic mite-sensitized subjects and normal control individuals bound with similar affinity to both native and pepsin-hydrolyzed Der p 1.100
Other approaches use specific mutations of the allergen that decrease their affinity for IgE while retaining the ability to induce T-cell proliferation, as successfully demonstrated for Bet v 1.93 Modifying T cell epitopes of Cry j 1 yielded a candidate for peptide-based immunotherapy of Japanese cedar pollinosis.101 A mimotope gene of a major IgE epitope of Phl p 5, when used to immunize mice, did not generate an IgE response and prevented activation of the epitope-specific T cells.102 Another interesting recent approach was to couple a hypoallergenic peptide from the major grass pollen allergen Phl p 1 to the VP1 surface protein of a human rhinovirus (HRV). Mice and rabbits immunized with this chimeric antigen produced IgG antibodies that recognized group 1 allergens from different grass species, blocked allergic patients’ IgE reactivity to Phl p 1, and prevented Phl p 1-induced basophil degranulation.103 Further, antibodies were also detected against VP1, suggesting that this vaccine might also be useful in vaccinating against HRV, which can cause serious infections in asthmatics. These successful efforts in modifying allergens by mutations and using genetically engineered allergen derivatives and fusion constructs with reduced allergenic activity for specific immunotherapy (SIT) may result in more convenient and safer forms of immunotherapy in the near future.
Conclusion
Some specific characteristics of allergenic proteins have been detected by combining experimental results with bioinformatics studies. The presence of multiple allergens from the same Pfam database family in many different plants and animals can explain many observed clinical cross-reactivities. However, more structural information on allergen specific motifs and conformational epitopes is needed to make reliable in silico predictions about cross-reactivities. Fortunately, the progress in producing recombinant allergen proteins in sufficient quantities and purity for 3D structural analysis by X-ray crystallography and NMR techniques have made it possible to obtain detailed structural information on conformational IgG and IgE epitopes in several clinically important allergens. Thanks to a combination of X-ray, NMR and reliable 3D-modelling on a large scale, it is now possible to prepare complete maps of conformational IgE epitopes of all major allergens based on high quality 3D experimental structures or models. The ability to compare the 3D structures and amino acid sequences of allergens with improved bioinformatics tools can also provide a rational basis for regulatory agencies to establish new rules for food labelling and for accepting novel recombinant proteins as food products. Detailed structural data for allergens will aid in designing individual proteins for SIT that have reduced allergenicity while retaining the ability to generate a protective immune response. Progress in this area will depend on further definition of the common properties of the major families of allergens portrayed here.
Acknowledgments
This work is supported by grants NIH R01 AI 064913, U.S. EPA RD 833137 and a contract from the U.S. Food and Drug Administration (HHSF223200710011I) to WB, a grant U.S. EPA RE-83406601 to CHS and by grants NIH R01AI1052428 and Advanced Technology Program from THECB to RMG. The article has not been formally reviewed by the EPA, and the views expressed in this document are solely those of the authors.
Footnotes
Disclosures
This manuscript has been read and approved by all authors. This paper is unique and not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material.
