Abstract
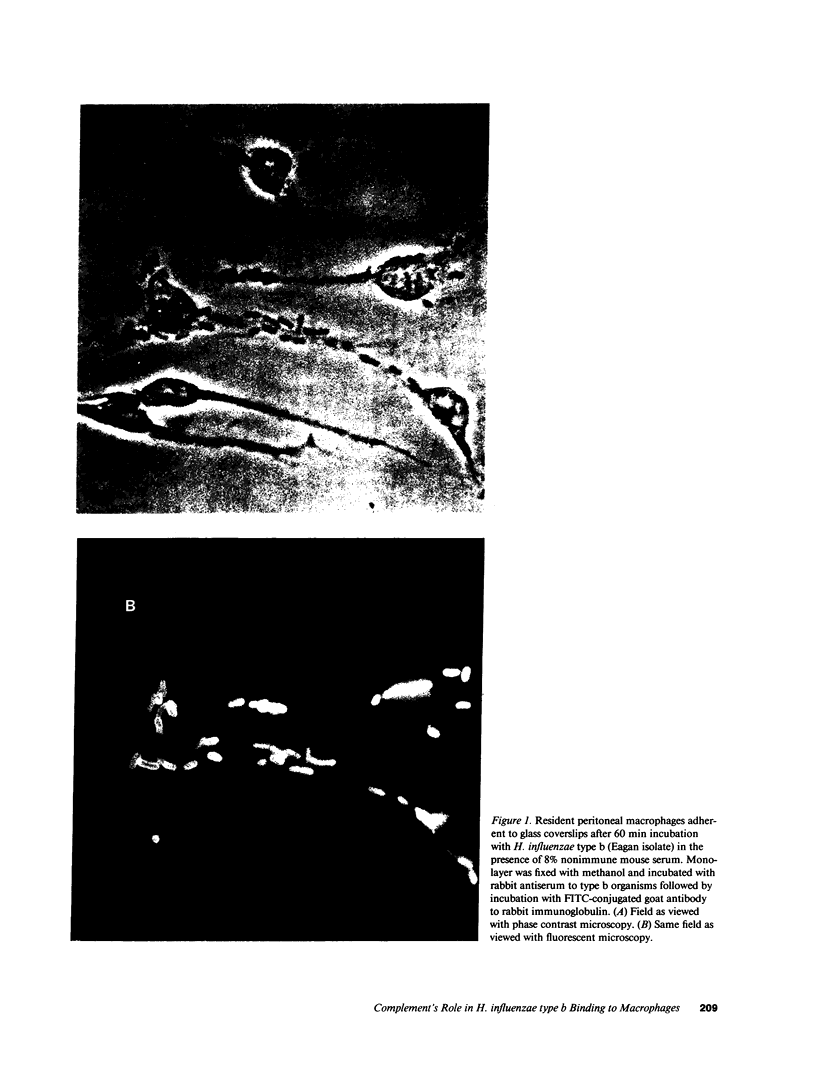
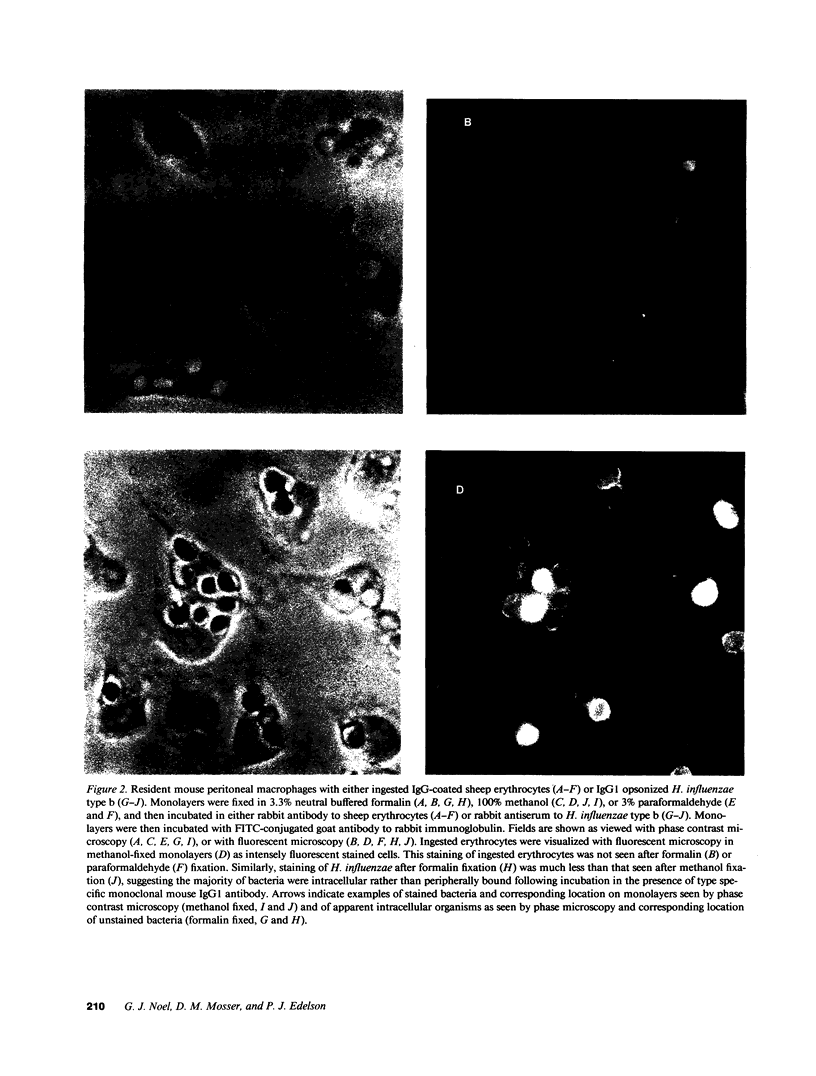
Previous in vivo studies demonstrated that clearance of encapsulated Haemophilus influenzae from blood is associated with the deposition of C3 on these bacteria and is independent of the later complement components (C5-C9). Since clearance of encapsulated bacteria is determined by phagocytosis of bacteria by fixed tissue macrophages, we studied the interaction of H. influenzae type b with macrophages in vitro. Organisms bound to macrophages in the presence of nonimmune serum. Binding was not evident in heat-treated serum or in serum from complement depleted animals and was inhibited by F(ab')2 fragments of antibody to C3 and by blockade of the macrophage complement receptor type 3. The majority of organisms bound in the presence of complement alone remained extracellular. Antibody in the form of convalescent serum or an IgG1 monoclonal to type b capsule did not increase the total number of organisms associated with macrophages, but did increase the number of organisms ingested. Furthermore, complement enhanced antibody-mediated ingestion. This in vitro study demonstrates that complement largely mediates binding of H. influenzae to macrophages. This binding may be critical in determining the early clearance of these bacteria from blood and may be an important mechanism of defense in the nonimmune, as well as the immune host.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cates K. L., Marsh K. H., Granoff D. M. Serum opsonic activity after immunization of adults with Haemophilus influenzae type b-diphtheria toxoid conjugate vaccine. Infect Immun. 1985 Apr;48(1):183–189. doi: 10.1128/iai.48.1.183-189.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cates K. L. Serum opsonic activity for Haemophilus influenzae type b in infants immunized with polysaccharide-protein conjugate vaccines. J Infect Dis. 1985 Nov;152(5):1076–1077. doi: 10.1093/infdis/152.5.1076. [DOI] [PubMed] [Google Scholar]
