Abstract
Theory of mind (ToM) has been defined as our ability to predict behaviors of others in terms of their underlying intentions. While the developmental trajectory of ToM had been thought to be invariant across cultures, several ToM studies conducted outside the Anglo-American cultural or linguistic milieus have obtained mixed results. To examine effects of culture/language on the development of neural bases of ToM, we studied 12 American monolingual children and 12 Japanese bilingual children with second-order false-belief story and cartoon tasks, using functional magnetic resonance imaging (fMRI). While a few brain regions such as ventro-medial prefrontal cortex (vmPFC) and precuneus were recruited by the both cultural/linguistic groups, several brain areas including inferior frontal gyrus (IFG) and temporo-parietal junction (TPJ) were employed in a culture/language-dependent manner during the ToM tasks. These results suggest that the neural correlates of ToM may begin to vary depending upon cultural/linguistic background from early in life.
1. Introduction
Despite our geographical and cultural differences, we all live in a world of communication and socialization. Thus, the ability to understand that others’ intentions and beliefs can be different from one’s own (i.e., ‘Theory of Mind’ [ToM]) (Premack and Woodruff, 1978; Flavell, 1999), is a critical human capacity in all parts the world. ToM has been tested extensively with false-belief tasks in normally developing (Wimmer and Perner, 1983) and atypical pediatric populations (Baron-Cohen et al., 1985). In a typical false-belief task, ‘Sally-Ann’ task (Baron-Cohen et al., 1985), a character (Sally) is described as having placed a toy in a box, but while she is away, another character (Ann) moves it into a different place. The key question concerns where Sally will look for the toy upon her return. Nearly universally observed results of these tests are that normally developing 3-year-olds fail yet 4-year-olds pass the tests (Flavell, 1999; see also Wellman et al., 2001 for a review). Thus, it has been suggested that ToM universally develops sometime between the third and fourth birthdays (Wellman et al., 2001).
However, the universal ToM hypothesis has not been uncontested. Using verbal false-belief style paradigms, several ToM studies conducted outside the Anglo-American cultural or linguistic boundaries have obtained mixed results. Some of these cross-cultural studies have supported the universal developmental trajectory of ToM (Avis and Harris, 1991; Lee et al., 1999; Naito et al., 1994; Tardiff and Wellman, 2000; Yazdi et al., 2006), whereas others have found some delays in ToM for the non-English speaking children (Chen and Lin, 1994; Louis, 1998; Naito, 2003; Vinden, 1996). Authors of the latter cases have given linguistic or cultural differences as explanations for the below-chance performance of the non-English speaking children. For instance, Vinden (1996) attributed Junin Quechua children’s poor ToM performance to their lack of mental state verbs. Similarly, Naito (2003) attributed below-chance ToM performance in 4 and 5 year-old Japanese children to differences in American/European and Asian cultural attribution styles: specifically, people brought up in American/European cultures tend to attribute behaviors to internal causes, while people raised in Asian cultures tend to attribute them to external and contextual causes (Nisbett, 2003; Masuda and Nisbett, 2001). These findings lead to an important question. If there are some differences in ToM performance in children across cultural/linguistic boundaries, have these children developed different neural correlates of ToM depending on their cultural/linguistic backgrounds?
Several brain imaging studies have examined the neural correlates of ToM in adults (Brunet et al., 2000; Calarge et al., 2003; Fletcher et al., 1995; Gallagher et al., 2000; Goel et al., 1995; Happé et al., 1996; Sabbagh and Taylor, 2000; Saxe and Kanwisher, 2003; Saxe and Wexler, 2005; Vogeley et al., 2001). Many of these studies implicated medial prefrontal cortex (mPFC) (Brunet et al., 2000; Calarge et al., 2003; Flethcer et al., 1995; Gallagher et al., 2000; Goel et al., 1995; Happé et al., 1996; Sabbagh and Taylor, 2000; Saxe and Kanwisher, 2003; Vogeley et al., 2001) and temporo-parietal junction (TPJ) (Gallagher et al., 2000; Saxe and Kanwisher, 2003; Saxe and Wexler, 2005) for ToM understanding. In a recent event-related potential (ERP) study, the authors tested 6-year-old children with an animated false-belief task, and found somewhat diffused ventro-frontal activity (Liu et al., 2005).
Neurological studies that examined relationship between ToM and language have obtained mixed results. On the one hand, a severe aphasic patient with a wide-range of left hemisphere damage showed intact performance in some nonverbal ToM tasks, despite failing all other syntax-related tasks (Varley and Siegal, 2000). On the other hand, a few studies have found activations in brain areas that were normally dedicated to language (e.g., Broca’s area) when subjects imitated intentional behaviors that are considered to be a lower-level ToM processing (Iacoboni et al., 1999; see also Chaminade et al., 2002 for a review). Moreover, evidence suggests that processing of pragmatically coherent sentences primarily recruits the mPFC area (Ferstl and von Cramon, 2002). These results suggest that some aspects of language (e.g., grammar) may be independent from ToM (see Siegal and Varley, 2002 for a review), but other aspects of language (e.g., pragmatics and reading communicative intentions) may profoundly affect ToM throughout the development.
In a previous study (Kobayashi et al., 2006) with Japanese-English bilingual and American monolingual adults we found both culture/language-dependent and -independent ToM-related brain activity. The mPFC/anterior cingulate (ACC) brain region showed activity during ToM tasks in all groups despite differences in language and cultural background. In addition, there were some brain regions (including the IFG and temporal pole [TP] that showed differences in ToM-related activity among the groups. These results indicate that some of the neural bases of ToM may be universal whereas others may vary depending upon the person’s cultural or linguistic background. However, when and how these develop is still unknown.
The present study sought to examine the development of these possibly culturally/linguistically dependent and independent neural correlates of ToM. Using fMRI, we examined the hemodynamic response of 8–11 year old Japanese-English bilingual and English speaking monolingual children during second-order false-belief ToM stories in English (Fig. 1a) and Japanese (Fig. 1b). Non-ToM control stories and scrambled sentences were used as control and baseline conditions. In addition, we tested both groups of children with a cartoon-based nonverbal ToM task (Fig. 1c), with corresponding pictorial control and baseline conditions. We predicted that if ToM has a universal neural basis, some significant overlap in brain activation patterns would be found among the three story ToM task-groups (monolingual English-speaking children, bilingual children viewing Japanese stories [L1], and bilingual children viewing English stories [L2]) as well as between the two cartoon task-groups (American and Japanese children viewing exactly the same cartoons) in candidate ToM brain areas (e.g., the mPFC [Frith and Frith, 2003], the TPJ [Saxe and Kanwisher, 2003], and ventro-medial prefrontal area [Liu et al., 2005]). Furthermore, by comparing between the different linguistic/cultural groups, we wished to find neural correlates of ToM that might vary depending upon the cultural and/or linguistic background of the subject. Our specific hypotheses were as follows:
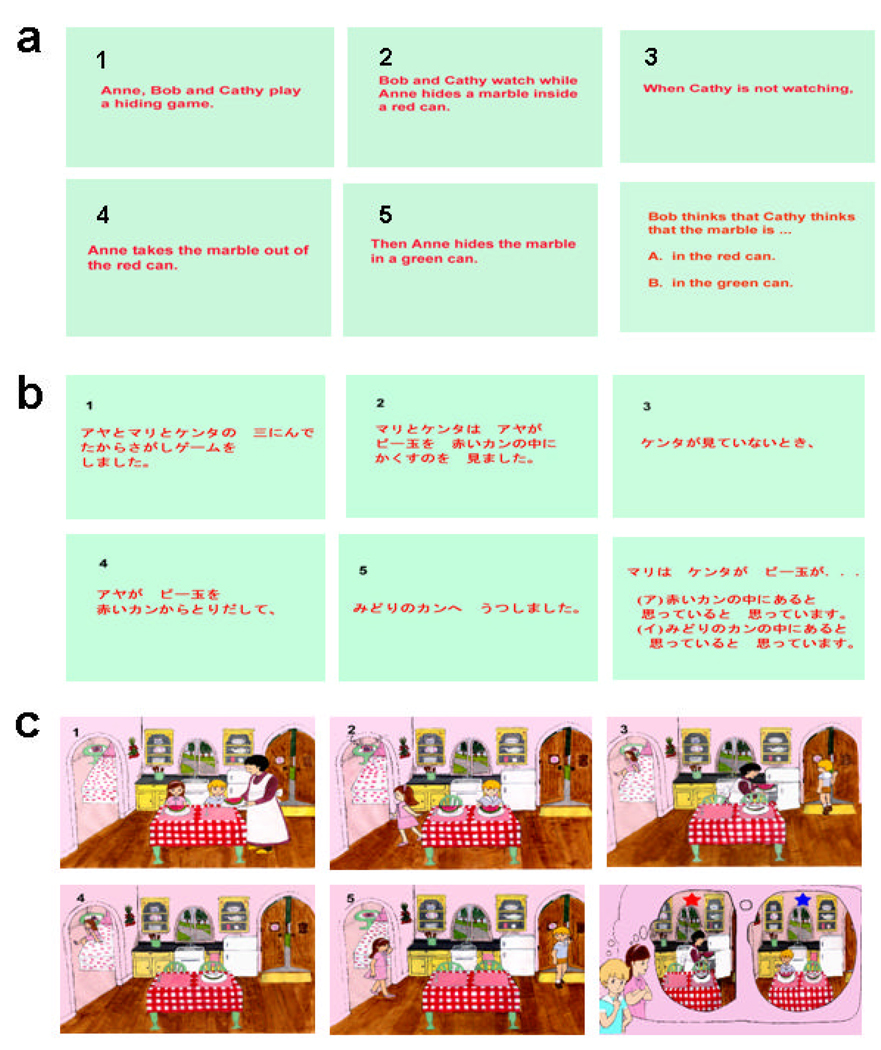
Fig. 1.