References
- 1.Selgrade MK, Bowman CC, Ladics GS, Privalle L, Laessig SA. Safety assessment of biotechnology products for potential risk of food allergy: implications of new research. Toxicol Sci. 2009 Jul;110(1):31–9. doi: 10.1093/toxsci/kfp075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aas K. What makes an allergen an allergen. Allergy. 1978 Feb;33(1):3–14. doi: 10.1111/j.1398-9995.1978.tb01501.x. [DOI] [PubMed] [Google Scholar]
- 3.Berman H, Henrick K, Nakamura H, Markley JL. The worldwide Protein Data Bank (wwPDB): ensuring a single, uniform archive of PDB data. Nucleic Acids Res. 2007 Jan;35(Database issue):D301–3. doi: 10.1093/nar/gkl971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gajhede M, Osmark P, Poulsen FM, et al. X-ray and NMR structure of Bet v 1, the origin of birch pollen allergy. Nat Struct Biol. 1996 Dec;3(12):1040–5. doi: 10.1038/nsb1296-1040. [DOI] [PubMed] [Google Scholar]
- 5.Gustchina A, Li M, Wunschmann S, Chapman MD, Pomes A, Wlodawer A. Crystal structure of cockroach allergen Bla g 2, an unusual zinc binding aspartic protease with a novel mode of self-inhibition. J Mol Biol. 2005 Apr 29;348(2):433–44. doi: 10.1016/j.jmb.2005.02.062. [DOI] [PubMed] [Google Scholar]
- 6.Trevino MA, Garcia-Mayoral MF, Barral P, et al. NMR solution structure of Ole e 6, a major allergen from olive tree pollen. J Biol Chem. 2004 Sep;279(37):39035–41. doi: 10.1074/jbc.M406045200. [DOI] [PubMed] [Google Scholar]
- 7.Yennawar NH, Li LC, Dudzinski DM, Tabuchi A, Cosgrove DJ. Crystal structure and activities of EXPB1 (Zea m 1), a beta-expansin and group-1 pollen allergen from maize. Proc Natl Acad Sci U S A. 2006 Oct 3;103(40):14664–71. doi: 10.1073/pnas.0605979103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Czerwinski EW, Midoro-Horiuti T, White MA, Brooks EG, Goldblum RM. Crystal structure of Jun a 1, the major cedar pollen allergen from Juniperus ashei, reveals a parallel beta-helical core. J Biol Chem. 2005 Feb;280(5):3740–6. doi: 10.1074/jbc.M409655200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oezguen N, Zhou B, Negi SS, et al. Comprehensive 3D-modeling of allergenic proteins and amino acid composition of potential conformational IgE epitopes. Mol Immunol. 2008 Aug;45(14):3740–7. doi: 10.1016/j.molimm.2008.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marsh DG, Goodfriend L, King TP, Lowenstein H, Platts-Mills TA. Allergen nomenclature. Bull World Health Org. 1986;64(5):767–74. [PMC free article] [PubMed] [Google Scholar]
- 11.King TP, Hoffman D, Lowenstein H, Marsh DG, Platts-Mills TA, Thomas W. Allergen nomenclature. WHO/IUIS Allergen Nomenclature Subcommittee. Int Arch Allergy Immunol. 1994 Nov;105(3):224–33. doi: 10.1159/000236761. [DOI] [PubMed] [Google Scholar]
- 12.Pearson WR. Using the FASTA program to search protein and DNA sequence databases. Methods Mol Biol. 1994;25:365–89. doi: 10.1385/0-89603-276-0:365. [DOI] [PubMed] [Google Scholar]
- 13.Altschul SF, Madden TL, Schaffer AA, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25(17):3389–402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bindsley-Jensen C, Sten E, Earl LK, et al. Assessment of the potential allergenicity of ice structuring protein type III HPLC 12 using the FAO/WHO 2001 decision tree for novel foods. Food Chem Toxicol. 2003 Jan;41(1):81–7. doi: 10.1016/s0278-6915(02)00212-0. [DOI] [PubMed] [Google Scholar]
- 15.WHO . Joint FAO/WHO Food Standards Programme. Codex Ad Hoc Intergovernmental Task Force on Foods Derived from Biotechnology, http://www.codexalimentarius.net/ Yokohama: World Health Organization; 2003. [Google Scholar]
