Abstract
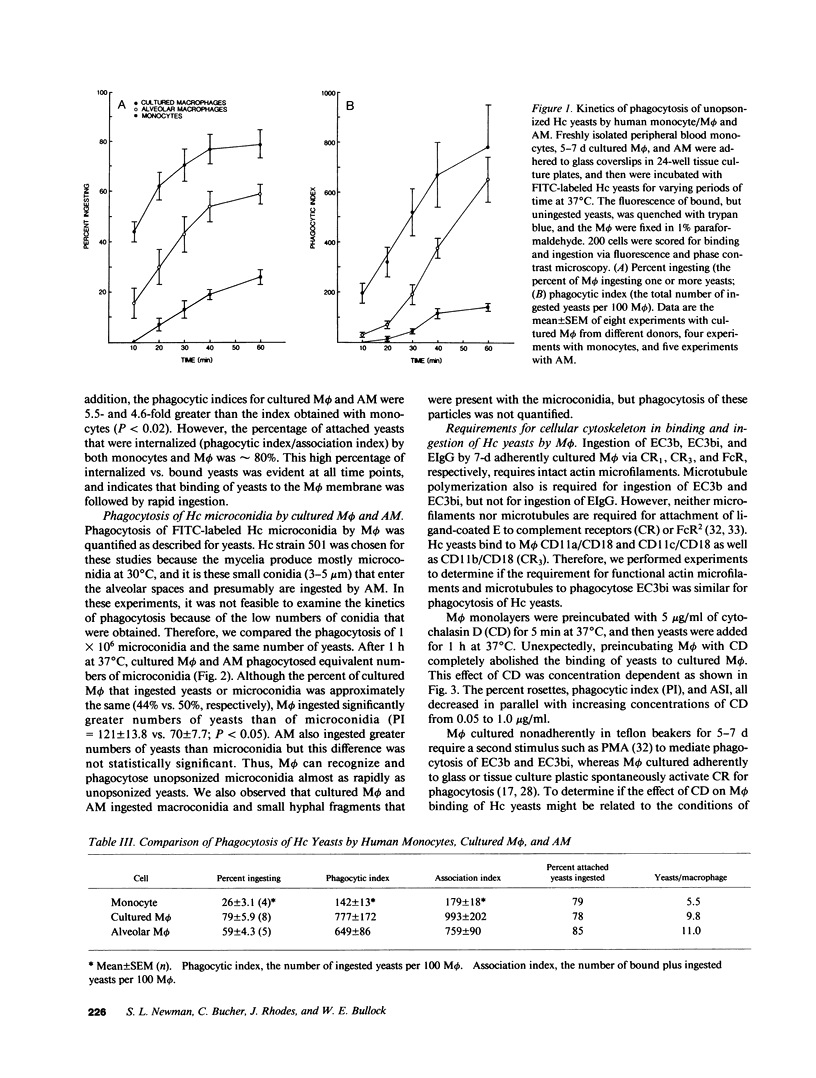
Phagocytosis of Histoplasma capsulatum (Hc) yeasts and microconidia by human macrophages (M phi) was quantified by a fluorescence quenching technique. Phagocytosis of unopsonized Hc yeasts by monocyte-derived M phi and human alveolar M phi (AM) was rapid. After 60 min, 79% of cultured M phi and 59% of AM had ingested an average of 9.8 and 11 yeasts/M phi, respectively. In contrast, only 26% of monocytes ingested 4.5 yeasts/cell after 60 min. Phagocytosis of unopsonized microconidia by cultured M phi and by AM was equivalent. Monoclonal antibodies specific for the alpha-chains and beta-chain of the CD18 family of adhesion receptors inhibited the binding of Hc yeasts and microconidia to cultured M phi and AM. Thus, the M phi CD18 complex mediates recognition of both phases of this dimorphic fungus. Disruption of actin microfilaments with cytochalasin D inhibited both attachment and ingestion of yeasts by M phi. In contrast, nocodazole, which prevents polymerization of microtubules, did not inhibit binding or ingestion. Both drugs inhibited ingestion, but neither drug inhibited binding of C3b- and C3bi-coated sheep erythrocytes to complement receptors type one (CR1) or type three (CR3), respectively. Therefore, different signal transducing mechanisms for phagocytosis appear to be triggered by the binding of Hc yeasts to CD18, and by the binding of EC3bi to CD11b/CD18, respectively.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artz R. P., Bullock W. E. Immunoregulatory responses in experimental disseminated histoplasmosis: depression of T-cell-dependent and T-effectory responses by activation of splenic suppressor cells. Infect Immun. 1979 Mar;23(3):893–902. doi: 10.1128/iai.23.3.893-902.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughman R. P., Kim C. K., Vinegar A., Hendricks D. E., Schmidt D. J., Bullock W. E. The pathogenesis of experimental pulmonary histoplasmosis. Correlative studies of histopathology, bronchoalveolar lavage, and respiratory function. Am Rev Respir Dis. 1986 Oct;134(4):771–776. doi: 10.1164/arrd.1986.134.4.771. [DOI] [PubMed] [Google Scholar]
- Berry C. L. The production of disseminated histoplasmosis in the mouse: the eff4ects of changes in reticulo-endothelial function. J Pathol. 1969 Mar;97(3):441–457. doi: 10.1002/path.1710970304. [DOI] [PubMed] [Google Scholar]
- Bullock W. E., Wright S. D. Role of the adherence-promoting receptors, CR3, LFA-1, and p150,95, in binding of Histoplasma capsulatum by human macrophages. J Exp Med. 1987 Jan 1;165(1):195–210. doi: 10.1084/jem.165.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dana N., Styrt B., Griffin J. D., Todd R. F., 3rd, Klempner M. S., Arnaout M. A. Two functional domains in the phagocyte membrane glycoprotein Mo1 identified with monoclonal antibodies. J Immunol. 1986 Nov 15;137(10):3259–3263. [PubMed] [Google Scholar]
- De Brabander M. J., Van de Veire R. M., Aerts F. E., Borgers M., Janssen P. A. The effects of methyl (5-(2-thienylcarbonyl)-1H-benzimidazol-2-yl) carbamate, (R 17934; NSC 238159), a new synthetic antitumoral drug interfering with microtubules, on mammalian cells cultured in vitro. Cancer Res. 1976 Mar;36(3):905–916. [PubMed] [Google Scholar]
- Dohn M. N., Baughman R. P. Effect of changing instilled volume for bronchoalveolar lavage in patients with interstitial lung disease. Am Rev Respir Dis. 1985 Aug;132(2):390–392. doi: 10.1164/arrd.1985.132.2.390. [DOI] [PubMed] [Google Scholar]
