Abstract
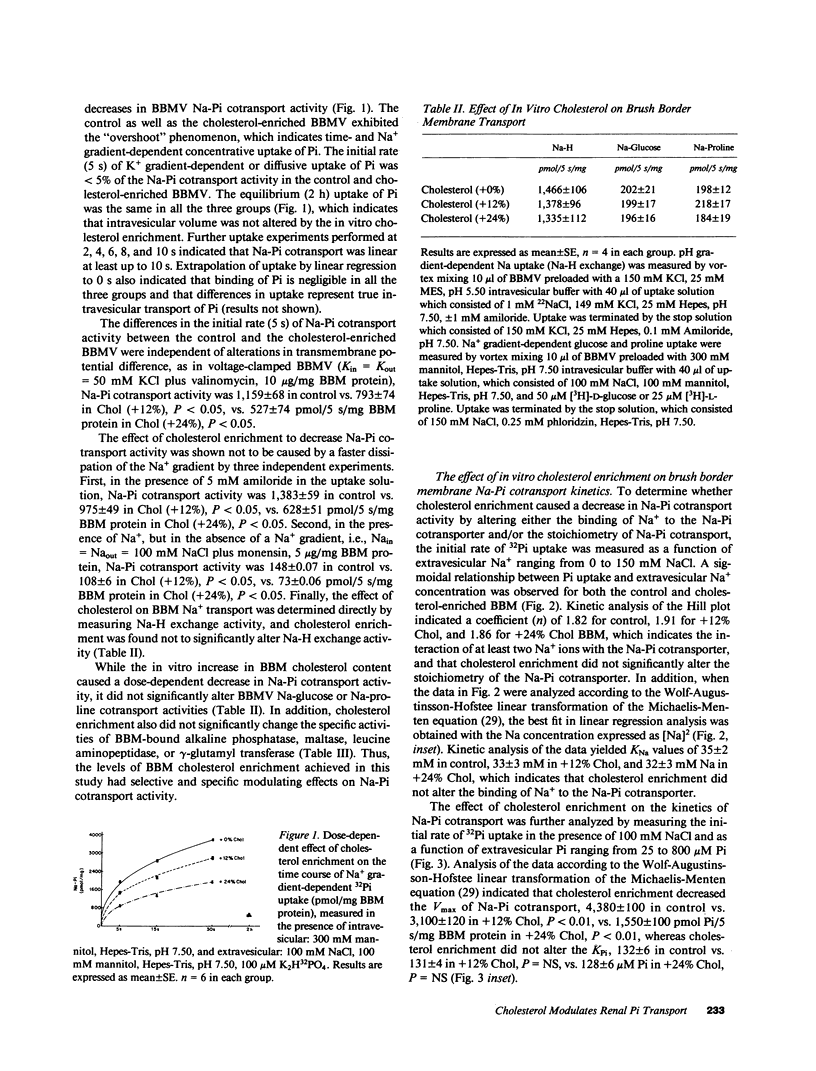
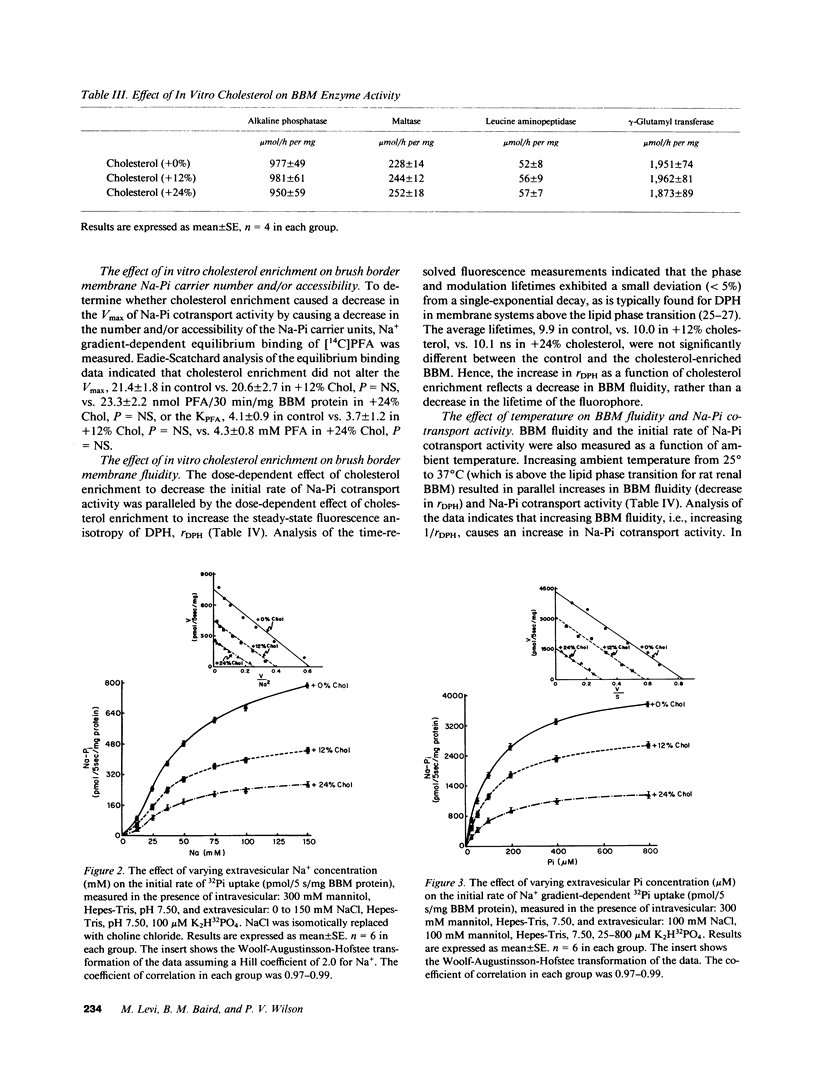
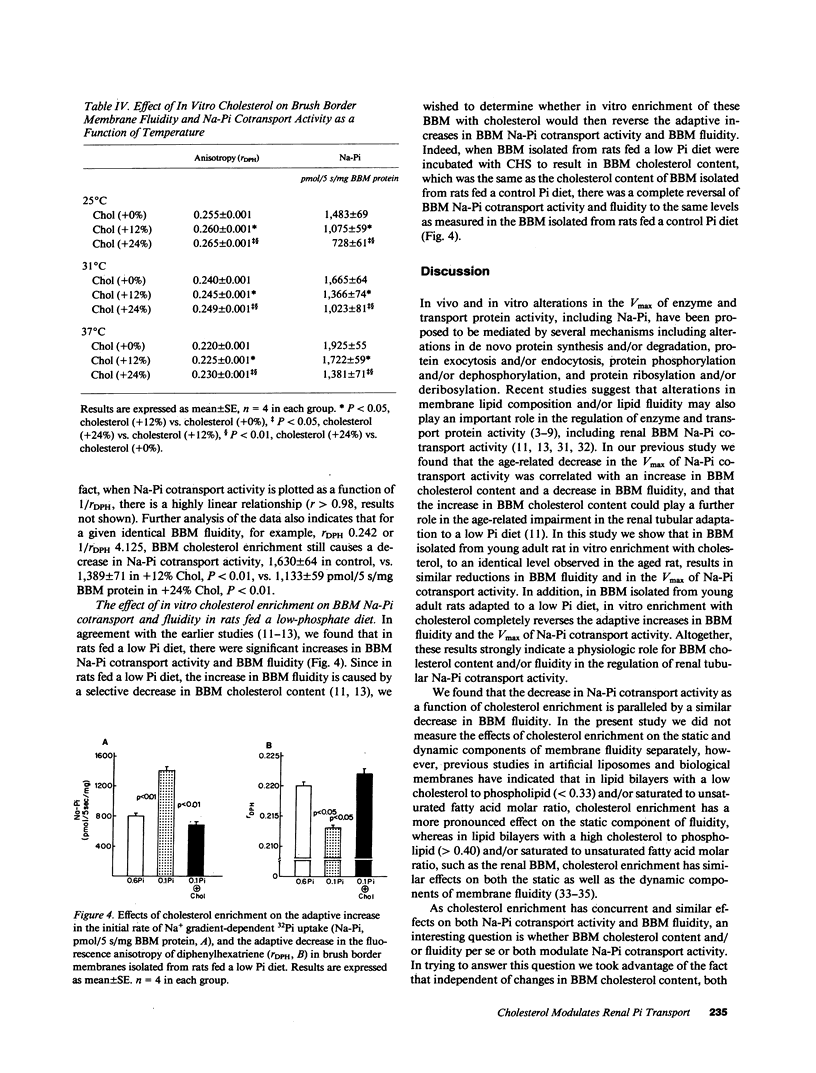
In dietary phosphate (Pi) deprivation and in aging there is an inverse correlation between renal proximal tubular brush border membrane (BBM) cholesterol (Chol) content, BBM fluidity, and BBM sodium gradient-dependent Pi transport activity (Na-Pi cotransport). The purpose of this study was to determine whether in vitro enrichment of renal BBM with Chol has a direct modulating effect on Na-Pi cotransport. 12 and 24 mol % increases in Chol content caused dose-dependent decreases in Na-Pi cotransport activity, 2,000 in control, vs. 1,450 in Chol (+12%), vs. 900 pmol/5 s/mg BBM protein in Chol (+24%), all P less than 0.01, which was paralleled by dose-dependent increases in the fluorescence anisotropy of diphenylhexatriene, rDPH, i.e., decrease in BBM fluidity, 0.203 in control, vs. 0.210 in Chol (+12%), vs. 0.219 in Chol (+24%), all P less than 0.01. We found that increasing ambient temperature, which increases BBM fluidity independent of changes in Chol content, increased Na-Pi cotransport. When Na-Pi cotransport was analyzed as a function of BBM fluidity, 1/rDPH, we found that at an equivalent BBM fluidity BBM Chol enrichment still resulted in a dose-dependent decrease in Na-Pi cotransport. Finally, in BBM isolated from rats fed a low Pi diet in vitro enrichment with Chol completely reversed the adaptive increases in Na-Pi cotransport and fluidity. Our study therefore, indicates that Chol is a direct modulator of renal BBM Na-Pi cotransport activity, and that in vivo alterations in BBM Chol content most likely plays an important role in the regulation of renal tubular Pi transport.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Alcala J. R., Gratton E., Prendergast F. G. Resolvability of fluorescence lifetime distributions using phase fluorometry. Biophys J. 1987 Apr;51(4):587–596. doi: 10.1016/S0006-3495(87)83383-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Baldassare J. J., Saito Y., Silbert D. F. Effect of sterol depletion on LM cell sterol mutants. Changes in the lipid composition of the plasma membrane and their effects on 3-O-methlglucose transport. J Biol Chem. 1979 Feb 25;254(4):1108–1113. [PubMed] [Google Scholar]
