Abstract
In a series of 301 renal homograft recipients, 17 (5.6%) had acute pancreatitis at some time after transplantation. Eleven of these patients died, for a mortality of 64.7%. In each instance, pancreatitis was a major factor in a complex chain of lethal events to which immunosuppression invariably contributed. An additional 43 patients (14.3%) developed asymptomatic hyperamylasemia after transplantation and, undoubtedly, some of these recipients also had pancreatitis. The factors causing pancreatitis in the renal transplantation patient include uremia, hyperparathyroidism, pancreatic injury by drugs, infections resulting from chronic immunosuppression, gallstones, and operative trauma to the pancreas. In cases of preexisting pancreatitis, transplantation is not necessarily precluded, but efforts should be made to find a specific cause of the pancreatitis and take corrective measures, such as biliary tract surgery or parathyroidectomy if indicated, in advance of transplantation.
A number of reports have appeared in the literature indicating that pancreatitis is a complication of renal homotransplantation.1–6 However, many questions require clarification, including the incidence and causes of pancreatitis, the significance of hyperamylasemia in renal homograft recipients; the prognosis with post-transplantation pancreatitis; and the outlook of patients with preexisting pancreatitis. In an attempt to answer some of these questions, 301 kidney recipients treated at the University of Colorado Medical Center and the Denver Veterans Administration Hospital were studied.
Clinical Material
The case records were reviewed of 301 recipients of 362 renal homografts who were treated between November 1962 and January 1971. The patients were 3 to 57 years of age; 207 were male and 94 were female. Two hundred thirty recipients obtained their primary grafts from related donors, while unrelated living volunteers and cadavers were used in 31 and 40 instances, respectively. Most of the grafts for retransplantation were from cadavers. Of the 301 recipients, 240 had trans-abdominal bilateral nephrectomies and splenectomy done at the time of transplantation. A few other patients had this operation at some time prior to or after the transplantation, while the remaining recipients did not undergo the procedure. Immunosuppression was provided with prednisone and azathioprine (Imuran) in 117 patients, and with these same two agents plus antilymphocyte globulin (ALG) in the remainder.4, 7
Evidence of pancreatitis was sought for by the notation of suggestive symptoms and physical signs, radiologic changes, elevation of the serum amylase level, laparotomy findings, and autopsy reports. In addition, possible specific etiologic factors were looked for including operative trauma to the pancreas, systemic infections, hyperparathyroidism, and gallbladder disease.
In the majority of the patients, serum amylase levels were measured in either Caraway units8 (normal, 60 to 160/100 ml) or iodometric units9 (normal, 45 to 150/100 ml). Since the range of these units was very similar, the results will be used interchangeably for the purposes of this communication. In a few indicated cases, Close-Street units10 were used (normal, 6 to 33/100 ml).
Results
Preexisting Pancreatitis
In five patients (four males, one female; aged 17 to 45 years) pancreatitis was diagnosed before or at the time of transplantation (incidence 1.7%). One of these recipients had recurrent clinical and chemical signs of pancreatitis before the onset of renal failure. Another was found to have widespread fat necrosis and calcification in the pancreas at the nephrectomy and splenectomy six months before renal transplantation; a simultaneous finding of gallstones prompted cholecystectomy. In the third patient, who underwent bilateral nephrectomy concomitant with the transplantation, the tail of the pancreas was found necrotic and had to be removed. A fourth patient had several nearly fatal bouts of pancreatitis while undergoing dialysis. Hyperparathyroidism was suspected and at exploratory operation hyperplastic glands were found and resected. Following this, pancreatitis did not recur and the patient underwent transplantation three months later.
After transplantation, four of the above recipients had an uneventful early convalescence, although hyper-amylasemia (236 to 1,010 units) occurred. The first patient eventually developed very brittle diabetes mellitus for which he now requires 135 units of insulin per day, 26 months after transplantation. The second and fourth recipients are well 16 and 13 months after transplantation, respectively. The third patient died of causes unrelated to pancreatitis 29 months after transplantation.
