Abstract
Recently, it has become clear that the actin cytoskeleton is involved in clathrin-mediated endocytosis. During clathrin-mediated endocytosis, clathrin triskelions and adaptor proteins assemble into lattices, forming clathrin-coated pits. These coated pits invaginate and detach from the membrane, a process that requires dynamic actin polymerization. We found an unexpected role for the clathrin adaptor epsin in regulating actin dynamics during this late stage of coated vesicle formation. In Dictyostelium cells, epsin is required for both the membrane recruitment and phosphorylation of the actin- and clathrin-binding protein Hip1r. Epsin-null and Hip1r-null cells exhibit deficiencies in the timing and organization of actin filaments at clathrin-coated pits. Consequently, clathrin structures persist on the membranes of epsin and Hip1r mutants and the internalization of clathrin structures is delayed. We conclude that epsin works with Hip1r to regulate actin dynamics by controlling the spatial and temporal coupling of actin filaments to clathrin-coated pits. Specific residues in the ENTH domain of epsin that are required for the membrane recruitment and phosphorylation of Hip1r are also required for normal actin and clathrin dynamics at the plasma membrane. We propose that epsin promotes the membrane recruitment and phosphorylation of Hip1r, which in turn regulates actin polymerization at clathrin-coated pits.
Keywords: Clathrin, Clathrin adaptor, Clathrin-mediated endocytosis, Cytoskeleton, ENTH
Introduction
Clathrin-mediated endocytosis is an essential cellular process involved in nutrient uptake, processing of extracellular signals, and membrane remodeling. During endocytosis, clathrin triskelions assemble together to form a coated pit that surrounds transmembrane cargo on the plasma membrane in association with several adaptor proteins. These adaptors help organize the clathrin-coated pit by promoting clathrin assembly, regulating the size of the coated pit, and physically linking clathrin triskelions with endocytic cargo and with the plasma membrane (Owen et al., 2004; Robinson, 2004). Coated pits then bud off into the cell, creating clathrin-coated vesicles.
Clathrin adaptors are targeted to the membrane by several independent interactions, forming a loose network mediated by many low-affinity interactions (Chen et al., 1999; Ford et al., 2001; Mishra et al., 2002; Aguilar et al., 2003; Traub, 2003; Legendre-Guillemin et al., 2004; Schmid et al., 2006). The specific contributions of unique adaptors to this network can be difficult to define. In general, studies that use RNA interference (RNAi) or genetic disruption to remove an individual adaptor from the cellular repertoire find that the interactions within network of assembled clathrin and adaptors are weakened, but not completely disrupted (Hinrichsen et al., 2003; Motley et al., 2003; Sigismund et al., 2005; Stavrou and O'Halloran, 2006; Repass et al., 2007; Brady et al., 2008). A unique exception to this rule is the absolute requirement of the clathrin adaptor protein epsin for both the recruitment and phosphorylation of another adaptor, Hip1r, to clathrin pits on the membrane of Dictyostelium cells (Repass et al., 2007).
Epsin is a clathrin adaptor that binds to the plasma membrane via an N-terminal ENTH (epsin N-terminal homology) domain and also binds to clathrin, AP2 and Eps15 homology (EH) domain-containing proteins via several C-terminal binding motifs (Chen et al., 1998; Owen et al., 1999; Traub et al., 1999; Drake et al., 2000; Itoh et al., 2001). In Dictyostelium, the loss of epsin does not prevent the formation of clathrin-coated pits on the plasma membrane, nor is epsin required for the localization of adaptors such as AP2 or AP180 on the plasma membrane (Stavrou and O'Halloran, 2006; Brady et al., 2008). However, Hip1r is unable to localize to clathrin pits or become phosphorylated in the absence of epsin (Repass et al., 2007). Although Hip1r requires epsin to concentrate in coated pits in Dictyostelium cells, how Hip1r and epsin interact to contribute to clathrin-mediated endocytosis is not known.
Actin polymerization also plays a role in clathrin-mediated endocytosis, possibly by providing the mechanical force necessary for the final stages of coated pit internalization (Giardini et al., 2003; Upadhyaya et al., 2003; Merrifield et al., 2005; Yarar et al., 2005). The polymerization of actin must be controlled both temporally and spatially so that actin filaments associate with assembling coated pits just before vesicle scission (Merrifield et al., 2002; Merrifield et al., 2005). However, the mechanisms governing the temporal and spatial coordination between actin and clathrin remain obscure. Hip1r might be involved in regulating this step, because both yeast and metazoan cells with reduced expression of Hip1r display altered actin polymerization at clathrin-coated pits (Kaksonen et al., 2003; Engqvist-Goldstein et al., 2004; Le Clainche et al., 2007). Vertebrate Hip1r has been proposed to be a negative regulator of actin polymerization at sites of clathrin assembly because of its ability to bind the actin regulator cortactin (Le Clainche et al., 2007). However, because Hip1, another member of the vertebrate Hip1 family, and both yeast sla2p and Dictyostelium Hip1r lack the cortactin-binding domain, the ability to negatively regulate actin through cortactin cannot be a universal mechanism for all members of the Hip1 family. Thus, open questions remain about both the identities and the functions of proteins that coordinate a focused band of actin filaments with sites of clathrin-coated pit invagination. Potentially, the interaction between Dictyostelium epsin and Hip1r might play a role in the regulation of dynamic actin during clathrin-mediated endocytosis in Dictyostelium cells and other eukaryotes.
Here, we provide evidence that epsin and Hip1r are necessary for coordinating both the timing and the organization of polymerized actin filaments as they couple to clathrin-coated pits in Dictyostelium. We have identified residues in the ENTH domain of epsin that are crucial for this function. We propose that, through its ENTH domain, epsin promotes the membrane recruitment and phosphorylation of Hip1r, which in turn coordinates the timing and morphology of actin filaments with coated pits on the plasma membrane. Epsin and Hip1r thus constitute a novel regulatory pathway for the temporal and spatial coordination of actin polymerization in clathrin-mediated endocytosis.
Results
Actin is coupled to clathrin-coated pit dynamics in Dictyostelium
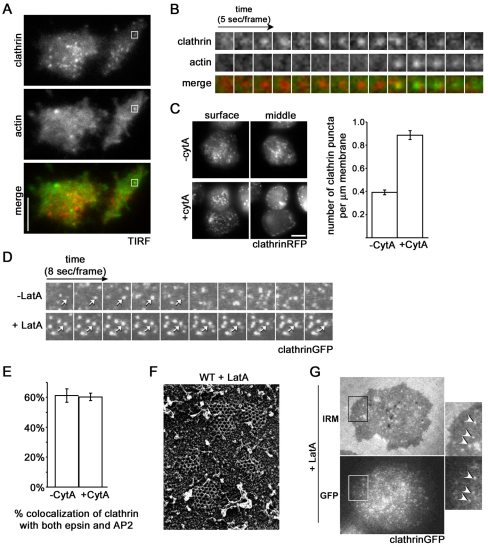
An array of Dictyostelium strains that are mutant in clathrin heavy and light chains and clathrin accessory proteins have been generated (O'Halloran and Anderson, 1992; Wang et al., 2003; Stavrou and O'Halloran, 2006; Repass et al., 2007; Brady et al., 2008; Wen et al., 2009), but the dynamic assembly of clathrin on the plasma membrane has not yet been visualized in these mutants. To visualize this process, we expressed clathrinRFP in Dictyostelium amoeba and monitored clathrin with total internal reflection fluorescence (TIRF) microscopy. We found that clathrin formed distinct but transient puncta on the plasma membrane (Fig. 1A). These puncta increased in intensity, persisted at a maximal intensity, and then rapidly disappeared from the membrane (Fig. 1B; supplementary material Movie S1). The average lifetime of a clathrin puncta on the plasma membrane was 39±2 seconds (mean ± s.e.m.).
Fig. 1.