Example of English (a) and Japanese (b) story ToM tasks. All the ToM tasks were the second-order false belief tasks in the form of “x thinks that y thinks that …” Japanese task was the exact translation of English task. All slides were presented serially. There were six slides in each story. On the sixth slide, children were asked to choose from two possible answers, A or B. The monolingual group completed the English story task and the bilingual group completed two story tasks (English and Japanese story tasks). Children in both cultural groups completed a ToM cartoon task (an example is shown in c). All the episodes of the cartoon ToM task depicted the second-order false-belief situation. On the sixth slide, children were asked to choose from possible answers, the red star or the blue star.
Cultural effects on ToM
Any brain regions, which show a greater activity during the Japanese cartoon and/or L2 (English) task than during the American monolinguals’ cartoon and/or story task, may be important for understanding ToM for the Japanese culture. Conversely, any brain regions with greater activity during the American monolinguals’ cartoon and/or story task than during the Japanese cartoon and/or L2 task may be important for understanding ToM for the American culture. Since the two groups of children perform exactly the same cartoon task, any difference in brain activity between the groups during the cartoon task may be attributed to purely cultural sources that are unrelated to language.
Linguistic effects on ToM
Any brain regions with greater activity during the English ToM task than during the Japanese ToM task (i.e., bilingual L1), may be important for processing ToM in English. Conversely, any brain regions, which have a greater activity during Japanese ToM task (L1) than during the English ToM task (monolingual and L2), may be important for processing ToM in Japanese.
2. Results
2.1. Behavioral results
2.1.1. Story task
For the story task, accuracy for all the monolingual and the bilingual task-groups was at the above 50% chance-level for the ToM and non-ToM conditions (Monolingual [max = 10]: M = 7.75, SD = 2.09, t(11) = 4.55, p < 0.001; L1 [Japanese]: M = 7.33, SD = 1.92, t(11) = 4.20, p < 0.005; L2 [English]: M = 8.17, SD = 1.64, t(11) = 6.68, p < 0.0001). Subjects in all the three task groups also performed at the above 50% chance-level for scrambled stories (Monolingual [max = 5]: t(11) = 2.70 p < 0.05; L1: t(11) = 7.37, p < 0.0001; L2: t(11) = 6.66, p < 0.0001). All subjects’ accuracy for ToM condition did not differ from accuracy for the non-ToM condition (p > 0.5). There was no difference among the three groups in average performances for the ToM condition (p > 0.5) and for the non-ToM condition (p > 0.05). Moreover, average reaction time (RT) (during the sixth slide) for the ToM condition did not differ significantly from that for the non-ToM conditions for either task-group (Monolingual: p > 0.1; L1: p > 0.1; L2: p > 0.5). There was no difference among the three groups in average RTs for the ToM condition (p > 0.1) and for the non-ToM condition (p > 0.1).
2.1.2. Cartoon task
For the cartoon task, accuracy of both the monolingual and the bilingual groups was at the above 50% chance-level for the ToM and non-ToM conditions (Monolingual [max = 10]: M = 6.92, SD = 1.98, t(11) = 3.36, p < 0.01; Bilingual: M = 7.46, SD = 1.56, t(11) = 5.45, p < 0.0005). Subjects in both groups also performed at the above 50% chance-level for scrambled stories (Monolingual [max = 5]: t(11) = 5.18 p < 0.01; Bilingual: t(11) = 7.37, p < 0.0001). All subjects’ accuracy for ToM condition did not differ from accuracy for the non-ToM condition (p > 0.5). There was no difference between the groups in average performances for the ToM condition (p > 0.5). Moreover, average RT for the ToM condition did not differ significantly from that for the non-ToM conditions for either group (Monolingual: p > 0.5; Bilingual: p > 0.1). There was no difference between groups in average RTs for the ToM condition (p > 0.5) and for the non-ToM condition (p > 0.5).
2.1.3. Story versus cartoon tasks
Mean accuracy for the story did not differ from that for the cartoon in either task-group (Monolingual [max = 10]: p > 0.05; L1: p > 0.5; L2: p > 0.05). Also, each task-group’s average RT for the story task did not differ from that for the cartoon task (Monolingual: p > 0.1; L1: p > 0.5; L2: p > 0.05).
2.1.4. Task performance related to gender
Mean accuracy of the male subjects in both groups did not differ significantly from that of the female subjects for the English story (p > 0.05), Japanese story (Japanese group only: p > 0.5), and cartoon (p > 0.5) tasks.
2.1.5. Correlational analyses
There was no correlation between task performance on the ToM task and indices to assess language abilities (i.e., verbal IQ, number of years of speaking English, time spent in the US and in other English speaking countries, and the proficiency level) in either group.
2.2. Brain imaging results
2.2.1. Within-group – brain activity during ToM story condition relative to non-ToM story condition
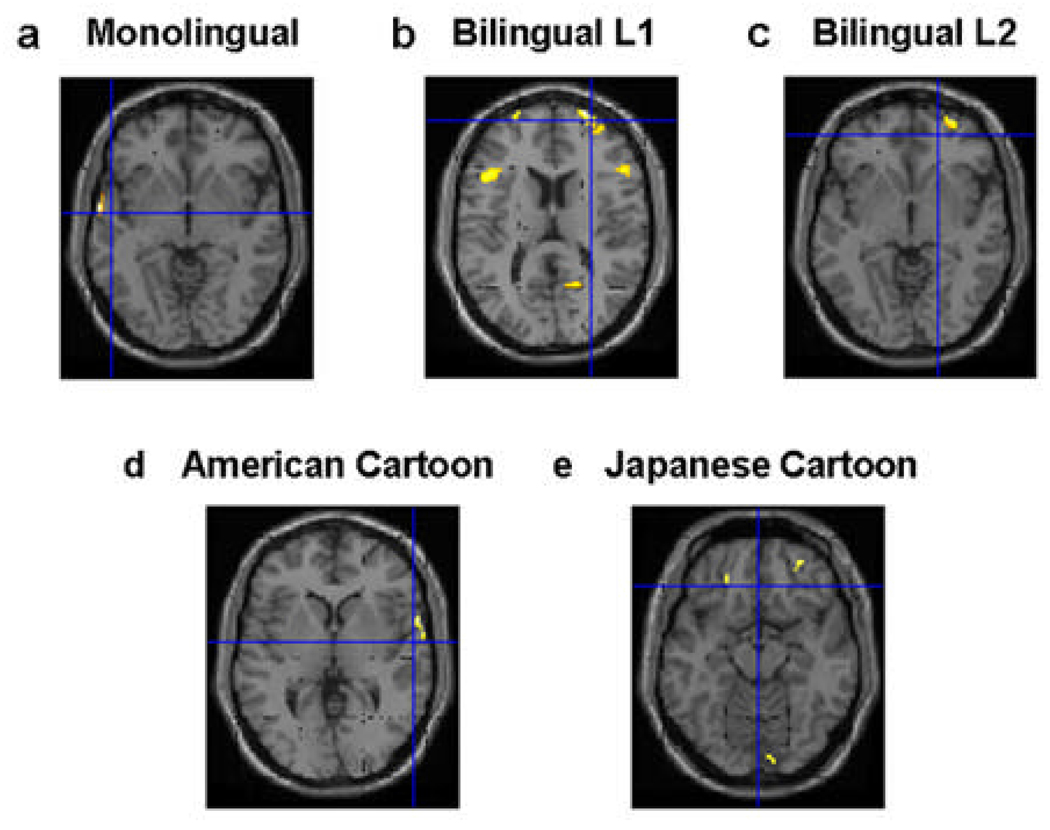
As listed in Table 1, a number of brain regions showed significant differences in ToM versus non-ToM comparison for each group. During the story task, the monolinguals had more brain activity in the left superior temporal gyrus (STG), IFG, and right vmPFC (Fig. 2a). For the bilingual L1 (Japanese) task, significant differences were seen in several brain regions, including the ACC, left inferior temporal gyrus (ITG), and right vmPFC/ventro-middle frontal gyrus (vMFG) (Fig. 2b). For the bilingual L2 (English) task, significant differences were seen in eight brain regions, including the bilateral mPFC/middle frontal gyrus (MFG), IFG, and mPFC (Fig. 2c).
Table 1.