The final patient was treated in 1963 with a cadaver kidney which never functioned. Abdominal pain and a serum amylase level of 850 iodometric units/100 ml suggested a diagnosis of pancreatitis shortly before operation. The patient died of bronchopneumonia and uremia 25 days later. At autopsy, the pancreas was fibrotic and infiltrated with histocytes and lymphocytes.
Pancreatitis Diagnosed After Transplantation
Definite Cases
There were 12 patients (nine males, three females; aged 11 to 49 years, mean, 33 years) in whom pancreatitis was diagnosed from one day to 44 months after the transplantation, and then subsequently confirmed at either laparotomy or autopsy (4%) (Table 1). In two instances, the pancreatitis was probably caused by injury to the tail of the pancreas during left nephrectomy or splenectomy. Both of these patients had delayed distal pancreatectomy but died with multiple intra-abdominal complications. A third patient was found to have a small abscess in the tail of the pancreas at autopsy.
Table 1.
Summary Data on 12 Patients With Definite Pancreatitis After Renal Transplantation*
| No. of Cases | ||||
|---|---|---|---|---|
| Diagnosis established at | Laparotomy | Autopsy | ||
| 4 | 8 | |||
| Localization of lesions | Whole pancreas | Distal part only | ||
| 9 | 3 | |||
| Time of diagnosis after transplantation | <1 mo | 1 mo-1 yr | >1 yr | |
| 4 | 4 | 4 | ||
| Serum amylase, highest value† | <200 units | 200–1,000 units | >1,000 units | |
| 1 | 3 | 6 | ||
| Creatinine clearance at time of diagnosis | None | <40 ml/min | >40 ml/min | |
| 3 | 5 | 4 | ||
| Treatment of pancreatitis | Conservative | Drainage | Distal pancreatic resection | |
| 8 | 1 | 3‡ | ||
| Outcome | Recovered | Died of pancreatitis | Died with multiple complications | |
| 1 | 5 | 6 | ||
All underwent bilateral nephrectomies and splenectomy concomitant with the transplantation.
Caraway or iodometric units except in one case with a level of 1,370 Close-Street units. In two patients no amylase data are available.
In one of the patients the whole pancreas was diseased.
In the other nine recipients, nephrectomies and splenectomy had also been performed on the same day as transplantation. However, the circumstances of the pancreatitis were against the possibility that pancreatic injury had occurred during removal of the organs. For example, the whole organ was involved in all nine recipients, and in eight of the patients it was months to years after operation when pancreatitis appeared.
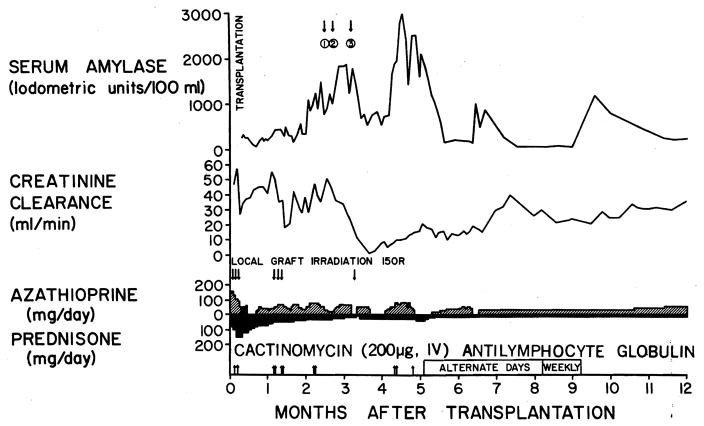
Treatment was conservative in eight of the 12 patients. A ninth patient (Fig 1 and 2) required drainage of a very extensive retroperitoneal abscess which developed as a complication of pancreatitis. Figure 1 shows the first year’s course of this 20-year-old woman following bilateral nephrectomy, splenectomy, and renal transplantation from her mother. The pancreatitis was suspected because of complaints of upper abdominal pain, hyper-amylasemia, and suggestive radiologic changes. Currently, the patient is at 72 months after transplantation with a stable creatinine clearance of 26 ml/min. The other three patients underwent distal pancreatic resections (Table 1).
