Abstract
Basic mechanisms through which men and women self-regulate arousal have received little attention in human experimental addiction research although stress-response-dampening and craving theories suggest an important role of emotional arousal in motivating alcohol use. This study examined gender differences in the effects of acute alcohol intoxication on psychophysiological and self-reported arousal in response to emotionally negative, positive, and neutral, and alcohol-related, picture cues. Thirty-six social drinkers (16 women) were randomly assigned to an alcohol, placebo, or control beverage group, and exposed to picture cues every 10 s (0.1 Hz presentation frequency). Psychophysiological arousal was assessed via a 0.1-Hz heart rate variability (HRV) index. A statistically significant beverage group-by-gender interaction effect on psychophysiological, but not self-reported, arousal was found. 0.1-Hz HRV responses to picture cues were suppressed by alcohol only in men. This gender-specific suppression pattern did not differ significantly across picture cue types. There were no significant gender differences in the placebo or control group. Greater dampening of arousal by alcohol intoxication in men, compared to women, may contribute to men's greater tendency to use alcohol to cope with stress.
Keywords: Gender, Emotion regulation, Heart Rate Variability (HRV), Alcohol Use, Stress
Individual differences in the self-regulation of arousal and attendant emotional experience have profound effects across the lifespan on many aspects of physical and psychological health (Appelhans & Lucken, 2007; Giardino, Lehrer, & Feldman, 2000). Not surprisingly, several fundamental, yet distinct research questions in the addictions literature share a common focus on better understanding mechanisms that support self-regulation of arousal, and individual differences that influence emotional regulatory systems. For example, since Conger (1956) first proposed a stress-response dampening (SRD) theory, the dampening effects of alcohol on negative emotional arousal have been hypothesized to explain drinking behavior motivated by the desire to cope with stressful emotional states. Similarly, theories of craving implicate physiological arousal in response to appetitive environmental cues as playing a role in eliciting drug seeking behaviors and in contributing to relapse (Carter & Tiffany, 1999; Tiffany, 1995). There are multiple systems involved in regulation of emotion. The autonomic nervous system (ANS) components of emotion serve a preparatory function, which involves general arousal that prepares an organism as a whole for action (e.g., increase in heart rate), and specific arousal that prepares the organism for a particular behavior (e.g., fleeing from danger, lighting a cigarette) (Iversen, Kupfermann, & Kandel, 2000). Together with endocrine response and cortical processing of salient environmental stimuli, individual differences in ANS arousal and its modulation contribute to the experience of emotional states that are relevant to understanding both SRD and craving. The focus of this study was on a basic psychophysiological mechanism that supports self-regulation of arousal in real-time in response to emotional and alcohol cues in the environment, how this mechanism is affected by alcohol intoxication, and how alcohol effects on arousal may conceivably play a role in differentially motivating alcohol consumption in men and women.
Alcohol Intoxication and Dampening of Arousal
Much research has examined how alcohol modifies emotional experience that is stressful in nature. Alcohol intoxication has been shown to affect psychophysiological indicators of emotional arousal by dampening increases in autonomic nervous system (ANS) reactivity, such as heart rate (HR) and galvanic skin response (GSR), in response to a wide variety of stressors such as negative emotional visual stimuli, pain, and the social discomfort of public speaking or making good impression to the opposite sex (Greeley & Oei, 1999; Strizke, Patrick, & Lang, 1995). Self-report studies further suggest that men are more likely than women to use alcohol to cope with stress (Frone, Cooper, & Rusell, 1994; San Jose, van Oers, van de Mheen, Garretsen, & Mackenback, 2000).
Many studies of acute alcohol effects on the ANS in response to stressors excluded women, however, and those that did examine gender differences reported inconsistent findings. Some studies found no gender differences in SRD effects of alcohol, primarily using reductions in HR as the indicator of reduced arousal (Hoaken, Campbell, Stewart, & Pihl, 2003; Levenson, Oyama, & Meek, 1987), while others have found gender differences. Sinha, Robinson, and O'Malley (1998) found significant SRD effects of alcohol on HR in women with a family history of alcohol use and/or anxiety disorders, compared to women without positive family histories and women in a placebo condition. Men did not show SRD effects, regardless of family history. On the other hand, Croissant, Rist, Demmel, and Olbrich (2006) reported that sons of alcoholics, but not daughters, showed strong SRD effects on HR following alcohol consumption, compared with drinking a non-alcoholic beverage. Neither study examined gender-by-alcohol interaction effects on ANS reactivity. Thus, gender differences in stress-dampening effects of alcohol across various levels of risk for problematic alcohol use remain unclear. The inconsistency in HR findings may also be due in part to differences between studies in methodology, such as the timing of HR measurements following alcohol consumption and baseline levels of HR (see Sayette, 1993, for a review).
Furthermore, it is unclear whether arousal dampening effects of alcohol are limited primarily to stress-inducing stimuli or apply more broadly to arousing stimuli irrespective of emotional valence. Due to the importance of “stress-response” dampening to etiological theories of alcohol use (Cooper, Frone, Russell, & Mudar, 1995; Cox & Klinger, 1988; Greeley & Oei, 1999), many previous studies included only stress-inducing stimulus conditions and emotionally neutral conditions for comparison. The study of a broader range of stimulus conditions is desirable, as shown in a study by Strizke and colleagues (1995) that found alcohol reduced GSR in response to both negative and positive emotional stimuli. It thus appears useful to carefully delineate arousal and valence components of ANS arousal regulatory systems.
Alcohol cue reactivity research has focused on alcohol dependent individuals who typically self-report craving and show increased autonomic arousal, such as increased HR and GSR, in response to both visual and olfactory alcohol cues (see Carter & Tiffany, 1999; Drummond, Cooper, & Glautier, 1990 for reviews). Cue reactivity induced by alcohol-related cues is not limited to alcohol dependent drinkers, however. Increased self-report of urges to drink (Cooney, Gillespie, Baker, & Kaplan, 1987) and increased physiological arousal responses to alcohol-related cues (Walitzer & Sher, 1990) have also been demonstrated in social drinkers. In cue reactivity studies, alcohol exposure typically does not involve administration of alcohol beyond the level of priming or tasting. Furthermore, the limited empirical evidence is equivocal as to whether alcohol cue reactivity during negative mood states differs for men and women who are alcohol dependent (Rubonis et al., 1994) or heavy social drinkers (Nesic & Duka, 2006). It is important to understand how alcohol administration may influence alcohol cue reactivity, and whether it influences cue reactivity differentially for men and women in response to emotional and alcohol-related cues. Therefore, the present study included alcohol-related picture cues, in addition to emotionally arousing cues, to examine the effects of alcohol in a broader context of arousal response.
Real-time Change in Heart Rate Variability as a Measure of Autonomic Arousal
While HR and GSR are useful indicators of changes in arousal and emotional reactivity, they do not directly characterize dynamic aspects of self-regulation (El-Sheikh, 2001). In line with a focus on modulation of arousal and the use of alcohol to self-regulate emotional response, the current study examined a component of heart rate variability (HRV), derived from cardiac beat-to-beat R-to-R intervals (RRI), to capture continuous, fine-grained adjustments that occur in HR in response to emotional and alcohol-related picture cues. Quantitative parameters of HRV characterize the adaptive capacity of the cardiovascular system, working together with the neural circuitry involved in the central autonomic network (Benarroch, 1997), and thus are well suited to quantify dynamic aspects of emotional self-regulation when stimulated (Porges, 2007; Thayer & Lane, 2000).