Brain areas activated during ToM condition relative to non-ToM condition Within-group comparison
| Coordinates | |||||
|---|---|---|---|---|---|
| Region (Brodmann area) | x | y | z | Z-value | P-value |
| American Child Story (English): | |||||
| Left STG/STS (21/22) | −61 | −2 | −3 | 3.18 | 0.001 |
| Right IFG (47) | 42 | 17 | −14 | 2.74 | 0.003 |
| Right vmPFC (11) | 24 | 50 | −13 | 2.34 | 0.01* |
| Japanese Child Story (L1 Japanese): | |||||
| ACC (24) | 4 | 20 | 17 | 3.62 | < 0.0005 |
| Left ITG (20) | −50 | −18 | −19 | 3.24 | 0.001 |
| Right vmPFC/vMFG (10) | 30 | 58 | −6 | 3.20 | 0.001 |
| Right IOG (18) | 32 | −93 | −4 | 2.71 | 0.003 |
| mPFC (9/10) | 6 | 63 | 26 | 2.45 | 0.007* |
| Japanese Child Story (L2 English): | |||||
| Right mPFC/MFG (10) | 26 | 65 | 12 | 3.71 | < 0.0005 |
| Right IFG (45) | 54 | 24 | 8 | 3.38 | < 0.0005 |
| Right mPFC (10) | 12 | 65 | 23 | 3.26 | 0.001 |
| Left mPFC/MFG (10) | −24 | 65 | 10 | 3.21 | 0.001 |
| Precuneus (7) | 0 | −60 | 42 | 3.15 | 0.001 |
| Left IFG (45) | −44 | 20 | 12 | 3.08 | 0.001 |
| Left MTG (21) | −48 | 40 | −14 | 2.94 | 0.002 |
| Right cerebellum | 28 | −84 | −18 | 2.81 | 0.002 |
| American Child Cartoon: | |||||
| Right STG/IFG (22/44) | 63 | 10 | 5 | 3.05 | 0.001 |
| Left cuneus (19) | −12 | −83 | 40 | 2.84 | 0.002 |
| Right MFG/DLPFC (46) | 36 | 47 | 14 | 2.74 | 0.003 |
| mPFC (32) | 2 | 10 | 36 | 2.66 | 0.004 |
| Japanese Child Cartoon: | |||||
| Right vmPFC/vMFG (10) | 28 | 58 | −5 | 3.02 | 0.001 |
| Precuneus (7) | 0 | −74 | 44 | 2.74 | 0.003 |
| Right IFG (44) | 65 | 9 | 18 | 2.62 | 0.004 |
| Left vmPFC (11) | −20 | 38 | −14 | 2.59 | 0.005 |
Abbreviations ACC = anterior cingulate cortex, DLPFC = dorso-lateral prefrontal cortex, IFG = inferior frontal gyrus, ITG = inferior temporal gyrus, IOG = inferior occipital gyrus, MFG = middle frontal gyrus, mPFC = medial prefrontal cortex, MTG = middle temporal gyrus, SFG = superior frontal gyrus, STG = superior temporal gyrus, STS = superior temporal sulcus, TPJ = temporo-parietal junction, vMFG = ventro-middle frontal gyrus, vmPFC = ventro-medial prefrontal gyrus.
Significant activations were recognized at p < 0.005, uncorrected. However, for those regions, in which we had primary interest, significant activations were recognized at p < 0.01, uncorrected.
Fig. 2.
Brain activation in ToM relative to non-ToM condition for the within-group comparison (Table 1). During the story task, for the monolingual group, significant activations were found in the left STG/STS, right IFG, and right vmPFC (a). For bilinguals L1 task-group, significant activations were found in several brain areas including the ACC, left ITG, and right vMFG (b). For the L2 group, significant activity was found in several brain areas including the right MFG, right IFG, and right mPFC (c). During the cartoon task, for the monolingual group, significant activations were found in the right STG (d). For the Japanese group, significant activity was found in the right vmPFC, precuneus, and right IFG (e).
2.2.2. Within-group – brain activity during ToM cartoon condition relative to non-ToM cartoon condition
During the cartoon ToM task, the American monolinguals had more activity during the ToM condition in the right STG, left cuneus, right MFG/dorsolateral prefrontal cortex (DLPFC) and mPFC than during the non-ToM condition (Fig. 2d). Japanese group had more activity in the bilateral vmPFC/vMFG, precuneus, and right IFG (Fig.2e).
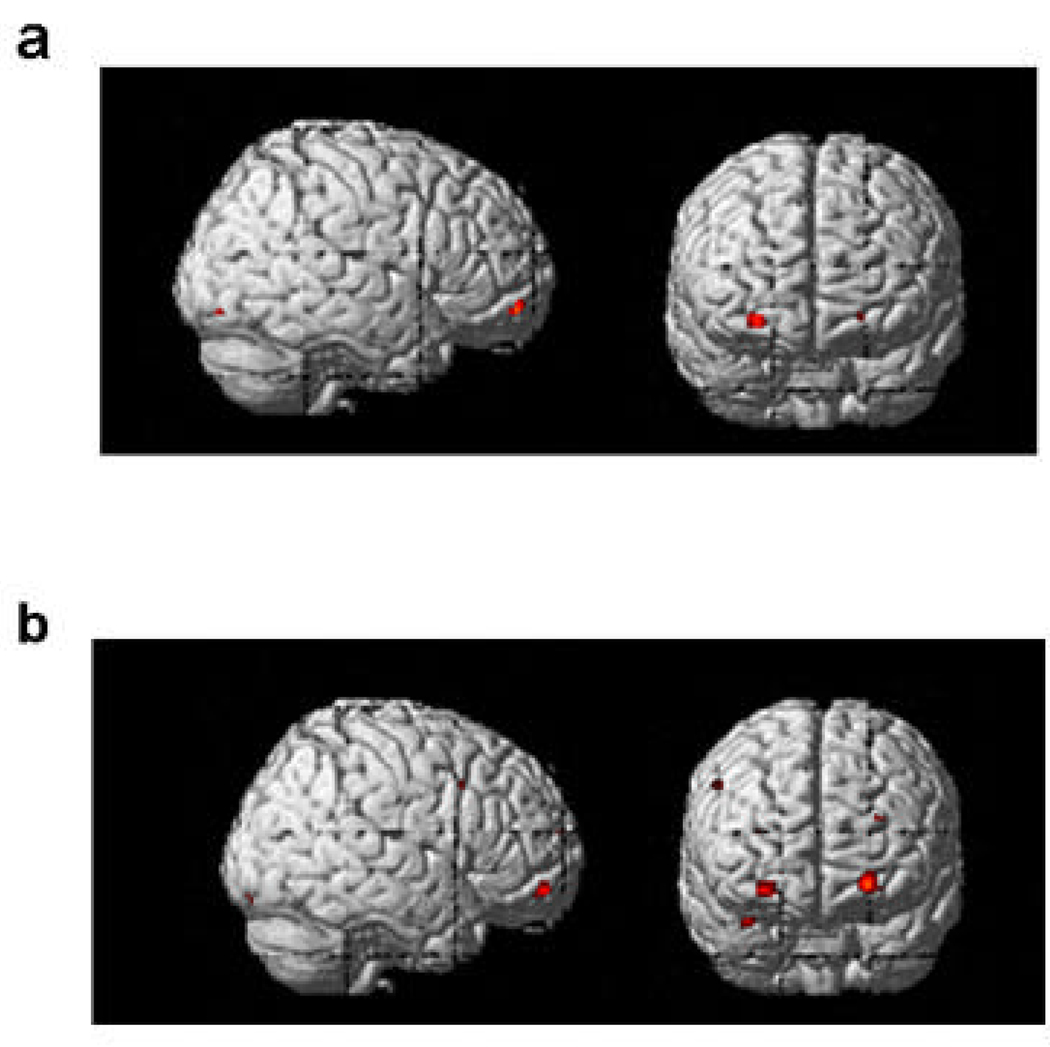
2.2.3 Conjunction analyses for story and cartoon task-groups
Listed in Table 2 are results of conjunction analyses among the three story groups that were done to explore the brain areas that may be specialized for language/culture-independent ToM. Monolingual, L2, and L1 groups’ story ToM-specific (ToM minus non-ToM contrasts) activations converged in the right IFG, right MFG, and mPFC. The two cultural groups’ cartoon ToM-specific activity converged in the right anterior STS (aSTS)/TP and right vMFG. American and Japanese children’s cartoon and English/Japanese L1 story ToM-specific activity converged in the left ITG, precuneus, and bilateral vmPFC (Fig. 3a). Through the same analysis with the L2 story task, we found significant convergences in the bilateral vmPFC, precuneus, right aSTS/TP, and left TPJ (Fig. 3b).
Table 2.
Brain areas activated during ToM condition relative to nonToM condition Conjunction among the task-groups
| Coordinates | |||||
|---|---|---|---|---|---|
| Region (BA) | x | y | z | Z-value | P-value |
| Monolingual Story + Bilingual L1 + Bilingual L2: | |||||
| Right IFG (45) | 59 | 22 | 4 | 2.93 | 0.002 |
| Right mPFC/MFG (10) | 30 | 63 | 13 | 2.67 | 0.004 |
| mPFC (10) | 4 | 45 | 46 | 2.59 | 0.005 |
| American & Japanese Cartoon: | |||||
| Right aSTS/TP (38) | 38 | 5 | −22 | 3.00 | 0.001 |
| Right vMFG (11) | 30 | 54 | −11 | 2.57 | 0.005 |
| American & Japanese Cartoon + Story (L1): | |||||
| Left ITG (20) | −50 | −20 | −19 | 3.39 | < 0.0005 |
| Precuneus (7) | −2 | −80 | 39 | 3.32 | < 0.0005 |
| Right vmPFC (11) | 28 | 54 | −11 | 3.10 | 0.001 |
| Left vmPFC (10) | −20 | 54 | −8 | 2.70 | 0.003 |
| American & Japanese Cartoon + Story (L2): | |||||
| Left vmPFC (11) | −22 | 52 | −11 | 3.71 | < 0.0005 |
| Precuneus (7) | 0 | −75 | 46 | 3.55 | 0.001 |
| Right aSTS/TP (38) | 36 | 5 | −20 | 3.26 | 0.001 |
| Right vmPFC (11) | 26 | 52 | −13 | 3.17 | 0.001 |
| Right MFG (10) | 34 | 61 | 12 | 2.82 | 0.002 |
Abbreviations: aSTS = anterior STS, TP = temporal pole (see Table 1 for others).
