Abstract
The generation of planar cell polarity (PCP) and tissue shape during morphogenesis is tightly linked, but it is not clear how. Aigouy et al. (2010) now show in the developing Drosophila wing that PCP initially has a radial orientation that becomes realigned to the proximal-distal axis of organ shape by mechanical forces mediated by Fat/Dachsous signaling.
Most tissues and organs composed of and organized as epithelial cell layers display, in addition to the common apical-basal epithelial polarity, a polarity within the epithelial plane. This is commonly referred to as planar cell polarity (PCP). Genetic studies in the fruit fly Drosophila melanogaster have established that there are two molecular systems coordinating the cellular asymmetries in the plane of tissues. These include the Frizzled/PCP signaling pathway containing the VanGogh (Vang, a.k.a Strabismus/Stbm) protein and other factors (Strutt, 2003; Seifert and Mlodzik, 2007) and a pathway mediated by the protocadherins Fat and Dachsous (Lawrence et al., 2007). Although the molecular relationships between these two systems are unclear, there is strong evidence that they act in parallel, likely affect different effectors and may compensate for each other in some tissues (Lawrence et al., 2007; Wu and Mlodzik, 2009).
In the developing Drosophila wing, cellular asymmetries in the plane of the epithelium are first detected at later pupal stages along the proximal-distal axis (at around 24–30 hours after puparium formation). The core Frizzled/PCP factors form two distinct complexes that become localized asymmetrically to either the proximal (Vang/Stbm and associated proteins) or the distal side of pupal wing cells (Frizzled and associated proteins). These complexes are stabilized by feedback loop interactions among themselves (Seifert and Mlodzik, 2007; Strutt, 2003). Moreover, Frizzled and Vang/Stbm protein complexes may be required earlier to coordinate global tissue polarity/PCP within the wing epithelium (Classen et al., 2005; Wu and Mlodzik, 2009).
Supporting this idea, the new study by Aigouy et al. (2010) in this issue of Cell helps to establish that subcellular asymmetries among the Frizzled/PCP core group proteins are already present at early pupal stages during wing development (14–15 hours after puparium formation or earlier). Strikingly, the Frizzled/PCP complexes display radial polarity that is perpendicular to the wing margin (Figure 1A), confirming that coordination of global Frizzled/PCP signaling is established early in pupal fly wings. The authors further demonstrate that these early asymmetries indeed depend on Frizzled-Vang/Stbm signaling as the non-autonomous behavior of frizzled mutant cell patches (clones) affecting the polarity of wild-type cells flanking the frizzled mutant cells (Vinson and Adler, 1987) is already observed at this stage. Strikingly, in contrast to the non-autonomous effects observed at the distal side of frizzled mutant cell patches in late pupal and adult wings (Vinson and Adler, 1987), early frizzled clones influence the polarity of wild-type cells residing between the wing margin and the clone within the radial polarity axis. This confirms a “signaling axis” towards the wing margin at early stages. Taken together, the observations of Aigouy et al. (2010) indicate that (1) PCP, mediated by Frizzled-Vang/Stbm signaling, is established during late-larval/early-pupal stages in a radial axis perpendicular to the margin, and (2) the polarity/PCP seen in the adult wing is a result of cellular rearrangements during wing morphogenesis within the proximal-distal axis that are dependent on Dachsous.