Various indices of HRV predict cardiovascular mortality (Schmidt et al., 2005; Stein et al., 2006), children's health, resiliency, and social competence (Doussard-Roosevelt, Porges, Scanlon, Alemi, & Scanlon, 1997; El-Sheikh, Harger, & Whitson, 2001; Katz & Gottman, 1995, 1997), and the use of active coping skills in adults (Fabes & Eisenberg, 1997; O'Connor, Allen, & Kaszniak, 2002). HRV indices have also demonstrated utility in the study of emotional regulation (Appelhans & Luecken, 2006; Berntson et al., 1997) in disorders such as depression (Nahshoni et al., 2004), anxiety (Dishman et al., 2000; Thayer, Friedman, & Borkovec, 1996), panic disorder (Cohen et al., 2000; Friedman & Thayer, 1998), and chronic heavy alcohol use (Thayer, Hall, Sollers, & Fischer, 2006). HRV has most often been operationalized in terms of characteristic background levels of ANS function (i.e., baseline HRV measured at rest), which are subject to suppression by acute and chronic stressors in healthy adults (Giardino et al., 2000), and by both acute (Bennett et al., 2001; Koskinen, Virolainen, & Kupari, 1994; Reed, Porges, & Newlin, 1999; Rossinen et al., 1997) and chronic (DePetrillo, White, & Liu, Hommer, & Goldman, 1999; Ingjaldsson, Laberg, & Thayer, 2003; Murata et al., 1994; Yokoyama et al., 1991) alcohol consumption.
These strong predictive links between background levels of HRV and physical and emotional health may reflect the cumulative outcomes of individual differences in arousal and modulation of arousal in response to environmental challenges as they occur in real-time (Appelhans & Luecken, 2006; Berntson et al., 1997). The only alcohol administration study of which we are aware that actually examined real-time changes in HRV in response to visual cues found that alcohol suppressed reactivity to cocaine cues in nine cocaine abusing men (Reed et al., 1999).
The Present Study
The current study examined alcohol and placebo effects on real-time changes in HRV in response to provocative visual cues as a method for evaluating how men and women modulate their arousal when they are stimulated with environmental cues. Based on the results of our previous comparative evaluation of multiple HRV indices using a subset of the data from the current study (HRV responses to emotionally valenced cues only, Vaschillo et al., 2008), a 0.1-Hz HRV index was selected as a sensitive measure of ANS regulation. Resonance effects maximize the amplitude of 0.1-Hz oscillations when the cardiovascular system is stimulated at this frequency (Vaschillo, Lehrer, Rishe, & Konstantinov, 2002; Vaschillo, Vaschillo, & Lehrer, 2006). In Vaschillo et al. (2008), we introduced a novel methodology of stimulating the cardiovascular system at its resonance frequency (0.1 Hz, i.e., one cue every 10 s), a distinctive feature of a 0.1-Hz HRV index, and quantitatively evaluated the relative sensitivity of the new 0.1-Hz HRV index, compared to several other traditional HRV indices, to negative, positive, and neutral picture cues. While all the evaluated HRV indices were sensitive to alcohol effects, the 0.1-Hz HRV index was found to be sensitive to placebo challenge, while other HRV indices were not, and sensitive to the valence of the emotional picture cues. Convergent evidence suggests that the 0.1-Hz HRV index evaluates both moment-to-moment ANS reaction to various external interventions (Nickel & Nachreiner, 2003), as well as activation of the baroreflex (Cevese, Gulli, Polati, Gotti, & Grasso, 2001), a reflex that modulates level of arousal.
In the present study, we used the 0.1-Hz HRV index to determine whether arousal in response to emotionally positive, negative, and neutral picture cues, and alcohol-related picture cues differ for men and women following alcohol administration, and compared psychophysiological arousal to subjective self-reports of arousal in response to the four stimulus cue types. We hypothesized that alcohol would reduce the 0.1-Hz HRV index of ANS reactivity to alcohol-related as well as emotionally-valenced cues. Men were predicted to show more dampening in the 0.1-Hz HRV index of autonomic nervous system reactivity to negative emotional stimuli during acute alcohol intoxication than would women, based on the self-report literature (Frone et al., 1994; San Jose et al., 2000). Examination of gender differences in response to positive emotional and alcohol-related cues was exploratory. Finally, we expected that the 0.1-Hz HRV index, relative to self-reports of arousal, would better model the relationships between arousal, exposure to alcohol, and stimuli among men and women than would self-report level of arousal, due in part to lower levels of errors in measurement relative to self-report measures.
Method
Participants
Participants were 20 men and 16 women, between 21 to 24 years of age, who were recruited through advertisements for social drinkers posted on bulletin boards and in university and community newspapers. Interested individuals called the lab for details about the study, and if interested, provided oral consent to complete a standardized telephone screening interview (Ray, Bates, & Bly, 2004; Tracy & Bates, 1999) to initially determine study eligibility. Based on the screening questions and a more in-depth laboratory assessment, individuals were excluded if positive for a history of psychiatric or neurological disorder or treatment; alcohol dependence; history of any substance abuse treatment; lifetime diagnosis of any substance use disorder on the part of the prospective participant's biological mother (to rule out prenatal exposure effects); medical conditions that preclude alcohol administration or confound interpretation of HRV (e.g., diabetes, heart disease, abnormal HR pattern); 20% over- and under-weight from the ideal for age and gender; and for women, pregnancy determined via urinalysis. In addition, individuals who reported weekly use of other illicit or prescribed drugs were not eligible for the study. Few participants reported use of any drugs other than marijuana (n = 10) in the past 30 days. Eleven individuals reported use of cigarettes in the past 30 days with four reporting regular use. To avoid exposure of participants to an alcohol dose substantially greater than their routine consumption levels in the natural environment, we also excluded men who didn't consume at least four standard alcohol drinks per occasion, and women who didn't consume at least three standard drinks per occasion, at least twice per month in the past year.
The majority of the participants were non-Hispanic White (61%); 22% were Asian, 6% were Hispanic White, and the remaining were African American and other (11%). Although we recruited in the university and surrounding communities, participants meeting the study inclusion criteria were all college students, with 14.6 years of education on average (SD = 1.2). The mean age was 21.8 years (SD = 0.98) and majority of the participants (85.7%) reported a family income of more than $41,000.
Measures and Procedures
Stimuli
The picture cue exposure tasks included four categories of picture cue blocks that are the focus of the present study: negative emotional, positive emotional, neutral, and alcohol-related as well as two exploratory picture cue blocks that were not included in the present study (marijuana and polydrug). There were 15 pictures per each picture cue type. Emotional pictures were selected from the International Affective Picture System (IAPS; Lang, Bradley, & Cuthbert, 2001); negative and positive pictures were matched on standardized ratings of arousal, but varied in valence. Alcohol-related picture stimuli were from the Normative Appetitive Picture System (NAPS; Stritzke, Breiner, Curtin, & Lang, 2004), as well as from Tapert et al. (2003), with additional alcohol-related pictures developed in our lab.