- Eddy A., Newman S. L., Cosio F., LeBien T., Michael A. The distribution of the CR3 receptor on human cells and tissue as revealed by a monoclonal antibody. Clin Immunol Immunopathol. 1984 Jun;31(3):371–389. doi: 10.1016/0090-1229(84)90090-4. [DOI] [PubMed] [Google Scholar]
- Fleit H. B., Wright S. D., Unkeless J. C. Human neutrophil Fc gamma receptor distribution and structure. Proc Natl Acad Sci U S A. 1982 May;79(10):3275–3279. doi: 10.1073/pnas.79.10.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gmelig-Meyling F., Waldmann T. A. Separation of human blood monocytes and lymphocytes on a continuous Percoll gradient. J Immunol Methods. 1980;33(1):1–9. doi: 10.1016/0022-1759(80)90077-0. [DOI] [PubMed] [Google Scholar]
- Griffin F. M., Jr, Mullinax P. J. Augmentation of macrophage complement receptor function in vitro. III. C3b receptors that promote phagocytosis migrate within the plane of the macrophage plasma membrane. J Exp Med. 1981 Aug 1;154(2):291–305. doi: 10.1084/jem.154.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWARD D. H. INTRACELLULAR BEHAVIOR OF HISTOPLASMA CAPSULATUM. J Bacteriol. 1964 Jan;87:33–38. doi: 10.1128/jb.87.1.33-38.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hed J., Hallden G., Johansson S. G., Larsson P. The use of fluorescence quenching in flow cytofluorometry to measure the attachment and ingestion phases in phagocytosis in peripheral blood without prior cell separation. J Immunol Methods. 1987 Jul 16;101(1):119–125. doi: 10.1016/0022-1759(87)90224-9. [DOI] [PubMed] [Google Scholar]
- Howard D. H. Further studies on the inhibition of Histoplasma capsulatum within macrophages from immunized animals. Infect Immun. 1973 Oct;8(4):577–581. doi: 10.1128/iai.8.4.577-581.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard D. H., Otto V., Gupta R. K. Lymphocyte-mediated cellular immunity in histoplasmosis. Infect Immun. 1971 Nov;4(5):605–610. doi: 10.1128/iai.4.5.605-610.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberlin C. L., Hariri A. R., Hempel H. O., Goodman N. L. Interactions between Histoplasma capsulatum and macrophages from normal and treated mice: comparison of the mycelial and yeast phases in alveolar and peritoneal macrophages. Infect Immun. 1981 Oct;34(1):6–10. doi: 10.1128/iai.34.1.6-10.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier L. L., Arnaout M. A., Schwarting R., Warner N. L., Ross G. D. p150/95, Third member of the LFA-1/CR3 polypeptide family identified by anti-Leu M5 monoclonal antibody. Eur J Immunol. 1985 Jul;15(7):713–718. doi: 10.1002/eji.1830150714. [DOI] [PubMed] [Google Scholar]
- Myones B. L., Dalzell J. G., Hogg N., Ross G. D. Neutrophil and monocyte cell surface p150,95 has iC3b-receptor (CR4) activity resembling CR3. J Clin Invest. 1988 Aug;82(2):640–651. doi: 10.1172/JCI113643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newberry W. M., Jr, Chandler J. W., Jr, Chin T. D., Kirkpatrick C. H. Immunology of the mycoses. I. Depressed lymphocyte transformation in chronic histoplasmosis. J Immunol. 1968 Feb;100(2):436–443. [PubMed] [Google Scholar]
- Newman S. L., Devery-Pocius J. E., Ross G. D., Henson P. M. Phagocytosis by human monocyte-derived macrophages. Independent function of receptors for C3b (CR1) and iC3b (CR3). Complement. 1984;1(4):213–227. [PubMed] [Google Scholar]