Fig 1.
In this patient, abscess in left labium majus was drained 75 days after transplantation ➀. Sinogram demonstrated an extensive proximal extension of abscess (Fig 2), which was drained through incision in left flank ➁. Large retroperitoneal abscess arising from pancreas was drained 16 days later ➂. All three abscesses communicated. Serum amylase level remained elevated for prolonged periods. Graft function deteriorated during treatment of abscesses.
Fig 2.
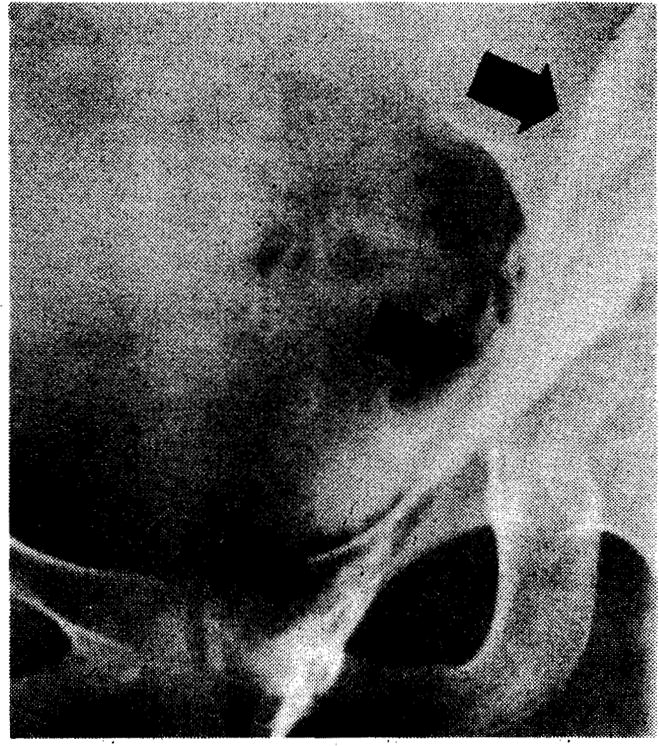
Sinogram demonstrating a tract (arrows) extending from left labium majus retroperitoneally into left flank. Same patient as Fig 1.
Pancreatitis in this group of 12 patients had a poor prognosis. It was the major cause of death or a significant contributing factor in 11 patients, while the survivor had a stormy convalescence before she eventually recovered from the pancreatitis and its complications.
Suspected Cases
Five of the patients (three males, two females; aged 19 to 37 years) had suggestive clinical and laboratory findings of pancreatitis (1.7%). All had nephrectomies and splenectomy at the time of transplantation. In two patients the disorder occurred within 4 and 11 days of operation and could have been aggravated by operative trauma. In two cases the evidence of pancreatitis developed after seven weeks; it occurred during a bout of acute hepatitis in one of the patients. In the final case, the diagnosis was made during an episode of streptococcal septicemia five months after transplantation. In two of the recipients, renal function was poor at the time of the suspected pancreatitis but in the other three it was satisfactory or good. The range for the highest serum amylase value was 104 to 325 iodometric units before the operation, and 400 to 3,500 units after the operation. All five patients are alive after follow-up studies ranging from 29 to 78 months.
If the 12 definite and five probable pancreatitis cases are pooled, the incidence of postoperative pancreatitis in the 301 patients is 5.6%.
Hyperamylasemia After Transplantation
Forty-three (26 males and 17 females; aged 10 to 44 years; mean, 26 years) of the 301 patients had asymptomatic hyperamylasemia during the postoperative period (14.3%) (Table 2). In these recipients, hyperamylasemia was very commonly present before operative intervention.
Table 2.