Procedures
Eligible participants were stratified by gender and then randomly assigned to an alcohol, placebo, or no alcohol control group (n = 12 per each group). Participants were asked to eat a light low-fat meal (e.g., cereal, oatmeal) 3 hrs prior to reporting to the lab, and to refrain from drinking alcohol or taking any drugs for 24 hrs before the session (except cigarettes and caffeine to avoid withdrawal symptoms during the experimental session). The session was scheduled during weekdays (i.e., Mondays through Fridays), and began between 10 a.m. and 2 p.m. to minimize biological circadian variations in alcohol metabolism and behavioral effects. All participants provided written informed consent and were compensated $10.00 per hr with a maximum of $50.00 for time spent in the lab, which included the time to return to a BAC of zero for the alcohol group. After providing consent, participants completed a series of questionnaires, including a brief version of the Profile of Mood States (POMS; McNair, Lorr, & Droppleman, 1992), which asked “how they feel right now,” and standardized Alcohol and Drug Use Questionnaires (Rutgers Health and Human Development Project; Pandina, Labouvie, & White, 1984). Then, the participants moved to the testing room. After the sensors for physiological recording were attached, the participant was seated in a comfortable chair located 2.5 m in front of a TV screen in a sound-attenuated, dimly-lit room. Before beverage administration, participants performed a standardized low-demand task, the “plain vanilla task” (Jennings, Kamarck, Stewart, Eddy, & Johnson, 1992) for 5 min to equate mental load across individuals. During this task, the participants sequentially viewed colored rectangles on a computer screen and silently counted the number of blue rectangles. Physiological reactivity during this period served as a pre-drinking baseline.
Alcohol doses to achieve a target blood alcohol concentration (BAC) of 90 mg/dl were calculated based on weight (0.90 ml/kg for men, 0.75ml/kg for women), and mixed with an orange juice mixer in a ratio of 4 parts mixer to 1 part ethanol. The beverage was divided into 3 equal drinks, and each drink was consumed during a consecutive 5-min interval. All participants consumed 3 volume-controlled drinks that were 100% mixer (told no alcohol and received no alcohol = control), mixer with 100μl ethanol float per each cup and other olfactory cues (told alcohol and received no alcohol = placebo), or mixer plus 95% ethanol dose. When a BAC of ∼60 mg/dl was reached on the ascending limb of the BAC curve (or after 10 min in placebo and control sessions), participants again performed the plain vanilla task, followed by picture cue presentation. Each participant individually completed one session, including a picture cue exposure phase (the focus of the current study), followed by a picture memory phase, as a part of another study. The entire experimental session lasted approximately 3 1/2 hrs. Participants in the alcohol condition remained in the laboratory until their BAC reached zero.
Picture presentation and arousal ratings
Each picture cue type was presented in a blocked manner. The blocks were presented in counterbalanced order following the 24 patterns of block orders generated using SAS Proc Plan (SAS Institute, 2000-2006). In each of the six blocks, a set of 15 pictures per block was presented twice for a total of 30 pictures per block. The order of picture presentation was randomized within each of six sets. Each picture was presented for 5 s with a 5 s inter-picture interval, resulting in a 0.1-Hz frequency of picture presentation. Each block lasted for 5 min with a 30 s inter-block interval. Using the Self-Assessment Manikin (SAM; Lang et al., 2001), participants verbally provided a liking (not used in this study) or an arousal rating during the 5 s picture-off interval, with the order of ratings counterbalanced across sets within blocks. Their verbal responses were coded and averaged across 15 picture cues per each of six picture cue blocks. Participants were instructed to rate each picture on a scale of 1 = calm or relaxed to 9 = excited, jittery, or awake for arousal ratings.
Physiological record
Electrocardiogram (ECG) activity was recorded during the pre-drinking baseline and presentation of six picture blocks. The ECG record was collected with a sampling rate of 1,000 per second by a Powerlab Acquisition system (ADInstruments, Colorado Springs, CO). Ag-AgCl ECG electrodes were placed on the right arm (active), left arm (ground), and left leg (active). Recorded data were exported to a WinCPRS software program (Absolute Aliens Oy, Turku, Finland) for analyses. The program measured beat-to-beat RR intervals (RRI) of ECG, segmented succession of RRI into 5-min blocks, and calculated RRI spectra through Fourier analysis (Cooke et al., 1999; Taylor, Carr, Myers, & Eckberg, 1998). The 0.1-Hz HRV index was calculated as the power of the RRI spectrum at 0.1-Hz for the pre-drinking baseline and each picture block. Prior to analysis, the 0.1-Hz HRV index scores were transformed using the natural logarithm.
Statistical analysis
Within-individual change scores were calculated by subtracting baseline (pre-drinking) 0.1-Hz HRV index scores from 0.1-Hz HRV index scores for each of four stimulus cue types to adjust for individual differences in HRV at baseline. Analysis of variance (ANOVA) and chi-square tests were used to examine whether gender differences existed in potential confounding variables, including age, pre-test POMS score, the 0.1-Hz HRV index at baseline, and alcohol and other drug use. For the main analysis, a repeated measures ANOVA was used to examine two between-subject main effects of group and gender, an interaction effect between group and gender on changes (from baseline) in the 0.1-Hz HRV index in response to four picture cue types (within-subject effects), and three between-subject by within-subject interaction effects (group × picture cue type, gender × picture cue type, and group × gender × picture cue type). Arousal ratings were also analyzed using the same analytic approach. Finally, correspondence between the 0.1-Hz HRV index and subjective self-report ratings of arousal was examined by Pearson's correlation analysis.
Results
Participant Characteristics across Men and Women
We initially examined whether men and women were equivalent in the 0.1-Hz HRV index at baseline as well as other individual characteristics, including age, alcohol use, and BAC levels (see Table 1). There were no significant gender differences in the 0.1-Hz HRV index at baseline. Men reported consuming significantly more standardized drinks per occasion in past 30 days than did women. This was expected due to the different study inclusion criteria for men and women based on typical quantity of alcohol consumed (see also the Participants section). There were no other significant gender differences.
Table 1. Participant Characteristics: Age, Alcohol Use, and Pre-test POMS score.
| Women (n = 16) |
Men (n = 20) |
t Statistics | |
|---|---|---|---|
| Age | 21.6 (.96) | 22.0 (1.00) | t (34) = -0.99 |
| 0.1-Hz HRV index (baseline) | 8.87 (1.14) | 9.24 (1.08) | t (34) = -1.00 |
| Alcohol use (past 30 days) | |||
| Quantity (per occasion)1 | 2.4 (1.5) | 5.3 (3.1) | t (28.3) = -3.66 a |
| Frequency (per week) | 1.6 (1.6) | 2.6 (1.8) | t (34) = -1.73 |
| POMS (pre-test) | .094 (7.28) | -1.00 (7.33) | t (34) = 0.79 |
| BAC (alcohol condition – pre-test) | .074 (.013) | .079 (.034) | t (34) = -0.31 |
| BAC (alcohol condition – post-test) | .064 (.008) | .054 (.011) | t (34) = 1.74 |
Notes.