Fig. 3.
Convergence of ToM-specific brain activations for the three task-groups (Table 2). For both cultural groups, the cartoon and the (L1 or L2) story activated overlapping brain regions in the vmPFC bilaterally. When the L1 (Japanese) task was used for the conjunction analysis, however, more right-lateralized vmPFC activity was found (a). When the L2 (English) story was used for the same analysis instead of the L1, more bi-lateralized vmPFC activity was found (b).
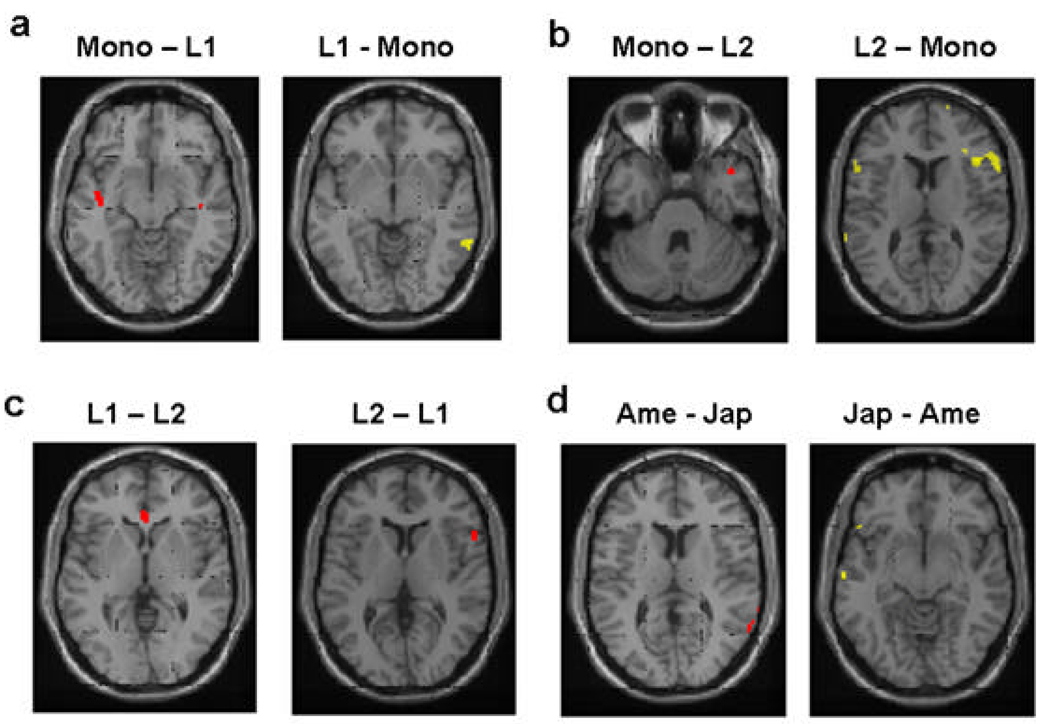
2.2.4. Between-group comparisons – story task-groups
Direct comparisons between the groups were done to examine differences between the task-groups in the story ToM-specific (ToM minus non-ToM contrasts) activations (Table 3). Compared to the L1 (Japanese) group, the monolingual group had slightly more activity in the left aSTS/TP (Fig. 4a, left). The opposite contrast revealed left ITG, with L1 group showing more activity in these areas relative to the monolingual group (Fig. 4a, right). In the comparison between the monolingual group and the L2 group (here both groups saw English) a slightly greater activity was found in the right STG/TP (Fig. 4b, left), with the monolingual group showing more activity there than the L2 group. The opposite contrast revealed significant differences in the MRI signal in the bilateral IFG and right mPFC/superior frontal gyrus (SFG), with the L2 group exhibiting greater activations than the monolingual group (Fig. 4b, right). Comparisons between the L1 and L2 tasks (Japanese versus English stories for the same bilingual subjects) detected small differences in the ACC through the L1 minus L2 contrast (Fig. 4c, left), and in the right IFG through the L2 minus L1 contrast (Fig. 4c).
Table 3.
Brain areas activated during ToM condition relative to nonToM condition Comparing between groups
| Coordinates | |||||
|---|---|---|---|---|---|
| Region (BA) | x | y | z | Z-value | P-value |
| Monolingual (English) minus Bilingual L1(Japanese): | |||||
| Left STS (21) | −42 | −8 | −10 | 1.93 | 0.027* |
| Bilingual L1(Japanese) minus Monolingual(English): | |||||
| Left ITG (20) | −50 | −22 | −19 | 2.84 | 0.002 |
| Monolingual(English) minus Bilingual L2 (English): | |||||
| Right aSTG/TP (38) | 44 | 12 | −26 | 1.98 | 0.024* |
| Bilingual L2(English) minus Monolingual(English): | |||||
| Right IFG (46) | 44 | 24 | 8 | 3.32 | < 0.0005 |
| Right mPFC/SFG (10) | 20 | 67 | 11 | 2.96 | 0.002 |
| Left IFG (45) | −55 | 16 | 7 | 2.75 | 0.003 |
| Bilingual L1(Japanese) minus L2(English): | |||||
| ACC (24) | 0 | 31 | −2 | 2.07 | 0.019* |
| Bilingual L2(English) minus L1(Japanese): | |||||
| Right IFG (45) | 58 | 16 | 3 | 2.25 | 0.012* |
| Cartoon --American minus Japanese: | |||||
| Right TPJ (42) | 61 | −54 | 14 | 2.20 | 0.014* |
| Cartoon – Japanese minus American: | |||||
| Left STS (21) | −63 | −14 | −3 | 2.63 | 0.004 |
| Right IFG (47) | 22 | 15 | −18 | 2.52 | 0.005 |
Fig. 4.
Brain activation in ToM relative to non-ToM condition for the between-group comparison (Table 3)*. The monolingual story group had slightly more activity in the left STS/TP than the L1 group (a, left) The opposite contrast revealed the right ITG, with L1 group showing significantly more activity in these areas than the monolingual group (a, right). The monolingual group, compared to the L2 group (here both groups saw English) had slightly greater activity in the right STS/TP (b, left). The opposite contrast revealed significant difference in MRI signal in the right IFG, right mPFC/SFG, with the L2 group exhibiting greater activity than the monolingual group (b, right). The direct comparison between the L1 and L2 tasks (Japanese versus English stories for the same bilingual subjects) detected small difference in MRI signal in the ACC (c, left), and right IFG through L2 minus L1 comparison (c, right). During the cartoon ToM task, American child group had slightly more activity in the right IPL/TPJ (d, left), and Japanese child group had slightly more activity in the left STS/TP and left IFG (d, right).
2.2.5. Between-group comparisons – cartoon task-groups
To examine culture-specific variation in the neural bases of ToM, that might be free from linguistic influence, we compared hemodynamic responses of the two cultural groups for the same cartoon ToM task (Table 3). The ToM minus the non-ToM contrast revealed slightly more brain activations in the right TPJ in the American monolingual than the Japanese group (Fig. 4d, left). The Japanese group had significantly more activity in the left STS/TP and slightly more activity in the left IFG (Fig. 4d).
2.2.7. Within-task comparisons – cartoon and story tasks
To further examine the within-group differences in brain function that may be culture- or language-related, we also compared cartoon and story ToM-specific activations within each group (Table 4). The American group had a greater activity in the left IFG during the cartoon ToM than during the story ToM task. The opposite contrast revealed greater activity in the bilateral aSTS/TP during the story ToM. The Japanese L1 task-group had a significantly greater activity in the right IFG during the cartoon ToM than during the story ToM task. The opposite comparison revealed greater activity in the aSTS/TP bilaterally during the story ToM. The same contrasts within the Japanese cartoon and the Japanese L2 task-groups revealed a slightly greater activity in the right TPJ during the cartoon ToM and in the left aSTS during the L2 story ToM.
Table 4.