- 16.EFSA . Guidance document of the GMO Panel for the risk assessment of genetically modified plants and derived food and feed, http://www.efsa.eu.int/science/gmo/gmo_guidance/660_en.html. European Food Safety Authority; 2004. [Google Scholar]
- 17.Zhang ZH, Tan SC, Koh JL, Falus A, Brusic V. ALLERDB database and integrated bioinformatic tools for assessment of allergenicity and allergic cross-reactivity. Cell Immunol. 2006 Dec;244(2):90–6. doi: 10.1016/j.cellimm.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Gendel SM, Jenkins JA. Allergen sequence databases. Mol Nutr Food Res. 2006 Jul;50(7):633–7. doi: 10.1002/mnfr.200500271. [DOI] [PubMed] [Google Scholar]
- 19.Mari A. Importance of databases in experimental and clinical allergology. Int Arch Allergy Immunol. 2005;138(1):88–96. doi: 10.1159/000087848. [DOI] [PubMed] [Google Scholar]
- 20.Ivanciuc O, Schein CH, Braun W. SDAP: database and computational tools for allergenic proteins. Nucleic Acids Res. 2003;31(1):359–62. doi: 10.1093/nar/gkg010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brusic V, Millot M, Petrovsky N, Gendel SM, Gigonzac O, Stelman SJ. Allergen databases. Allergy. 2003 Nov;58(11):1093–100. doi: 10.1034/j.1398-9995.2003.00248.x. [DOI] [PubMed] [Google Scholar]
- 22.Hileman RE, Silvanovich A, Goodman RE, et al. Bioinformatic methods for allergenicity assessment using a comprehensive allergen database. Int Arch Allergy Immunol. 2002 Aug;128(4):280–91. doi: 10.1159/000063861. [DOI] [PubMed] [Google Scholar]
- 23.Ivanciuc O, Schein CH, Braun W. Data mining of sequences and 3D structures of allergenic proteins. Bioinformatics. 2002;18(10):1358–64. doi: 10.1093/bioinformatics/18.10.1358. [DOI] [PubMed] [Google Scholar]
- 24.Ivanciuc O, Schein CH, Garcia T, Oezguen N, Negi SS, Braun W. Structural analysis of linear and conformational epitopes of allergens. Regul Toxicol Pharmacol. 2009 Aug;54(Suppl 3):S11–9. doi: 10.1016/j.yrtph.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas K, Herouet-Guicheney C, Ladics G, et al. Current and future methods for evaluating the allergenic potential of proteins: international workshop report 23–25 Oct 2007. Food Chem Toxicol. 2008 Sep;46(9):3219–25. doi: 10.1016/j.fct.2008.06.078. [DOI] [PubMed] [Google Scholar]
- 26.Ladics GS, Bardina L, Cressman RF, Mattsson JL, Sampson HA. Lack of cross-reactivity between the Bacillus thuringiensis derived protein Cry1F in maize grain and dust mite Der p7 protein with human sera positive for Der p7-IgE. Regul Toxicol Pharmacol. 2006 Mar;44(2):136–43. doi: 10.1016/j.yrtph.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 27.Schein CH, Ivanciuc O, Braun W. Bioinformatics approaches to classifying allergens and predicting cross-reactivity. Immunol Allergy Clin North Am. 2007 Feb;27(1):1–27. doi: 10.1016/j.iac.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fiers M, Kleter GA, Nijland H, Peijnenburg A, Nap JP, van Ham R. Allermatch (TM), a webtool for the prediction of potential allergenicity according to current FAO/WHO Codex alimentarius guidelines. BMC Bioinformatics. 2004 Sep 16;5:133. doi: 10.1186/1471-2105-5-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Finn RD, Tate J, Mistry J, et al. The Pfam protein families database. Nucleic Acids Res. 2008 Jan;36(Database issue):D281–8. doi: 10.1093/nar/gkm960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benson D, Karsch-Mizrachi I, Lipman D, Ostell J, Wheeler D. Genbank. Nucleic Acids Res. 2006;34:D16–20. doi: 10.1093/nar/gkj157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mari A, Rasi C, Palazzo P, Scala E. Allergen databases: current status and perspectives. Curr Allergy Asthma Rep. 2009 Sep;9(5):376–83. doi: 10.1007/s11882-009-0055-9. [DOI] [PubMed] [Google Scholar]