Numbers in parentheses indicate standard deviations.
Average number of standard drinks per occasion; POMS = Profile of Mood States (McNair et al., 1992); BAC = Blood Alcohol Concentration;
Statistically significant differences between genders (p < .05).
Physiological Response to Emotional and Alcohol-related Stimuli
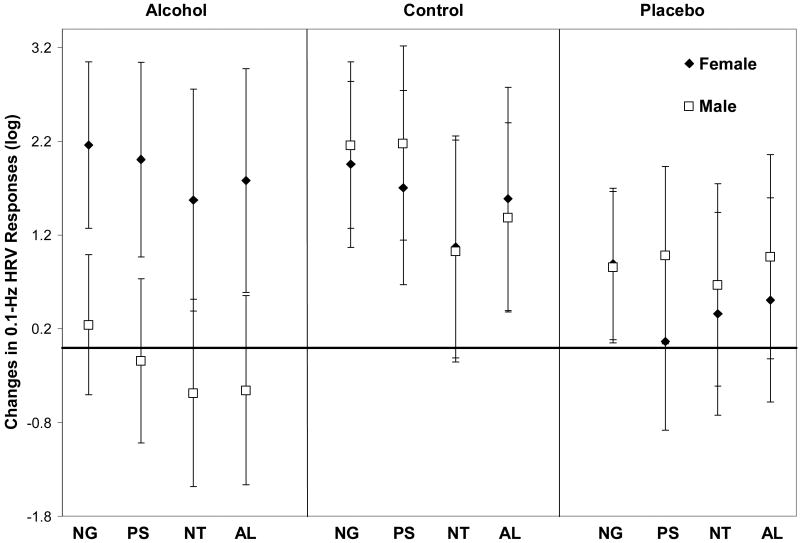
Repeated measures ANOVA (the sphericity assumption was met, Mauchly's criterion = .73, Chi-square = 8.88, with df = 5, ns) results revealed an interaction effect between group and gender on the 0.1 Hz-HRV index, F(2, 30) = 5.03, p < .05, partial η2 = .25 (see Figure 1). Men showed significantly less increase in the 0.1-Hz HRV index following alcohol challenge than did women. The main effect of gender was not significant, F(1, 30) = 2.20, p = .14, partial η2= .07, and the main effect of group was significant at the level of a trend, F(2, 30) = 2.90, p = .07, partial η2 = .16. The alcohol and placebo groups (men and women combined) tended to show lower levels of HRV responses, compared to the control group (p = .07). The alcohol and placebo groups did not differ from each other (p = .69). Among the within-subject effects, only the main effect of picture cue type was significant, F(3, 90) = 6.85, p <.05, partial η2 = .19. Significantly higher levels of HRV responses were observed for negative and positive picture cues but not alcohol-related cues, compared to those for neutral picture cues. All within-subject by between-subject interaction effects were not significant, including the interaction effect between group, gender, and picture cue type. This indicated that the dampened 0.1-Hz HRV response by men in the alcohol group was consistent across different picture cue types.1
Figure 1.
Means and 95% confidence intervals for 0.1-Hz HRV responses to emotional and alcohol-related picture cues. NG = Emotional Negative, PS = Emotional Positive, NT = Emotional Neutral, AL = Alcohol-related. The horizontal x-axis line at y = 0 indicates the reference line for no within-person change in 0.1 Hz HRV. The 0.1-Hz HRV index response score above y = 0 indicates increased response to the picture cues from baseline. The 0.1-Hz HRV index score below y = 0 indicates decreased response to the picture cues from baseline.
Subjective Ratings of Arousal
Participants rated their subjective perceptions of arousal to the different cue types in the same order of magnitude as obtained for their physiological responses. The absolute mean of the 0.1-Hz HRV index, without subtraction of baseline, was greatest in response to negative emotional cues (mean = 10.41, SD = 1.08), followed by positive (mean = 10.17, SD = 1.06), alcohol (mean = 9.97, SD = 1.11), and neutral (mean = 9.72, SD = 0.88). In parallel, participants subjectively rated negative emotional cues as most arousing (mean = 5.59, SD = 1.83), followed by positive (mean = 5.04, SD = 1.59), alcohol (mean = 3.91, SD = 1.66), and neutral (mean = 2.40, SD = 1.21) cues. To account for potential response biases due to rater (e.g., raters' leniency biases or social desirability biases; see Podsakoff, MacKenzie, Lee, & Podsakoff, 2003 for a review), the average self-report rating of arousal in response to the neutral picture cue block (arousal ratings were not assessed during baseline) was subtracted from average negative, positive, and alcohol picture cue block ratings. Repeated measures ANOVA results indicated a statistically significant main effect of picture cue type, F(2, 60) = 30.38, p < .05, partial η2 = 0.40, but no other effects.2 The negative picture cues were rated significantly greater in arousal than the positive and alcohol picture cues, and the positive picture cues were rated significantly greater than the alcohol picture cues.
Overall the correspondence between subjective ratings and the 0.1-Hz HRV index was rather weak and inconsistent as shown by Pearson's correlations between the 0.1-Hz HRV change scores and both raw subjective arousal ratings (i.e., without subtracting neutral), and change in subjective rating scores (see Table 2). Thus, the link between subjective arousal ratings and physiological responses may be influenced by factors other than stimulus cue properties.
Table 2. Zero-Order Correlation between the 0.1-Hz HRV Index and Subjective Ratings of Arousals (N = 36).
| Negative | Positive | Alcohol | Neutral | |
|---|---|---|---|---|
| Alcohol (n = 12) |
.15 (.19) |
-.24 (-.03) |
-.30 (-.46) |
.07 (N/A) |
| Control (n = 12) |
-.31 (-.34) |
-.23 (-.29) |
.19 (-.18) |
.16 (N/A) |
| Placebo (n = 12) |
-.19 (-.11) |
.33 (.35) |
.08 (-.07) |
-.01 (N/A) |
Notes. Upper numbers indicate correlations between the 0.1-Hz HRV change scores (baseline 0.1-Hz HRV subtracted) and original scores (i.e., without subtracting average ratings for neutral picture cues) of subjective ratings of arousals. Lower numbers in parentheses indicate correlations between the 0.1-Hz HRV change scores (baseline 0.1-Hz HRV subtracted) and change scores for the subjective ratings of arousal after subtracting ratings for neutral picture cues. All correlations were statistically non-significant at p < .05.
Discussion
The present study had two main findings. First, we found that alcohol intoxication significantly dampened emotional arousal in men, compared with women. To our knowledge, this is the first study to use an index of HRV to demonstrate differential ANS arousal response in men and women following alcohol consumption. Second, this dampening effect of alcohol on the 0.1-Hz index of arousal modulation in men was not selective to picture cues with negative emotional valence, suggesting that alcohol depressed the arousal rather than the valence component of emotional response. This finding corresponds with Strizke et al. (1995), who found attenuated skin conductance response to both negative and positive picture cues in a combined sample of men and women. The present study further showed the effects of alcohol on general arousal by showing dampened reactivity to alcohol, neutral, and positive cues. Suppressed alcohol cue reactivity by alcohol in men is consistent with Reed and colleagues' (1999) finding that acute alcohol intoxication suppressed increases in RSA during cocaine cue exposure in male cocaine abusers. Future research is needed to determine the generality of alcohol's dampening of men's arousal response to emotional challenges other than picture cues, as well as to other ANS regulatory mechanisms involving sympathetic and parasympathetic components. It may be speculated that, at least with respect to the 0.1-Hz HRV index, alcohol decreased men's general preparatory arousal, rather than interfering with specific arousal for a particular behavior that would be needed to respond to stressful or unpleasant environmental cues.
