Abstract
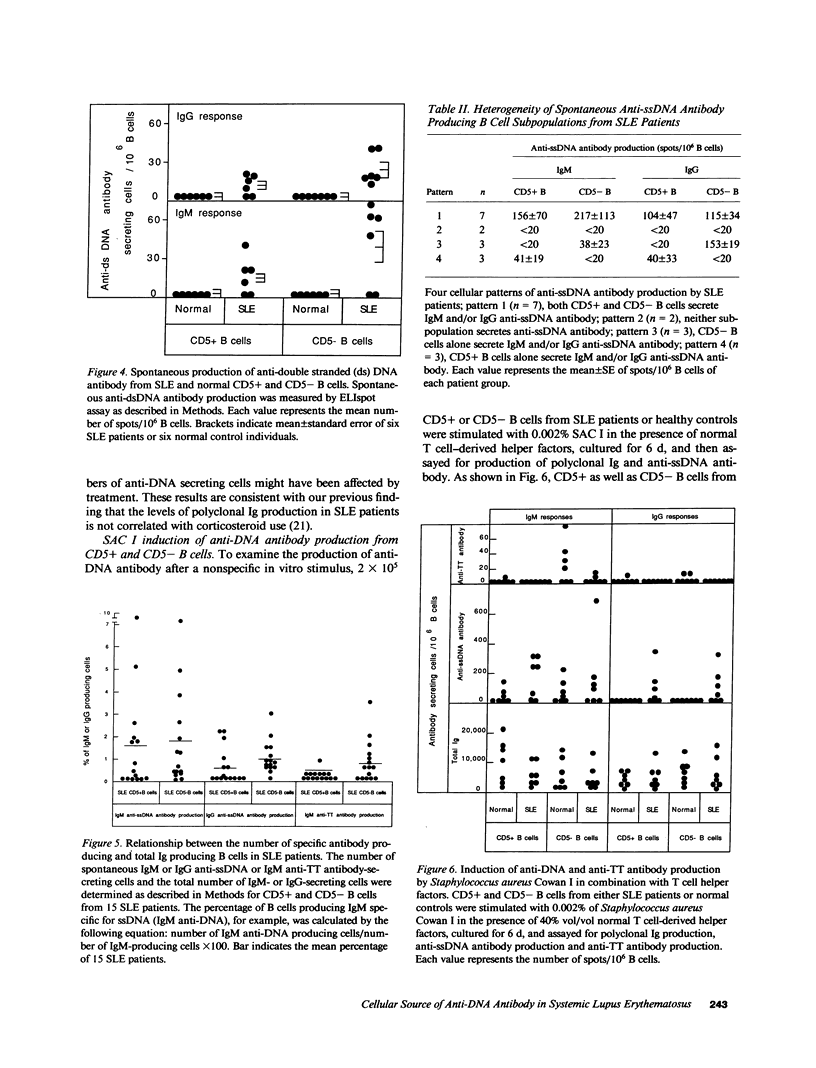
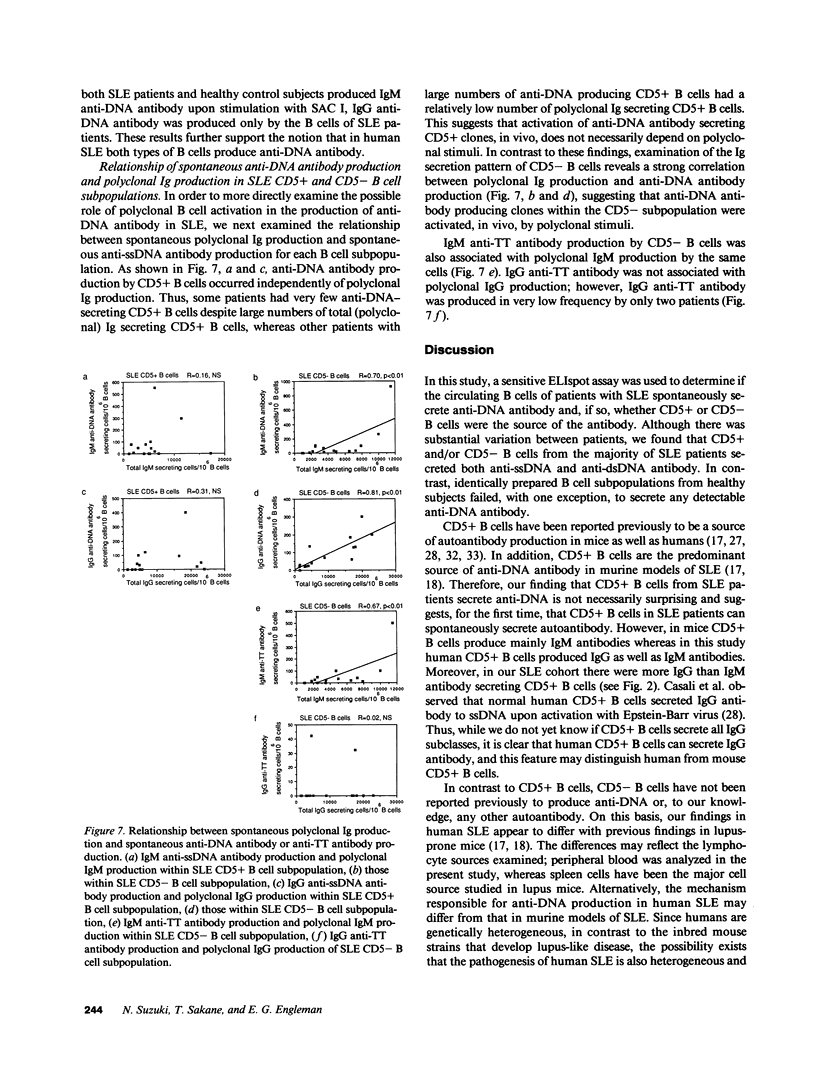
Although the presence of anti-DNA antibody is a hallmark of systemic lupus erythematosus (SLE), neither the subsets of B cells that secrete anti-DNA antibody nor the stimuli responsible for the induction of anti-DNA secretion is known. In particular, the role of CD5+ B cells in human SLE, a distinct subpopulation of antibody-secreting cells shown previously to be a source of anti-DNA antibody in murine models of SLE, is unknown. To approach these questions, we developed a sensitive enzyme-linked immunospot (ELIspot) assay to measure spontaneous secretion of antibody to single-stranded (ss) DNA, double-stranded (ds) DNA, tetanus toxoid, and polyclonal immunoglobulin (Ig) by purified CD5+ and CD5- B cells of 15 SLE patients and 15 healthy control subjects. The B cells of only 1 of 15 healthy subjects secreted a significant level of anti-ssDNA antibody, and none secreted anti-dsDNA. By contrast, in the majority of SLE patients both CD5+ and CD5- B cells secreted IgG and/or IgM anti-ssDNA as well as anti-dsDNA antibody. Further analysis of the anti-ssDNA response revealed that the level of IgG and IgM anti-DNA antibody secretion by CD5- B cells correlated closely with the level of polyclonal Ig production by the same subpopulation (r = 0.81 and 0.70, respectively). In contrast, production of anti-DNA by CD5+ B cells occurred independently of polyclonal Ig production by both CD5+ and CD5- B cell subpopulations. These results suggest that in human SLE there exist two anti-DNA antibody-producing B cell subpopulations with distinct induction mechanisms: one (CD5+), which independently secretes anti-DNA, and another (CD5-), which produces anti-DNA as an apparent consequence of polyclonal B cell activation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdou N. I., Sagawa A., Pascual E., Hebert J., Sadeghee S. Suppressor T-cell abnormality in idiopathic systemic lupus erythematosus. Clin Immunol Immunopathol. 1976 Sep;6(2):192–199. doi: 10.1016/0090-1229(76)90110-0. [DOI] [PubMed] [Google Scholar]