- 32.Vita R, Zarebski L, Greenbaum JA, et al. The immune epitope database 2.0. Nucleic Acids Res. 2010 Jan;38(Database issue):D854–62. doi: 10.1093/nar/gkp1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Radauer C, Bublin M, Wagner S, Mari A, Breiteneder H. Allergens are distributed into few protein families and possess a restricted number of biochemical functions. J Allergy Clin Immunol. 2008 Apr;121(4):847–52. e847. doi: 10.1016/j.jaci.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 34.Ivanciuc O, Garcia T, Torres M, Schein CH, Braun W. Characteristic motifs for families of allergenic proteins. Mol Immunol. 2009 Feb;46(4):559–68. doi: 10.1016/j.molimm.2008.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abagyan RA, Batalov S. Do aligned sequences share the same fold. J Mol Biol. 1997 Oct 17;273(1):355–68. doi: 10.1006/jmbi.1997.1287. [DOI] [PubMed] [Google Scholar]
- 36.Midoro-Horiuti T, Schein CH, Mathura V, et al. Structural basis for epitope sharing between group 1 allergens of cedar pollen. Mol Immunol. 2006 Feb;43(6):509–18. doi: 10.1016/j.molimm.2005.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ayuso R, Reese G, Leong-Kee S, Plante M, Lehrer SB. Molecular basis of arthropod cross-reactivity: IgE-binding cross-reactive epitopes of shrimp, house dust mite and cockroach tropomyosins. Int Arch Allergy Immunol. 2002 Sep;129(1):38–48. doi: 10.1159/000065172. [DOI] [PubMed] [Google Scholar]
- 38.Reese G, Schicktanz S, Lauer I, et al. Structural, immunological and functional properties of natural recombinant Pen a 1, the major allergen of Brown Shrimp. Penaeus aztecus Clin Exp Allergy. 2006 Apr;36(4):517–24. doi: 10.1111/j.1365-2222.2006.02454.x. [DOI] [PubMed] [Google Scholar]
- 39.Radauer C, Breiteneder H. Pollen allergens are restricted to few protein families and show distinct patterns of species distribution. J Allergy Clin Immunol. 2006 Jan;117(1):141–7. doi: 10.1016/j.jaci.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 40.Bonds RS, Midoro-Horiuti T, Goldblum R. A structural basis for food allergy: the role of cross-reactivity. Curr Opin Allergy Clin Immunol. 2008 Feb;8(1):82–6. doi: 10.1097/ACI.0b013e3282f4177e. [DOI] [PubMed] [Google Scholar]
- 41.Teuber S, Beyer K, Comstock S, Wallowitz M. The big eight foods: clinical and epidemiological overview. In: Malecki S, editor. Food Allergy. Washington DC: ASM Press; 2006. pp. 49–79. [Google Scholar]
- 42.Shreffler WG, Beyer K, Chu TH, Burks AW, Sampson HA. Microarray immunoassay: association of clinical history, in vitro IgE function, and heterogeneity of allergenic peanut epitopes. J Allergy Clin Immunol. 2004 Apr;113(4):776–82. doi: 10.1016/j.jaci.2003.12.588. [DOI] [PubMed] [Google Scholar]
- 43.Klinglmayr E, Hauser M, Zimmermann F, et al. Identification of B-cell epitopes of Bet v 1 involved in cross-reactivity with food allergens. Allergy. 2009 Apr;64(4):647–51. doi: 10.1111/j.1398-9995.2008.01844.x. [DOI] [PubMed] [Google Scholar]
- 44.Wiche R, Gubesch M, Konig H, et al. Molecular basis of pollen-related food allergy: identification of a second cross-reactive IgE epitope on Pru av 1, the major cherry (Prunus avium) allergen. Biochem J. 2005 Jan 1;385(Pt 1):319–27. doi: 10.1042/BJ20040842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wangorsch A, Ballmer-Weber BK, Rosch P, Holzhauser T, Vieths S. Mutational epitope analysis and cross-reactivity of two isoforms of Api g 1, the major celery allergen. Mol Immunol. 2007 Apr;44(10):2518–27. doi: 10.1016/j.molimm.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 46.Bohle B. The impact of pollen-related food allergens on pollen allergy. Allergy. 2007 Jan;62(1):3–10. doi: 10.1111/j.1398-9995.2006.01258.x. [DOI] [PubMed] [Google Scholar]