Findings in 43 Patients With Asymptomatic Hyperamylasemia After Renal Transplantation*
| Operation | No. of Cases | Serum Amylase, Highest Value, Units† | Postoperative Hyperamylasemia | ||
|---|---|---|---|---|---|
| Before Operation | After Operation | Onset, Days After Operation | Duration, Days | ||
| Bilateral nephrectomies and splenectomy, transplantation | 27 | 75–678 (225) | 300–5,500 (1,097) | 1–18 (4) | 1–69 (11) |
| Transplantation only‡ | 16 | 44–275 (144) | 350–1,422 (872) | 1–43 (6) | 1–28 (8) |
Range and mean values are given.
Caraway or iodometric units.
Eleven patients had undergone preliminary bilateral nephrectomies and splenectomy; two patients received second transplants 28 and 74 months after having undergone the combined procedure; three patients never had nephrectomies and splenectomy.
Of the 240 patients that underwent the composite operation of bilateral nephrectomy, splenectomy, and renal homotransplantation on the same day, with the possibility of operative pancreatic trauma, 27 had significant elevations of serum amylase level (11.3%) (Table 2). Following the other 122 transplantations where the kidneys and spleen were not removed concomitantly, there were 16 instances of hyperamylasemia after transplantation (13.1%). In these cases, the possibility of injury to the pancreas at the time of transplantation was eliminated. The timing, magnitude, and duration of hyperamylasemia before and after transplantation was similar to that in the group described above (Table 2).
Among the 43 patients with asymptomatic hyperamylasemia, 15 had absent or poor renal function at the time of the biochemical abnormalities. Twelve of the 43 patients eventually died. Postmortem examination in ten of these showed either a normal pancreas or insignificant histologic changes. Autopsy was not performed in the other two cases.
Etiologic Associations
Hypercalcemia and Hyperparathyroidism
Among the five patients with pre-existing pancreatitis, hyperparathyroidism was demonstrated by operation in one. Four of the 12 patients with proven pancreatitis after transplantation had elevated serum calcium levels which ranged from 11 mg/100 ml to 16.6 mg/100 ml and which spontaneously returned to normal in all but one instance. Ultimately, all four of the patients died and autopsy was performed in three. Two of these three patients had parathyroid hyperplasia.
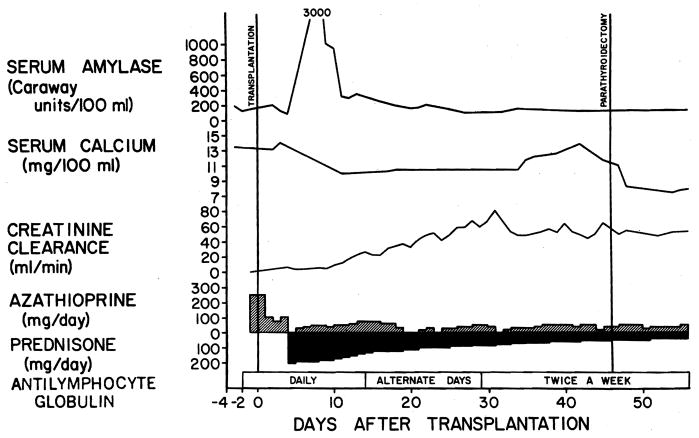
In addition, five of the 43 patients with asymptomatic hyperamylasemia had postoperative hypercalcemia, with the maximum calcium concentration being 16.3 mg/100 ml. One of these latter recipients required an emergency parathyroidectomy for hyperplasia 46 days after transplantation (Fig 3). Currently, this patient is at 46 months after transplantation, with normal serum amylase and calcium levels.
Fig 3.
Course of 24-year-old woman who underwent bilateral nephrectomies, splenectomy, and renal transplantation from her father. Major asymptomatic rise in serum amylase level followed operation. One month after operation, enzyme levels returned to normal. Maximal amylase elevations occurred when renal function was poor. Hypercalcemia necessitated emergency parathyroidectomy 46 days after transplantation.