- Ando D. G., Ebling F. M., Hahn B. H. Detection of native and denatured DNA antibody forming cells by the enzyme-linked immunospot assay. A clinical study of (New Zealand black x New Zealand white)F1 mice. Arthritis Rheum. 1986 Sep;29(9):1139–1146. doi: 10.1002/art.1780290912. [DOI] [PubMed] [Google Scholar]
- Antin J. H., Emerson S. G., Martin P., Gadol N., Ault K. A. Leu-1+ (CD5+) B cells. A major lymphoid subpopulation in human fetal spleen: phenotypic and functional studies. J Immunol. 1986 Jan;136(2):505–510. [PubMed] [Google Scholar]
- Budman D. R., Merchant E. B., Steinberg A. D., Doft B., Gershwin M. E., Lizzio E., Reeves J. P. Increased spontaneous activity of antibody-forming cells in the peripheral blood of patients with active SLE. Arthritis Rheum. 1977 Apr;20(3):829–833. doi: 10.1002/art.1780200312. [DOI] [PubMed] [Google Scholar]
- Casali P., Burastero S. E., Nakamura M., Inghirami G., Notkins A. L. Human lymphocytes making rheumatoid factor and antibody to ssDNA belong to Leu-1+ B-cell subset. Science. 1987 Apr 3;236(4797):77–81. doi: 10.1126/science.3105056. [DOI] [PubMed] [Google Scholar]
- Caton A. J., Brownlee G. G., Staudt L. M., Gerhard W. Structural and functional implications of a restricted antibody response to a defined antigenic region on the influenza virus hemagglutinin. EMBO J. 1986 Jul;5(7):1577–1587. doi: 10.1002/j.1460-2075.1986.tb04399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chused T. M., McCoy K. L., Lal R. B., Brown E. M., Baker P. J. Multigenic basis of autoimmune disease in New Zealand mice. Concepts Immunopathol. 1987;4:129–143. [PubMed] [Google Scholar]
- Clarke S. H., Huppi K., Ruezinsky D., Staudt L., Gerhard W., Weigert M. Inter- and intraclonal diversity in the antibody response to influenza hemagglutinin. J Exp Med. 1985 Apr 1;161(4):687–704. doi: 10.1084/jem.161.4.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfraissy J. F., Segond P., Galanaud P., Wallon C., Massias P., Dormont J. Depressed primary in vitro antibody response in untreated systemic lupus erythematosus. T helper cell defect and lack of defective suppressor cell function. J Clin Invest. 