- 47.Jenkins JA, Griffiths-Jones S, Shewry PR, Breiteneder H, Mills EN. Structural relatedness of plant food allergens with specific reference to cross-reactive allergens: an in silico analysis. J Allergy Clin Immunol. 2005 Jan;115(1):163–70. doi: 10.1016/j.jaci.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 48.Breiteneder H, Mills C. Structural bioinformatic approaches to understand cross-reactivity. Mol Nutr Food Res. 2006 Jul;50(7):628–32. doi: 10.1002/mnfr.200500274. [DOI] [PubMed] [Google Scholar]
- 49.Aalberse RC. Assessment of allergen cross-reactivity. Clin Mol Allergy. 2007;5:2. doi: 10.1186/1476-7961-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lauer I, Miguel-Moncin MS, Abel T, et al. Identification of a plane pollen lipid transfer protein (Pla a 3) and its immunological relation to the peach lipid-transfer protein, Pru p 3. Clin Exp Allergy. 2007 Feb;37(2):261–9. doi: 10.1111/j.1365-2222.2007.02653.x. [DOI] [PubMed] [Google Scholar]
- 51.Ganglberger E, Radauer C, Wagner S, et al. Hev b 8, the Hevea brasiliensis latex profilin, is a cross-reactive allergen of latex, plant foods and pollen. Int Arch Allergy Immunol. 2001 Jul;125(3):216–27. doi: 10.1159/000053819. [DOI] [PubMed] [Google Scholar]
- 52.Barre A, Culerrier R, Granier C, et al. Mapping of IgE-binding epitopes on the major latex allergen Hev b 2 and the cross-reacting 1,3 beta-glucanase fruit allergens as a molecular basis for the latex-fruit syndrome. Mol Immunol. 2009 May;(8–9):46. 1595–604. doi: 10.1016/j.molimm.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 53.Radauer C, Willerroider M, Fuchs H, et al. Cross-reactive and species-specific immunoglobulin E epitopes of plant profilins: an experimental and structure-based analysis. Clin Exp Allergy. 2006 Jul;36(7):920–9. doi: 10.1111/j.1365-2222.2006.02521.x. [DOI] [PubMed] [Google Scholar]
- 54.Verdino P, Barderas R, Villalba M, et al. Three-dimensional structure of the cross-reactive pollen allergen Che a 3: visualizing cross-reactivity on the molecular surfaces of weed, grass, and tree pollen allergens. J Immunol. 2008 Feb 15;180(4):2313–21. doi: 10.4049/jimmunol.180.4.2313. [DOI] [PubMed] [Google Scholar]
- 55.Castillo RM, Mizuguchi K, Dhanaraj V, Albert A, Blundell TL, Murzin AG. A six-stranded double-psi beta barrel is shared by several protein superfamilies. Structure. 1999 Feb 15;7(2):227–36. doi: 10.1016/s0969-2126(99)80028-8. [DOI] [PubMed] [Google Scholar]
- 56.Egger M, Mutschlechner S, Wopfner N, Gadermaier G, Briza P, Ferreira F. Pollen-food syndromes associated with weed pollinosis: an update from the molecular point of view. Allergy. 2006 Apr;61(4):461–76. doi: 10.1111/j.1398-9995.2006.00994.x. [DOI] [PubMed] [Google Scholar]
- 57.Scala E, Alessandri C, Bernardi ML, et al. Cross-sectional survey on immunoglobulin E reactivity in 23,077 subjects using an allergenic molecule-based microarray detection system. Clin Exp Allergy. 2010 Jun;40(6):911–21. doi: 10.1111/j.1365-2222.2010.03470.x. [DOI] [PubMed] [Google Scholar]
- 58.Marti P, Truffer R, Stadler MB, et al. Allergen motifs and the prediction of allergenicity. Immunol Lett. 2007 Mar 15;109(1):47–55. doi: 10.1016/j.imlet.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 59.Stadler MB, Stadler BM. Allergenicity prediction by protein sequence. FASEB J. 2003 Apr;17(6):1141–3. doi: 10.1096/fj.02-1052fje. [DOI] [PubMed] [Google Scholar]
- 60.Bailey TL, Elkan C. Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proc Int Conf Intell Syst Mol Biol. 1994;2:28–36. [PubMed] [Google Scholar]
- 61.Mathura VS, Schein CH, Braun W. Identifying property based sequence motifs in protein families and superfamilies: application to DNase-1 related endonucleases. Bioinformatics. 2003 Jul 22;19(11):138190. doi: 10.1093/bioinformatics/btg164. [DOI] [PubMed] [Google Scholar]