Several potential explanations for men's differential response to alcohol were examined. Differences between men and women were not likely due to differences in mood, as the pre-test POMS scores indicated that across beverage conditions, women and men were not significantly different in mood state. It is also unlikely that the arousal dampening effects of alcohol in men were due to gender differences in overall HRV, as men and women did not differ in the 0.1-Hz HRV index at baseline. Previous studies of young adults' background levels of HRV have reported equivocal gender differences across various HRV indices. Some studies reported women exhibit greater vagally-mediated HRV, compared to men (Evans et al., 2001; Rossy & Thayer, 1998), while others found the opposite (Umetani, Singer, McCraty, & Atkinson, 1998).
Placebo groups are traditionally used to differentiate the cognitive influence of alcohol outcome expectancies from the acute pharmacological effects of alcohol (Martin & Sayette, 1993). Our previous study (Vaschillo et al., 2008) showed that in the combined sample of men and women, the 0.1-Hz HRV responses by the placebo group were not distinguishable from those of the alcohol group, but different from those of the control group. The present study additionally included 0.1-Hz HRV responses to alcohol-related cues and beverage group by gender interaction effects in the model. In this context, the group main effect was at the level of trend. Thus, we speculate that a cognitively mediated reduction of HRV by alcohol likely exists in the combined sample of men and women. Together with the observed gender-specific dampening effects of alcohol, this pattern of cognitively mediated effects suggests that women may expect alcohol to suppress arousal in much the same way as men do, yet they do not appear to experience the same pharmacological effects of alcohol on the ANS reactivity as did men. The 0.1-Hz HRV index captures a fundamental component of ANS reactivity and modulation, yet does not differentiate sympathetic and parasympathetic nervous system activation and other ANS mechanisms involved in differentiated emotional response. Therefore, it is possible that women experience pharmacological dampening effects of alcohol on other aspects of ANS regulation. Disentangling the potential mechanism of differential effects of alcohol and placebo in women is beyond the scope of current study and requires further research with larger samples.
A gender-by-group interaction effect on self-reported levels of arousal to the picture cues was not found, suggesting that self-reports do not provide a sensitive indicant of alcohol's selective suppression of 0.1-Hz HRV arousal in men. Further, there were weak and inconsistent correlations between the 0.1-Hz HRV index and the subjective ratings. Weak correlations may conceivably be related to the difference in the base values used to calculate change scores between the 0.1-Hz HRV index (pre-drinking baseline) and the subjective ratings of arousal (post-drinking response to neutral pictures). Nonetheless, it is not uncommon to find modest coherence between physiological measures and subjective reports of mood or emotion, perhaps due to factors such as the nature and intensity of the emotional experience (Mauss, Levenson, McCarter, Wilhelm, & Gross, 2005) and individual differences in interoceptive awareness (i.e., sensitivity to visceral activity; Pollatos, Herbert, Matthias, & Schandry, 2007).
Several limitations of this study needed to be considered when interpreting the results. First, the modest sample size limited statistical power for identifying group effects. Second, the current findings may not generalize to populations other than young adult social drinkers. Restrictions in the range of age and alcohol use behaviors may have attenuated the true magnitude of association and limited variability in HRV responses to alcohol-related picture cues. Finally, across human and animal studies involving alcohol administration, females are often excluded due to difficulties with controlling potential confounds. For example, it has been suggested that alcohol pharmacokinetics in women may fluctuate with the menstrual cycle potentially due to changing sex steroid hormone levels, although the literature is equivocal (e.g., Cole-Harding & Wilson, 1987; Jones & Jones, 1984; Lammers, Mainzer, & Breteler, 1995; Sutker, Goist, & King, 1987). Further study is needed to examine alcohol effects on psychophysiological reactivity of women at different phases of the menstrual cycle, as this was not assessed in the present study.
Despite these limitations, the results point to the likelihood of potentially meaningful gender differences in alcohol effects on the modulation of arousal with men, but not women, experiencing dysregulated emotional preparatory response to stimulus cues during acute intoxication. More broadly, the present study demonstrates that a specific emotional regulatory process involving moment-to-moment ANS reactivity and modulation can be objectively quantified when prefrontal function is intact or compromised by alcohol challenge. This basic behavioral approach to assessing ANS participation in emotional experience in real time presents a new opportunity for treatment research for at least two reasons. First, HRV as a background capacity measure of self-regulation as typically studied may be helpful for identifying those at risk for various physical and emotional health conditions. As a potential screening tool, however, HRV indices taken at rest (without stimulation) cast a very wide net. In contrast, it may be possible to screen those with atypical self-regulation of emotional arousal with respect to salient environmental cues from those with poor general health conditions, using a real-time HRV assessment approach. Second, stimulating the cardiovascular system at its resonance frequency via paced breathing has been used to improve ANS regulation in several clinical groups that share difficulties in modulating arousal (Hassett et al., 2007; Karavidas et al., 2007; Lehrer et al., 2003, 2004). Paced breathing, or perhaps the related HRV approach used in this study, involving resonance frequency stimulation with visual cues, may potentially be useful to improve self-regulation among those at risk or in treatment for substance use disorders. Unlike stationary HRV measured at rest, the HRV paradigm used here has the potential to be manipulated and adapted as a behavioral method to exercise activation of baroreflex, a reflex involved in maintenance of homeostasis, which in turn improves emotional self-regulation through enhancing autonomic modulatory flexibility.
Acknowledgments
This study was supported in part by NIAAA grants R01 AA015248 and K02 AA00325 and by NIDA grants P20 DA017552 and 3P20 DA017552-05S1. The authors thank Jamie Kingeter for assistance in data collection and analysis. This study was conducted in partial fulfillment of the requirements for a Ph.D. in public health.
Footnotes
Preliminary findings were presented at the 15th Annual Meeting of the Society for Prevention Research (SPR), Washington, D.C. in May 2007.
The within-subject by between-subject interaction effects between picture cue type and group, between picture cue type and gender, and between picture cue type, group, and gender were statistically non-significant, F(6, 90) = 1.22, ns, partial η2 = 0.08; F(3, 90) = 0.72, ns, partial η2 = 0.02; and F(6, 90) = 0.57, ns, partial η2 = 0.04, respectively.
The within-subject by between-subject interaction effects between picture cue type and group, between picture cue type and gender, and between picture cue type, group, and gender were statistically non-significant, F(4, 60) = 1.36, ns, partial η2 = 0.08; F(2, 60) = 2.67, ns, partial η2 = 0.08; and F(4, 60) = 0.90, ns, partial η2 = 0.06, respectively. All between-subject effects (group, gender, and group × gender effects) were not statistically significant, F(2, 30) = 1.37, ns, partial η2 = 0.08; F(1, 30) = 2.23, ns, partial η2 = 0.07; and F(2, 30) = 0.12, ns, partial η2 = 0.01, respectively.