Brain areas activated during ToM condition relative to non-ToM condition Within-group comparisons of brain activity during story and cartoon tasks
| Coordinates | |||||
|---|---|---|---|---|---|
| Region (BA) | x | y | z | Z-value | P-value |
| American – Cartoon minus Story: | |||||
| Left IFG (44) | −55 | 16 | 8 | 2.69 | 0.004 |
| American – Story minus Cartoon: | |||||
| Left STS (21) | −61 | −6 | −5 | 3.14 | 0.001 |
| Right aSTS/TP (21/38) | 48 | 5 | −19 | 3.04 | 0.001 |
| Japanese - Cartoon minus L1(Japanese): | |||||
| Right IFG (44) | 65 | 7 | 18 | 2.94 | 0.002 |
| Japanese - L1(Japanese) minus Cartoon: | |||||
| Left aSTS/TP (21/38) | −38 | 9 | −19 | 3.34 | <0.0005 |
| Right aSTS/TP (21/38) | 38 | 7 | −19 | 3.17 | 0.001 |
| Japanese – Cartoon minus L2(English): | |||||
| Right TPJ (39) | 59 | −56 | 14 | 2.16 | 0.015* |
| Japanese – L2(English) minus Cartoon: | |||||
| Left aSTG/TP (38) | −48 | 16 | −21 | 1.83 | 0.034* |
3. Discussion
This study is the first to examine the hypothesized language- or culture-dependent and independent neural bases of ToM development. Our findings suggest that there are both culture/language-independent and -dependent brain functions associated with ToM development. Specifically, the bilateral vmPFC showed activity during ToM tasks in all the task-groups regardless of linguistic or cultural backgrounds.
The finding in the ventral frontal area is consistent with results of Liu et al. (2005) that tested 6-year-old children with an animation-based false-belief task using ERP. Activity in the vmPFC has been found in several imaging studies which tested people’s ability of reading socio-emotional cues from others (Baron-Cohen et al., 1999; Moll et al., 2002; Winston et al., 2002). It has also been suggested that the ventro-prefrontal area plays an essential role for conceptualizing emotions in socially meaningful ways (Gainotti, 2001), which is, in fact, a defining capacity of ToM. Thus, the culture- and language-independent activity in this area found in our study reinforces the effects of the ‘higher-order’ socio-emotional function (see Damasio, 1994, and Stuss and Benson, 1986 for reviews) throughout the ToM development. In addition, as Figure 3a and 3b show, convergence of the two groups between the story and cartoon ToM-specific activity occurred predominantly in the right vmPFC when the L1 (Japanese) story task-group was used for the analysis but in the bilateral vmPFC regions when the L2 (English) story task-group was used (with other task-groups unchanged). These results further suggest that the bilingual children recruit the vmPFC area in a language/culture-specific manner to understand presumably affective aspects of ToM.
One notable difference between the present study and previous brain imaging studies on ToM, including our own with American monolingual and Japanese bilingual adults (Kobayashi et al., 2006), was that in the present study we found more convergent activity among the groups in the ventral medial prefrontal area than in the dorsal medial prefrontal area. Also, in an additional study (manuscript under review), in which we examined age-related differences in neural bases of ToM, we found significantly greater activity in children in several brain regions including the vmPFC than in adults. These results suggest ventral medial prefrontal area is more important for the universal understanding of ToM during childhood than during adulthood. It has been suggested that the dorsal cingulate area is primarily dedicated to cognitive aspects of behaviors yet the ventral cingulate area is more dedicated to emotional aspects of behaviors (see Bush et al., 2000, for a review). In line with these results, a recent ERP study (Sabbagh, 2003) found vmPFC/orbito-frontal activity while their subject encoded others’ emotions from eye-gazes, but dorsal mPFC activity when they engaged in the cognition-based standard ToM task. Moreover, a recent study has shown that the ventro-medial frontal damage causes the most severe impairments in the affective facets of ToM but not in the cognitive facets (Shamay-Tsoory et al., 2005). These results suggest that ToM may require more emotional processing for children but more cognitive processing for adults.
The between-group contrasts revealed more ToM-specific activation in right TPJ in the American group than the Japanese group. The difference in this area may represent a specific way of ToM processing unique to the American culture during childhood, since American group had more activity in this area then the Japanese group even though both groups were viewing exactly the same cartoon ToM task. Although most of the earlier ToM brain imaging studies in adults with various cultural backgrounds found brain activations in the medial frontal regions (Brunet et al., 2000; Fletcher et al., 1995; Gallagher et al., 2000; Goel et al., 1995; Happé et al., 1996; Vogeley et al., 2001), several more recent studies on English-speaking American or English adults found significant brain activity in the TPJ during the mental attribution tasks (Gallagher et al., 2000; Saxe and Kanwisher, 2003; Saxe and Wexler, 2005). Thus, these results imply that the ToM-related activation in the posterior STS/TPJ region may be culture-specific.
It has been suggested that this area might be involved in the more general ability of distinguishing self-agency from others (Blakemore and Frith, 2003; Jackson and Decety, 2004; see also Decety and Grézes, 2006 for a review). Increasing evidence from socio-psychological studies suggests that Japanese and other Asian cultures encourage the use of group-agency more than individualistic self-agency to account for various kinds of human behaviors (Ames et al., 2001; Nisbett, 2003). The diminished activity in the TPJ area in Japanese adults and children during the ToM tasks might represent the demoted sense of self-agency in the Japanese culture.
The opposite contrast (Japanese cartoon minus American cartoon) revealed more brain activity in left aSTS/TP. However, since the American monolingual group had more activity in the same area during the story ToM task than the bilingual group, the difference in this area may reflect some difference in the ways in which verbal and nonverbal ToM tasks are processed during childhood (rather than being a cultural difference). In fact, our previous study on adults found more activity in this area for the American group than the Japanese group when they processed story-based ToM tasks (Kobayashi et al., 2006). The TP area has been suggested to integrate all the sensory modalities and limbic inputs (Moran et al., 1987) and play a major role in connecting past experiences with currently processed material (Frith and Frith, 2003). Thus, it is possible that Japanese children had to integrate sensory and limbic inputs more for the cartoon-based ToM than the American children who needed the same capacity more for the story-based ToM.
Compared to the between group comparisons of the cartoon ToM-specific activations those of the story ToM-specific activations revealed only small differences among the three story task-groups. This may be because the cultural difference in understanding ToM is greater than the linguistic one during childhood. Nonetheless, we did find a significantly greater brain activity in left ITG for the bilingual L1 group than the monolingual group. The differential activity in the ITG was not found in the same comparison for the three adult groups (Kobayashi et al., 2006). Thus the Japanese language-specific recruitment of the ITG may only persist during childhood. The ITG area has been hypothesized to be involved in semantic analysis of visually presented words (Brunswick et al., 1999). In addition, several studies have found activation in this area when subjects processed Japanese orthography or kanji characters (Nakamura et al., 2000; Sakurai et al., 2000; Uchida et al., 1999). It is possible that the Japanese ToM task demanded orthography-related semantic analyses more than the English ToM task for children. However, this is unlikely because we confirmed that the Japanese children could read and comprehend all the kanji characters that appeared in the Japanese ToM task with ease prior to the experiment. Also, our non-ToM stories (as well as the baseline stories) included as many kanji characters as our ToM stories. Thus, the difference in the ITG activation may reflect a greater difficulty in doing more general semantic analyses in the Japanese ToM task relative to the English one.
The same comparison between the bilingual L2 group and the monolingual group revealed more activity in the bilateral IFG in the L2 group. Notably, the difference in the right IFG was the most significant (p < 0.0005). Since we also found slightly more activity in the homologous area in the left hemisphere in the Japanese group during the cartoon task, the difference in these areas may reflect some culture-specific way(s) of understanding ToM that are related to language or pragmatics. The difference in the right IFG activity has also been found in the same comparison in adult groups (Kobayashi et al., 2006). Also, a recent brain imaging study found a correlation between fear-related emotion and brain activity in the IFG area in Japanese adults but not in Caucasian adults (Moriguchi et al., 2005). Thus, it is possible that the stronger activity in the IFG region reflects the culturally unique style of understanding some emotional aspects of verbal ToM.
As with the monolingual minus the Japanese story (L1) comparisons, the L1 minus L2 and L2 minus L1 comparisons revealed little differences. This similarity across groups might have been due to the fact that the bilingual children in this study were all balanced bilinguals (i.e., they acquired the two languages simultaneously and spoke both languages equally well). The similar ways of understanding ToM of the two cultural groups are also reflected in the results of the within-group comparisons for the cartoon ToM versus story ToM tasks. Both cultural groups employed IFG regions more for processing the cartoon ToM than the story ToM. Besides the aforementioned functions, the IFG regions have been suggested to be involved in the inhibitory control (see Aron et al., 2004, for a review). Thus it is possible that the cartoon task demanded inhibitory control more than the story task for both cultural groups. Moreover, despite the linguistic differences, both cultural groups recruited the bilateral aSTS/TP areas more during the story ToM task than during the cartoon ToM task. Since these anterior parts of STS or TP areas are often found to be involved in processing phonologically oriented language processing (see Price, 2000, for a review), this difference may represent general linguistic demands in the ToM story task relative to the ToM cartoon task.
There are limitations in the present study. One limitation is that even though we have matched the English and Japanese false-belief story tasks in semantics, the two are syntactically different. For example, unlike English false-belief sentences, Japanese false-belief sentences have a center-embedded structure (in which a relative clause is placed in between the subject and verb of the sentence). Most linguists agree that, in general, center-embedded sentences are harder to parse for both adults (Kimball, 1973; Mazuka, 1998) and children (Hakuta, 1981). Even though our behavioral results did not indicate a difference in the task difficulty, it is possible that the difference in syntax has accounted for the differences in the brain activity during the ToM story tasks. Thus, we suggest a future study on speakers of languages that have a false-belief sentential structure similar to English.