- 62.Ivanciuc O, Midoro-Horiuti T, Schein CH, et al. The property distance index PD predicts peptides that cross-react with IgE antibodies. Mol Immunol. 2009 Feb;46(5):873–83. doi: 10.1016/j.molimm.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chruszcz M, Chapman MD, Vailes LD, et al. Crystal structures of mite allergens Der f 1 and Der p 1 reveal differences in surface-exposed residues that may influence antibody binding. J Mol Biol. 2009 Feb;386(2):520–30. doi: 10.1016/j.jmb.2008.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fedorov AA, Ball T, Mahoney NM, Valenta R, Almo SC. The molecular basis for allergen cross-reactivity: crystal structure and IgE epitope mapping of birch pollen profilin. Structure. 1997;5(1):33–45. doi: 10.1016/s0969-2126(97)00164-0. [DOI] [PubMed] [Google Scholar]
- 65.Garman SC, Kinet JP, Jardetzky TS. Crystal structure of the human high-affinity IgE receptor. Cell. 1998 Dec 23;95(7):951–61. doi: 10.1016/s0092-8674(00)81719-5. [DOI] [PubMed] [Google Scholar]
- 66.Li M, Gustchina A, Alexandratos J, et al. Crystal structure of a dimerized cockroach allergen Bla g 2 complexed with a monoclonal antibody. J Biol Chem. 2008 Aug 15;283(33):22806–14. doi: 10.1074/jbc.M800937200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Spangfort MD, Mirza O, Holm J, Larsen JN, Ipsen H, Lowenstein H. The structure of major birch pollen allergens—epitopes, reactivity and cross-reactivity. Allergy. 1999;50:23–6. doi: 10.1111/j.1398-9995.1999.tb05021.x. [DOI] [PubMed] [Google Scholar]
- 68.Silvanovich A, Nemeth MA, Song P, Herman R, Tagliani L, Bannon GA. The value of short amino acid sequence matches for prediction of protein allergenicity. Toxicol Sci. 2006 Mar;90(1):252–8. doi: 10.1093/toxsci/kfj068. [DOI] [PubMed] [Google Scholar]
- 69.Saha S, Raghava GP. AlgPred: prediction of allergenic proteins and mapping of IgE epitopes. Nucleic Acids Res. 2006 Jul 1;34(Web Server issue):W202–9. doi: 10.1093/nar/gkl343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Spangfort MD, Mirza O, Ipsen H, van Neerven RJ, Gajhede M, Larsen JN. Dominating IgE-binding epitope of Bet v 1, the major allergen of birch pollen, characterized by X-ray crystallography and site-directed mutagenesis. J Immunol. 2003 Sep 15;171(6):3084–90. doi: 10.4049/jimmunol.171.6.3084. [DOI] [PubMed] [Google Scholar]
- 71.Niemi M, Jylha S, Laukkanen ML, et al. Molecular interactions between a recombinant IgE antibody and the beta-lactoglobulin allergen. Structure. 2007 Nov;15(11):1413–21. doi: 10.1016/j.str.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 72.Padavattan S, Schirmer T, Schmidt M, et al. Identification of a B-cell epitope of hyaluronidase, a major bee venom allergen, from its crystal structure in complex with a specific Fab. J Mol Biol. 2007 May 4;368(3):742–52. doi: 10.1016/j.jmb.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 73.Padavattan S, Flicker S, Schirmer T, et al. High-affinity IgE recognition of a conformational epitope of the major respiratory allergen Phl p 2 as revealed by X-ray crystallography. J Immunol. 2009 Feb 15;182(4):2141–51. doi: 10.4049/jimmunol.0803018. [DOI] [PubMed] [Google Scholar]
- 74.Mirza O, Henriksen A, Ipsen H, et al. Dominant epitopes and allergic cross-reactivity: complex formation between a Fab fragment of a monoclonal murine IgG antibody and the major allergen from birch pollen Bet v 1. J Immunol. 2000 Jul 1;165(1):331–8. doi: 10.4049/jimmunol.165.1.331. [DOI] [PubMed] [Google Scholar]
- 75.Johnson P, Marsh DG. ‘Isoallergens’ from rye grass pollen. Nature. 1965 May 29;206(987):935–7. doi: 10.1038/206935b0. [DOI] [PubMed] [Google Scholar]