Actin polymerization functions in the late stages of clathrin-mediated endocytosis in Dictyostelium. (A) TIRF images of the membrane of wild-type cells expressing clathrinRFP (clathrin) and limEΔcoilGFP (actin). Scale bar: 5 μm. (B) Time-lapse images of the individual clathrinRFP punctum (clathrin) identified in A from appearance to internalization accompanied by a punctum of actin labeled by limEΔcoil (actin). (C) Treatment with the actin depolymerizing drug cytochalasin A leads to an accumulation of clathrin puncta on the plasma membrane. Epifluorescence images from surface and middle focal planes of wild-type cells expressing clathrinRFP. Cells treated with cytochalasin A (+cytA) show increased numbers of clathrin puncta on the plasma membrane, 0.89±0.04 puncta per μm, compared to control cells (−cytA), 0.39±0.02 puncta per μm. Mean ± s.e.m., n=30 cells. Scale bar: 5 μm. (D) Treatment with the actin depolymerizing drug Latrunculin A treatment causes clathrin pits to persist on the membrane. TIRF time-lapse images of cells expressing clathrinGFP (clathrinGFP) with (+LatA) and without (−LatA) treatment. (E) Quantification of clathrin pits colocalizing with epsin and AP2 simultaneously in the presence (60±2%; n=12 cells) and absence (61±4%; n=12 cells) of cytochalasin A treatment. (F) Quick-freeze deep etch scanning electron micrograph of assembled clathrin lattices in wild-type cells treated with Latrunculin A (WT + LatA) show that the morphology of clathrin lattices is unaffected. (G) Latrunculin A treatment causes an accumulation of invaginated clathrin-coated pits. Wild-type cells expressing clathrinGFP were treated with Latrunculin A and imaged under IRM and epifluorescence microscopy (GFP). Arrows indicate colocalization between clathrin signal and deeply invaginated pits. All quantification was performed on cells from three independent experiments for each condition.
In many organisms, short bursts of actin polymerization accompany the internalization of clathrin puncta from the membrane (Merrifield et al., 2002; Merrifield et al., 2005; Newpher et al., 2005). To examine the coordination of dynamic actin with clathrin-coated pits in Dictyostelium cells, we coexpressed a fragment of an actin-binding protein, limEΔcoilGFP, that preferentially labels filamentous actin (Bretschneider et al., 2004), and examined the cells by TIRF microscopy. We found that the loss of signal from a clathrin punctum was frequently associated with a brief burst of actin polymerization lasting 13.7±0.9 seconds (mean ± s.e.m.; Fig. 1A,B; supplementary material Movie S1). About 78±10% of disappearing clathrin puncta were associated with actin puncta, suggesting that actin polymerization plays a role in the late stages of clathrin-mediated endocytosis in Dictyostelium, similar to endocytosis in both yeast and vertebrates (Merrifield et al., 2002; Kaksonen et al., 2003; Merrifield et al., 2005). Although a small fraction of clathrin puncta disappeared without actin, in all cases the association of actin with a clathrin punctum resulted in the disappearance of the clathrin punctum. The coincidence between the arrival of actin and the departure of clathrin supports the idea that a primary function of these transient actin puncta could be to assist in the late stages of clathrin-coated pit internalization.
If actin is important for the final stages of clathrin-coated pit internalization in Dictyostelium, then inhibiting actin polymerization should arrest clathrin pits on the plasma membrane. To test this idea, we treated cells expressing clathrinRFP with the actin depolymerizing drug cytochalasin A. Clathrin normally formed puncta at the plasma membrane and in the cytoplasm, and strongly labeled the perinuclear region of the cell (Fig. 1C). After cytochalasin A treatment, the number of clathrin puncta increased significantly at the plasma membrane, with fewer clathrin puncta in the cytoplasm (Fig. 1C). Additionally, cytochalasin A treatment abolished the localization of clathrin to the perinuclear region of the cell, suggesting that the requirements for actin in clathrin dynamics at intracellular membranes are distinct from those of the plasma membrane. Clathrin also accumulated on the membranes of cells treated with latrunculin A, a drug that results in depolymerization of actin by a different mechanism (Fig. 1D; supplementary material Fig. S1).
Three lines of evidence indicated that the clathrin structures that accumulated after blocking dynamic actin represented bona fide clathrin-coated pits. First, the clathrin puncta on the plasma membrane colocalize with multiple clathrin-associated proteins typical of coated pits (Fig. 1E). Second, electron micrographs of the membrane showed that the accumulated clathrin structures represented lattices of triskelions assembled normally into the hexagons and pentagons typical of clathrin-coated pits (Fig. 1F). Finally, interference reflection microscopy (IRM) demonstrated that the accumulated clathrin was associated with white areas of the membrane, embedded in the gray of the surrounding plasma membrane (Fig. 1G). In IRM imaging of the membrane, invaginated portions of the membrane, which are further from the coverslip, appear as white areas, whereas the plasma membrane in contact with the cover slip is gray (Izzard and Lochner, 1976). The coincidence of the clathrinGFP with the white areas indicated that the clathrin was associated with invaginated pits attached to the membrane and not with coated vesicles detached from the membrane. Because the cytochalasin-A-induced clathrinGFP puncta that accumulated on the plasma membrane contained clathrin adaptor proteins, were of normal architecture, and were associated with invaginated areas of the plasma membrane, we concluded that treatment with actin-depolymerizing drugs arrests clathrin pits at the late stages of their maturation. This suggests that actin polymerization is a crucial prerequisite to vesicle scission.
Epsin and Hip1r mutants display abnormal clathrin and actin dynamics at the plasma membrane
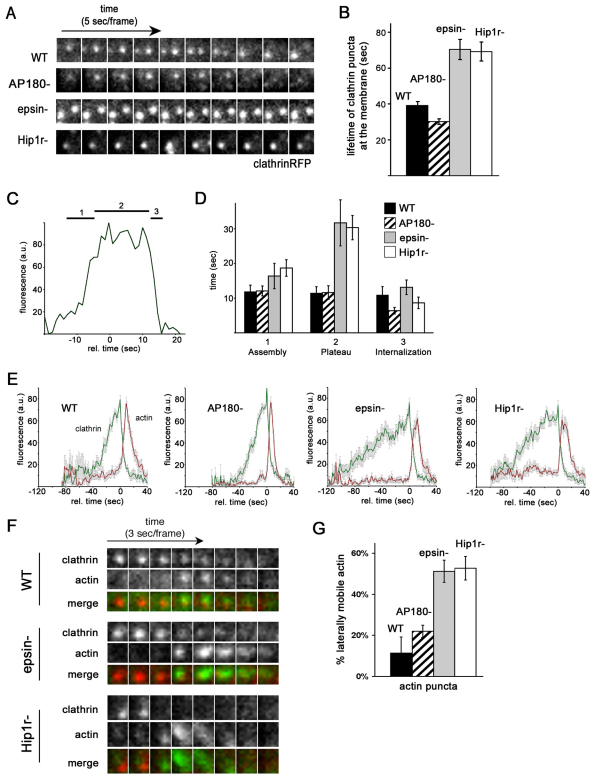
To examine how different clathrin accessory proteins influenced coated pit formation, we coexpressed our reporters for clathrin (clathrinRFP) and actin (limEΔcoilGFP) in Dictyostelium mutants that carried deletions in different clathrin accessory proteins. We then imaged the plasma membranes of the mutant cells with TIRF microscopy (Fig. 2). In mutants deleted for AP180, a monomeric clathrin-associated protein, clathrin puncta formed similarly to those of wild-type cells (Fig. 2A). As seen in wild-type cells, clathrin signal increased in intensity as it assembled into puncta on the plasma membrane of AP180-null cells and persisted at a maximal intensity before disappearing from the plasma membrane (Fig. 2A). The average lifetime of a clathrin punctum in AP180-null mutants was 30±2 seconds for AP180-null cells, similar to the life of 39±2 seconds for wild-type cells (Fig. 2B). However, in epsin-null cells and Hip1r-null cells, the assembled clathrin persisted at the membrane before disappearing for much longer than puncta observed in wild-type cells (Fig. 2A; supplementary material Movies S2 and S3). In contrast to the shorter lifetime of clathrin puncta in wild-type or AP180-null cells (30–39 seconds), clathrin puncta lasted 70±6 seconds on the membrane of epsin-null cells and 68±4 seconds on the membrane of Hip1r-null cells (Fig. 2B).
