Although there are a dozen or more etiologies for aortic insufficiency, the ultimate problem is similar in most cases. There is a disparity between the area of the aortic orifice and the area of valve leaflet with which this orifice must be covered during diastole. Recently, efforts have been made under direct vision to establish a more favorable orifice/valve leaflet ratio by excision of the noncoronary cusp and conversion of the valve to a bicuspid structure.1–4, 6, 7 Early results by Bailey with the clinical application of this method appear to warrant a more extensive clinical trial.1, 2
Concerning the physiologic consequence of this procedure, several problems remained unsettled. Since bicuspidization of the valve reduces the orifice by more than 50 per cent, the effect of the relative stenosis on the left ventricle is of interest. It is also important to know if it is necessary to excise the leaflet of the noncoronary cusp or if this leaflet can simply be defunctionalized. The present investigation is concerned with these questions and with a description of a simplified method for bicuspidization of the aortic valve.
METHODS
Eleven mongrel dogs (8 to 15 Kg.) were used. The operative procedure and all subsequent tests were performed under sodium pentobarbital anesthesia (25 to 30 mg. per kilogram). Surgery was done under hypothermia (27° to 32° C.), cooling and rewarming being done by immersion in water baths of appropriate temperature. Simultaneous pressures were obtained postoperatively by transcarotid catheters and left ventricular puncture, respectively, and were recorded with an inductance manometer which had a frequency response in excess of 30 cycles per second. Heart sounds and electrocardiogram were recorded with a commercial phonocardiogram.
DESCRIPTION OF OPERATIVE PROCEDURE
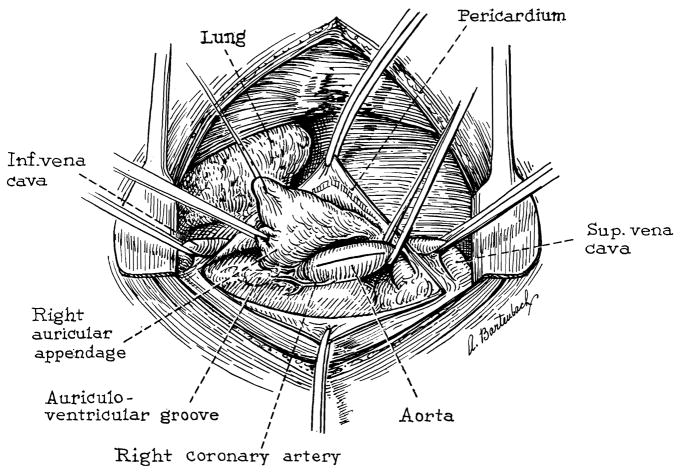
Thoracotomy is performed through the right fourth intercostal space. The azygos vein is ligated and the superior and inferior venae cavae encircled with tapes. The pericardium is opened and a stay suture in the auricular appendage is used to retract the right atrium inferiorly (Fig. 1). The cleavage plane between the right atrium and the noncoronary sinus of Valsalva is developed. Finally, the origin of the right coronary artery is identified (Fig. 1). Both venae cavae are then occluded with Rumel-Belmont tourniquets and the ascending aorta cross-clamped. A longitudinal ascending aortotomy is made and extended proximally through the annulus to the base of the noncoronary cusp (Figs. 1 and 2).
Fig. 1.
View of operative field through right thoracotomy.
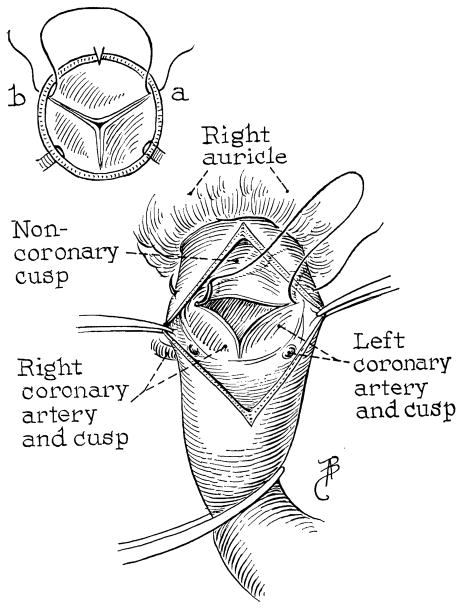
Fig. 2.
Suture at insertions of noncoronary leaflet.