- 76.Marsh DG, Milner FH, Johnson P. The allergenic activity and stability of purified allergens from the pollen of common rye grass (Lolium perenne) Int Arch Allergy Appl Immunol. 1966;29(6):521–35. doi: 10.1159/000229739. [DOI] [PubMed] [Google Scholar]
- 77.Weber RW. Cross-reactivity of pollen allergens: impact on allergen immunotherapy. Ann Allergy Asthma Immunol. 2007 Sep;99(3):203–12. doi: 10.1016/S1081-1206(10)60654-0. [DOI] [PubMed] [Google Scholar]
- 78.Jensen-Jarolim E, Leitner A, Kalchhauser H, et al. Peptide mimotopes displayed by phage inhibit antibody binding to Bet v 1, the major birch pollen allergen, and induce specific IgG response in mice. FASEB J. 1998 Dec;12(15):1635–42. doi: 10.1096/fasebj.12.15.1635. [DOI] [PubMed] [Google Scholar]
- 79.Szalai K, Fuhrmann J, Pavkov T, et al. Mimotopes identify conformational B-cell epitopes on the two major house dust mite allergens Der p 1 and Der p 2. Mol Immunol. 2008 Mar;45(5):1308–17. doi: 10.1016/j.molimm.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 80.Sookrung N, Chaicumpa W, Tungtrongchitr A, et al. Periplaneta americana arginine kinase as a major cockroach allergen among Thai patients with major cockroach allergies. Environ Health Perspect. 2006 Jun;114(6):875–80. doi: 10.1289/ehp.8650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Leitner A, Vogel M, Radauer C, et al. A mimotope defined by phage display inhibits IgE binding to the plant panallergen profilin. Eur J Immunol. 1998 Sep;28(9):2921–7. doi: 10.1002/(SICI)1521-4141(199809)28:09<2921::AID-IMMU2921>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 82.Pacios LF, Tordesillas L, Cuesta-Herranz J, et al. Mimotope mapping as a complementary strategy to define allergen IgE-epitopes: Peach Pru p 3 allergen as a model. Mol Immunol. 2008 Apr;45(8):2269–76. doi: 10.1016/j.molimm.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 83.Negi S, Braun W. Automated detection of conformational epitopes using phage display peptide sequences. Bioinform Biol Insights. 2009;3:71–81. doi: 10.4137/bbi.s2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mayrose I, Shlomi T, Rubinstein ND, et al. Epitope mapping using combinatorial phage-display libraries: a graph-based algorithm. Nucleic Acids Res. 2007;35(1):69–78. doi: 10.1093/nar/gkl975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huang J, Gutteridge A, Honda W, Kanehisa M. MIMOX: a web tool for phage display based epitope mapping. BMC Bioinformatics. 2006;7:451. doi: 10.1186/1471-2105-7-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Christensen LH, Holm J, Lund G, Riise E, Lund K. Several distinct properties of the IgE repertoire determine effector cell degranulation in response to allergen challenge. J Allergy Clin Immunol. 2008 Aug;122(2):298–304. doi: 10.1016/j.jaci.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 87.Ibarrola I, Sanz ML, Gamboa PM, et al. Biological characterization of glutaraldehyde-modified Parietaria judaica pollen extracts. Clin Exp Allergy. 2004 Feb;34(2):303–9. doi: 10.1111/j.1365-2222.2004.01859.x. [DOI] [PubMed] [Google Scholar]
- 88.Norman PS, King TP, Alexander JF, Jr, Kagey-Sobotka A, Lichtenstein LM. Immunologic responses to conjugates of antigen E in patients with ragweed hay fever. J Allergy Clin Immunol. 1984 Jun;73(6):782–9. doi: 10.1016/0091-6749(84)90448-2. [DOI] [PubMed] [Google Scholar]
- 89.Vrtala S, Akdis CA, Budak F, et al. T cell epitope-containing hypoallergenic recombinant fragments of the major birch pollen allergen, Bet v 1, induce blocking antibodies. J Immunol. 2000 Dec 1;165(11):6653–9. doi: 10.4049/jimmunol.165.11.6653. [DOI] [PubMed] [Google Scholar]