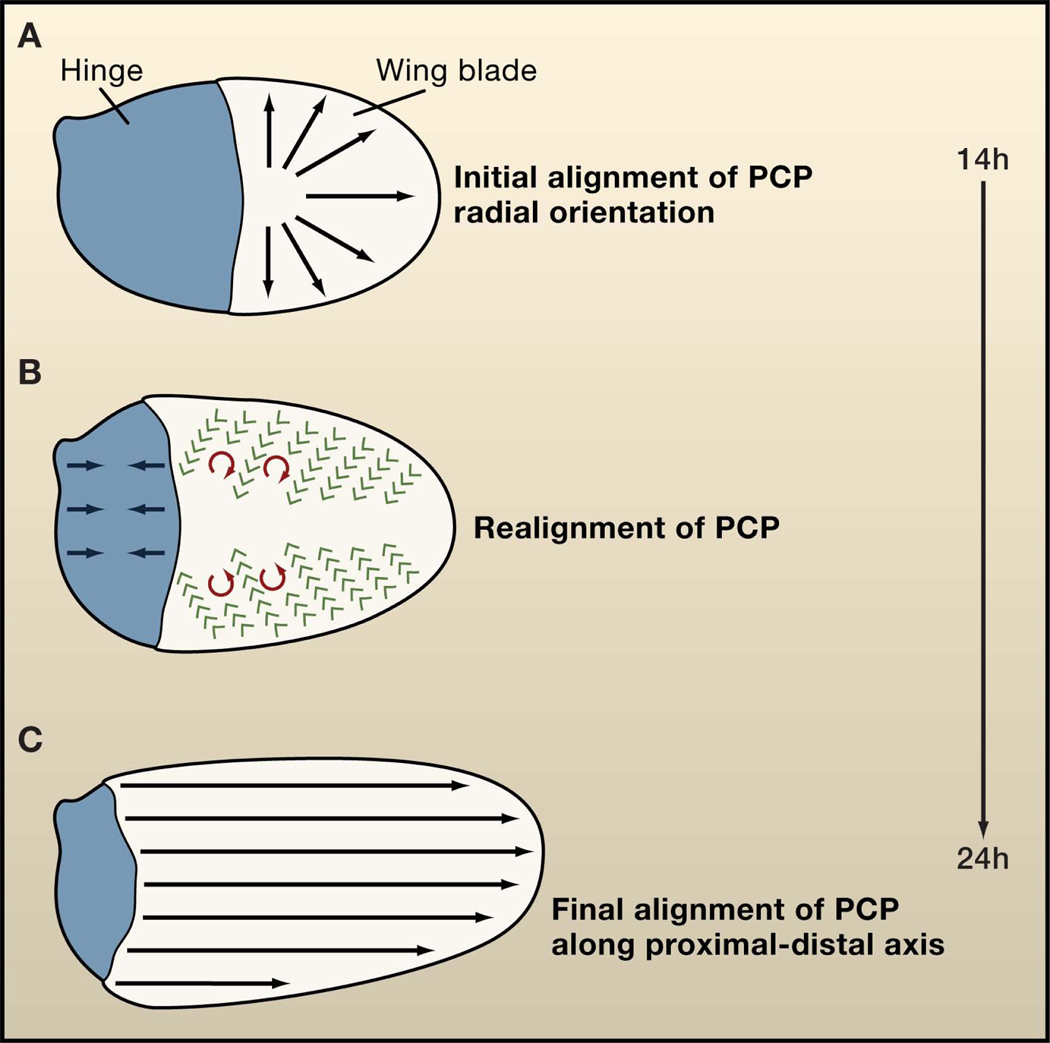
Figure 1. Planar cell polarity in the fly wing.
(A) During fly pupal development, the initial axis of planar cell polarity (PCP) is radial, that is, oriented towards the wing margin (black arrows). (B) As development proceeds, the hinge region (blue) contracts creating an anisotropic mechanical stress on the wing blade resulting in movements of wing cells and realignment of PCP to the proximal-distal axis. (C) The final orientation of PCP is in the proximal-distal axis in late pupal/adult fly wings (black arrows). Green arrows indicate the direction of cell movement, and red arrows show the direction of cellular rotations. These processes take place between 14 and 24 hours after puparium formation.
How is polarity realigned along the proximal-distal axis as morphogenesis proceeds? As PCP is already established at early stages, its final alignment from the radial orientation to the proximal-distal axis must be redirected through active relocalization of Frizzled and Vang/Stbm complexes and/or through the shifting or moving of the cells as a whole. For example, polarity could be achieved by rotating the cells toward the distal axis as happens with the rotation of photoreceptor cell clusters (ommatidia) in the Drosophila eye towards the anterior-posterior axis (Seifert and Mlodzik, 2007). But how would such a rotation be regulated? At early stages of pupal development, the proximal half of the wing epithelium (hinge) and the wing blade are similar in size (Figure 1A). Subsequently, preceding and coinciding with PCP realignment, the hinge contracts and generates an anisotropic mechanical stress on the blade, which leads to its elongation in the proximal-distal axis (Figure 1B). Strikingly, epithelial cell elongation could drive the realignment of cortical microtubules with the proximal-distal axis, which appears essential for the delivery of Frizzled to the distal side of cells (Shimada et al., 2006). New work appearing in Developmental Cell by Uemura and colleagues (Harumoto et al., 2010) shows that prior to hinge contraction, cortical microtubules align perpendicular to the margin in the proximal region of the wing blade. This supports a general role for the orientation of cortical microtubules in PCP and in the realignment of PCP later in development.