Gallstones
One patient with pre-existing pancreatitis, another with definite posttransplantation pancreatitis, and a third with asymptomatic hyperamylasemia had gallstones. In the first and third instances, the gallbladder was removed before transplantation at the time of nephrectomies. The other patient died of generalized sepsis and pancreatitis 44 months after transplantation and was found at autopsy to have a stone in the common bile duct.
Systemic Infections
Four cases of proven and one of suspected pancreatitis occurred during episodes of septicemia or fungemia, or both. Only the patient with the suspected pancreatitis survived.
Viral Hepatitis
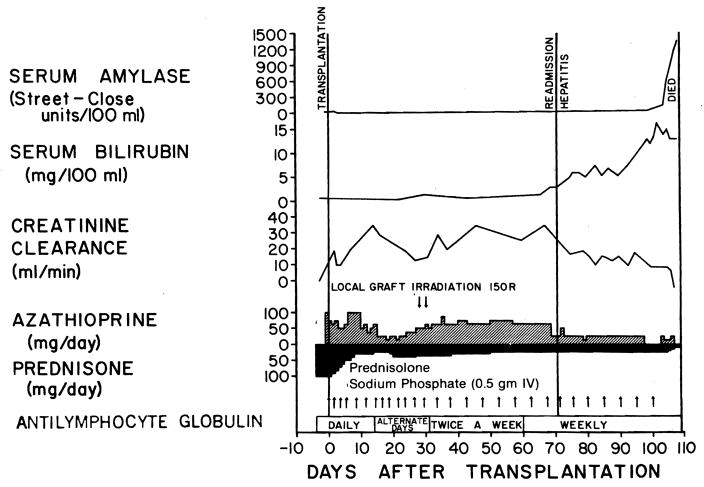
Two instances of proved and one of suspected acute pancreatitis became manifest during episodes of acute hepatitis. Two of the three patients died (Fig 4). The third recovered from the pancreatitis, but has had slowly deteriorating hepatic function during the 8½ years of life after transplantation.
Fig 4.
Course of 11-year-old girl who underwent transplantation for renal failure caused by cystinosis. Donor (maternal grandmother) developed hepatitis 30 days after operation. Forty-one days later the patient was readmitted with same diagnosis. Pancreatitis developed approximately 100 days after transplantation. Severe hypocalcemia (serum calcium level below 2.4 mEq/liter), marked metabolic acidosis, and falling hematocrit were observed. Patient died 109 days after transplantation. Autopsy confirmed presence of hepatitis and acute hemorrhagic pancreatitis with fat necrosis.
Comment
In the first report of pancreatitis after renal homotransplantation,4 there were three proven examples in 42 consecutive recipients (7.1%), and two additional suspected cases for a total incidence of 11.9%. In the present series, which is an extension of the original one, there were 17 (5.6%) of 301 patients with proven or suspected pancreatitis, as well as another 43 (14.3%) with posttransplantation hyperamylasemia. Such figures make it clear that pancreatitis is a very significant problem after renal homotransplantation, and that it can be expected far more frequently than at the 2% incidence found in the survey of transplantation centers made by Johnson and Nabseth.2
There are undoubtedly many factors that contribute to pancreatitis in the complex setting of clinical renal homotransplantation. To begin with, inflammatory changes in the pancreas have been said to be very common in uremia.11–13 In the course of performing bilateral nephrectomy and splenectomy in our patients, it often has been noted that the pancreas is harder or larger than normal, and in three cases, definite evidence of old pancreatitis was found.
Why uremia should cause pancreatitis is not known. One possible mechanism is by the secondary hyperparathyroidism which is a well-recognized complication of chronic renal failure.14 In our patients, there were only three examples of the combination of proven hyperparathyroidism and pancreatitis, but more subtle forms of hyperparathyroidism than those associated with high serum calcium levels may have gone undiagnosed.