An additional limitation is that we tested the bilingual subjects twice with both L1 and L2 tasks because we foresaw some advantages in having the stimuli content be the same. In so doing, we might have given the bilinguals more chance to practice than the monolinguals. Nevertheless, we did not see any attenuation in the MRI signal that often accompanies this kind of practice (Maccotta & Buckner, 2004; Yi & Chun, 2005) in either the L1 or the L2 group. In addition, the order of the languages was counter-balanced across bilingual subjects so any possible practice effects would have been distributed across the L1 and L2 results.
Another limitation in the present study is that we used two different relative height thresholds to recognize significant differences in some of the between-group comparisons. Potentially, significant differences detected through the height threshold of p < 0.05 (uncorrected) may be regarded as weak results. However, we wished to report those regions with lesser significance in order to avoid possible type 2 error. Given this is the first study to examine ToM associated brain function in children of different cultures, we felt this was warranted. Future work will surely need to be done to verify these results. .
In sum, the present study examined development of the neural correlates of culture/language-dependent and -independent ToM. Our study identified both culture/language-dependent and -independent neural correlates of ToM in English speaking monolingual and Japanese/English bilingual children. Our results suggest that the vmPFC may be involved in culturally and linguistically independent processing of ToM during the childhood. However, as with our study in adults (Kobayashi et al., 2006), the results in children demonstrated that several different brain regions are activated during the ToM tasks depending upon the cultural/linguistic backgrounds of the subjects. These results indicate that some of the neural correlates of ToM begin to vary depending upon the person’s cultural/linguistic background from early periods in life.
4. Experimental Procedure
4.1. Participants
Twelve Japanese-English speaking bilingual children (6 males and 6 females) and 12 English speaking monolingual children (6 males and 6 female) from the New York Metropolitan area with mean age of 9;11 ± 1;2 SD (range 8 to 11;11) participated in the experiment. Fourteen monolingual children were recruited initially. However, both behavioral and fMRI data for two of those children were removed because they generated too much movement (more than 5 mm) during the fMRI scans. All participants were early bilinguals (acquired English and Japanese simultaneously before the age of 5). All bilingual participants spoke Japanese as their primary language (L1) and English as their secondary language (L2). Ten bilingual children had two Japanese parents, and two bilingual children had a Japanese parent and an American parent. All participants were healthy and right-handed. IQs of the subjects were assessed through Wechsler Abbreviated Scale of Intelligence™ (WASI™, The Psychological Corporation®, Harcourt Assessment Inc., San Antonio, TX). In addition, all subjects were tested for their knowledge of syntax through a subtest (Sentence Combining test) in Test of Early Language Development, Intermediate – 3rd Edition [TELD-I:3; Hammill and Newcomer, 1999]. Japanese bilingual children were also tested for their proficiency in Japanese with a home-made Japanese test (made by CK), which was similar to the TELD-I:3. Also, we confirmed that all subjects could read and comprehend all the Japanese kanji characters which appeared in the task prior to the experiment. There was no significant difference between the groups in the average IQ score (p > 0.1) or in the average score in the TELD-I:3 subtest (p > 0.1). In addition, all Japanese bilingual children did at above chance on the Japanese proficiency test (M = 99.17, SD = 2.89, t[11] = 59, p < 0.0005). Parents of all participants signed written consent forms and child participants themselves signed written assent forms. Both forms were approved by our Institutional Review Board.
4.2. Materials
Subjects completed three conditions – an experimental ToM, a non-ToM control condition, and a baseline condition – in a standard block design (Posner et al., 1988) for each of the verbal (story) and pictorial (cartoon) versions of the task. Bilingual subjects did two versions of the story tasks in Japanese (L1) and English (L2). The ToM condition consisted of second-order false-belief stories (in the form of ‘x thinks that y thinks that …’) (Perner and Wimmer, 1985). We used the second-order format because we wished to test the subjects with a paradigm which is difficult enough to keep them engaged while in the MRI scanner. It has been shown that the first-order false-belief tasks (in the form of ‘x thinks that …’) are usually passed by normally developing 4–5 year-old children and children with high-functioning autism, but the second-order false-belief tasks are more difficult and cannot be passed until 6–7 years of age (Astington et al., 2002). The non-ToM stories described physical causal situations (as in Fletcher et al., 1995) and were in the same sentential form with a complement as the ToM stories. However, unlike the ToM condition, the non-ToM condition contained perceptual verbs (e.g., ‘sees’ and ‘hears’) instead of mental verbs so that subjects were required to understand physical causal reasoning and not the mental causal reasoning during this condition. The baseline condition consisted of sentences that were presented unlinked (or scrambled) so that they did not make a coherent story as a whole. To test Japanese-English bilinguals, exact translations of the English sentences were used. However, the characters in the stories were given Japanese names to control for the familiarity difference between the two cultures. The Japanese translation was back-translated by another translator to confirm accuracy of the initial translation. Length and semantics of each Japanese sentence (Fig. 1b) were checked by a linguist to ensure that they matched with the corresponding English sentences (Fig. 1a). These story tasks were the same as those used to test adult groups (Kobayashi et al., 2006). As shown in Figure 1c, cartoon ToM task depicted the characteristics of the second-order false-belief task by enclosing the first person’s thought-bubble in the second person’s thought-bubble. As the story version, the cartoon version of the non-ToM task depicted the physical (non-mental) causalities. All the cartoons and stories were colored. Each story was preceded by a 2 second-prompt to indicate “What are they thinking?” (ToM), “What is happening?” (non-ToM), or “Scrambled sentences” (baseline). Each cartoon was preceded by a 2 second-prompt that showed either ‘a picture of a boy thinking’ (to represent ToM), ‘a picture of a woman falling while skiing’ (to represent non-ToM), or ‘a picture of colored puzzles’ (to represent scrambled pictures). These pictures for the prompts were downloaded from commercially available clip-art provided by MS Powerpoint® software (Microsoft Corporation). All the cartoon episodes were matched with the story episodes in content and duration. The same cartoon task was used to test both groups of children.
Example of “What are they thinking?” story (ToM):
John and Paul are watching the World Cup Soccer on TV.
At first France is winning by a lot.
Paul gets up and goes to the bathroom.
While Paul is gone, John sees the USA win the game.
Paul comes back after the game is over.
[Outcome slide] John thinks that Paul thinks that …
the USA wins.
France wins.
Example of “What is happening?” story (non-ToM) :
In a village, there are two men named Nightman and Dayman.
They fight whenever they meet.
One time they meet during the day and Dayman wins.
Next time they meet at night and Nightman wins.
They meet next in the morning.
[Outcome slide] After the fight, newspaper says that …
Dayman wins.
Nightman wins.
Example of Scrambled sentences (baseline) :
Teddy buys red roses for Mary’s birthday.
Mike likes his new car.
Mary’s cat eats all the cookies.
Ted thinks that Cathy thinks that he wears a blue shirt.
Bob sees Italy winning by a lot.
[Question slide (subjects were asked to choose a sentence that had appeared in the preceding 5 slides.)]
John thinks that Paul thinks that his car is new.
Teddy buys red roses for Mary’s birthday.
4.3. Imaging Procedure
There were five stories or cartoons for each condition. Each story or cartoon consisted of five slides followed by a sixth slide showing two different outcomes. The subject’s task was to choose the correct outcome by pressing one of two keys for either possible outcome. The baseline condition simply had the subject choose which of two sentences or pictures had appeared in the preceding five slides. Each of the first five slides of the story or cartoon was shown for 4 seconds, and the sixth outcome slide was shown for 10 seconds, with a total time of 32 seconds per story or cartoon episode (including the 2 second-prompt). An entire run lasted for 8 minutes 8 seconds (excluding a 30 second-instruction before each run and including the 2 second-prompt). Each block was consisted of exactly the three different conditions, so that each condition was easily differentiated later in the design matrix for the data-analysis. All participants had been acclimated with the MRI scanner environment with a simulator housed in Sackler Institute, Weill Medical College of Cornell University, before they were tested in the real MRI scanner. Inside the simulator, the subjects completed short example stimuli. These examples were similar but different from the actual tasks that subjects performed in the scanner. In the actual scanner, the bilingual children were scanned during both English and Japanese versions of the task, with order of language counter-balanced across subjects. Stimuli presentation was also counter-balanced by condition and gender.
4.4. Brain imaging data acquisition
Brain image slices were acquired on a 3-T GE Signa scanner (General Electric Medical Systems, Milwaukee, WI). A three-dimensional spoiled gradient recalled (SPGR) echo in the steady state imaging sequence (repetition time [TR] = 23 ms, echo time [TE] = Minimum Full, Flip angle 20°, 124 slices, 1.4 mm slice thickness, field of view [FOV] = 240 mm in-plane resolution of 0.9 mm by 1.3 mm) were used to acquire T1*-weighted images. In addition, we acquired T2*-weighted two-dimensional axial anatomical images with a Fast spin-echo (FSE) sequence (TR = 6000 ms, TE = 68, Flip angle = 90°, 29 slices, 5 mm slice thickness, FOV = 200 mm). Functional blood oxygen level-dependent (BOLD) images were acquired using an in-out Spiral sequence (Glover & Lai, 1998) (TR = 2000 ms, TE = 30 ms, FOV = 200 mm, Flip angle=90° and 64 mm × 64 mm matrix). The center of the 29 axial 5 mm thick slices was positioned along the anterior commissure-posterior commissure (AC-PC) line to cover the whole brain. For each run, 244 functional scans were acquired.