- Barrow D. A., Lentz B. R. Membrane structural domains. Resolution limits using diphenylhexatriene fluorescence decay. Biophys J. 1985 Aug;48(2):221–234. doi: 10.1016/S0006-3495(85)83775-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasitus T. A., Dahiya R., Dudeja P. K., Bissonnette B. M. Cholesterol modulates alkaline phosphatase activity of rat intestinal microvillus membranes. J Biol Chem. 1988 Jun 25;263(18):8592–8597. [PubMed] [Google Scholar]
- Carrière B., Le Grimellec C. Effects of benzyl alcohol on enzyme activities and D-glucose transport in kidney brush-border membranes. Biochim Biophys Acta. 1986 May 28;857(2):131–138. doi: 10.1016/0005-2736(86)90340-8. [DOI] [PubMed] [Google Scholar]
- Cheng L., Liang C. T., Sacktor B. Phosphate uptake by renal membrane vesicles of rabbits adapted to high and low phosphorus diets. Am J Physiol. 1983 Aug;245(2):F175–F180. doi: 10.1152/ajprenal.1983.245.2.F175. [DOI] [PubMed] [Google Scholar]
- De Smedt H., Kinne R. Temperature dependence of solute transport and enzyme activities in hog renal brush border membrane vesicles. Biochim Biophys Acta. 1981 Nov 6;648(2):247–253. doi: 10.1016/0005-2736(81)90040-7. [DOI] [PubMed] [Google Scholar]
- Deuticke B., Haest C. W. Lipid modulation of transport proteins in vertebrate cell membranes. Annu Rev Physiol. 1987;49:221–235. doi: 10.1146/annurev.ph.49.030187.001253. [DOI] [PubMed] [Google Scholar]
- Esko J. D., Raetz C. R. Mutants of Chinese hamster ovary cells with altered membrane phospholipid composition. Replacement of phosphatidylinositol by phosphatidylglycerol in a myo-inositol auxotroph. J Biol Chem. 1980 May 25;255(10):4474–4480. [PubMed] [Google Scholar]
- Fiorini R., Valentino M., Wang S., Glaser M., Gratton E. Fluorescence lifetime distributions of 1,6-diphenyl-1,3,5-hexatriene in phospholipid vesicles. Biochemistry. 1987 Jun 30;26(13):3864–3870. doi: 10.1021/bi00387a019. [DOI] [PubMed] [Google Scholar]