Fig. 2.
Hip1r and epsin, but not AP180, are required for normal clathrin and actin dynamics at the plasma membrane. (A) Clathrin puncta persist at the membrane of epsin and Hip1r-null cells. Time-lapse TIRF images of wild-type (WT), AP180-null (AP180−), epsin-null (epsin−), and Hip1r-null (Hip1r−) cells expressing clathrinRFP. (B) Quantification of the lifetime of clathrin puncta identified at the beginning of TIRF time-lapse acquisition. Wild-type (39±2 seconds, n=49; AP180-null (AP180−) 30±2 seconds, n=35; epsin-null (epsin−) 70±6 seconds, n=31; and Hip1r-null (Hip1r−) 68±4 seconds, n=29. (C) Representative plot showing the fluorescence intensity of a wild-type clathrinRFP (clathrin) punctum over time; 1, 2 and 3 mark the assembly, plateau and internalization phases, respectively. (D) Quantification of the average lifetime (seconds) of clathrin puncta from wild-type (n=20), AP180-null (n=20), epsin-null (n=16), and Hip1r-null (n=20) cells. Cells are in the assembly phase (1) with normalized intensities of 25–75 a.u.; plateau phase (2) with intensities above 75 a.u.; and internalization phase (3) with intensities of 75–25 a.u. (E) Average plots of the intensity of clathrinRFP (clathrin) puncta over time, with the accompanying actin puncta as labeled by limEΔcoilGFP (actin) in wild-type, AP180-null, epsin-null, and Hip1r-null cells; n=16–20 per cell line. (F) Time-lapse TIRF images of individual clathrin and actin puncta in wild-type, AP180-null, epsin-null, and Hip1r-null cells coexpressing clathrinRFP (clathrin) and limEΔcoilGFP (actin). (G) Quantification of laterally mobile actin puncta as labeled by limEΔcoilGFP: wild-type 11±8%, n=35 puncta on 5 cells; AP180-null 22±3%, n=39 puncta on 6 cells; epsin-null 56±6%, n=49 puncta on 5 cells; and Hip1r-null 53±6%, n=51 puncta on 6 cells. All values are mean ± s.e.m. All quantification was performed on cells from three independent experiments for each condition.
In order to further evaluate the dynamics of clathrin puncta in these backgrounds, we examined the intensity of fluorescence for each clathrin punctum over its entire lifetime (Fig. 2C,D). We found that the pattern of fluorescence intensity could be divided into three phases: an assembly phase characterized by a rapid increase in signal, a plateau phase where the intensity remained near a maximum, and an internalization phase characterized by a rapid loss of signal (Fig. 2C). In wild-type cells, the duration of the assembly phase was 12±1 seconds (Fig. 2D). The duration of the assembly phase for clathrin puncta in AP180 and epsin mutants was statistically similar to that for wild-type cells (AP180-null 12±1 seconds; epsin-null 16±4 seconds), whereas Hip1r mutants had a slightly longer assembly phase of 19±2 seconds (P=0.02). However, the plateau phase for clathrin puncta in epsin and Hip1r mutants was significantly longer than for wild-type cells. Clathrin puncta in wild-type cells and AP180 mutants had a plateau phase of 11±2 seconds. By contrast, the duration of the plateau phase for clathrin puncta in epsin and Hip1r mutants was 32±7 seconds (P=0.005) and 30±4 seconds (P<0.001), respectively (Fig. 2D). In contrast to the prolonged plateau phase, the internalization phase was brief and similar in wild-type cells, epsin mutants and Hip1r mutants (9–13 seconds) and slightly shorter in AP180-null cells (6.4 seconds). Thus, the increased lifetime of clathrin puncta seen for epsin and Hip1r mutants can be attributed largely to a prolonged persistence after assembly on the plasma membrane.
In wild-type cells and all mutant cells, the short internalization phase for clathrin puncta was characterized by a rapid loss of clathrin fluorescence, accompanied by a rapid increase in actin fluorescence (Fig. 2E). The engagement of actin with clathrin puncta invariably resulted in the disappearance of clathrin from the plasma membrane. Scanning electron micrographs of assembled clathrin lattices in wild-type and Hip1r-null cells revealed no appreciable difference in the static clathrin and actin morphology (supplementary material Fig. S2). However, close examination of the dynamic actin associated with clathrin puncta in live epsin-null and Hip1r-null cells revealed two key differences. First, the dynamic morphology of the actin structures associated with clathrin puncta in these mutants differed from that of wild-type cells. In contrast to the round focused spot of actin associated with clathrin structures in wild-type cells, actin structures in both epsin and Hip1r-null mutants were more diffuse, slightly larger and irregular in shape (Fig. 2F). Second, the actin structures in epsin and Hip1r mutants were more motile. Whereas the focused spots of actin in wild-type cells or AP180-null cells were relatively immobile, in epsin and Hip1r-null cells the actin structures often moved laterally on the membrane before disappearing (Fig. 2F; supplementary material Movies S1–S3). Approximately 51±5% of actin puncta in epsin-null cells and 53±6% of actin puncta in Hip1r-null cells were oblong and moved laterally along the plane of the membrane, whereas only 11±8% of actin puncta were mobile in wild-type cells (Fig. 2G). The similarities in their actin phenotypes suggested that epsin and Hip1r regulate in a similar way both the shape and the mobility of actin filaments associated with clathrin on the plasma membrane.
Blocking dynamic actin abrogates the requirement of epsin for Hip1r localization but not for Hip1r phosphorylation
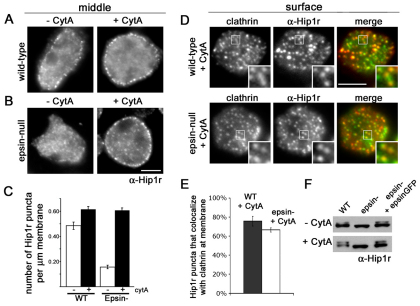
Previously, we demonstrated a relationship between epsin and Hip1r (Repass et al., 2007). Hip1r is phosphorylated and concentrates within clathrin structures on the plasma membrane in Dictyostelium cells. Epsin is required for both the phosphorylation and the association of Hip1r within clathrin structures on the membrane (Repass et al., 2007). To determine how actin influenced this relationship, we treated wild-type and epsin-null cells with cytochalasin A, then fixed and immunostained with anti-Hip1r antibodies (Fig. 3A). In untreated wild-type cells, Hip1r formed puncta on the cell membrane and not in the perinuclear region of the cell, consistent with the localization of Dictyostelium Hip1r in membrane-associated clathrin structures described previously (Repass et al., 2007) (data not shown). After treatment with cytochalasin A, the number of Hip1r puncta on the membrane increased in these cells (Fig. 3A,C). By contrast, Hip1r largely failed to form puncta on the membrane of untreated epsin-null cells (Fig. 3B). Remarkably, cytochalasin A treatment induced a dramatic increase in membrane-associated Hip1r puncta in epsin-null cells (Fig. 3B,C). Moreover, these induced Hip1r puncta colocalized with clathrin (Fig. 3D). Quantification showed that 76±5% of Hip1r in wild-type cells and 67±2% of Hip1r in epsin-null cells colocalized with clathrin on the plasma membrane after cytochalasin treatment (Fig. 3D,E). Thus, blocking actin polymerization abrogated the requirement for epsin in the localization of Hip1r within clathrin structures on the plasma membrane.
Fig. 3.