- 90.Vrtala S, Focke M, Kopec J, et al. Genetic engineering of the major timothy grass pollen allergen, Phl p 6, to reduce allergenic activity and preserve immunogenicity. J Immunol. 2007 Aug;179(3):1730–9. doi: 10.4049/jimmunol.179.3.1730. [DOI] [PubMed] [Google Scholar]
- 91.Purohit A, Niederberger V, Kronqvist M, et al. Clinical effects of immunotherapy with genetically modified recombinant birch pollen Bet v 1 derivatives. Clin Exp Allergy. 2008 Sep;38(9):1514–25. doi: 10.1111/j.1365-2222.2008.03042.x. [DOI] [PubMed] [Google Scholar]
- 92.Cocco RR, Jarvinen KM, Sampson HA, Beyer K. Mutational analysis of major, sequential IgE-binding epitopes in alpha(s1)-casein, a major cow’s milk allergen. J Allergy Clin Immunol. 2003 Aug;112(2):433–7. doi: 10.1067/mai.2003.1617. [DOI] [PubMed] [Google Scholar]
- 93.Ferreira F, Ebner C, Kramer B, et al. Modulation of IgE reactivity of allergens by site-directed mutagenesis: potential use of hypoallergenic variants for immunotherapy. FASEB J. 1998 Feb;12(2):231–42. doi: 10.1096/fasebj.12.2.231. [DOI] [PubMed] [Google Scholar]
- 94.Batard T, Laroze A, David B, Peltre G, Basuyaux B. Isotypic analysis of grass-pollen-specific antibodies in human plasma. III. Relationship to autoantibodies to IgE. Allergy. 1996 Jul;51(7):473–81. doi: 10.1111/j.1398-9995.1996.tb04653.x. [DOI] [PubMed] [Google Scholar]
- 95.Flicker S, Steinberger P, Eibensteiner PB, Lebecque S, Kraft D, Valenta R. Molecular characterization of a human immunoglobulin G4 antibody specific for the major birch pollen allergen, Bet v 1. Clin Exp Allergy. 2008 Feb;38(2):365–73. doi: 10.1111/j.1365-2222.2007.02883.x. [DOI] [PubMed] [Google Scholar]
- 96.Denepoux S, Eibensteiner PB, Steinberger P, et al. Molecular characterization of human IgG monoclonal antibodies specific for the major birch pollen allergen Bet v 1. Anti-allergen IgG can enhance the anaphylactic reaction. FEBS Lett. 2000 Jan 7;465(1):39–46. doi: 10.1016/s0014-5793(99)01703-2. [DOI] [PubMed] [Google Scholar]
- 97.Visco V, Dolecek C, Denepoux S, et al. Human IgG monoclonal antibodies that modulate the binding of specific IgE to birch pollen Bet v 1. J Immunol. 1996 Jul 15;157(2):956–62. [PubMed] [Google Scholar]
- 98.Cerecedo I, Zamora J, Shreffler WG, et al. Mapping of the IgE and IgG4 sequential epitopes of milk allergens with a peptide microarray-based immunoassay. J Allergy Clin Immunol. 2008 Sep;122(3):589–94. doi: 10.1016/j.jaci.2008.06.040. [DOI] [PubMed] [Google Scholar]
- 99.Bhalla PL, Singh MB. Engineered allergens for immunotherapy. Curr Opin Allergy Clin Immunol. 2004 Dec;4(6):569–73. doi: 10.1097/00130832-200412000-00016. [DOI] [PubMed] [Google Scholar]
- 100.Duchateau J, Michils A, Michel O, Baras L. Mite allergy is associated with a specific profile of IgG epitopes recognized on antigen p1 of Dermatophagoides pteronyssinus. Clin Exp Allergy. 1997 Mar;27(3):296–305. [PubMed] [Google Scholar]
- 101.Masuyama K, Chikamatsu K, Ikagawa S, et al. Analysis of helper T cell responses to Cry j 1-derived peptides in patients with nasal allergy: candidate for peptide-based immunotherapy of Japanese cedar pollinosis. Allergol Int. 2009 Mar;58(1):63–70. doi: 10.2332/allergolint.08-OA-0008. [DOI] [PubMed] [Google Scholar]
- 102.Wallmann J, Proell M, Stepanoska T, et al. A mimotope gene encoding the major IgE epitope of allergen Phl p 5 for epitope-specific immunization. Immunol Lett. 2009 Jan;122(1):68–75. doi: 10.1016/j.imlet.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Edlmayr J, Niespodziana K, Linhart B, et al. A combination vaccine for allergy and rhinovirus infections based on rhinovirus-derived surface protein VP1 and a nonallergenic peptide of the major timothy grass pollen allergen Phl p 1. J Immunol. 2009 May 15;182(10):6298–306. doi: 10.4049/jimmunol.0713622. [DOI] [PubMed] [Google Scholar]