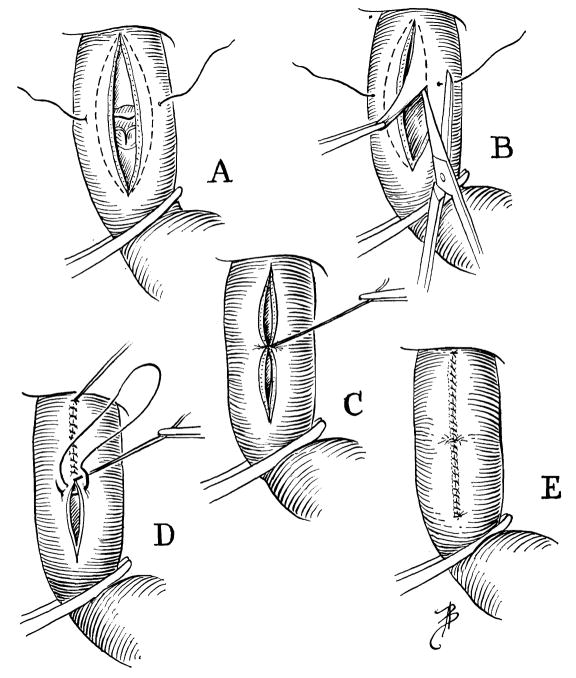
When the lips of the aortotomy are spread and residual blood sucked away, a splendid view of the valve is afforded (Fig. 2). The two points of insertion of the noncoronary leaflet are identified. A needle, swedged with 5-0 silk, is placed through these two points, passing from outside in on one side and inside out on the other side (Fig. 2). Having selected these two points for approximation (Fig. 2), an ellipse of excess aortic and annulus tissue is trimmed away on each side down to within 1 mm. of the previously placed suture (Fig. 3, B).
Fig. 3.
Excision of excess aorta, annulus, and cusp. Closure of aortotomy.
The suture is tied, closing a central point of the aortotomy (Fig. 3, C). The aortotomy is closed with a continuous suture, starting proximally and oversewing the guide stitch in the middle (Fig. 3, D). Before the last sutures are placed, the venae cavae are released to allow air in the left ventricle to be flushed out, and the aortic clamp is removed.
The period of caval occlusion in this series ranged from 7 to 11 minutes. Ten of the eleven dogs developed ventricular fibrillation, with successful resuscitation in all. In several instances, it was necessary to raise the heart temperature by lavaging the chest with warm saline solution before a good beat could be re-established. Blood transfusions were not given.
RESULTS
Operative Mortality and Morbidity
There was no mortality at the operating table. One dog died of heart failure (with acute pulmonary edema) 6 days after surgery. The 9 remaining dogs lived for chronic study and their behavior and activity were entirely normal.
Ascultatory Findings
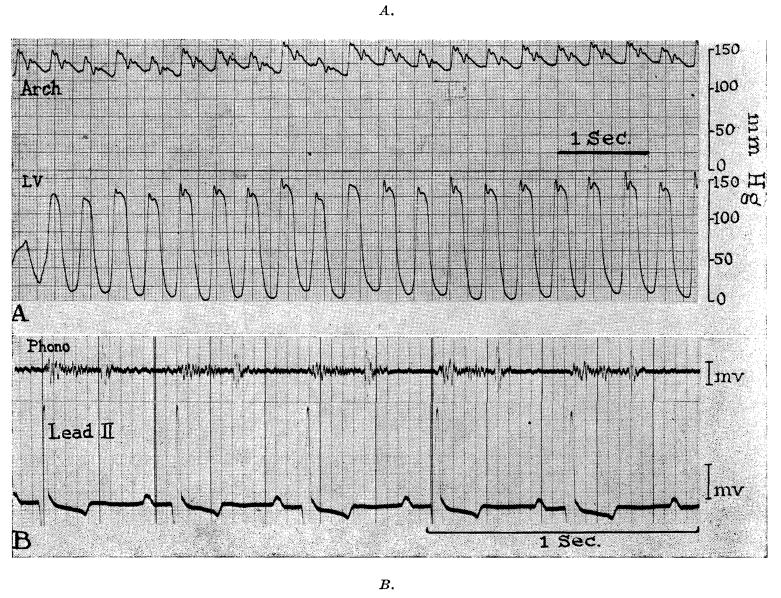
All chronic dogs were examined for heart murmurs at intervals postoperatively and at least one phonocardiographic recording obtained before they were sacrificed. The findings were similar in every case. There was a diamond-shaped systolic murmur which was heard most distinctly just to the left of the sternum in the second or third interspace (Fig. 4, B). In the same location, the second heart sound was invariably accentuated with a snapping tambour quality (Fig. 4, B). In no instance was a diastolic murmur present.