Disrupting actin polymerization affects Hip1r localization but not phosphorylation in epsin-null cells. (A) Epifluorescence images from a middle focal plane of wild-type cells (−CytA) and cells treated with cytochalasin A (+CytA), immunostained with anti-Hip1r (α-Hip1r) antibodies. Scale bar: 5 μm. (B) Epifluorescence images from a middle focal plane of epsin-null cells and epsin-null cells treated with cytochalasin A, immunostained with anti-Hip1r antibodies. Note the increased Hip1r localization to the membrane in epsin-null cells treated with cytochalasin A. Scale bar: 5 μm. (C) Quantification of Hip1r localization in wild-type (WT) and epsin-null cells (epsin−) with and without cytochalasin A treatment: WT −cytA, 0.48 ±0.03 puncta per μm; WT +cytA, 0.61±0.02 puncta per μm; epsin− −cytA, 0.16±0.01 puncta per μm; epsin− +cytA, 0.61±0.02 puncta per μm. All values are mean ± s.e.m., n=30 cells from three independent experiments for each condition. (D) Epifluorescence images from a surface focal plane of wild-type and epsin-null cells expressing clathrinGFP (clathrin), treated with cytochalasin A, and immunostained for Hip1r. Scale bar: 5 μm. (E) Quantification of colocalization between Hip1r and clathrin in wild-type and epsin-null cells treated with cytochalasin A: wild-type 76±5%, n=14 cells; Hip1r-null 67±2%, n=20 cells. (F) Western blots of whole cell lysates from wild-type, epsin-null, and epsin-null cells expressing epsinGFP (epsin− + epsinGFP) with or without cytochalasin A treatment. Blots were probed with anti-Hip1r antibodies. Note that cytochalasin A treatment does not induce a phosphorylated species of Hip1r in epsin-null cells.
Conceivably, inducing Hip1r puncta at the membrane of epsin-null cells with cytochalasin A could also eliminate the requirement for epsin in Hip1r phosphorylation. To test this possibility, we treated wild-type and epsin-null cells with cytochalasin A and analyzed the cell lysates by immunoblot using anti-Hip1r antibodies (Fig. 3F). As previously reported (Repass et al., 2007), blots of lysates from untreated wild-type cells displayed two species of Hip1r: an unphosphorylated lower band and a phosphorylated upper band (Fig. 3F, top row). Both species of Hip1r were also present in lysates of wild-type cells treated with cytochalasin A and in epsin-null cells complemented with epsinGFP (Fig. 3F, bottom row). By contrast, lysates from epsin-null cells contained only the unphosphorylated form of Hip1r (Fig. 3F, top row). Blots of lysates from epsin-null cells treated with cytochalasin A also displayed only the unphosphorylated form of Hip1r (Fig. 3F, bottom row). Thus, although cytochalasin A induces Hip1r to localize on the membrane of epsin-null cells, Hip1r remains unphosphorylated, demonstrating a strict requirement for epsin in Hip1r phosphorylation. Taken together, these data suggest that epsin functions in a pathway that promotes Hip1r phosphorylation and stabilizes Hip1r association with clathrin-coated structures when dynamic actin is present.
PtdIns(4,5)P2 binding by the ENTH domain of epsin is required for the membrane localization and phosphorylation of Hip1r
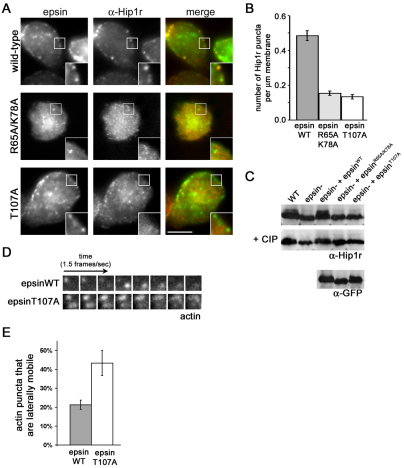
To further dissect the mechanism of how epsin influences Hip1r localization and phosphorylation, we examined two informative epsin mutant proteins. The first mutant, epsinR65A/K78A, carried mutations in amino acids R65 and K78 found within the ENTH domain of epsin. These residues are required for Dictyostelium epsin to bind to phosphatidylinositol (4,5)-bisphosphate [PtdIns(4,5)P2], and are necessary for rescuing epsin function during development (Brady et al., 2008). To determine whether the R65A/K78A mutations influence the ability of epsin to rescue Hip1r localization, we expressed epsinWTGFP or epsinR65A/K78AGFP in an epsin-null background and immunostained for Hip1r (Fig. 4A). Expression of epsinWTGFP in epsin-null cells rescued the formation of Hip1r puncta at the plasma membrane, a pattern indistinguishable from wild-type cells (Fig. 4A, top row; compare with Fig. 3A). On the other hand, expression of epsinR65A/K78AGFP did not associate with the plasma membrane and also failed to restore Hip1r localization to the plasma membrane (Fig. 4A, center row). Instead, Hip1r formed cytoplasmic puncta similar to the distribution of Hip1r in epsin-null cells. Moreover, epsin-null cells that expressed epsinR65A/K78AGFP also failed to rescue Hip1r phosphorylation, as revealed by immunoblot analysis (Fig. 4C). These deficiencies suggested that the ability of epsin to bind PtdIns(4,5)P2 is important for the capacity of epsin to promote both the membrane localization and the phosphorylation of Hip1r.
Fig. 4.
EpsinT107A does not rescue the Hip1r and actin-related phenotypes of epsin-null cells. (A) Epifluorescence images from a middle focal plane of epsin-null cells expressing epsinWTGFP (WT), epsinR65A/K78AGFP (R65A/K78A), or epsinT107AGFP (T107A) and immunostained for Hip1r (α-Hip1r). Scale bar: 5 μm. (B) Quantification of Hip1r puncta at the membrane of epsin-null cells expressing epsinWTGFP (epsinWT, 0.40±0.03 puncta per μm), epsinR65A/K78AGFP (epsinR65A/K78A, 0.15±0.01 puncta per μm) epsinT107AGFP (epsinT107A, 13±0.01 puncta per μm); n=30 cells from three independent experiments for each cell line. (C) Immunoblots of whole cell lysates from wild-type (WT) and epsin-null (epsin−) cells, and epsin-null cells expressing epsinWTGFP (epsin− + epsinWT), epsinR65A/K78AGFP (epsin− + epsinR65A/K78A), or epsinT107AGFP (epsin− + epsinT107A). Blots were probed with anti-Hip1r (α-Hip1r) or anti-GFP (α-GFP) antibodies. Center row indicates whole-cell lysates treated with calf intestinal phosphatase (CIP). (D) Time-lapse TIRF images of epsin-null cells coexpressing limEΔcoilGFP and either epsinWTGFP or epsinT107AGFP. (E) Quantification of lateral mobility of actin puncta labeled by limEΔcoilGFP in epsin-null cells expressing either epsinWTGFP (21±2%, n=23 puncta on three cells) or epsinT107AGFP (43±7%, n=30 puncta on three cells).
The epsin ENTH domain regulates Hip1r via a residue that is independent of PtdIns(4,5)P2 binding
We next tested the ability of a second epsin mutant protein, epsinT107A, to rescue Hip1r localization and phosphorylation. Epsin that carries the T107A point mutation in the ENTH domain, epsinT107A, retains the ability to bind to PtdIns(4,5)P2, but cannot rescue epsin developmental deficiencies (Brady et al., 2008). We expressed epsinT107AGFP in epsin-null cells and examined its localization. EpsinT107AGFP formed puncta on the membrane that associated with clathrin and clathrin adaptors (supplementary material Fig. S3). However, despite the ability of this mutant epsin to associate with clathrin at the plasma membrane, it could not recruit Hip1r to the same clathrin-coated structures. Immunostaining the cells expressing epsinT107A with anti-Hip1r antibodies showed that Hip1r failed to localize within membrane puncta (Fig. 4A, bottom row; Fig. 3B). Instead, Hip1r localized to the cytoplasm, as seen in epsin-null cells (Fig. 4A, bottom row; compare with Fig. 3B, left). Thus, epsinT107AGFP was not capable of restoring the membrane localization of Hip1r in epsin-null cells. Moreover, when we analyzed lysates of epsin-null cells expressing epsinT107GFP by immunoblot analysis, we found only the unphosphorylated species of Hip1r (Fig. 4C). This demonstrated that the T107A mutation in epsin abolished the ability of epsin to facilitate the phosphorylation of Hip1r. These results indicate that residue T107 contributes to an essential function of the ENTH domain, distinct from binding PtdIns(4,5)P2, that is required for epsin to facilitate the membrane localization as well as phosphorylation of Hip1r.