- Friedlander G., Shahedi M., Le Grimellec C., Amiel C. Increase in membrane fluidity and opening of tight junctions have similar effects on sodium-coupled uptakes in renal epithelial cells. J Biol Chem. 1988 Aug 15;263(23):11183–11188. [PubMed] [Google Scholar]
- Gratton E., Limkeman M. A continuously variable frequency cross-correlation phase fluorometer with picosecond resolution. Biophys J. 1983 Dec;44(3):315–324. doi: 10.1016/S0006-3495(83)84305-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illsley N. P., Lin H. Y., Verkman A. S. Lipid domain structure correlated with membrane protein function in placental microvillus vesicles. Biochemistry. 1987 Jan 27;26(2):446–454. doi: 10.1021/bi00376a016. [DOI] [PubMed] [Google Scholar]
- Ives H. E., Verkman A. S. Effects of membrane fluidizing agents on renal brush border proton permeability. Am J Physiol. 1985 Dec;249(6 Pt 2):F933–F940. doi: 10.1152/ajprenal.1985.249.6.F933. [DOI] [PubMed] [Google Scholar]
- Jameson D. M., Gratton E., Eccleston J. F. Intrinsic fluorescence of elongation factor Tu in its complexes with GDP and elongation factor Ts. Biochemistry. 1987 Jun 30;26(13):3894–3901. doi: 10.1021/bi00387a023. [DOI] [PubMed] [Google Scholar]
- Karnovsky M. J., Kleinfeld A. M., Hoover R. L., Klausner R. D. The concept of lipid domains in membranes. J Cell Biol. 1982 Jul;94(1):1–6. doi: 10.1083/jcb.94.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefee E. B., Scharschmidt B. F., Blankenship N. M., Ockner R. K. Studies of relationship among bile flow, liver plasma membrane NaK-ATPase, and membrane microviscosity in the rat. J Clin Invest. 1979 Dec;64(6):1590–1598. doi: 10.1172/JCI109620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiebzak G. M., Sacktor B. Effect of age on renal conservation of phosphate in the rat. Am J Physiol. 1986 Sep;251(3 Pt 2):F399–F407. doi: 10.1152/ajprenal.1986.251.3.F399. [DOI] [PubMed] [Google Scholar]