After transplantation, the drugs that are administered probably play a far-reaching etiologic role and one which is not easily analyzable. Corticosteroids have been shown in rabbits15,16 and man12,17–19 to produce pancreatitis and, in addition, this complication has been ascribed in isolated cases to azathioprine20 and to the diuretic, chlorothiazide.19
Apart from their intrinsic toxicity to the pancreas, the immunosuppressive agents required to control rejection could contribute to pancreatitis by predisposing to infection. Four of our proven and one of our suspected cases of pancreatitis occurred during septicemia or fungemia, or both. The association of pancreatitis with systemic infections is recognized even in patients who have not undergone im-munosuppression.12,19
Similarly, three of the proven or suspected cases of pancreatitis occurred during a bout of hepatitis. The association of pancreatitis with hepatitis has been described by Blumenthal and Probstein12 and Elliot and Williams.21 In turn, it has recently been shown that acute and chronic hepatitis are very common in the period after transplantation, to the extent that patients who have undergone long-term immunosuppression may actually represent a public health problem.22
Under conditions of immunosuppression, chronic infestations with viruses in addition to the virus responsible for serum hepatitis has been well documented. Herpesvirus and cytomegalovirus (CMV) have been particularly well studied.1,23 Conceivably, there could be direct colonization of the pancreas by such viruses. In two cases of posttransplantation pancreatitis reported by Tilney5 and van Geertruyden and Toussaint,6 the CMV virus was found in the organ at autopsy. Whether or not the microorganism contributed to the development of pancreatitis is speculative.
The extent to which immunosuppression affects the outcome of pancreatitis after it is established is not known. On the one hand, one might hope that an “autoimmune component,” such as speculated upon by Thal,24–26 secondary to pancreatic tissue, injury would be minimized by host immunologic paralysis. On the other hand, Apostolou et al27 have suggested that host immune responses may help limit the disease of pancreatitis, perhaps by the elaboration of anti-enzyme antibodies. If this latter view were true, immunologic invalidism would be an adverse circumstance. The latter hypothesis would be consistent with the fact that 11 of our 12 patients with proven pancreatitis died. This heavy mortality also characterized Johnson and Nabseth’s compilation,2 in which more than half the patients died.
In addition to the special metabolic or pharmacologic factors that apply generally to posttransplantation patients, there were also examples of pancreatitis from a predominantly mechanical cause. In our series, gallstones probably were contributory in three patients. Injury to the tail of the pancreas was definitely responsible for regional pancreatitis in two others. Finally, the asymptomatic hyperamylasemia, so commonly noted in our cases, may have been inadvertently caused during the nephrectomies and splenectomy. A high incidence of amylase elevation has been noted in non immunosuppressed patients submitted to various kinds of upper-abdominal surgery.21,28,29 Against operative trauma being a common etiologic factor, however, is the finding that hyperamylasemia was equally frequent in those of our patients whose upper abdomen was not operated on at the time of transplantation. Since retention of the enzyme occurs in renal failure30–34 poor graft function could have contributed to the elevated enzyme levels.
Four of the five patients with pre-existing pancreatitis survived after operation without any early complications directly attributable to the abnormal pancreas, although one of the recipients subsequently developed rather severe diabetes. These results may be fortuitous but they do indicate that there are hopes for success, particularly if factors predisposing to pancreatitis can be corrected. Conditions that would be easily rectified include biliary tract disease and, possibly, parathyroid hyperplasia. The avoidance of pancreatitis by these means prior to transplantation would seem particularly worthwhile since after transplantation there is little that can be done for suspected pancreatitis except to take pains that the diagnosis is correct and to provide general supportive care including antibiotics. Reduction in the immunosuppressive therapy, particularly in the dosage of prednisone, should be considered.
Acknowledgments
This investigation was supported by research grants from the Veterans Administration, by grants RR-00051 and RR-00069 from the general clinical research centers program of the Division of Research Resources from the National Institutes of Health, and by Public Health Service research grants AI-10176-01, AI-AM-08898, AM-07772, and HE-09110.
Nonproprietary and Trade Names of Drug
- Cactinomycin
Sanamycin
Footnotes
Read before the 29th annual meeting of the Central Surgical Association, Chicago, March 2, 1972.