Quantitative analyses of time-lapse imaging of the pupal wing by Aigouy et al. (2010) show that, in response to the anisotropic stress, cells move with respect to each other in a proximal direction and inwards with different velocities (Figure 1B). This behavior causes shear and the local rotation of cells, mainly clockwise in the anterior and anticlockwise in the posterior half of the blade (Figure 1B). As a consequence, PCP is reoriented from a radial to a proximal-distal axis within the wing (Figure 1C). Interestingly, during this remodeling process, the global coordination/long-range coherence of PCP is diminished, which may be the reason why previous studies of PCP during development have missed the early asymmetry/polarity of PCP core proteins. As pupal wing cells repack as they acquire a hexagonal shape, the global coordination/long-range coherence of PCP increases again. This phenomenon is a consequence of the persistence of the Vang/Stbm and Frizzled complexes at boundaries formed in the early stages of development and of the proximal-distal alignment of new boundaries. On the other hand, the Frizzled/PCP core factors are required for hexagonal cell packing, probably by polarizing membrane trafficking along the proximal-distal axis (Classen et al., 2005). Thus, both early polarity and cellular packing would feed in to one another to shape and repolarize the epithelia.
It has been proposed that the Fat/Dachsous system would provide “global cues” that orient the initial polarity of Frizzled/PCP complexes, but this hypothesis has been challenged by strong genetic evidence showing that the Fat/Dachsous and Frizzled/PCP systems act in parallel (Lawrence et al., 2007). During fly larval stages, the Fat/Dachsous system is required to regulate the growth and shape of the wing; the latter (at least in part) by orienting the axis of cell division perpendicular to the margin (Baena-Lopez et al., 2005). Aigouy et al. (2010) show that high expression of Dachsous in the hinge region is not required for its contraction, but correct Dachsous levels in the wing blade are required for the wing blade to respond to the anisotropic mechanical stress that orients cell elongation along the proximal-distal axis. Moreover, cell polarity defects correlate with the inversion of local tissue rotation in wild-type wings, with altered levels of Dachsous in the posterior compartment, suggesting that Dachsous imbues cells with the ability to respond coordinately to mechanical stress. Interestingly, Harumoto et al. (2010) show that Dachsous and Fat are required to align cortical microtubules along the proximal-distal axis and that Dachsous biases the direction of microtubule growth from high to low Dachsous levels, similar to its role in cell orientation. Whether the Fat/Dachsous system regulates cell remodeling by controlling the polarity of cortical microtubules or vice versa remains to be resolved.
In their elegant new study, Aigouy et al. (2010) analyzed the timeline of events for the establishment of PCP in the developing Drosophila wing. They have provided evidence that the early Frizzled/PCP core polarization towards the wing margin (in a radial orientation) is realigned along the proximal-distal axis by anisotropic mechanical stress and Dachsous-mediated tissue remodeling. These conclusions are consistent with, and supported by, the phenotypic PCP features of Frizzled/PCP core group genes on one side and that of the Fat/Dachsous system on the other. Flies carrying mutations in Frizzled/PCP core proteins exhibit defects in PCP throughout the wing. In contrast, the Fat/Dachsous system mainly affects polarity in the proximal half of the wing, as this area strongly depends on cellular realignment and rotation during the switch to the proximal-distal PCP axis. Together, these observations provide an exciting new framework for understanding the generation of PCP and its relation to new mechanisms that sculpt the shape of organs in general.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aigouy B, Farhadifar R, Staple DB, Sagner A, Röper J, Jüulicher F, Eaton S. Cell. 2010 doi: 10.1016/j.cell.2010.07.042. in press. [DOI] [PubMed] [Google Scholar]
- Baena-Lopez LA, Baonza A, Garcia-Bellido A. Curr Biol. 2005;15:1640–1644. doi: 10.1016/j.cub.2005.07.062. [DOI] [PubMed] [Google Scholar]
- Classen AK, Anderson KI, Marois E, Eaton S. Dev Cell. 2005;9:805–817. doi: 10.1016/j.devcel.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Harumoto T, Ito M, Shimada Y, Kobayashi TJ, Ueda HR, Lu B, Uemura T. Developmental Cell. 2010 doi: 10.1016/j.devcel.2010.08.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence PA, Struhl G, Casal J. Nat Rev Genet. 2007;8:555–563. doi: 10.1038/nrg2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert JR, Mlodzik M. Nat Rev Genet. 2007;8:126–138. doi: 10.1038/nrg2042. [DOI] [PubMed] [Google Scholar]
- Shimada Y, Yonemura S, Ohkura H, Strutt D, Uemura T. Dev Cell. 2006;10:209–222. doi: 10.1016/j.devcel.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Strutt D. Development. 2003;130:4501–4513. doi: 10.1242/dev.00695. [DOI] [PubMed] [Google Scholar]
- Vinson CR, Adler PN. Nature. 1987;329:549–551. doi: 10.1038/329549a0. [DOI] [PubMed] [Google Scholar]
- Wu J, Mlodzik M. Trends Cell Biol. 2009;19:295–305. doi: 10.1016/j.tcb.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]