1980 Jul;66(1):141–148. doi: 10.1172/JCI109827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziarski R. Anti-immunoglobulin autoantibodies are not preferentially induced in polyclonal activation of human and mouse lymphocytes, and more anti-DNA and anti-erythrocyte autoantibodies are induced in polyclonal activation of mouse than human lymphocytes. J Immunol. 1984 Nov;133(5):2537–2544. [PubMed] [Google Scholar]
- Dziarski R. Autoimmunity: polyclonal activation or antigen induction? Immunol Today. 1988 Nov;9(11):340–342. doi: 10.1016/0167-5699(88)91333-3. [DOI] [PubMed] [Google Scholar]
- Dziarski R. Preferential induction of autoantibody secretion in polyclonal activation by peptidoglycan and lipopolysaccharide. I. In vitro studies. J Immunol. 1982 Mar;128(3):1018–1025. [PubMed] [Google Scholar]
- Eaton R. B., Schnneider G., Schur P. H. Enzyme immunoassay for antibodies to native DNA. Specificity and quality of antibodies. Arthritis Rheum. 1983 Jan;26(1):52–62. doi: 10.1002/art.1780260109. [DOI] [PubMed] [Google Scholar]
- Engleman E. G., Benike C. J., Grumet F. C., Evans R. L. Activation of human T lymphocyte subsets: helper and suppressor/cytotoxic T cells recognize and respond to distinct histocompatibility antigens. J Immunol. 1981 Nov;127(5):2124–2129. [PubMed] [Google Scholar]
- Fauci A. S., Steinberg A. D., Haynes B. F., Whalen G. Immunoregulatory aberrations in systemic lupus erythematosus. J Immunol. 1978 Oct;121(4):1473–1479. [PubMed] [Google Scholar]
- Fish F., Ziff M. The in vitro and in vivo induction of anti-double-stranded DNA antibodies in normal and autoimmune mice. J Immunol. 1982 Jan;128(1):409–414. [PubMed] [Google Scholar]
- Gadol N., Ault K. A. Phenotypic and functional characterization of human Leu1 (CD5) B cells. Immunol Rev. 1986 Oct;93:23–34. doi: 10.1111/j.1600-065x.1986.tb01500.x. [DOI] [PubMed] [Google Scholar]