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/journals/adb
References
- Appelhans BM, Luecken LJ. Heart rate variability as an index of regulated emotional responding. Review of General Psychology. 2006;10(3):229–240. [Google Scholar]
- Banarroch EE. The central autonomic network. In: Low PA, editor. Clinical autonomic disorders. 2nd. Philadelphia, PA: Lippincott-Raven; 1997. pp. 17–24. [Google Scholar]
- Bennett AJ, Sponberg AC, Graham T, Suomi SJ, Higley JD, DePetrillo PB. Initial ethanol exposure results in decreased heart rate variability in ethanol-naive rhesus monkeys. European Journal of Pharmacology. 2001;433:169–172. doi: 10.1016/s0014-2999(01)01445-5. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Bigger JT, Eckberg DL, Grossman P, Kaufmann PH, Malik M, et al. Heart rate variability: Origins, methods and interpretive caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94(3):327–340. [PubMed] [Google Scholar]
- Cevese A, Gulli G, Polati E, Gottin L, Grasso R. Baroreflex and oscillation of heart period at 0.1 Hz studied by alpha-blockade and cross-spectral analysis in healthy humans. Journal of Physiology. 2001;531:235–244. doi: 10.1111/j.1469-7793.2001.0235j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H, Benjamin J, Geva AB, Matar MA, Kaplan Z, Kotler M. Autonomic dysregulation in panic disorder and in posttraumatic stress disorder: Application of power spectrum analysis of heart rate variability and in response to recollection of trauma or panic attacks. Psychiatry Research. 2000;96(1):1–13. doi: 10.1016/s0165-1781(00)00195-5. [DOI] [PubMed] [Google Scholar]
- Cole-Harding S, Wilson JR. Ethanol metabolisms in men and women. Journal of Studies on Alcohol. 1987;48(4):380–387. doi: 10.15288/jsa.1987.48.380. [DOI] [PubMed] [Google Scholar]
- Conger JJ. Alcoholism: theory, problem and challenge II. Reinforcement theory and the dynamics of alcoholism. Quarterly Journal of Studies on Alcohol. 1956;17(2):296–305. [PubMed] [Google Scholar]
- Cooke WH, Hoag JB, Crossman AA, Kuusela TA, Tahvanainen KUO, Eckberg DL. Human response to upright tilt: A window on central autonomic integration. The Journal of Physiology. 1999;517:617–628. doi: 10.1111/j.1469-7793.1999.0617t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney NL, Gillespie RA, Baker LH, Kaplan RF. Cognitive changes after alcohol cue exposure. Journal of Consulting and Clinical Psychology. 1987;55(2):150–155. doi: 10.1037//0022-006x.55.2.150. [DOI] [PubMed] [Google Scholar]
- Cooper ML, Frone MR, Russell M, Mudar P. Drinking to regulate positive and negative emotions: A motivational model of alcohol use. Journal of Personality and Social Psychology. 1995;69(5):990–1005. doi: 10.1037//0022-3514.69.5.990. [DOI] [PubMed] [Google Scholar]
- Cox M, Klinger E. A motivational model of alcohol use. Journal of Abnormal Psychology. 1988;97:168–180. doi: 10.1037//0021-843x.97.2.168. [DOI] [PubMed] [Google Scholar]
- Croissant B, Rist F, Demmel R, Olbrich R. Alcohol-induced heart rate response dampening during aversive and rewarding stress paradigms in subjects at risk for alcoholism. International Journal of Psychophysiology. 2006;61:253–261. doi: 10.1016/j.ijpsycho.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Dishman RK, Nakamura Y, Garcia ME, Thompson RW, Dunn AL, Blair SN. Heart rate variability, trait anxiety, and perceived stress among physically fit men and women. International Journal of Psychophysiology. 2000;37:121–133. doi: 10.1016/s0167-8760(00)00085-4. [DOI] [PubMed] [Google Scholar]
- DePetrillo PB, White KV, Liu M, Hommer D, Goldman D. Effects of alcohol use and gender on the dynamics of EKG time-series data. Alcoholism: Clinical and Experimental Research. 1999;23(4):745–750. [PubMed] [Google Scholar]
- Doussard-Roosevelt JA, Porges SW, Scanlon JW, Alemi B, Scanlon KB. Vagal regulation of heart rate in the prediction of developmental outcome for very low birth weight preterm infants. Child Development. 1997;68(2):173–186. [PubMed] [Google Scholar]
- Drummond DC, Cooper T, Glautier SP. Conditioned learning in alcohol dependence: Implications for cue exposure treatment. British Journal of Addiction. 1990;85:725–743. doi: 10.1111/j.1360-0443.1990.tb01685.x. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M. Parental drinking problems and children's adjustment: Vagal regulation and emotional reactivity as pathways and moderators of risk. Journal of Abnormal Psychology. 2001;110(4):499–515. doi: 10.1037//0021-843x.110.4.499. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Harger J, Whitson SM. Exposure to interparental conflict and children's adjustment and physical health: The moderating role of vagal tone. Child Development. 2001;72(6):1617–1636. doi: 10.1111/1467-8624.00369. [DOI] [PubMed] [Google Scholar]
- Evans JM, Ziegler MG, Patwardhan AR, Ott JB, Kim CS, Leonelli FM, et al. Gender differences in autonomic cardiovascular regulation: Spectral, hormonal, and hemodynamic indexes. Journal of Applied Physiology. 2001;91(6):2611–2618. doi: 10.1152/jappl.2001.91.6.2611. [DOI] [PubMed] [Google Scholar]
- Fabes RA, Eisenberg N. Regulatory control and adults' stress-related responses to daily life events. Journal of Personality and Social Psychology. 1997;73(5):1107–1117. doi: 10.1037//0022-3514.73.5.1107. [DOI] [PubMed] [Google Scholar]
- Friedman BH, Thayer JF. Autonomic balance revisited: Panic anxiety and heart rate variability. Journal of Psychosomatic Research. 1998;44:133–151. doi: 10.1016/s0022-3999(97)00202-x. [DOI] [PubMed] [Google Scholar]
- Frone M, Cooper L, Rusell M. Stressful life events, gender, and substance use: An application of tobit regression. Psychology of Addictive Behaviors. 1994;8(2):59–69. [Google Scholar]
- Giardino N, Lehrer PM, Feldman J. The role of oscillations in self-regulation: Their contribution to homeostasis. In: Kenney D, McGuigan FJ, editors. Stress and health: Research and clinical applications. London: Harwood Publishers; 2000. pp. 27–52. [Google Scholar]
- Greeley J, Oei T. Alcohol and tension reduction. In: Leonard KE, Blane HL, editors. Psychological theories of drinking and alcoholism. 2nd. New York: Guilford Press; 1999. pp. 14–53. [Google Scholar]