- Kutchai H., Chandler L. H., Zavoico G. B. Effects of cholesterol on acyl chain dynamics in multilamellar vesicles of various phosphatidylcholines. Biochim Biophys Acta. 1983 Dec 21;736(2):137–149. doi: 10.1016/0005-2736(83)90277-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lakowicz J. R., Prendergast F. G., Hogen D. Differential polarized phase fluorometric investigations of diphenylhexatriene in lipid bilayers. Quantitation of hindered depolarizing rotations. Biochemistry. 1979 Feb 6;18(3):508–519. doi: 10.1021/bi00570a021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi M., Jameson D. M., van der Meer B. W. Role of BBM lipid composition and fluidity in impaired renal Pi transport in aged rat. Am J Physiol. 1989 Jan;256(1 Pt 2):F85–F94. doi: 10.1152/ajprenal.1989.256.1.F85. [DOI] [PubMed] [Google Scholar]
- Levi M., Molitoris B. A., Burke T. J., Schrier R. W., Simon F. R. Effects of vitamin D-induced chronic hypercalcemia on rat renal cortical plasma membranes and mitochondria. Am J Physiol. 1987 Feb;252(2 Pt 2):F267–F275. doi: 10.1152/ajprenal.1987.252.2.F267. [DOI] [PubMed] [Google Scholar]
- Molitoris B. A., Alfrey A. C., Harris R. A., Simon F. R. Renal apical membrane cholesterol and fluidity in regulation of phosphate transport. Am J Physiol. 1985 Jul;249(1 Pt 2):F12–F19. doi: 10.1152/ajprenal.1985.249.1.F12. [DOI] [PubMed] [Google Scholar]
- Molitoris B. A., Kinne R. Ischemia induces surface membrane dysfunction. Mechanism of altered Na+-dependent glucose transport. J Clin Invest. 1987 Sep;80(3):647–654. doi: 10.1172/JCI113117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y., Silbert D. F. Selective effects of membrane sterol depletion on surface function thymidine and 3-O-methyl-D-glucose transport in a sterol auxotroph. J Biol Chem. 1979 Feb 25;254(4):1102–1107. [PubMed] [Google Scholar]