- Gharavi A. E., Chu J. L., Elkon K. B. Autoantibodies to intracellular proteins in human systemic lupus erythematosus are not due to random polyclonal B cell activation. Arthritis Rheum. 1988 Nov;31(11):1337–1345. doi: 10.1002/art.1780311101. [DOI] [PubMed] [Google Scholar]
- Ginsburg W. W., Finkelman F. D., Lipsky P. E. Circulating and pokeweed mitogen-induced immunoglobulin-secreting cells in systemic lupus erythematosus. Clin Exp Immunol. 1979 Jan;35(1):76–88. [PMC free article] [PubMed] [Google Scholar]
- Hardin J. A. The lupus autoantigens and the pathogenesis of systemic lupus erythematosus. Arthritis Rheum. 1986 Apr;29(4):457–460. doi: 10.1002/art.1780290401. [DOI] [PubMed] [Google Scholar]
- Hardy R. R., Hayakawa K. Development and physiology of Ly-1 B and its human homolog, Leu-1 B. Immunol Rev. 1986 Oct;93:53–79. doi: 10.1111/j.1600-065x.1986.tb01502.x. [DOI] [PubMed] [Google Scholar]
- Hardy R. R., Hayakawa K., Shimizu M., Yamasaki K., Kishimoto T. Rheumatoid factor secretion from human Leu-1+ B cells. Science. 1987 Apr 3;236(4797):81–83. doi: 10.1126/science.3105057. [DOI] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Herzenberg L. A., Herzenberg L. A. Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J Exp Med. 1985 Jun 1;161(6):1554–1568. doi: 10.1084/jem.161.6.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Honda M., Herzenberg L. A., Steinberg A. D., Herzenberg L. A. Ly-1 B cells: functionally distinct lymphocytes that secrete IgM autoantibodies. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2494–2498. doi: 10.1073/pnas.81.8.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izui S., McConahey P. J., Dixon F. J. Increased spontaneous polyclonal activation of B lymphocytes in mice with spontaneous autoimmune disease. J Immunol. 1978 Dec;121(6):2213–2219. [PubMed] [Google Scholar]
- Kipps T. J., Vaughan J. H. Genetic influence on the levels of circulating CD5 B lymphocytes. J Immunol. 1987 Aug 15;139(4):1060–1064. [PubMed] [Google Scholar]
- Klinman D. M., Ishigatsubo Y., Steinberg A. D. Acquisition and maturation of expressed B cell repertoires in normal and autoimmune mice. J Immunol. 1988 Aug 1;141(3):801–806. [PubMed] [Google Scholar]