4.5. Analysis and statistics
For preprocessing the acquired brain images, we used statistical parametric mapping software (SPM2: Wellcome Department of Cognitive Neurology, London, UK) implemented in MATLAB 6.1 (Mathworks, Natick, MA). The first four acquisitions of each series were discarded in order to avoid intensity variation due to magnetization non-equilibrium effects in the Spiral pulse sequence used to acquire BOLD data. All functional images were realigned to the initial image to generate a mean functional image, which was used to determine estimated motion for each individual. The mean functional image was then co-registered with the anatomical images for overlaying the functional image and an anatomical image later in the process. The functional images were then normalized to a Montreal Neurological Institute (MNI) template image. The normalized images were then smoothed using an isotropic Gaussian filter kernel having a full-width half-maximum of twice the normalized voxel size (3.125 mm × 3.125 mm × 5 mm).
Individual analyses were performed using a fixed-effect model where data were best fitted at every voxel, using the General Linear Model (Friston et al., 1999) to describe the variability in the data in terms of the effects of interest. At the single subject level, there were six contrasts of interest for story (L1 and L2 for bilinguals) and cartoon: ToM minus baseline, non-ToM minus baseline, ToM minus non-ToM, and three other contrasts of the opposite subtractions. Next, a group-level analysis was performed using a random-effect model that enabled statistical inferences of population levels (Friston et al., 1999). Contrast images were made for each subject for the six contrasts listed above for story and cartoon. A t-test was performed for each contrast to identify significantly activated brain regions specific to each contrast within each group. To compare activity between groups, two-sample t-tests were used for specific contrasts (e.g., monolingual group versus bilingual L1 group). Paired t-tests were performed to compare brain activation patterns within each group doing two separate tasks (e.g., the cartoon and story tasks). In addition, conjunction analyses (based on the ToM versus non-ToM contrasts) were performed to find convergences of brain activations among the groups. For both within- and between-group comparisons, we used a significance level of p < 0.005 without correction for multiple comparisons, unless otherwise indicated. However, for those comparisons, in which we could not find any brain regions that were significantly different at p < 0.005 (uncorrected), we used more lenient height threshold of p < 0.05 (uncorrected) to recognize the significant differences (actual p-values for these cases are shown in each Table). The stereotactic coordinates of the voxels that showed significant activations were then matched with the anatomical localizations of the local maxima on the standard brain atlas (Talairach and Tournoux, 1988). Before the matching, the MNI coordinates of the normalized functional images were converted to Talairach coordinates using a Matlab function (Brett, 2006).
Acknowledgments
The present study was supported by a grant from NAAR(44519/A001) to ET, as well as from NIH(P41-RR0974) to GHG. We thank Dr. Barbara Lust, Dr. Michael J. Spivey, and Dr. John Whitman for discussion. We also thank Dr. Matthew Davidson, Dr. Bruce D. McCandliss, Dr. Henning U. Voss, Dr. Barbara L. Ganzel, Victor Laczo, and Michiko Sullivan for assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
For some of these between-group contrasts, significant differences in brain activity were recognized only at a more lenient height threshold (p < 0.05, uncorrected). These small differences are shown here in red blobs.
References
- Ames DR, Knowles ED, Morris MW, Kalish CW, Rosati AD, Gopnik A. The social folk theorist: Insights from social and cultural psychology on the contents and contexts of folk theorizing. In: Malle BF, Moses LJ, Baldwin DA, editors. Intentions and intentionality. Cambridge, MA: MIT Press; 2001. pp. 208–329. [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn. Sci. 2004;8(4):170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Astington JW, Pelletier J, Homer B. Theory of mind and epistemological development: the relation between children’s second-order false-belief understanding and their ability to reason about evidence. New Ideas Psychol. 2002;20:131–144. [Google Scholar]
- Avis J, Harris PL. Belief-desire reasoning among Baka children: Evidence for a universal conception of mind. Child Dev. 1991;62:460–467. [Google Scholar]
- Baron-Cohen S, Leslie AM, Frith U. Does the autistic child have a “theory of mind”? Cognition. 1985;21:37–46. doi: 10.1016/0010-0277(85)90022-8. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Ring HA, Wheelwright S, Bullmore ET, Brammer MJ, Simmons A, Williams SC. Social intelligence in the normal and autistic brain: an fMRI study. Eur. J. Neurosci. 1999;11:1891–1898. doi: 10.1046/j.1460-9568.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- Blakemore S-J, Frith CD. Self-awareness and action. Cur. Op. Neurobiol. 2003;13:219–224. doi: 10.1016/s0959-4388(03)00043-6. [DOI] [PubMed] [Google Scholar]
- Brett M. The MNI brain and the Talairach atlas. [Accessed December 1, 2003];2006 Available from http://www.mrc.cam.ac.uk/Imaging/Common/mnispace.shtml.
- Brunet E, Sarfati Y, Hardy-Baylé M-C, Decety J. A PET investigation of the attribution of intentions with a nonverbal task. NeuroImage. 2000;11:157–166. doi: 10.1006/nimg.1999.0525. [DOI] [PubMed] [Google Scholar]
- Brunswick N, McCrory E, Price CJ, Frith CD, Frith U. Explicit and implicit processing of words and pseudowords by adult developmental dyslexics: a search for Wernicke’s Wortschatz. Brain. 1999;122:1901–1917. doi: 10.1093/brain/122.10.1901. [DOI] [PubMed] [Google Scholar]
- Luu Bush, G, Posner MJ. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. 2000;4(6):215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Calarge C, Andreasen NC, O’Leary DS. Visualizing how one brain understands another: A PET study of theory of mind. Am. J. Psychiatry. 2003;160(11):1954–1964. doi: 10.1176/appi.ajp.160.11.1954. [DOI] [PubMed] [Google Scholar]
- Chaminade T, Meltzoff AN, Decety J. Does the end justify the mean? A PET exploration of the mechanisms involved in human imitation. NeuroImage. 2002;15:318–328. doi: 10.1006/nimg.2001.0981. [DOI] [PubMed] [Google Scholar]
- Chen MJ, Lin ZX. Chinese preschoolers’ difficulty with theory-of-mind tests. Bull. Hong Kong Psychol. Soc. 1994;32/33:34–46. [Google Scholar]
- Damasio AR. Descartes’ error: Emotion, research and the human brain. New York: Avon; 1994. [Google Scholar]
- Decety J, Grèzes J. The power of simulation: imaging one’s own and other’s behavior. Brain Res. 2006 doi: 10.1016/j.brainres.2005.12.115. (in press) [DOI] [PubMed] [Google Scholar]
- Ferstl EC, von Cramon DY. What does the frontomedian cortex contribute to language processing: coherence or theory of mind? NeuroImage. 2002;17:1599–1612. doi: 10.1006/nimg.2002.1247. [DOI] [PubMed] [Google Scholar]
- Flavell JH. Cognitive development: Children’s knowledge about the mind. Ann. Rev. Psychol. 1999;50:21–45. doi: 10.1146/annurev.psych.50.1.21. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Happé F, Frith U, Baker SC, Dolan RJ, Frackwiak RS, Frith CD. Other minds in the brain: a functional imaging study of “theory of mind” in story comprehension. Cognition. 1995;57:109–128. doi: 10.1016/0010-0277(95)00692-r. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Price CJ, Buchel C, Worsley KJ. Multisubject fMRI studies and conjunction analyses. NeuroImage. 1999;10(4):385–396. doi: 10.1006/nimg.1999.0484. [DOI] [PubMed] [Google Scholar]
- Frith U, Frith CD. Development and neurophysiology and mentalizing. Phil. Trans. R. Soc. London, Ser. B. 2003;358:459–473. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainotti G. Disorders of emotional behaviour. Journal of Neurology. 2001;248:743–749. doi: 10.1007/s004150170088. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Frith CD. Functional imaging of ‘theory of mind.’. Trends Cogn. Sci. 2003;7(2):77–83. doi: 10.1016/s1364-6613(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Happé F, Frunswick N, Fletcher PC, Frith U, Frith CD. Reading the mind in cartoons and stories: an fMRI study of ‘theory of mind’ in verbal and nonverbal tasks. Neuropsychologia. 2000;38:11–21. doi: 10.1016/s0028-3932(99)00053-6. [DOI] [PubMed] [Google Scholar]
- Glover GH, Lai S. Self-navigated spiral fMRI: interleaved versus single-shot. Magn. Reson. Med. 1998;39(3):361–368. doi: 10.1002/mrm.1910390305. [DOI] [PubMed] [Google Scholar]