- Crosson F. J., Jr, Winkelstein J. A., Moxon E. R. Participation of complement in the nonimmune host defense against experimental Haemophilus influenzae type b septicemia and meningitis. Infect Immun. 1976 Oct;14(4):882–887. doi: 10.1128/iai.14.4.882-887.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlenberger A. G., Nussenzweig V. The role of membrane receptors for C3b and C3d in phagocytosis. J Exp Med. 1977 Feb 1;145(2):357–371. doi: 10.1084/jem.145.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D. L., Johnson G. M., Hostetter M. K. Ligand-receptor interactions in the phagocytosis of virulent Streptococcus pneumoniae by polymorphonuclear leukocytes. J Infect Dis. 1986 Oct;154(4):619–626. doi: 10.1093/infdis/154.4.619. [DOI] [PubMed] [Google Scholar]
- Griffin F. M., Jr, Bianco C., Silverstein S. C. Characterization of the macrophage receptro for complement and demonstration of its functional independence from the receptor for the Fc portion of immunoglobulin G. J Exp Med. 1975 Jun 1;141(6):1269–1277. doi: 10.1084/jem.141.6.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A., Silverstein S. C. Influence of the Escherichia coli capsule on complement fixation and on phagocytosis and killing by human phagocytes. J Clin Invest. 1980 Jan;65(1):82–94. doi: 10.1172/JCI109663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosea S. W., Brown E. J., Frank M. M. The critical role of complement in experimental pneumococcal sepsis. J Infect Dis. 1980 Dec;142(6):903–909. doi: 10.1093/infdis/142.6.903. [DOI] [PubMed] [Google Scholar]
- Kinoshita T., Takeda J., Hong K., Kozono H., Sakai H., Inoue K. Monoclonal antibodies to mouse complement receptor type 1 (CR1). Their use in a distribution study showing that mouse erythrocytes and platelets are CR1-negative. J Immunol. 1988 May 1;140(9):3066–3072. [PubMed] [Google Scholar]
- Mosser D. M., Edelson P. J. The mouse macrophage receptor for C3bi (CR3) is a major mechanism in the phagocytosis of Leishmania promastigotes. J Immunol. 1985 Oct;135(4):2785–2789. [PubMed] [Google Scholar]
- Moxon E. R., Smith A. L., Averill D. R., Smith D. H. Haemophilus influenzae meningitis in infant rats after intranasal inoculation. J Infect Dis. 1974 Feb;129(2):154–162. doi: 10.1093/infdis/129.2.154. [DOI] [PubMed] [Google Scholar]
- Musher D. M., Watson D. A., Lepow M. L., McVerry P., Hamill R., Baughn R. E. Vaccination of 18-month-old children with conjugated polyribosyl ribitol phosphate stimulates production of functional antibody to Haemophilus influenzae type b. Pediatr Infect Dis J. 1988 Mar;7(3):156–159. doi: 10.1097/00006454-198803000-00004. [DOI] [PubMed] [Google Scholar]