References
- 1.Hill RB, Jr, Dahrling BE, II, Starzl TE, et al. Death after transplantation. An analysis of sixty cases. Amer J Med. 1967;42:327–334. doi: 10.1016/0002-9343(67)90260-4. [DOI] [PubMed] [Google Scholar]
- 2.Johnson WC, Nabseth DC. Pancreatitis in renal transplantation. Ann Surg. 1970;171:309–314. doi: 10.1097/00000658-197002000-00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Penn I, Groth CG, Brettschneider L, et al. Surgically correctable intra-abdominal complications before and after renal homotransplantation. Ann Surg. 1968;168:865–870. doi: 10.1097/00000658-196811000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Starzl TE. Experience in Renal Transplantation. Philadelphia: WB Saunders Co; 1964. [Google Scholar]
- 5.Tilney NL, Collins JJ, Jr, Wilson RE. Hemorrhagic pancreatitis. A fatal complication of renal transplantation. New Eng J Med. 1966;274:1051–1057. doi: 10.1056/NEJM196605122741903. [DOI] [PubMed] [Google Scholar]
- 6.van Geertruyden J, Toussaint C. Pancréatite aigue après transplantation renale. Acta Chir Belg. 1967;66:271–280. [PubMed] [Google Scholar]
- 7.Starzl TE. Experience in Hepatic Transplantation. Philadelphia: WB Saunders Co; 1969. [Google Scholar]
- 8.Caraway WT. A stable starch substrate for the determination of amylase in serum and other body fluids. Amer J Clin Path. 1959;32:97–99. doi: 10.1093/ajcp/32.1_ts.97. [DOI] [PubMed] [Google Scholar]
- 9.Harms DR, Camfield RN. An automated iodometric method for the determination of amylase. Amer J Med Tech. 1966;32:341–347. [PubMed] [Google Scholar]
- 10.Street HV, Close JR. Determination of amylase activity in biological fluids. Clin Chim Acta. 1956;1:256–268. doi: 10.1016/0009-8981(56)90072-9. [DOI] [PubMed] [Google Scholar]
- 11.Baggenstoss AH. The pancreas in uremia. A histopathologic study. Amer J Path. 1948;24:1003–1011. [PMC free article] [PubMed] [Google Scholar]
- 12.Blumenthal HT, Probstein JG. A Clinical-pathologic Correlation. Springfield, Ill: Charles C. Thomas, Publishers; Pancreatitis. [Google Scholar]
- 13.Merrill JP, Hampers CL. Uremia. New Eng J Med. 1970;282:953–961. doi: 10.1056/NEJM197004232821706. [DOI] [PubMed] [Google Scholar]
- 14.Katz AI, Hampers CL, Merrill JP. Secondary hyperparathyroidism and renal osteodystrophy in chronic renal failure. Analysis of 195 patients, with observations on the effects of chronic dialysis, kidney transplantation and subtotal parathyroidectomy. Medicine. 1969;48:333–374. [PubMed] [Google Scholar]
- 15.Lazarus SS, Bencosme SA. Development and regression of cortisone-induced lesions in rabbit pancreas. Amer J Clin Path. 1956;26:1146–1156. doi: 10.1093/ajcp/26.10.1146. [DOI] [PubMed] [Google Scholar]
- 16.Stumpf HH, Wilens SL, Somoza C. Pancreatic lesions and peripancreatic fat necrosis in cortisone-treated rabbits. Lab Invest. 1956;5:224–235. [PubMed] [Google Scholar]
- 17.Baar HS, Wolff OH. Pancreatic necrosis in cortisone-treated children. Lancet. 1957;1:812–815. doi: 10.1016/s0140-6736(57)90974-1. [DOI] [PubMed] [Google Scholar]
- 18.Carone FA, Liebow AA. Acute pancreatic lesions in patients treated with A.C.T.H. and adrenal corticoids. New Eng J Med. 1957;257:690–697. doi: 10.1056/NEJM195710102571502. [DOI] [PubMed] [Google Scholar]