- Klinman D. M., Steinberg A. D. Proliferation of anti-DNA-producing NZB B cells in a non-autoimmune environment. J Immunol. 1986 Jul 1;137(1):69–75. [PubMed] [Google Scholar]
- Klinman D. M., Steinberg A. D. Systemic autoimmune disease arises from polyclonal B cell activation. J Exp Med. 1987 Jun 1;165(6):1755–1760. doi: 10.1084/jem.165.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
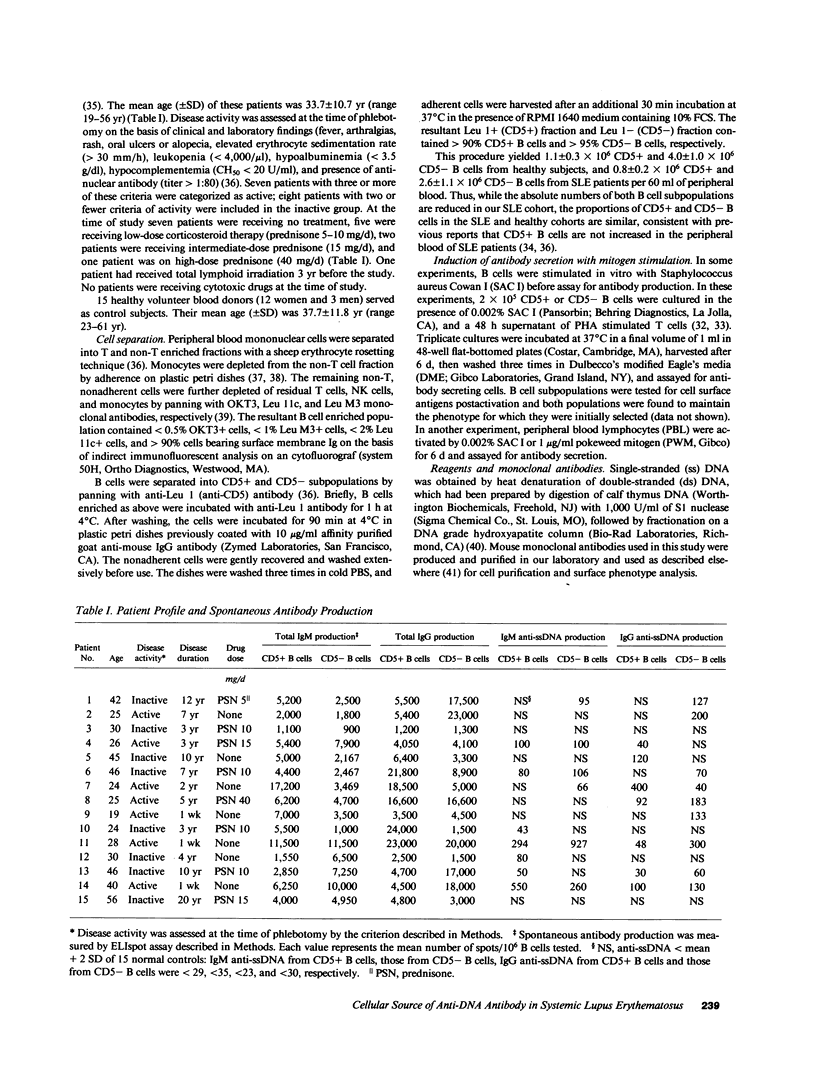
- Locker J. D., Medof M. E., Bennett R. M., Sukhupunyaraksa S. Characterization of DNA used to assay sera for anti-DNA antibodies; determination of the specificities of anti-DNA antibodies in SLE and non-SLE rheumatic disease states. J Immunol. 1977 Feb;118(2):694–701. [PubMed] [Google Scholar]
- Logtenberg T., Jonker M., Kroon A., Gmelig-Meyling F. H., Ballieux R. E. Enumeration of (auto)antibody producing cells in human using the "spot-ELISA". Immunol Lett. 1985;9(6):343–347. doi: 10.1016/0165-2478(85)90060-4. [DOI] [PubMed] [Google Scholar]
- Logtenberg T., Melissen P. M., Kroon A., Gmelig-Meyling F. H., Ballieux R. E. Autoreactive B cells in normal humans. Autoantibody production upon lymphocyte stimulation with autoantigen-xenoantigen conjugates. J Immunol. 1988 Jan 15;140(2):446–450. [PubMed] [Google Scholar]
- Morimoto C., Steinberg A. D., Letvin N. L., Hagan M., Takeuchi T., Daley J., Levine H., Schlossman S. F. A defect of immunoregulatory T cell subsets in systemic lupus erythematosus patients demonstrated with anti-2H4 antibody. J Clin Invest. 1987 Mar;79(3):762–768. doi: 10.1172/JCI112882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M., Burastero S. E., Notkins A. L., Casal P. Human monoclonal rheumatoid factor-like antibodies from CD5 (Leu-1)+ B cells are polyreactive. J Immunol. 1988 Jun 15;140(12):4180–4186. [PubMed] [Google Scholar]