Fig. 4.
Pressures, phonocardiogram, and electrocardiogram 3 months postoperatively (Dog 9).
Left Ventricular and Aortic Arch Pressures
The animals were lightly anesthetized with sodium pentobarbital before they were sacrificed, and simultaneous pressures obtained from the aortic arch (by retrograde catheterization) and left ventricle (by direct puncture). In no case was a gradient demonstrated between the ventricle and the arch (Fig. 4, A). In every case, the aortic pressure complex included a sharp dichrotic notch (Fig. 4, A), strengthening the belief that significant regurgitation had not been produced.
Effect of the Operative Procedure on Left Ventricular Weight
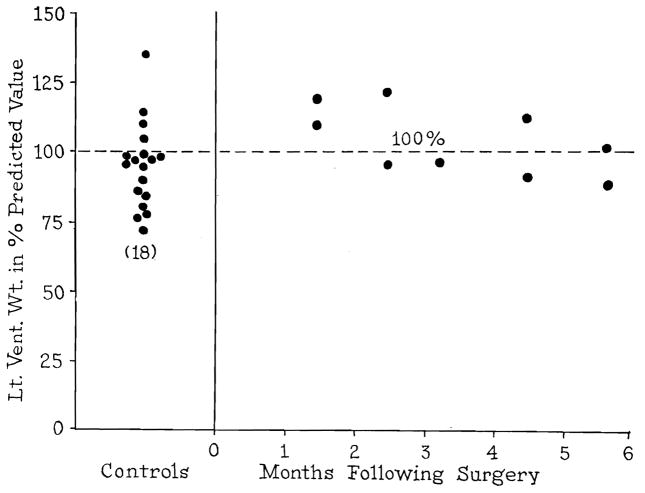
Before sacrificing the animals, accurate body weights were obtained. At autopsy, dissection on the left ventricle was performed by the method of Goodale and Hackel8 and the dissected left ventricle weighed. The actual weight of the left ventricle was compared with the left ventricular weight which was anticipated on the basis of body weight according to the Goodale-Hackel coefficient. As a control, hearts from 18 dogs sacrificed in various acute experiments were also weighed (Fig. 5).
Fig. 5.
Left ventricular weights at autopsy, expressed as per cent predicted value. Control dogs are at left.
The left ventricular weights of the normal control dogs are seen on the left in Fig. 5. On the right, expressed in per cent of predicted weight, are depicted the left ventricular weights of the 9 chronic dogs at various times after aortic valve bicuspidization. It is seen that in 4 cases the weights were slightly less than predicted, in 4 cases slightly greater, and in one case exactly the same. Thus, in the series studied, there is no evidence of hypertrophy up to 6 months postoperatively.
Autopsy Findings
Two animals died 6 days postoperatively, one as a result of empyema and the other of heart failure. Nine animals were electively sacrificed at 6 weeks to 5½ months postoperatively. The reconstructed valves were examined with care and sections of liver and lung taken for histologic study.
In all dogs except one (Case 10), the valves had remained bicuspid. The prolapsed noncoronary cusp was found to be delicate and to be the seat of vegetations in no case. In Case 10, the suture line had spread approximately 2 mm. (Fig. 6). It is interesting that pressures from this animal were entirely normal and that there was no evidence of a diastolic murmur on the phonocardiogram, suggesting that the noncoronary cusp had resumed function and prevented the development of aortic insufficiency.
Fig. 6.
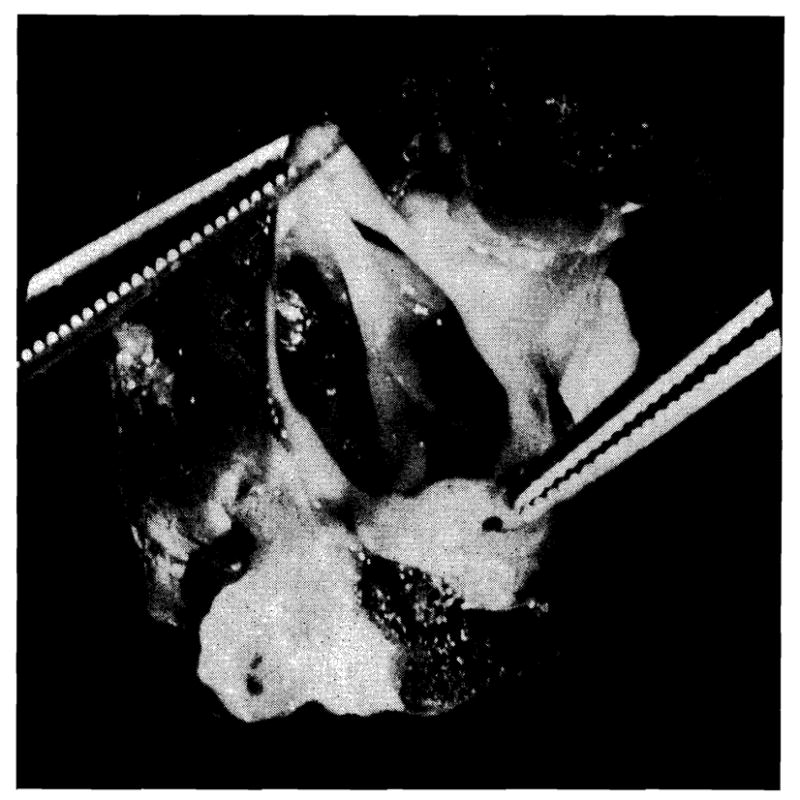
Aortic valve in dog (Case 10) in which spread of aortotomy suture line had allowed valve to resume partially tricuspid structure.