- Hassett AL, Radvanski DC, Vaschillo EG, Vaschillo B, Sigal LH, Karavidas MK, et al. A pilot study of the efficacy of heart rate variability (HRV) biofeedback in patients with fibromyalgia. Applied Psychophysiology and Biofeedback. 2007;32(1):1–10. doi: 10.1007/s10484-006-9028-0. [DOI] [PubMed] [Google Scholar]
- Hoaken PNS, Campbell T, Stewart SH, Pihl RO. Effects of alcohol on cardiovascular reactivity and the mediation of aggressive behavior in adult men and women. Alcohol and Alcoholism. 2003;38(1):84–92. doi: 10.1093/alcalc/agg022. [DOI] [PubMed] [Google Scholar]
- Ingjaldsson JT, Laberg JC, Thayer JF. Reduced heart rate variability in chronic alcohol abuse: Relationship with negative mood, chronic thought suppression, and compulsive drinking. Biological Psychiatry. 2003;54:1427–1436. doi: 10.1016/s0006-3223(02)01926-1. [DOI] [PubMed] [Google Scholar]
- Iversen S, Kupfermann I, Kandel ER. Emotional States and Feelings. In: Kandel ER, Schwartz JH, Jessell TM, editors. Principles of neural science. 4th. New York: McGraw-Hill; 2000. pp. 982–996. [Google Scholar]
- Jennings JR, Kamarck T, Stewart C, Eddy M, Johnson P. Alternate cardiovascular baseline assessment techniques: Vanilla or resting baseline. Psychophysiology. 1992;29(6):742–750. doi: 10.1111/j.1469-8986.1992.tb02052.x. [DOI] [PubMed] [Google Scholar]
- Jones MK, Jones BM. Ethanol metabolism in women taking oral contraceptives. Alcoholism: Clinical and Experimental Research. 1984;8(1):24–28. doi: 10.1111/j.1530-0277.1984.tb05026.x. [DOI] [PubMed] [Google Scholar]
- Karavidas MK, Lehrer PM, Vaschillo E, Vaschillo B, Marin H, Buyske S, et al. Preliminary results of an open label study of heart rate variability biofeedback for the treatment of major depression. Applied Psychophysiology and Biofeedback. 2007;32(1):19–30. doi: 10.1007/s10484-006-9029-z. [DOI] [PubMed] [Google Scholar]
- Katz LF, Gottman JM. Vagal tone protects children from marital conflict. Development and Psychopathology. 1995;7(1):83–92. [Google Scholar]
- Katz LF, Gottman JM. Buffering children from marital conflict and dissolution. Journal of Clinical Child Psychology. 1997;26(2):157–171. doi: 10.1207/s15374424jccp2602_4. [DOI] [PubMed] [Google Scholar]
- Koskinen P, Virolainen J, Kupari M. Acute alcohol intake decreases short-term heart rate variability in healthy subjects. Clinical Science. 1994;87(2):225–230. doi: 10.1042/cs0870225. [DOI] [PubMed] [Google Scholar]
- Lammers SM, Mainzer DE, Breteler MH. Do alcohol pharmacokinetics in women vary due to the menstrual cycle? Addiction. 1995;90(1):23–30. doi: 10.1046/j.1360-0443.1995.901235.x. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Instruction manual and affective ratings (Technical Report A-4) Gainesville, FL: The Center for Research in Psychophysiology, University of Florida; 2001. [Google Scholar]
- Lehrer PM, Vaschillo E, Vaschillo B, Lu SE, Eckberg DL, Edelberg R, et al. Heart rate variability biofeedback increases baroreflex gain and peak expiratory flow. Psychosomatic Medicine. 2003;65(5):796–805. doi: 10.1097/01.psy.0000089200.81962.19. [DOI] [PubMed] [Google Scholar]
- Lehrer PM, Vaschillo E, Vaschillo B, Lu SE, Scardella A, Siddique M, et al. Biofeedback treatment for asthma. Chest. 2004;126(2):352–361. doi: 10.1378/chest.126.2.352. [DOI] [PubMed] [Google Scholar]
- Levenson RW, Oyama ON, Meek PS. Greater reinforcement from alcohol for those at risk: Parental risk, personality risk, and sex. Journal of Abnormal Psychology. 1987;96:242–253. doi: 10.1037//0021-843x.96.3.242. [DOI] [PubMed] [Google Scholar]
- Martin CS, Sayette MA. Experimental design in alcohol administration research: Limitations and alternatives in the manipulation of dosage-set. Journal of Studies on Alcohol. 1993;54(6):750–761. doi: 10.15288/jsa.1993.54.750. [DOI] [PubMed] [Google Scholar]
- Mauss IB, Levenson RW, McCarter L, Wilhelm FH, Gross JJ. The tie that binds? Coherence among emotion experience, behavior, and physiology. Emotion. 2005;5(2):175–190. doi: 10.1037/1528-3542.5.2.175. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. Profile of Mood States Manual. North Tonawanda, NY: Multi-Health Systems; 1992. [Google Scholar]
- Murata K, Araki S, Yokoyama K, Sata F, Yamashita K, Ono Y. Autonomic neurotoxicity of alcohol assessed by heart rate variability. Journal of the Autonomic Nervous System. 1994;48(2):105–111. doi: 10.1016/0165-1838(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Nahshoni E, Aravot D, Aizenberg D, Sigler M, Zalsman G, Strasberg B, et al. Heart rate variability in patients with major depression. Psychosomatics. 2004;45:129–134. doi: 10.1176/appi.psy.45.2.129. [DOI] [PubMed] [Google Scholar]
- Nesic J, Duka T. Gender specific effects of a mild stressor on alcohol cue reactivity in heavy social drinkers. Pharmacology, Biochemistry and Behavior. 2006;83:239–248. doi: 10.1016/j.pbb.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Nickel P, Nachreiner F. Sensitivity and diagnosticity of the 0.1-Hz component of heart rate variability as an indicator of mental workload. Human Factors. 2003;45:575–590. doi: 10.1518/hfes.45.4.575.27094. [DOI] [PubMed] [Google Scholar]
- O'Connor MF, Allen JJB, Kaszniak AW. Autonomic and emotion regulation in bereavement and depression. Journal of Psychosomatic Research. 2002;52:182–185. doi: 10.1016/s0022-3999(02)00292-1. [DOI] [PubMed] [Google Scholar]
- Pandina RJ, Labouvie EW, White HR. Potential contribution of the life span development approach to the study of adolescent alcohol and drug use: The Rutgers Health and Human Development Project, a working model. Journal of Drug Issues. 1984;14:253–265. [Google Scholar]
- Pollatos O, Herbert BM, Matthias E, Schandry R. Heart rate response after emotional picture presentation is modulated by interoceptive awareness. International Journal of Psychophysiology. 2007;63:117–124. doi: 10.1016/j.ijpsycho.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biological Psychology. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Bates ME, Bly BM. Alcohol's dissociation of implicit and explicit memory processes: Implication of a parallel distributed processing model of semantic priming. Experimental & Clinical Psychopharmacology. 2004;12:118–125. doi: 10.1037/1064-1297.12.2.118. [DOI] [PubMed] [Google Scholar]
- Reed SF, Porges SW, Newlin DB. Effect of alcohol on vagal regulation of cardiovascular function: Contributions of the polyvagal theory to the psychophysiology of alcohol. Experiment and Clinical Psychopharmacology. 1999;7(4):484–492. doi: 10.1037//1064-1297.7.4.484. [DOI] [PubMed] [Google Scholar]