- Newman S. L., Musson R. A., Henson P. M. Development of functional complement receptors during in vitro maturation of human monocytes into macrophages. J Immunol. 1980 Nov;125(5):2236–2244. [PubMed] [Google Scholar]
- PROCKNOW J. J., PAGE M. I., LOOSLI C. G. Early pathogenesis of experimental histoplasmosis. Arch Pathol. 1960 Apr;69:413–426. [PubMed] [Google Scholar]
- Reynolds H. Y., Atkinson J. P., Newball H. H., Frank M. M. Receptors for immunoglobulin and complement on human alveolar macrophages. J Immunol. 1975 Jun;114(6):1813–1819. [PubMed] [Google Scholar]
- Ross G. D., Cain J. A., Lachmann P. J. Membrane complement receptor type three (CR3) has lectin-like properties analogous to bovine conglutinin as functions as a receptor for zymosan and rabbit erythrocytes as well as a receptor for iC3b. J Immunol. 1985 May;134(5):3307–3315. [PubMed] [Google Scholar]
- SALVIN S. B. Acquired resistance in experimental histoplasmosis. Trans N Y Acad Sci. 1956 Mar;18(5):462–468. doi: 10.1111/j.2164-0947.1956.tb00469.x. [DOI] [PubMed] [Google Scholar]
- Sanchez-Madrid F., Nagy J. A., Robbins E., Simon P., Springer T. A. A human leukocyte differentiation antigen family with distinct alpha-subunits and a common beta-subunit: the lymphocyte function-associated antigen (LFA-1), the C3bi complement receptor (OKM1/Mac-1), and the p150,95 molecule. J Exp Med. 1983 Dec 1;158(6):1785–1803. doi: 10.1084/jem.158.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Voorhis W. C., Steinman R. M., Hair L. S., Luban J., Witmer M. D., Koide S., Cohn Z. A. Specific antimononuclear phagocyte monoclonal antibodies. Application to the purification of dendritic cells and the tissue localization of macrophages. J Exp Med. 1983 Jul 1;158(1):126–145. doi: 10.1084/jem.158.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Levin S. M., Jong M. T., Chad Z., Kabbash L. G. CR3 (CD11b/CD18) expresses one binding site for Arg-Gly-Asp-containing peptides and a second site for bacterial lipopolysaccharide. J Exp Med. 1989 Jan 1;169(1):175–183. doi: 10.1084/jem.169.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Silverstein S. C. Receptors for C3b and C3bi promote phagocytosis but not the release of toxic oxygen from human phagocytes. J Exp Med. 1983 Dec 1;158(6):2016–2023. doi: 10.1084/jem.158.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Silverstein S. C. Tumor-promoting phorbol esters stimulate C3b and C3b' receptor-mediated phagocytosis in cultured human monocytes. J Exp Med. 1982 Oct 1;156(4):1149–1164. doi: 10.1084/jem.156.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu-Hsieh B. A., Howard D. H. Inhibition of the intracellular growth of Histoplasma capsulatum by recombinant murine gamma interferon. Infect Immun. 1987 Apr;55(4):1014–1016. doi: 10.1128/iai.55.4.1014-1016.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu-Hsieh B., Howard D. H. Inhibition of growth of Histoplasma capsulatum by lymphokine-stimulated macrophages. J Immunol. 1984 May;132(5):2593–2597. [PubMed] [Google Scholar]
- van der Meer J. W., Bulterman D., van Zwet T. L., Elzenga-Claasen I., van Furth R. Culture of mononuclear phagocytes on a teflon surface to prevent adherence. J Exp Med. 1978 Jan 1;147(1):271–276. doi: 10.1084/jem.147.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]