- Sinensky M., Pinkerton F., Sutherland E., Simon F. R. Rate limitation of (Na+ + K+)-stimulated adenosinetriphosphatase by membrane acyl chain ordering. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4893–4897. doi: 10.1073/pnas.76.10.4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotte J. P., Bierman E. L. Depletion of plasma-membrane sphingomyelin rapidly alters the distribution of cholesterol between plasma membranes and intracellular cholesterol pools in cultured fibroblasts. Biochem J. 1988 Mar 15;250(3):653–658. doi: 10.1042/bj2500653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector A. A., Yorek M. A. Membrane lipid composition and cellular function. J Lipid Res. 1985 Sep;26(9):1015–1035. [PubMed] [Google Scholar]
- Spinedi A., Rufini S., Luly P., Farias R. N. The temperature-dependence of human erythrocyte acetylcholinesterase activity is not affected by membrane cholesterol enrichment. Biochem J. 1988 Oct 15;255(2):547–551. [PMC free article] [PubMed] [Google Scholar]
- Sweet W. D., Wood W. G., Schroeder F. Charged anesthetics selectively alter plasma membrane order. Biochemistry. 1987 May 19;26(10):2828–2835. doi: 10.1021/bi00384a026. [DOI] [PubMed] [Google Scholar]
- Szczepanska-Konkel M., Yusufi A. N., Dousa T. P. Interactions of [14C]phosphonoformic acid with renal cortical brush-border membranes. Relationship to the Na+-phosphate co-transporter. J Biol Chem. 1987 Jun 15;262(17):8000–8010. [PubMed] [Google Scholar]
- Yeagle P. L. Lipid regulation of cell membrane structure and function. FASEB J. 1989 May;3(7):1833–1842. [PubMed] [Google Scholar]
- Yeagle P. L., Young J., Rice D. Effects of cholesterol on (Na+,K+)-ATPase ATP hydrolyzing activity in bovine kidney. Biochemistry. 1988 Aug 23;27(17):6449–6452. doi: 10.1021/bi00417a037. [DOI] [PubMed] [Google Scholar]
- Yuli I., Wilbrandt W., Shinitzky M. Glucose transport through cell membranes of modified lipid fluidity. Biochemistry. 1981 Jul 21;20(15):4250–4256. doi: 10.1021/bi00518a003. [DOI] [PubMed] [Google Scholar]
- van Blitterswijk W. J., van der Meer B. W., Hilkmann H. Quantitative contributions of cholesterol and the individual classes of phospholipids and their degree of fatty acyl (un)saturation to membrane fluidity measured by fluorescence polarization. Biochemistry. 1987 Mar 24;26(6):1746–1756. doi: 10.1021/bi00380a038. [DOI] [PubMed] [Google Scholar]