- Musher D., Goree A., Murphy T., Chapman A., Zahradnik J., Apicella M., Baughn R. Immunity to Haemophilus influenzae type b in young adults: correlation of bactericidal and opsonizing activity of serum with antibody to polyribosylribitol phosphate and lipooligosaccharide before and after vaccination. J Infect Dis. 1986 Dec;154(6):935–943. doi: 10.1093/infdis/154.6.935. [DOI] [PubMed] [Google Scholar]
- Newman S. L., Mikus L. K. Deposition of C3b and iC3b onto particulate activators of the human complement system. Quantitation with monoclonal antibodies to human C3. J Exp Med. 1985 Jun 1;161(6):1414–1431. doi: 10.1084/jem.161.6.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel G. J., Katz S., Edelson P. J. Complement-mediated early clearance of Haemophilus influenzae type b from blood is independent of serum lytic activity. J Infect Dis. 1988 Jan;157(1):85–90. doi: 10.1093/infdis/157.1.85. [DOI] [PubMed] [Google Scholar]
- Paul M. S., Aegerter M., O'Brien S. E., Kurtz C. B., Weis J. H. The murine complement receptor gene family. Analysis of mCRY gene products and their homology to human CR1. J Immunol. 1989 Jan 15;142(2):582–589. [PubMed] [Google Scholar]
- Payne N. R., Horwitz M. A. Phagocytosis of Legionella pneumophila is mediated by human monocyte complement receptors. J Exp Med. 1987 Nov 1;166(5):1377–1389. doi: 10.1084/jem.166.5.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelton S. I., Shurin P. A., Klein J. O., Finland M. Quantitative inhibition of Haemophilus influenzae by trimethoprim/sulfamethoxazole. Antimicrob Agents Chemother. 1977 Dec;12(6):649–654. doi: 10.1128/aac.12.6.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn P. H., Crosson F. J., Jr, Winkelstein J. A., Moxon E. R. Activation of the alternative complement pathway by Haemophilus influenzae type B. Infect Immun. 1977 Apr;16(1):400–402. doi: 10.1128/iai.16.1.400-402.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROGERS D. E. Host mechanisms which act to remove bacteria from the blood stream. Bacteriol Rev. 1960 Mar;24(1):50–66. doi: 10.1128/br.24.1.50-66.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross G. D., Cain J. A., Lachmann P. J. Membrane complement receptor type three (CR3) has lectin-like properties analogous to bovine conglutinin as functions as a receptor for zymosan and rabbit erythrocytes as well as a receptor for iC3b. J Immunol. 1985 May;134(5):3307–3315. [PubMed] [Google Scholar]