- Nakamura M., Burastero S. E., Ueki Y., Larrick J. W., Notkins A. L., Casali P. Probing the normal and autoimmune B cell repertoire with Epstein-Barr virus. Frequency of B cells producing monoreactive high affinity autoantibodies in patients with Hashimoto's disease and systemic lupus erythematosus. J Immunol. 1988 Dec 15;141(12):4165–4172. [PubMed] [Google Scholar]
- Nelson J. L., Nardella F. A., Oppliger I. R., Mannik M. Rheumatoid factors from patients with rheumatoid arthritis possess private repertoires of idiotypes. J Immunol. 1987 Mar 1;138(5):1391–1396. [PubMed] [Google Scholar]
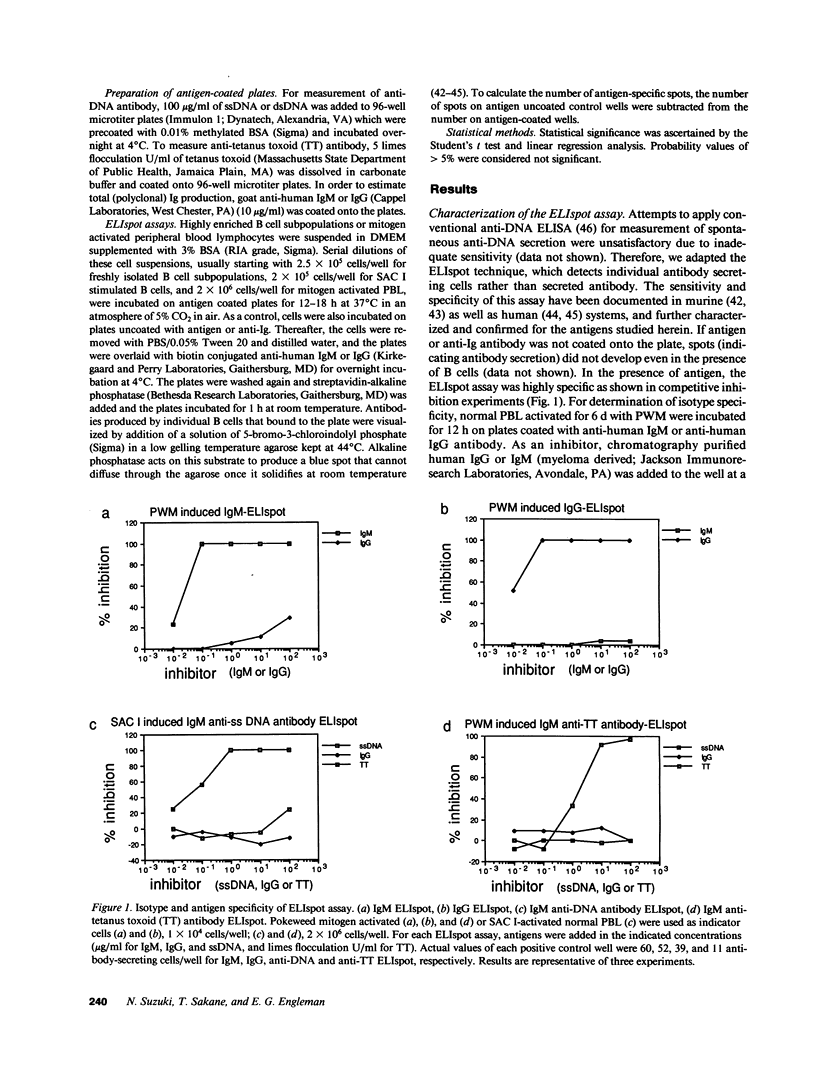
- Portanova J. P., Claman H. N., Kotzin B. L. Autoimmunization in murine graft-vs-host disease. I. Selective production of antibodies to histones and DNA. J Immunol. 1985 Dec;135(6):3850–3856. [PubMed] [Google Scholar]
- Prud'homme G. J., Balderas R. S., Dixon F. J., Theofilopoulos A. N. B cell dependence on and response to accessory signals in murine lupus strains. J Exp Med. 1983 Jun 1;157(6):1815–1827. doi: 10.1084/jem.157.6.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard Y., Leprince C., Dugas B., Treton D., Galanaud P. Reactivity of Leu-1+ tonsillar B cells to a high molecular weight B cell growth factor. J Immunol. 1987 Sep 1;139(5):1563–1567. [PubMed] [Google Scholar]
- Rivas A., Koide J., Cleary M. L., Engleman E. G. Evidence for involvement of the gamma, delta T cell antigen receptor in cytotoxicity mediated by human alloantigen-specific T cell clones. J Immunol. 1989 Mar 15;142(6):1840–1846. [PubMed] [Google Scholar]
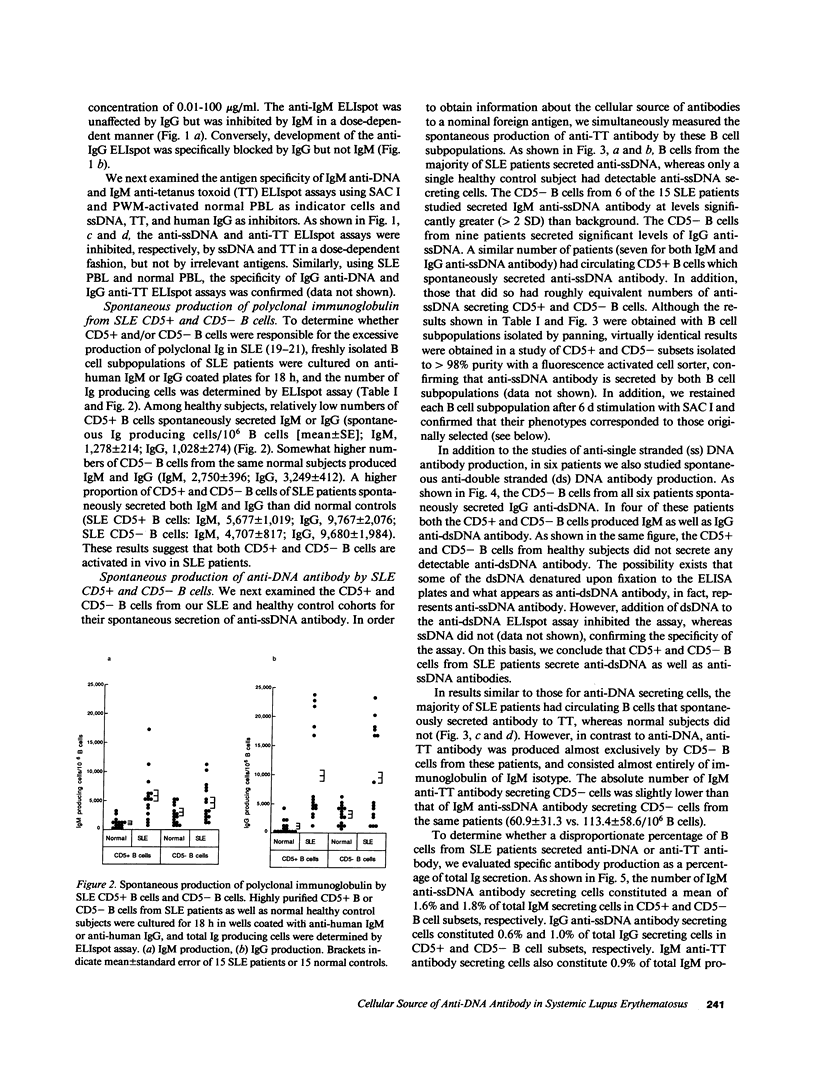
- Sakane T., Suzuki N., Takada S., Ueda Y., Murakawa Y., Tsuchida T., Yamauchi Y., Kishimoto T. B cell hyperactivity and its relation to distinct clinical features and the degree of disease activity in patients with systemic lupus erythematosus. Arthritis Rheum. 1988 Jan;31(1):80–87. doi: 10.1002/art.1780310112. [DOI] [PubMed] [Google Scholar]
- Sakane T., Takada S., Suzuki N., Tsuchida T., Murakawa Y., Ueda Y. Deficiencies in suppressor T cell activity seen in patients with active systemic lupus erythematosus are due to the dilution of normally functioning suppressor T cells by nonsuppressor T cells. J Immunol. 1986 Dec 15;137(12):3809–3813. [PubMed] [Google Scholar]