- Goel V, Grafman J, Sadato N, Hallet M. Modeling other minds. NeuroReport. 1995;6(13):1741–1746. doi: 10.1097/00001756-199509000-00009. [DOI] [PubMed] [Google Scholar]
- Hakuta K. Grammatical description vs. configurational arrangement in language acquisition: The case of relative clause in Japanese. Cognition. 1981;9:197–236. doi: 10.1016/0010-0277(81)90001-9. [DOI] [PubMed] [Google Scholar]
- Hammill DD, Newcommer PL. TOLD-I:3, Test of language development: Intermediate. Austin, TX: Pro-ed; 1999. [Google Scholar]
- Happé F, Ehler S, Fletcher P, Frith U, Johansson M, Gillberg C, et al. ‘Theory of mind’ in the brain: evidence from a PET scan study of Asperger syndrome. NeuroReport. 1996;8:197–201. doi: 10.1097/00001756-199612200-00040. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC, Rizzolatti G. Cortical mechanisms of human imitation. Science. 1999;286:2526–2528. doi: 10.1126/science.286.5449.2526. [DOI] [PubMed] [Google Scholar]
- Jackson PL, Decety J. Motor cognition: a new paradigm to study self other interactions. Cur. Op. Neurobiol. 2004;14:259–263. doi: 10.1016/j.conb.2004.01.020. [DOI] [PubMed] [Google Scholar]
- Kimball J. Seven principles of surface structure parsing in natural language. Cognition. 1973;2(1):15–47. [Google Scholar]
- Kobayashi C, Glover GH, Temple E. Cultural and linguistic influence on neural bases of ‘Theory of mind’: an fMRI study with Japanese bilinguals. Brain Lang. 2006;98(2):210–220. doi: 10.1016/j.bandl.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Lee K, Olson DR, Torrance N. Chinese children’s understanding of false-beliefs: The role of language. J. Child Lang. 1999;26:1–21. doi: 10.1017/s0305000998003626. [DOI] [PubMed] [Google Scholar]
- Liu D, Sabbagh MA, Gehring WJ, Wellman HM. An ERP study of 6-year-olds’ theory of mind; Poster presented at the 2005 annual meeting of the Cognitive Neuroscience Society; New York City, NY. 2005. [Google Scholar]
- Louis BJ. Psychology Ph.D. Thesis. New Haven, CT: Yale University; 1998. Does Childhood Bilingualism facilitate the Development of Theory of Mind? [Google Scholar]
- Maccotta L, Buckner RL. Evidence for neural effects of repetition that directly correlate with bahavioral priming. J. Cogn. Neurosci. 2004;16(9):1625–1632. doi: 10.1162/0898929042568451. [DOI] [PubMed] [Google Scholar]
- Masuda T, Nisbett RE. Attending holistically vs. analytically: Comparing the context sensitivity of Japanese and Americans. J. Personality Soc. Psychol. 2001;81:922–934. doi: 10.1037//0022-3514.81.5.922. [DOI] [PubMed] [Google Scholar]
- Mazuka R. Development of language process strategies. Mahwah, NJ: Lawrence Erlbaum; 1998. [Google Scholar]
- Moll J, de Oliveira-Souza R, Bramati IE, Grafman J. Functional networks in emotional moral and nonmoral social judgments. NeuroImage. 2002;16:696–703. doi: 10.1006/nimg.2002.1118. [DOI] [PubMed] [Google Scholar]
- Moran MA, Musfson EJ, Mesulam MM. Neural inputs into the temporopolar cortex of the rhesus monkey. J. Comp. Neurol. 1987;256:88–103. doi: 10.1002/cne.902560108. [DOI] [PubMed] [Google Scholar]
- Moriguchi Y, Ohnishi T, Kawachi T, Mori T, Hirakawa M, Yamada M, Matsuda H, Komaki G. Specific brain activation in Japanese and Caucasian people to fearful faces. NeuroReport. 2005;16(2):133–136. doi: 10.1097/00001756-200502080-00012. [DOI] [PubMed] [Google Scholar]
- Naito M. The relationship between theory of mind and episodic memory: Evidence for the development of autonoetic consciousness. J. Exp. Child Psychol. 2003;85:312–336. doi: 10.1016/s0022-0965(03)00075-4. [DOI] [PubMed] [Google Scholar]
- Naito M, Komatsu S, Fuke T. Normal and autistic children’s understanding of their own and others’ false-belief: A study from Japan. Br. J. Dev. Psychol. 2004;12:403–416. [Google Scholar]
- Nakamura K, Honda M, Okada T, Hanakawa T, Toma K, Fukuyama H, Konishi J, Shibasaki H. Participation of the left posterior inferior temporal cortex in writing and mental recall of Kanji orthography: a functional fMRI study. Brain. 2000;123:954–967. doi: 10.1093/brain/123.5.954. [DOI] [PubMed] [Google Scholar]
- Nisbett RE. The geography of thought. New York: The Free Press; 2003. [Google Scholar]
- Perner J, Wimmer H. “John thinks that Mary thinks that…” attribution of second-order beliefs by 5- to10-year-old children. J. Exp. Child Psychol. 1985;38:437–471. [Google Scholar]
- Posner MI, Peterson SE, Fox PT, Raichle ME. Localization of cognitive operations in the human brain. Sem. Clin. Neuropsychiatry. 1988;1:76–88. doi: 10.1126/science.3289116. [DOI] [PubMed] [Google Scholar]
- Premack D, Woodruff G. Does the chimpanzee have a theory of mind? Behav. Brain Sci. 1978;1:515–526. [Google Scholar]
- Price CJ. The anatomy of language: contributions from functional neuroimaging. J. Anat. 2000;197:335–359. doi: 10.1046/j.1469-7580.2000.19730335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbagh MA. Understanding orbitofrontal contributions to theory-of-mind reasoning: Implications for autism. Brain Cogn. 2003;55:209–219. doi: 10.1016/j.bandc.2003.04.002. [DOI] [PubMed] [Google Scholar]
- Sabbagh MA, Taylor M. Neural correlates of theory-of-mind reasoning: an event-related potential study. Psychol. Sci. 2000;11(1):46–50. doi: 10.1111/1467-9280.00213. [DOI] [PubMed] [Google Scholar]
- Sakurai Y, Momose T, Iwata M, Sudo Y, Ohmoto K, Kanazawa I. Different cortical activity in reading Kanji words, Kana words and Kans nonwords. Cogn. Brain Res. 2000;9:111–115. doi: 10.1016/s0926-6410(99)00052-x. [DOI] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N. People thinking about thinking people: the role of the temporo-parietal junction in “theory of mind.”. NeuroImage. 2003;19:1835–1842. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- Saxe R, Wexler A. Making sense of another mind: the role of the right temporo-parietal junction. Neuropsychologia. 2005;43:1391–1399. doi: 10.1016/j.neuropsychologia.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Siegal M, Varley R. Neural systems involved in ‘theory of mind’. Nature Rev. 2002;3:463–471. doi: 10.1038/nrn844. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Tomer R, Berger BD, Goldsher D, Aharon-Peretz J. Impaired “affective theory of mind” is associated with right ventromedial prefrontal damage. Cog. Behav. Neurol. 2005;18(1):55–67. doi: 10.1097/01.wnn.0000152228.90129.99. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Benson DF. The frontal lobes. New York: Raven Press; 1986. [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- Tardiff T, Wellman HM. Acquisition of mental state language in Mandarin- and Cantonese-speaking children. Dev. Psychol. 2000;36(1):25–43. doi: 10.1037//0012-1649.36.1.25. [DOI] [PubMed] [Google Scholar]
- Uchida I, Kikyo H, Nakajima K, Konishi S, Sekihara K, Miyashima Y. Activation of lateral extrastriate areas during orthographic processing of Japanese characters studied with fMRI. NeuroImage. 1999;9(2):208–215. doi: 10.1006/nimg.1999.0400. [DOI] [PubMed] [Google Scholar]
- Varley R, Siegal M. Evidence for cognition without grammar from causal reasoning and ‘theory of mind’ in an agrammatic aphasic patient. Cur. Biol. 2000;10:723–726. doi: 10.1016/s0960-9822(00)00538-8. [DOI] [PubMed] [Google Scholar]
- Vinden P. Junin Quechua children’s understanding of mind. Child Dev. 1996;67:1707–1716. [Google Scholar]
- Vogeley K, Bussfeld P, Newen A, Herrmann S, Happé F, Falkai P, Maier W, Shah NJ, Fink GR, Zilles K. Mind reading: Neural mechanisms of theory of mind and self-perspective. NeuroImage. 2001;14:170–181. doi: 10.1006/nimg.2001.0789. [DOI] [PubMed] [Google Scholar]
- Wellman HM, Cross DC, Watson J. Meta-analysis of theory of mind development: The truth about false belief. Child Dev. 2001;72(3):655–684. doi: 10.1111/1467-8624.00304. [DOI] [PubMed] [Google Scholar]
- Werner H, Kaplan B. Symbol formation. New York: John Wiley and Sons; 1963. [Google Scholar]
- Wimmer H, Perner J. Belief about belief: representation and constraining function of wrong beliefs in young children’s understanding of deception. Cognition. 1983;13:103–128. doi: 10.1016/0010-0277(83)90004-5. [DOI] [PubMed] [Google Scholar]
- Winston B, Strange BA, O’Doherty J, Dolan RJ. Automatic and intentional brain responses during evaluation of trustworthiness of faces. Nature Neuroscience. 2002;5:277–283. doi: 10.1038/nn816. [DOI] [PubMed] [Google Scholar]
- Yazdi AA, German TP, Defeyter MA, Siegal M. Competence and performance in belief-desire reasoning across two cultures: The truth, the whole truth and nothing but the truth about false belief? Cognition. 2006;100(2):343–368. doi: 10.1016/j.cognition.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Yi D-J, Chun MM. Attentional modulation of learning-related repetition attenuation effects in human parahippocampal cortex. J. Neurosci. 2005;25(14):3593–3600. doi: 10.1523/JNEUROSCI.4677-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]