- 19.Dreiling DA, Janowitz HD, Perrier CV. A Physiologic Approach. New York: Paul B. Hoeber, Inc; 1964. Pancreatic Inflammatory Disease; pp. 37–67. [Google Scholar]
- 20.Hume DM. Progress in clinical renal homotransplantation. In: Welch CE, editor. Advances in Surgery. Vol. 2. Chicago: Year Book Medical Publishers, Inc; 1966. pp. 419–498. [PubMed] [Google Scholar]
- 21.Elliot DW, Williams RD. A re-evaluation of serum amylase determinations. Arch Surf! 1961;83:130–137. doi: 10.1001/archsurg.1961.01300130134016. [DOI] [PubMed] [Google Scholar]
- 22.Torisu M, Yokoyama T, Amemiya H, et al. Immunosuppression, liver injury, and hepatitis in renal, hepatic, and cardiac homograft recipients. With particular reference to the Australia antigen. Ann Surg. 1971;174:620–639. doi: 10.1097/00000658-197110000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hill RB, Jr, Rowlands IT, Jr, Rifkind D. Infectious pulmonary disease in patients receiving immunosuppressive therapy for organ transplantation. New Eng J Med. 1964;271:1021–1027. doi: 10.1056/NEJM196411122712001. [DOI] [PubMed] [Google Scholar]
- 24.Murray MJ, Thal AP. Clinical significance of circulating pancreatic antibodies. Ann Intern Med. 1960;53:548–555. doi: 10.7326/0003-4819-53-3-548. [DOI] [PubMed] [Google Scholar]
- 25.Thal AP, Brackney EL. Acute hemorrhagic pancreatic necrosis produced by local Shwartzman reaction. Experimental study on pancreatitis. JAMA. 1954;155:569–574. doi: 10.1001/jama.1954.73690240003011. [DOI] [PubMed] [Google Scholar]
- 26.Thal A. Studies on pancreatitis. II. Acute pancreatitic necrosis produced experimentally by the Arthus sensitization reaction. Surgery. 1955;37:911–917. [PubMed] [Google Scholar]
- 27.Apostolou K, Nabseth DC, Shapiro DH, et al. Immunity to lethal effects of acute pancreatitis. Surg Forum. 1965;26:382–384. [PubMed] [Google Scholar]
- 28.Dunphy JE, Brooks JR, Achroyd F. Acute postoperative pancreatitis. New Eng J Med. 1953;248:445–451. doi: 10.1056/NEJM195303122481102. [DOI] [PubMed] [Google Scholar]
- 29.Perryman RD, Hoerr SO. Observations on postoperative pancreatitis and postoperative elevation of the serum amylase. Amer J Surg. 1954;88:417–420. doi: 10.1016/0002-9610(54)90359-1. [DOI] [PubMed] [Google Scholar]
- 30.Bailey GL, Katz AI, Hampers CL, et al. Alterations in serum enzymes in chronic renal failure. JAMA. 1970;213:2263–2265. [PubMed] [Google Scholar]
- 31.Janowitz HD, Drieling DA. The plasma amylase. Source, regulation and diagnostic significance. Amer J Med. 1959;27:924–935. doi: 10.1016/0002-9343(59)90176-7. [DOI] [PubMed] [Google Scholar]
- 32.Heifetz CJ, Probstein JG, Gray LH. Clinical studies on blood diastase. II. Significance of increased blood diastase. Arch Intern Med. 1941;67:819–827. [Google Scholar]
- 33.Levitt MD, Rapoport M, Cooperband SR. The renal clearance of amylase in renal insufficiency, acute pancreatitis, and macroamylasemia. Ann Intern Med. 1969;71:919–925. doi: 10.7326/0003-4819-71-5-919. [DOI] [PubMed] [Google Scholar]
- 34.Meroney WH, Lawson NL, Rubini ME, et al. Some observations of the behavior of amylase in relation to acute renal insufficiency. New Eng J Med. 1956;255:315–320. doi: 10.1056/NEJM195608162550702. [DOI] [PubMed] [Google Scholar]