- Sanz I., Dang H., Takei M., Talal N., Capra J. D. VH sequence of a human anti-Sm autoantibody. Evidence that autoantibodies can be unmutated copies of germline genes. J Immunol. 1989 Feb 1;142(3):883–887. [PubMed] [Google Scholar]
- Sedgwick J. D., Holt P. G. A solid-phase immunoenzymatic technique for the enumeration of specific antibody-secreting cells. J Immunol Methods. 1983 Feb 25;57(1-3):301–309. doi: 10.1016/0022-1759(83)90091-1. [DOI] [PubMed] [Google Scholar]
- Shlomchik M. J., Aucoin A. H., Pisetsky D. S., Weigert M. G. Structure and function of anti-DNA autoantibodies derived from a single autoimmune mouse. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9150–9154. doi: 10.1073/pnas.84.24.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlomchik M. J., Marshak-Rothstein A., Wolfowicz C. B., Rothstein T. L., Weigert M. G. The role of clonal selection and somatic mutation in autoimmunity. 1987 Aug 27-Sep 2Nature. 328(6133):805–811. doi: 10.1038/328805a0. [DOI] [PubMed] [Google Scholar]
- Shores E. W., Eisenberg R. A., Cohen P. L. Role of the Sm antigen in the generation of anti-Sm autoantibodies in the SLE-prone MRL mouse. J Immunol. 1986 May 15;136(10):3662–3667. [PubMed] [Google Scholar]
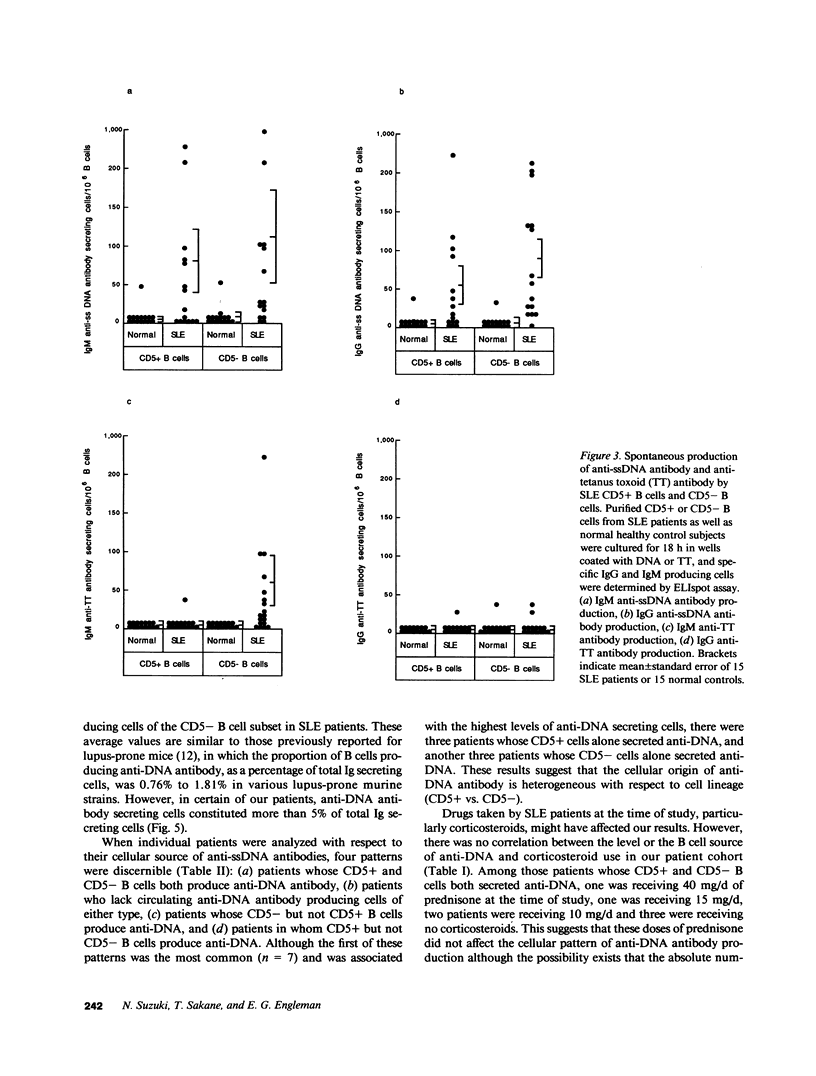
- Smith H. R., Steinberg A. D. Autoimmunity--a perspective. Annu Rev Immunol. 1983;1:175–210. doi: 10.1146/annurev.iy.01.040183.001135. [DOI] [PubMed] [Google Scholar]
- Suzuki N., Sakane T. Induction of excessive B cell proliferation and differentiation by an in vitro stimulus in culture in human systemic lupus erythematosus. J Clin Invest. 1989 Mar;83(3):937–944. doi: 10.1172/JCI113979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N., Sakane T., Ueda Y., Murakawa Y., Tsunematsu T. Implications for the role of cognate interactions in in vitro human B cell activation by Staphylococcus aureus Cowan I and pokeweed mitogen. J Clin Invest. 