Excepting the dog which died of pulmonary edema in the immediate postoperative period, there was no gross evidence of heart failure in any animal. One of the 9 animals studied chronically had histologic evidence of chronic passive congestion of the liver and lungs (Table I).
Table I.
| NO. | WEIGHT (KG.) | DURATION | L. VENTRICULAR AORTIC GRADIENT | SYSTOLIC MURMUR | DIASTOLIC MURMUR | AUTOPSY FINDINGS |
|---|---|---|---|---|---|---|
| 1 | 12.7 | 11 wk. | 0 | + | 0 | Valve bicuspid; no gross failure; chronic passive congestion histologically |
| 2 | 9.0 | 6 da. | Died of empyema; valve bicuspid | |||
| 3 | 9.1 | 18 wk. | 0 | + | 0 | Valve bicuspid; no gross or microscopic evidence of failure |
| 4 | 10.0 | 20 wk. | 0 | + | 0 | Valve bicuspid; no gross or microscopic evidence of failure |
| 5 | 9.0 | 17 wk. | 0 | + | 0 | Valve bicuspid; no gross or microscopic evidence of failure |
| 6 | 10.0 | 6 da. | Valve bicuspid; gross and histologic pulmonary edema | |||
| 7 | 11.0 | 22 wk. | 0 | + | 0 | Valve bicuspid; no gross or microscopic evidence of failure |
| 8 | 13.2 | 7 wk. | 0 | + | 0 | Valve bicuspid; no gross or histologic evidence of failure |
| 9 | 15.9 | 10 wk. | 0 | + | 0 | Valve bicuspid; no gross or histologic evidence of failure |
| 10 | 8.6 | 6 wk. | 0 | + | 0 | Valve had 2 mm. separation suture line and defunctionalized valve covered gap; no gross or histologic evidence of failure |
| 11 | 10.4 | 22 wk. | 0 | + | 0 | Valve bicuspid; no gross or microscopic evidence of failure |
DISCUSSION
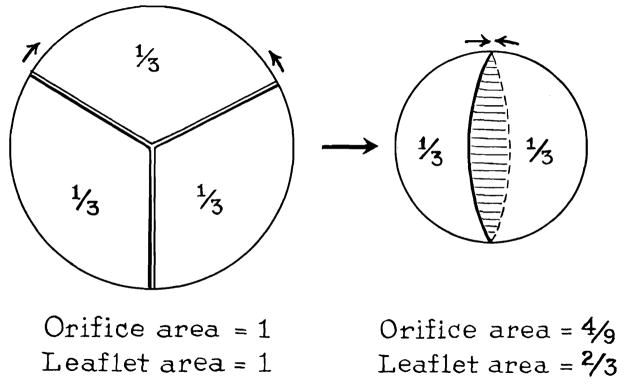
The fundamental geometric alteration produced by aortic valve bicuspidization is shown in Fig. 7. The effect on the orifice area of removing one third the circumference is expressed by the formula
Fig. 7.
Geometric changes after bicuspidization.
in which Ax = original orifice area = 1; Ay = reconstructed orifice area; Cx = original circumference = 1; and Cy = reconstructed circumference = ⅔.
Since the leaflet area which is defunctionalized or removed is one third (one leaflet), the end ratio of is or 1.49. Although the calculations indicate that the bicuspid valve now has 1.49 more leaflet area than orifice area, it is possible that deformation of the leaflet attachments reduces this ratio. It is quite clear, however, from examination of the gross valve, that the bicuspid valve has more leaflet than is necessary to cover the reconstructed orifice. It is probably the excess of leaflet tissue that accounts for the accentuated and tambour second heart sound after surgery.