EpsinT107A cannot rescue the dynamic actin defects of epsin-null cells
The previous experiments highlighted the requirement for residue T107 in the epsin ENTH domain for Hip1r localization and phosphorylation. If the functional interaction between epsin and Hip1r contributes to the promotion of actin polymerization at clathrin-coated pits, then the expression of the epsinT107A mutant protein should fail to rescue the dynamic actin defects found in epsin-null cells.
To determine whether the T107A mutation negatively influences the ability of epsin to regulate actin puncta at the cell surface, we coexpressed either epsinWTGFP or epsinT107GFP with LimEΔcoilRFP in epsin-null cells and examined the LimEΔcoil-labeled actin puncta under TIRF microscopy. We found that in epsin-null cells expressing epsinWTGFP, only 21±2% of the dynamic actin puncta were laterally mobile, a value similar to wild-type or AP180-null cells (Fig. 4D,E). On the other hand, 43±7% of actin puncta in epsin-null cells expressing epsinT107GFP were laterally mobile, a value similar to that found for epsin-null cells (Fig. 4D,E). Thus, the T107A mutation inhibited the ability of epsin to regulate actin dynamics at the plasma membrane. Taken together, our data indicate that the ENTH domain of epsin, via the T107 residue, functions in a pathway that facilitates the membrane recruitment and phosphorylation of Hip1r, which in turn promotes actin polymerization at clathrin-coated pits.
Discussion
In a wide range of simple and complex eukaryotic cells, actin polymerizes into a focused band of filaments that couples to a clathrin-coated pit just before the pit detaches from the plasma membrane (Merrifield et al., 2002; Kaksonen et al., 2003; Newpher and Lemmon, 2006; Le Clainche et al., 2007). This event must be coordinated temporally, so that actin filaments polymerize at coated pits at the appropriate time, and spatially, so that the actin filaments assemble a focused tail of filaments to efficiently propel the coated vesicle into the cytoplasm. The molecular mechanism and the proteins involved in coordinating the cytoskeleton and coated pits remain obscure. Using Dictyostelium cells as a model, we demonstrated crucial roles for both epsin and Hip1r in the temporal and spatial coupling of actin to a maturing coated pit. We propose that epsin, working through specific residues in its N-terminal ENTH domain, functions in a regulatory pathway that promotes the phosphorylation of Hip1r, which in turn regulates actin polymerization at endocytic sites.
Dictyostelium as a model system for clathrin and actin dynamics
We found that inhibiting actin polymerization arrested clathrin pits on the plasma membrane. IRM imaging revealed these clathrin pits to be arrested in an invaginated state. Thus, in Dictyostelium, as in other eukaryotes from yeast to mammalian cells, actin polymerization contributes to late stages of clathrin-mediated endocytosis, including the efficient maturation and scission of a coated pit (Merrifield et al., 2002; Engqvist-Goldstein and Drubin, 2003; Kaksonen et al., 2003; Merrifield et al., 2005; Yarar et al., 2005). In our system, in all instances where we visualized a localized burst of actin assembled with a clathrin puncta, the clathrin internalized. This connection suggests that clathrin-coated areas on the membrane are sites of single endocytic events and that, in contrast to cultured mammalian cells, multiple coated pits do not bud from a stable clathrin-coated patch that resides on the membrane (Merrifield et al., 2005; Rappoport et al., 2006). An open question concerning Dictyostelium and other systems is: what are the actin filaments doing at coated pits? One possibility could be that these localized bursts of polymerization provide the mechanical force necessary for coated pits to complete their scission from the plasma membrane. Alternatively, actin polymerization might be required to direct the clathrin-coated vesicle away from the membrane after scission. Such a mechanism is employed during phagocytosis in Dictyostelium, where actin polymerization propels the nascent phagosome away from the membrane (Clarke et al., 2006).
Epsin regulates actin dynamics through Hip1r
Although yeast epsin-null cells display disorganized cortical actin, (Wendland et al., 1999; Aguilar et al., 2006), epsin has not been shown previously to affect actin dynamics during clathrin-mediated endocytosis. Here, we have shown that, in Dictyostelium, epsin is important for efficiently coupling actin to the clathrin machinery. In epsin mutants, clathrin pits persisted longer at the membrane before associating with an actin puncta and internalizing. We also found that, once coupled to clathrin pits in epsin-null cells, the small bursts of polymerized actin were elongated and moved laterally in the membrane. The Hip1r mutant had similar phenotypes, suggesting that these two adaptors function in the same pathway. Epsin is expressed normally in Hip1r-null mutants and is functional, as indicated by the finding that Hip1r-null cells do not display epsin-specific developmental phenotypes (Brady et al., 2008). However, in epsin-null mutants, Hip1r is mislocalized and unphosphorylated, suggesting that epsin functions upstream of Hip1r in the regulation of actin polymerization at clathrin pits. Furthermore, we found that the epsinT107A mutant, which concentrates within clathrin-coated pits but fails to rescue Hip1r localization or phosphorylation, also failed to rescue the disorganized actin puncta. Therefore, we propose that epsin modulates actin dynamics at clathrin-coated pits by facilitating the recruitment and phosphorylation of Hip1r.
Although epsin is normally required for both the phosphorylation and the localization of Hip1r (Repass et al., 2007), we found that inhibition of actin polymerization allowed Hip1r to associate with coated pits, even in the absence of epsin. Thus, epsin-dependent phosphorylation might promote Hip1r localization at the membrane-bound coated pits, whereas later-acting dynamic actin might destabilize Hip1r localization. Epsin, which targets to clathrin-coated pits independently of Hip1r, might be necessary to recruit an intermediate kinase to the coated pit, which could then phosphorylate and stabilize Hip1r at the coated pit. Such phosphorylation could modulate the binding properties of Hip1r. Recent work with the mammalian Hip1r indicates that Hip1r cannot bind simultaneously to both clathrin and actin (Wilbur et al., 2008). Thus, by modulating affinities for clathrin and actin, the phosphorylation and dephosphorylation of Hip1r could promote a transient and dynamic remodeling of actin as the coated pit assembles and detaches from the plasma membrane as a vesicle.
A crucial residue in the ENTH domain of epsin is essential for both Hip1r phosphorylation and actin dynamics at the plasma membrane
The ENTH domain of epsin is sufficient to rescue all epsin-null phenotypes, including the membrane localization and phosphorylation of Hip1r (Repass et al., 2007; Brady et al., 2008). The ENTH domain binds PtdIns(4,5)P2, which is required for epsin activity. We also found that residue T107, which resides within the ENTH domain but is not involved in lipid-binding, was essential for rescuing the defects in actin dynamics at the membrane of epsin-null cells. In yeast, the residue analogous to T107 is found in a surface patch of residues within the ENTH domain and is important for binding to Rga1 and Rga2, which are cdc42 GAP proteins that regulate the actin cytoskeleton (Aguilar et al., 2006). Mutations in this patch of residues impair the function of yeast epsin and block binding to these regulatory proteins (Aguilar et al., 2006). Although Dictyostelium does not contain cdc42 or its GAP proteins, the T107 residue in the Dictyostelium ENTH domain could be part of a surface patch that similarly contributes to the actin cytoskeleton by interacting with regulatory proteins, possibly a kinase, that work through Hip1r to control actin dynamics at coated pits. Identifying the mechanism by which residue T107 affects ENTH function will be key in understanding the broader mechanism of the functional interaction between epsin and Hip1r.