1986 Jan;77(1):294–300. doi: 10.1172/JCI112290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N., Ueda Y., Sakane T. Differential mechanism for differentiation into immunoglobulin-secreting cells in human resting B lymphocyte subsets isolated on the basis of cell density. J Clin Invest. 1988 Jan;81(1):261–269. doi: 10.1172/JCI113304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan E. M., Cohen A. S., Fries J. F., Masi A. T., McShane D. J., Rothfield N. F., Schaller J. G., Talal N., Winchester R. J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982 Nov;25(11):1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- Taniguchi O., Miyajima H., Hirano T., Noguchi M., Ueda A., Hashimoto H., Hirose S., Okumura K. The Leu-1 B-cell subpopulation in patients with rheumatoid arthritis. J Clin Immunol. 1987 Nov;7(6):441–448. doi: 10.1007/BF00915053. [DOI] [PubMed] [Google Scholar]
- Tarlinton D., Stall A. M., Herzenberg L. A. Repetitive usage of immunoglobulin VH and D gene segments in CD5+ Ly-1 B clones of (NZB x NZW)F1 mice. EMBO J. 1988 Dec 1;7(12):3705–3710. doi: 10.1002/j.1460-2075.1988.tb03253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teodorescu M. Characterization and role in autoimmune diseases of the polyclonal B-cell activator produced by T-cells-the helper factor. Immunol Rev. 1981;55:155–178. doi: 10.1111/j.1600-065x.1981.tb00342.x. [DOI] [PubMed] [Google Scholar]
- Theofilopoulos A. N., Dixon F. J. Murine models of systemic lupus erythematosus. Adv Immunol. 1985;37:269–390. doi: 10.1016/s0065-2776(08)60342-9. [DOI] [PubMed] [Google Scholar]
- Theofilopoulos A. N., Shawler D. L., Eisenberg R. A., Dixon F. J. Splenic immunoglobulin-secreting cells and their regulation in autoimmune mice. J Exp Med. 1980 Feb 1;151(2):446–466. doi: 10.1084/jem.151.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rappard-Van der Veen F. M., Kiesel U., Poels L., Schuler W., Melief C. J., Landegent J., Gleichmann E. Further evidence against random polyclonal antibody formation in mice with lupus-like graft-vs-host disease. J Immunol. 1984 Apr;132(4):1814–1820. [PubMed] [Google Scholar]