- Ross S. C., Densen P. Complement deficiency states and infection: epidemiology, pathogenesis and consequences of neisserial and other infections in an immune deficiency. Medicine (Baltimore) 1984 Sep;63(5):243–273. [PubMed] [Google Scholar]
- Rubin L. G., Zwahlen A., Moxon E. R. Role of intravascular replication in the pathogenesis of experimental bacteremia due to Haemophilus influenzae type b. J Infect Dis. 1985 Aug;152(2):307–314. doi: 10.1093/infdis/152.2.307. [DOI] [PubMed] [Google Scholar]
- Schreiber J. R., Barrus V., Cates K. L., Siber G. R. Functional characterization of human IgG, IgM, and IgA antibody directed to the capsule of Haemophilus influenzae type b. J Infect Dis. 1986 Jan;153(1):8–16. doi: 10.1093/infdis/153.1.8. [DOI] [PubMed] [Google Scholar]
- Storrie B., Edelson P. J. Distribution of concanavalin A in fibroblasts: direct endocytosis versus surface capping. Cell. 1977 Jul;11(3):707–717. doi: 10.1016/0092-8674(77)90087-3. [DOI] [PubMed] [Google Scholar]
- Sutton A., Schneerson R., Kendall-Morris S., Robbins J. B. Differential complement resistance mediates virulence of Haemophilus influenzae type b. Infect Immun. 1982 Jan;35(1):95–104. doi: 10.1128/iai.35.1.95-104.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr P. I., Hosea S. W., Brown E. J., Schneerson R., Sutton A., Frank M. M. The requirement of specific anticapsular IgG for killing of Haemophilus influenzae by the alternative pathway of complement activation. J Immunol. 1982 Apr;128(4):1772–1775. [PubMed] [Google Scholar]
- Unkeless J. C. Characterization of a monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptors. J Exp Med. 1979 Sep 19;150(3):580–596. doi: 10.1084/jem.150.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D. Cellular strategies in receptor-mediated phagocytosis. Rev Infect Dis. 1985 May-Jun;7(3):395–397. doi: 10.1093/clinids/7.3.395. [DOI] [PubMed] [Google Scholar]
- Zwahlen A., Rubin L. G., Connelly C. J., Inzana T. J., Moxon E. R. Alteration of the cell wall of Haemophilus influenzae type b by transformation with cloned DNA: association with attenuated virulence. J Infect Dis. 1985 Sep;152(3):485–492. doi: 10.1093/infdis/152.3.485. [DOI] [PubMed] [Google Scholar]