Hip1r regulates the coupling of actin to clathrin-coated pits
All members of the Hip1r family contain sequential domains that bind the membrane, clathrin and actin, an organization that has led to the proposal that Hip1r acts as a bridge between actin and clathrin (Engqvist-Goldstein et al., 1999; Engqvist-Goldstein et al., 2001; Ford et al., 2002; Chen and Brodsky, 2005; Sun et al., 2005; Ybe et al., 2007). Hip1r also forms a homodimer (Engqvist-Goldstein et al., 2001), allowing it to bind more than one molecule of clathrin or actin simultaneously. The model of Hip1r as a bridge between actin and clathrin is consistent with our finding that clathrin puncta persist longer at the plasma membrane before associating with actin in Hip1r-null mutants. Similarly, yeast Hip1r mutants also display stabilized clathrin puncta on the plasma membrane (Newpher et al., 2005). However, in our study we found that clathrin puncta eventually disappeared from the membrane of Hip1r- and epsin-null cells, accompanied by a burst of actin polymerization. Thus, the mutant phenotype exhibited a delay in coupling coated pits with actin, not an absolute block as a bridge model would suggest.
A second indication that Hip1r does more than link clathrin with actin filaments is that, once coupled to clathrin-coated pits, the arrays of actin coupled to clathrin that we observed in Hip1r-null cells were more diffuse than wild-type actin and also spread laterally in the plane of the plasma membrane. Mammalian cultured cells with RNAi-mediated reduction of Hip1r expression exhibit long-lived clathrin pits coupled to oddly-shaped actin tails that wave in the cytoplasm (Engqvist-Goldstein et al., 2004). Although the specific alterations in actin morphology differ between these mammalian cultured cells and Dictyostelium cells, the commonality of defects at the interface of actin and clathrin when Hip1r activity is blocked suggests that the role for Hip1r in shaping and orienting actin filaments coupled to coated pits is conserved.
Taken together, our results are most consistent with cooperative roles for Dictyostelium epsin and Hip1r in promoting the temporal and spatial regulation of actin filaments at coated pits. Epsin-dependent phosphorylation could modulate the binding properties of Hip1r dynamically while the actin and clathrin remodel to liberate a coated vesicle from the plasma membrane. Epsin is not a kinase, and the epsin-dependent kinase that phosphorylates Hip1r remains to be identified. Once activated by phosphorylation, Hip1r could directly regulate and orient actin filament formation, or act as a scaffold to recruit other proteins that regulate actin. Whether direct or not, this pathway probably involves the protein Arp2/3, a key regulator of polymerization of actin filaments that interacts with many proteins involved in clathrin-mediated endocytosis via N-WASP and localizes to internalizing clathrin pits in Dictyostelium and a wide range of other eukaryotes (Schafer, 2002; Merrifield et al., 2004; Heinrich et al., 2008; Yamada et al., 2009). Understanding how this regulatory pathway functions will be key to understanding the complexity of how clathrin-coated pits are integrated with the dynamic actin cytoskeleton.
Materials and Methods
Strains and cell culture
Dictyostelium discoideum strains included Ax2, an axenic wild-type strain; 10G10 and 5B4, epsin-null strains derived from Ax2 (Brady et al., 2008); and 4F6, a Hip1r-null strain also derived from Ax2 (Repass et al., 2007). Cells were cultured on tissue culture plates with HL-5 medium (Sussman, 1987) supplemented with 60 U/ml penicillin and 60 μg/ml streptomycin (Invitrogen, Carlsbad, CA) at 18°C. Null cells grown under selection were supplemented with 5 μg/ml blasticidin (ICN Biomedicals, Irvine, CA), and cells carrying expression plasmids were supplemented with 20 μg/ml G418 (geneticin, Gibco-BRL, Invitrogen).
Cloning clathrinRFP
Clathrin light chain was cloned from pTxGFP:CLC (Wang et al., 2006) into p333-9 mRFPmars BsrH expression vector (kind gift of Annette Muller-Taubenberger, Ludwig Maximilians University Munich, Germany) with EcoRI and XhoI.
Dictyostelium transformation
Dictyostelium cell lines were transformed with various expression plasmids by electroporation. About 5×106 cells in 100 μl of buffer H-50 (20 mM HEPES, 50 mM KCl, 10 mM NaCl, 1 mM MgSO4, 5 mM NaHCO3, 1 mM NaH2PO4) were mixed with 10 μg of plasmid and electroporated using a Bio-Rad Gene Pulser (Bio-Rad, Hercules, CA) at 75 kV and 25 μF. For co-transformations, cells were mixed with 10 μg of each plasmid (20 μg total) before electroporation.
Live fluorescence microscopy
Cells expressing GFP or RFP expression plasmids were harvested and allowed to attach to glass coverslips for 10 minutes at 18°C and then incubated with low fluorescence media (Liu et al., 2002) for at least 20 minutes. Images were taken using an inverted Nikon Eclipse TE200 microscope (Nikon Instruments, Dallas, TX) with 100× 1.4 NA PlanFlour objective and a Quantix 57 camera (Roper Scientific, AZ) controlled by Metamorph software (Universal Images, PA). Confocal images were acquired on a Leica TCS-SP2 laser scanning confocal inverted microscope (Leica Microsystems, Wetzlar, Germany). Interference fluorescence microscopy images were acquired as described previously (Heuser et al., 1993). Images were processed using Metamorph (Molecular Devices, Sunnyvale, CA), Adobe Photoshop (Adobe Systems, San Jose, CA), and ImageJ (US National Institutes of Health, Bethesda, MD) software.
Fixed immunofluorescence microscopy
Cells were fixed with 2% formaldehyde and 0.01% Triton-X 100 in PDF medium (2 mM KCL, 1.1 mM K2HPO4, 1.32 mM KH2PO4, 0.1 mM CaCl2, 0.25 mM MgSO4, pH 6.7) at room temperature for 15 minutes and then in 100% methanol at −20°C for 5 minutes, then rinsed with PDF and mounted on glass slides. For immunostaining, cells on coverslips were blocked with 3% BSA (Fisher Scientific, Pittsburgh, PA) in PBS (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4, pH 7.4) for 20 minutes at 37°C, and then incubated with rabbit anti-CLC (Wang et al., 2003), rabbit anti-AP2 IgG or rabbit anti-Hip1r (Repass et al., 2007) for 1 hour. Coverslips were rinsed with PBS, incubated for 30 minutes with 30 μg/ml goat anti-rabbit IgG conjugated to Texas Red or Pacific Blue (Molecular Probes, Invitrogen), and rinsed again in PBS. Coverslips were then rinsed in sterile distilled water, mounted on glass slides and imaged as described above.
Quantification of colocalization
Images were cropped and prepared for analysis using Adobe Photoshop (Adobe Systems) and ImageJ (US National Institutes of Health). Images of individual cells were normalized by scaling to the same maximum and minimum intensities for each channel. Each pixel of a normalized image of a single cell was scored for its intensity in each channel above the mean image intensity for that channel. Colocalization was then expressed as a percentage of the total pixels above the mean intensity for a given channel that were also above the mean intensity of another channel.
Live cell imaging by TIRF microscopy
Cells were imaged by TIRF microscopy at the University of Texas Southwestern Medical Center Imaging Core Facility with the assistance of Kate Luby-Phelps. Images were acquired on a Zeiss AxioObserver microscope using 488 nm and 561 nm lasers with a Roper QuantEM camera controlled by Slidebook software (Intelligent Imaging Innovations, Denver, CO). Images were acquired approximately every 1.5 seconds.
Quantification of the duration of the lifetimes of clathrin and actin puncta was done manually. For plotting the intensity of individual clathrin and actin puncta, a region was drawn around the punctum with a radius of 5 pixels. The mean intensity of each channel was recorded for each frame. The mean intensity of a ring immediately surrounding the region was calculated and subtracted as background for each frame. Measurements that were below background levels were recorded as zero. Relative fluorescence (a.u.) was defined as the percentage of maximum intensity over the given time frame. Time point 0 seconds was defined as the maximum clathrin signal before dimming for each punctum.