Previous studies on aortic valve bicuspidization6, 7 have emphasized removal of the noncoronary leaflet as an integral part of valvuloplasty because of the fear that the prolapsed leaflet would cause aortic insufficiency. The present study suggests that excision of the leaflet is not uniformly necessary. Aortic insufficiency did not develop in any case in the present study. In fact, in one of the dogs (Case 10) some spreading of the aortotomy suture line occurred, leaving a gap at one end of the remaining two leaflets. This gap was covered by the prolapsed leaflet which had apparently resumed function.
The issue of whether or not the leaflet must invariably be removed would appear to be of more than academic interest in assessing the potential value of the described procedure in the treatment of clinical aortic insufficiency. In patients with regurgitation, the disparity between available leaflet and the orifice area seldom exceeds 25 per cent and is usually less. Restoration of valve competence would often not require such radical rearrangement of valve structure as with total bicuspidization. Rather, it would seem that treatment could consist of excision of a portion of the noncoronary annulus with retention of the tricuspid valve as suggested by Hufnagel.9 Improvement in the ratio would occur according to the amount of annular circumference excised and the reduction in orifice area could again be calculated from the formula as outlined above.
The operative procedure described would afford considerable versatility in the treatment of aortic insufficiency. According to the particular needs of the case, one could excise a portion of the noncoronary annulus, excise the entire noncoronary annulus with defunctionalization of the valve, or excise both the annulus and leaflet. In the last case, excision of the leaflet can be done with the addition of only a few seconds to the operating time.
The operative procedure in the present study was done with inflow occlusion and hypothermia, necessitating haste and the routine use of resuscitative measures. Undoubtedly, a more desirable approach in clinical application would be the use of extracorporeal circulation with coronary perfusion.
SUMMARY
A simplified method is described for conversion of the aortic valve from a tricuspid to a bicuspid structure. Under conditions of hypothermia and inflow occlusion, the operative mortality is 10 per cent.
The noncoronary cusp can be completely or partially removed with this technique and the valve leaflet either defunctionalized (as was done in this series) or removed.
In dogs studied chronically, there was no evidence of physiologically significant aortic stenosis or aortic insufficiency. Left ventricular weights up to 5½ months postoperatively were within normal limits.
The geometric alterations produced in the aortic valve after bicuspidization are subjected to analysis. The applicability of this technique in the treatment of clinical aortic insufficiency is discussed.
Acknowledgments
This work was supported by Grant No. 166 from the American Heart Association.
Footnotes
Reprinted from THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY St. Louis
References
- 1.Bailey CP. Personal communication. Oct, 1958.
- 2.Bailey CP, Zimmerman J. The Surgical Correction of Aortic Regurgitation. Am J Cardiol. 1959;3:6. doi: 10.1016/0002-9149(59)90390-x. [DOI] [PubMed] [Google Scholar]
- 3.Cooley DA. Discussion of Ref. 5 [Google Scholar]
- 4.Creech O. Discussion of Ref. 5 [Google Scholar]
- 5.Dye WS, Julian OC, Javid H, Grove WJ, Morehead DE, Prec O. Aortic Commissurotomy Under Direct Vision. Ann Surg. 1958;148:469. doi: 10.1097/00000658-195809000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garamella JJ, Anderson JG, Oropeza R. The Surgical Treatment of Aortic Insufficiency by Open Plastic Revision of the Tricuspid Aortic Valve to a Bicuspid Valve. Surg Gynec & Obst. 1958;106:679. [PubMed] [Google Scholar]
- 7.Garamella JJ, Anderson JG, Oropeza R, Veloso A, Naidu R. The Surgical Treatment of Aortic Insufficiency by Conversion of the Tricuspid Aortic Valve to a Bicuspid Valve. J Thoracic Surg. 1959;37:177. [PubMed] [Google Scholar]
- 8.Goodale WT, Hackel DB. Measurement of Coronary Blood Flow in Dogs and Man From Rate of Myocardial Nitrous Oxide Desaturation. Circulation Res. 1953;1:502. doi: 10.1161/01.res.1.6.502. [DOI] [PubMed] [Google Scholar]
- 9.Hufnagel CA, Vilkgas PD, Nahas H. Experiences With New Types of Aortic Valvular Prostheses. Ann Surg. 1958;147:636. doi: 10.1097/00000658-195805000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]