- Rossinen J, Viitasalo M, Partanen J, Koskinen P, Kupari M, Nieminen MS. Effects of acute alcohol ingestion on heart rate variability in patients with documented coronary artery disease and stable angina pectoris. American Journal of Cardiology. 1997;79:487–491. doi: 10.1016/s0002-9149(96)00790-4. [DOI] [PubMed] [Google Scholar]
- Rossy LA, Thayer JF. Fitness and gender-related differences in heart period variability. Psychosomatic Medicine. 1998;60(6):773–81. doi: 10.1097/00006842-199811000-00022. [DOI] [PubMed] [Google Scholar]
- Rubonis AV, Colby SM, Monti PM, Rohsenow DJ, Gulliver SB, Sirota AD. Alcohol cue reactivity and mood induction in male and female alcoholics. Journal of Studies on Alcohol. 1994;55(4):487–494. doi: 10.15288/jsa.1994.55.487. [DOI] [PubMed] [Google Scholar]
- San Jose B, van Oers HAM, van de Mheen HD, Garretsen HFL, Mackenback JP. Stressors and alcohol consumption. Alcohol and Alcoholism. 2000;35:307–312. doi: 10.1093/alcalc/35.3.307. [DOI] [PubMed] [Google Scholar]
- SAS Institute. SAS for Windows [Computer program] Cary, NC: Author; 2002-2006. [Google Scholar]
- Sayette MA. Heart rate as an index of stress response in alcohol administration research: A critical review. Alcoholism: Clinical and Experimental Research. 1993;17:802–809. doi: 10.1111/j.1530-0277.1993.tb00845.x. [DOI] [PubMed] [Google Scholar]
- Schmidt H, Muller-Werdan U, Hoffmann T, Francis DP, Piepoli MF, Rauchhaus M, et al. Autonomic dysfunction predicts mortality in patients with multiple organ dysfunction syndrome of different age groups. Critical Care Medicine. 2005;33(9):1994–2002. doi: 10.1097/01.ccm.0000178181.91250.99. [DOI] [PubMed] [Google Scholar]
- Sinha R, Robinson J, O'Malley S. Stress response dampening: Effects of gender and family history of alcoholism and anxiety disorders. Psychopharmacology. 1998;137:311–320. doi: 10.1007/s002130050624. [DOI] [PubMed] [Google Scholar]
- Stein PK, Barzilay J, Domitrovich PP, Chaves PHM, Gottdiener J, Rich MW, et al. Abnormal heart rate variability is a powerful independent predictor of long-term cardiovascular mortality in the elderly: The cardiovascular health study. Journal of the American College of Cardiology. 2006;47(4):28A–29A. [Google Scholar]
- Stritzke WG, Breiner MJ, Curtin JJ, Lang AR. Assessment of substance cue reactivity: Advances in reliability, specificity, and validity. Psychology of Addictive Behaviors. 2004;18(2):148–159. doi: 10.1037/0893-164X.18.2.148. [DOI] [PubMed] [Google Scholar]
- Strizke WG, Patrick CJ, Lang AR. Alcohol and human emotion: A multidimensional analysis incorporating startle-probe methodology. Journal of Abnormal Psychology. 1995;104(1):114–122. doi: 10.1037//0021-843x.104.1.114. [DOI] [PubMed] [Google Scholar]
- Sutker PB, Goist KC, Jr, King AR. Acute alcohol intoxication in women: Relationship to dose and menstrual cycle phase. Alcoholism: Clinical and Experimental Research. 1987;11(1):74–79. doi: 10.1111/j.1530-0277.1987.tb01266.x. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Cheung EH, Brown GG, Frank LR, Paulus MP, Schweinsburg AD, et al. Neural response to alcohol stimuli in adolescents with alcohol use disorder. Archives of General Psychiatry. 2003;60(7):727–735. doi: 10.1001/archpsyc.60.7.727. [DOI] [PubMed] [Google Scholar]
- Taylor JA, Carr DI, Myers CW, Eckberg DL. Mechanisms underlying very-low-frequency RR-interval oscillations in humans. Circulation. 1998;98:547–555. doi: 10.1161/01.cir.98.6.547. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Friedman BH, Borkovec TD. Autonomic characteristics of generalized anxiety disorder and worry. Biological Psychiatry. 1996;39:255–266. doi: 10.1016/0006-3223(95)00136-0. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Hall M, Sollers JJ, III, Fischer JE. Alcohol use, urinary cortisol, and heart rate variability in apparently healthy men: Evidence for impaired inhibitory control of the HPA axis in heavy drinkers. International Journal of Psychophysiology. 2006;59(3):244–250. doi: 10.1016/j.ijpsycho.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders. 2000;61:201–216. doi: 10.1016/s0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- Tiffany ST. Potential functions of classical conditioning in drug addiction. In: Drummond DC, Tiffany ST, Glautier S, Remington B, editors. Addictive behavior: Cue exposure theory and practice. New York: John Wiley & Sons; 1995. pp. 47–71. [Google Scholar]
- Tracy JI, Bates ME. The selective effects of alcohol on automatic and effortful memory processes. Neuropsychology. 1999;13:282–290. doi: 10.1037//0894-4105.13.2.282. [DOI] [PubMed] [Google Scholar]
- Umetani K, Singer DH, McCraty R, Atkinson M. Twenty-four-hour time domain heart rate variability and heart rate: Relation to age and gender over nine decades. Journal of American College of Cardiology. 1998;31(3):593–601. doi: 10.1016/s0735-1097(97)00554-8. [DOI] [PubMed] [Google Scholar]
- Vaschillo EG, Bates ME, Vaschillo B, Lehrer P, Udo T, Mun EY, et al. Heart rate variability response to alcohol, placebo, and emotional picture cue challenges: Effects of 0.1-Hz stimulation. Psychophysiology. 2008;45:847–858. doi: 10.1111/j.1469-8986.2008.00673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaschillo E, Lehrer P, Rishe N, Konstantinov M. Heart rate variability biofeedback as a method for assessing baroreflex function: A preliminary study of resonance in the cardiovascular system. Applied Psychophysiology and Biofeedback. 2002;27:1–27. doi: 10.1023/a:1014587304314. [DOI] [PubMed] [Google Scholar]
- Vaschillo EG, Vaschillo B, Lehrer PM. Characteristics of resonance in heart rate variability stimulated by biofeedback. Applied Psychophysiology and Biofeedback. 2006;31(2):129–142. doi: 10.1007/s10484-006-9009-3. [DOI] [PubMed] [Google Scholar]
- Walitzer KS, Sher KJ. Cue reactivity and ad lib drinking in young men at risk for alcoholism. Addictive Behaviors. 1990;15(1):29–46. doi: 10.1016/0306-4603(90)90005-i. [DOI] [PubMed] [Google Scholar]
- Yokoyama A, Takagi T, Ishii H, Muramatsu T, Akai J, Kato S, et al. Impaired autonomic nervous system in alcoholics assessed by heart rate variation. Alcoholism: Clinical and Experimental Research. 1991;15:761–765. doi: 10.1111/j.1530-0277.1991.tb00595.x. [DOI] [PubMed] [Google Scholar]