Treatment of cells with cytochalasin A
For microscopy, cells were harvested and allowed to attach to glass coverslips for 10 minutes at 18°C and incubated with low fluorescence media (Liu et al., 2002) for at least 20 minutes. Cells were then incubated with 10 or 20 μM cytochalasin A (Sigma) in low fluorescence media for 1 hour before being fixed and stained as described. For western blot, cells were harvested, washed in PDF, and resuspended at 2×106 cells/ml. Cells were treated with 20 μM cytochalasin A in PDF for 1 hour, centrifuged at 1500 g for 5 minutes, and loaded onto an SDS-PAGE gel for western blot analysis.
Supplementary Material
Acknowledgments
We thank Robyn Roth for generating the EM images, Annette Mueller-Taubenberger for the gift of the GFP-LimEΔcoil plasmid, and Kate Luby-Phelps at University of Texas Southwestern Medical Center Imaging Core Facility for assistance with TIRF microscopy. We also thank members of the O'Halloran, De Lozanne and Sisson laboratories for helpful discussions on our manuscript. This work is supported by NIH RO1 GM048625 to T.J.O. Deposited in PMC for release after 12 months.
Footnotes
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/123/21/3652/DC1
References
- Aguilar R. C., Watson H. A., Wendland B. (2003). The yeast Epsin Ent1 is recruited to membranes through multiple independent interactions. J. Biol. Chem. 278, 10737-10743 [DOI] [PubMed] [Google Scholar]
- Aguilar R. C., Longhi S. A., Shaw J. D., Yeh L. Y., Kim S., Schon A., Freire E., Hsu A., McCormick W. K., Watson H. A., et al. (2006). Epsin N-terminal homology domains perform an essential function regulating Cdc42 through binding Cdc42 GTPase-activating proteins. Proc. Natl. Acad. Sci. USA 103, 4116-4121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady R. J., Wen Y., O'Halloran T. J. (2008). The ENTH and C-terminal domains of Dictyostelium epsin cooperate to regulate the dynamic interaction with clathrin-coated pits. J. Cell Sci. 121, 3433-3444 [DOI] [PubMed] [Google Scholar]
- Bretschneider T., Diez S., Anderson K., Heuser J., Clarke M., Muller-Taubenberger A., Kohler J., Gerisch G. (2004). Dynamic actin patterns and Arp2/3 assembly at the substrate-attached surface of motile cells. Curr. Biol. 14, 1-10 [DOI] [PubMed] [Google Scholar]
- Chen C. Y., Brodsky F. M. (2005). Huntingtin-interacting protein 1 (Hip1) and Hip1-related protein (Hip1R) bind the conserved sequence of clathrin light chains and thereby influence clathrin assembly in vitro and actin distribution in vivo. J. Biol. Chem. 280, 6109-6117 [DOI] [PubMed] [Google Scholar]
- Chen H., Fre S., Slepnev V. I., Capua M. R., Takei K., Butler M. H., Di Fiore P. P., De Camilli P. (1998). Epsin is an EH-domain-binding protein implicated in clathrin-mediated endocytosis. Nature 394, 793-797 [DOI] [PubMed] [Google Scholar]
- Chen H., Slepnev V. I., Di Fiore P. P., De Camilli P. (1999). The interaction of epsin and Eps15 with the clathrin adaptor AP-2 is inhibited by mitotic phosphorylation and enhanced by stimulation-dependent dephosphorylation in nerve terminals. J. Biol. Chem. 274, 3257-3260 [DOI] [PubMed] [Google Scholar]
- Clarke M., Muller-Taubenberger A., Anderson K. I., Engel U., Gerisch G. (2006). Mechanically induced actin-mediated rocketing of phagosomes. Mol. Biol. Cell 17, 4866-4875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake M. T., Downs M. A., Traub L. M. (2000). Epsin binds to clathrin by associating directly with the clathrin-terminal domain. Evidence for cooperative binding through two discrete sites. J. Biol. Chem. 275, 6479-6489 [DOI] [PubMed] [Google Scholar]
- Engqvist-Goldstein A. E., Drubin D. G. (2003). Actin assembly and endocytosis: from yeast to mammals. Annu. Rev. Cell Dev. Biol. 19, 287-332 [DOI] [PubMed] [Google Scholar]
- Engqvist-Goldstein A. E., Kessels M. M., Chopra V. S., Hayden M. R., Drubin D. G. (1999). An actin-binding protein of the Sla2/Huntingtin interacting protein 1 family is a novel component of clathrin-coated pits and vesicles. J. Cell Biol. 147, 1503-1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engqvist-Goldstein A. E., Warren R. A., Kessels M. M., Keen J. H., Heuser J., Drubin D. G. (2001). The actin-binding protein Hip1R associates with clathrin during early stages of endocytosis and promotes clathrin assembly in vitro. J. Cell Biol. 154, 1209-1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engqvist-Goldstein A. E., Zhang C. X., Carreno S., Barroso C., Heuser J. E., Drubin D. G. (2004). RNAi-mediated Hip1R silencing results in stable association between the endocytic machinery and the actin assembly machinery. Mol. Biol. Cell 15, 1666-1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford M. G., Pearse B. M., Higgins M. K., Vallis Y., Owen D. J., Gibson A., Hopkins C. R., Evans P. R., McMahon H. T. (2001). Simultaneous binding of PtdIns(4,5)P2 and clathrin by AP180 in the nucleation of clathrin lattices on membranes. Science 291, 1051-1055 [DOI] [PubMed] [Google Scholar]
- Ford M. G., Mills I. G., Peter B. J., Vallis Y., Praefcke G. J., Evans P. R., McMahon H. T. (2002). Curvature of clathrin-coated pits driven by epsin. Nature 419, 361-366 [DOI] [PubMed] [Google Scholar]
- Giardini P. A., Fletcher D. A., Theriot J. A. (2003). Compression forces generated by actin comet tails on lipid vesicles. Proc. Natl. Acad. Sci. USA 100, 6493-6498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich D., Youssef S., Schroth-Diez B., Engel U., Aydin D., Blummel J., Spatz J. P., Gerisch G. (2008). Actin-cytoskeleton dynamics in non-monotonic cell spreading. Cell Adh. Migr. 2, 58-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J., Zhu Q., Clarke M. (1993). Proton pumps populate the contractile vacuoles of Dictyostelium amoebae. J. Cell Biol. 121, 1311-1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinrichsen L., Harborth J., Andrees L., Weber K., Ungewickell E. J. (2003). Effect of clathrin heavy chain- and alpha-adaptin-specific small inhibitory RNAs on endocytic accessory proteins and receptor trafficking in HeLa cells. J. Biol. Chem. 278, 45160-45170 [DOI] [PubMed] [Google Scholar]
- Itoh T., Koshiba S., Kigawa T., Kikuchi A., Yokoyama S., Takenawa T. (2001). Role of the ENTH domain in phosphatidylinositol-4,5-bisphosphate binding and endocytosis. Science 291, 1047-1051 [DOI] [PubMed] [Google Scholar]
- Izzard C. S., Lochner L. R. (1976). Cell-to-substrate contacts in living fibroblasts: an interference reflexion study with an evaluation of the technique. J. Cell Sci. 21, 129-159 [DOI] [PubMed] [Google Scholar]
- Kaksonen M., Sun Y., Drubin D. G. (2003). A pathway for association of receptors, adaptors, and actin during endocytic internalization. Cell 115, 475-487 [DOI] [PubMed] [Google Scholar]
- Le Clainche C., Pauly B. S., Zhang C. X., Engqvist-Goldstein A. E., Cunningham K., Drubin D. G. (2007). A Hip1R-cortactin complex negatively regulates actin assembly associated with endocytosis. EMBO J. 26, 1199-1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre-Guillemin V., Wasiak S., Hussain N. K., Angers A., McPherson P. S. (2004). ENTH/ANTH proteins and clathrin-mediated membrane budding. J. Cell Sci. 117, 9-18 [DOI] [PubMed] [Google Scholar]
- Liu T., Mirschberger C., Chooback L., Arana Q., Dal Sacco Z., MacWilliams H., Clarke M. (2002). Altered expression of the 100 kDa subunit of the Dictyostelium vacuolar proton pump impairs enzyme assembly, endocytic function and cytosolic pH regulation. J. Cell Sci. 115, 1907-1918 [DOI] [PubMed] [Google Scholar]
- Merrifield C. J., Feldman M. E., Wan L., Almers W. (2002). Imaging actin and dynamin recruitment during invagination of single clathrin-coated pits. Nat. Cell Biol. 4, 691-698 [DOI] [PubMed] [Google Scholar]
- Merrifield C. J., Qualmann B., Kessels M. M., Almers W. (2004). Neural Wiskott Aldrich Syndrome Protein (N-WASP) and the Arp2/3 complex are recruited to sites of clathrin-mediated endocytosis in cultured fibroblasts. Eur. J. Cell Biol. 83, 13-18 [DOI] [PubMed] [Google Scholar]
- Merrifield C. J., Perrais D., Zenisek D. (2005). Coupling between clathrin-coated-pit invagination, cortactin recruitment, and membrane scission observed in live cells. Cell 121, 593-606 [DOI] [PubMed] [Google Scholar]
- Mishra S. K., Keyel P. A., Hawryluk M. J., Agostinelli N. R., Watkins S. C., Traub L. M. (2002). Disabled-2 exhibits the properties of a cargo-selective endocytic clathrin adaptor. EMBO J. 21, 4915-4926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motley A., Bright N. A., Seaman M. N., Robinson M. S. (2003). Clathrin-mediated endocytosis in AP-2-depleted cells. J. Cell Biol. 162, 909-918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newpher T. M., Lemmon S. K. (2006). Clathrin is important for normal actin dynamics and progression of Sla2p-containing patches during endocytosis in yeast. Traffic 7, 574-588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newpher T. M., Smith R. P., Lemmon V., Lemmon S. K. (2005). In vivo dynamics of clathrin and its adaptor-dependent recruitment to the actin-based endocytic machinery in yeast. Dev. Cell 9, 87-98 [DOI] [PubMed] [Google Scholar]
- O'Halloran T. J., Anderson R. G. (1992). Clathrin heavy chain is required for pinocytosis, the presence of large vacuoles, and development in Dictyostelium. J. Cell Biol. 118, 1371-1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen D. J., Vallis Y., Noble M. E., Hunter J. B., Dafforn T. R., Evans P. R., McMahon H. T. (1999). A structural explanation for the binding of multiple ligands by the alpha-adaptin appendage domain. Cell 97, 805-815 [DOI] [PubMed] [Google Scholar]
- Owen D. J., Collins B. M., Evans P. R. (2004). Adaptors for clathrin coats: structure and function. Annu. Rev. Cell Dev. Biol. 20, 153-191 [DOI] [PubMed] [Google Scholar]
- Rappoport J. Z., Kemal S., Benmerah A., Simon S. M. (2006). Dynamics of clathrin and adaptor proteins during endocytosis. Am. J. Physiol. Cell Physiol. 291, C1072-C1081 [DOI] [PubMed] [Google Scholar]
- Repass S. L., Brady R. J., O'Halloran T. J. (2007). Dictyostelium Hip1r contributes to spore shape and requires epsin for phosphorylation and localization. J. Cell Sci. 120, 3977-3988 [DOI] [PubMed] [Google Scholar]
- Robinson M. S. (2004). Adaptable adaptors for coated vesicles. Trends Cell Biol. 14, 167-174 [DOI] [PubMed] [Google Scholar]
- Schafer D. A. (2002). Coupling actin dynamics and membrane dynamics during endocytosis. Curr. Opin. Cell Biol. 14, 76-81 [DOI] [PubMed] [Google Scholar]
- Schmid E. M., Ford M. G., Burtey A., Praefcke G. J., Peak-Chew S. Y., Mills I. G., Benmerah A., McMahon H. T. (2006). Role of the AP2 beta-appendage hub in recruiting partners for clathrin-coated vesicle assembly. PLoS Biol. 4, e262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigismund S., Woelk T., Puri C., Maspero E., Tacchetti C., Transidico P., Di Fiore P. P., Polo S. (2005). Clathrin-independent endocytosis of ubiquitinated cargos. Proc. Natl. Acad. Sci. USA 102, 2760-2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavrou I., O'Halloran T. J. (2006). The monomeric clathrin assembly protein, AP180, regulates contractile vacuole size in Dictyostelium discoideum. Mol. Biol. Cell 17, 5381-5389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Kaksonen M., Madden D. T., Schekman R., Drubin D. G. (2005). Interaction of Sla2p's ANTH domain with PtdIns(4,5)P2 is important for actin-dependent endocytic internalization. Mol. Biol. Cell 16, 717-730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman M. (1987). Cultivation and synchronous morphogenesis of Dictyostelium under controlled experimental conditions. Methods Cell Biol. 28, 9-29 [DOI] [PubMed] [Google Scholar]
- Traub L. M. (2003). Sorting it out: AP-2 and alternate clathrin adaptors in endocytic cargo selection. J. Cell Biol. 163, 203-208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub L. M., Downs M. A., Westrich J. L., Fremont D. H. (1999). Crystal structure of the alpha appendage of AP-2 reveals a recruitment platform for clathrin-coat assembly. Proc. Natl. Acad. Sci. USA 96, 8907-8912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyaya A., Chabot J. R., Andreeva A., Samadani A., van Oudenaarden A. (2003). Probing polymerization forces by using actin-propelled lipid vesicles. Proc. Natl. Acad. Sci. USA 100, 4521-4526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Virta V. C., Riddelle-Spencer K., O'Halloran T. J. (2003). Compromise of clathrin function and membrane association by clathrin light chain deletion. Traffic 4, 891-901 [DOI] [PubMed] [Google Scholar]
- Wang J., Wang Y., O'Halloran T. J. (2006). Clathrin light chain: importance of the conserved carboxy terminal domain to function in living cells. Traffic 7, 824-832 [DOI] [PubMed] [Google Scholar]
- Wen Y., Stavrou I., Bersuker K., Brady R. J., De Lozanne A., O'Halloran T. J. (2009). AP180-mediated trafficking of Vamp7B limits homotypic fusion of Dictyostelium contractile vacuoles. Mol. Biol. Cell 20, 4278-4288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendland B., Steece K. E., Emr S. D. (1999). Yeast epsins contain an essential N-terminal ENTH domain, bind clathrin and are required for endocytosis. EMBO J. 18, 4383-4393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbur J. D., Chen C. Y., Manalo V., Hwang P. K., Fletterick R. J., Brodsky F. M. (2008). Actin binding by Hip1 (huntingtin-interacting protein 1) and Hip1R (Hip1-related protein) is regulated by clathrin light chain. J. Biol. Chem. 283, 32870-32879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H., Padilla-Parra S., Park S. J., Itoh T., Chaineau M., Monaldi I., Cremona O., Benfenati F., De Camilli P., Coppey-Moisan M., et al. (2009). Dynamic interaction of amphiphysin with N-WASP regulates actin assembly. J. Biol. Chem. 284, 34244-34256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarar D., Waterman-Storer C. M., Schmid S. L. (2005). A dynamic actin cytoskeleton functions at multiple stages of clathrin-mediated endocytosis. Mol. Biol. Cell 16, 964-975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ybe J. A., Mishra S., Helms S., Nix J. (2007). Crystal structure at 2.8 A of the DLLRKN-containing coiled-coil domain of huntingtin-interacting protein 1 (HIP1) reveals a surface suitable for clathrin light chain binding. J. Mol. Biol. 367, 8-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.