Abstract
Emerging evidence suggests that eukaryotic gene transcription is regulated primarily at the elongation stage by association and dissociation of the inhibitory protein cardiac lineage protein 1 (CLP-1/HEXIM1) from the positive transcription elongation factor b (P-TEFb) complex. It was reported recently that P-TEFb interacts with skeletal muscle-specific regulatory factor, MyoD, suggesting a linkage between CLP-1-mediated control of transcription and skeletal myogenesis. To examine this, we produced CLP-1 knockdown skeletal muscle C2C12 cells by homologous recombination, and demonstrated that the C2C12 CLP-1 +/− cells failed to differentiate when challenged by low serum in the medium. We also showed that CLP-1 interacts with both MyoD and histone deacetylases (HDACs) maximally at the early stage of differentiation of C2C12 cells. This led us to hypothesize that the association might be crucial to inhibition of MyoD-target proliferative genes. Chromatin immunoprecipitation analysis revealed that the CLP-1/MyoD/HDAC complex binds to the promoter of the cyclin D1 gene, which is downregulated in differentiated muscle cells. These findings suggest a novel transcriptional paradigm whereby CLP-1, in conjunction with MyoD and HDAC, acts to inhibit growth-related gene expression, a requirement for myoblasts to exit the cell cycle and transit to myotubes.
Keywords: CLP-1, HEXIM1, MyoD, P-TEFb, Skeletal muscle
Introduction
Skeletal muscle development involves coordinated expression of transcription factors that control specification of mesodermal progenitors to the muscle fate and differentiation of committed myoblasts into myotubes (Stockdale, 1992). The development of skeletal muscle is directed by four myogenic regulatory factors (MRFs): MyoD, myf-5, MRF4 and myogenin (Sabourin and Rudnicki, 2000; Yun and Wold, 1996). MyoD and myf5 are expressed in proliferating myoblasts and influence lineage restriction, whereas myogenin, a target of MyoD, is induced upon differentiation (Rudnicki and Jaenisch, 1995). The MRFs heterodimerize with ubiquitous E-proteins, bind to conserved E-box sequences (CANNTG) in the promoter of muscle-specific genes, and control transcription (French et al., 1991). The transcriptional activity of MyoD is influenced by its interaction with an array of coactivators and corepressors (McKinsey et al., 2001). The repressor protein histone deacetylase 1 (HDAC1) was reported to interact with MyoD and deacetylate it, consequently suppressing the transcriptional activity of MyoD (Mal et al., 2001). Activator proteins, histone acetylases (HATs), on the other hand, are known to stimulate MyoD-dependent transcription by engaging histone acetylases p300 and PCAF, which, in turn, promote acetylation of MyoD itself and increase its affinity for target-gene promoters (Puri et al., 1997; Sartorelli et al., 1999). It is well known that the positive transcription elongation factor b (P-TEFb) complex, which consists of cdk9 and cyclin T1 (or the minor forms T2a and T2b), mediates the transcription of RNA polymerase II (Pol II) genes (Fu et al., 1999; Peng et al., 1998). P-TEFb phosphorylates the C-terminal domain (CTD) of the largest subunit of Pol II at serine 2 of a 52-tandem heptapeptide; this phosphorylation is required for transcription to change from the abortive to productive phase of transcriptional elongation (Price, 2000; Zhou et al., 2000).
We and others have demonstrated that the mouse cardiac lineage protein 1 (CLP-1) (Huang et al., 2004), and its human homolog HEXIM1 (Schulte et al., 2005), function as transcriptional repressors. In HeLa cells, P-TEFb exists in equilibrium between active and inactive forms by way of association and dissociation of HEXIM1 from the P-TEFb complex (Nguyen et al., 2001; Yang et al., 2001). Our laboratory knocked out the CLP-1 gene in mice, which resulted in lethality in late fetal stages (E17–E18) due to hypertrophic growth of the embryonic hearts (Huang et al., 2002; Huang et al., 2004). Subsequently, studies in our laboratory demonstrated that introduction of CLP-1 heterozygosity +/− in the background of cardiac-specific cyclin T1 overexpression enhanced RNA Pol II phosphorylation at serine 2 (Espinoza-Derout et al., 2009).
Skeletal muscle differentiation, characterized by silencing of the proliferative genes and up-regulation of muscle-specific genes, is prominently influenced by MyoD, which is known to target more than 300 genes controlling several subprograms of skeletal muscle gene expression (Bergstrom et al., 2002). The reported link between MyoD and P-TEFb prompted us to examine whether CLP-1 is involved in control of P-TEFb activity during the transition of myoblasts to myotubes. In this study, we report that CLP-1 associates with MyoD and HDAC proteins in the early phase of differentiation of C2C12 myoblasts and conclude that the CLP-1/MyoD/HDAC complex is crucial for control of P-TEFb activity and in regulation of skeletal muscle cell differentiation.
Results
Association of CLP-1 with P-TEFb and MyoD in C2C12 cell differentiation
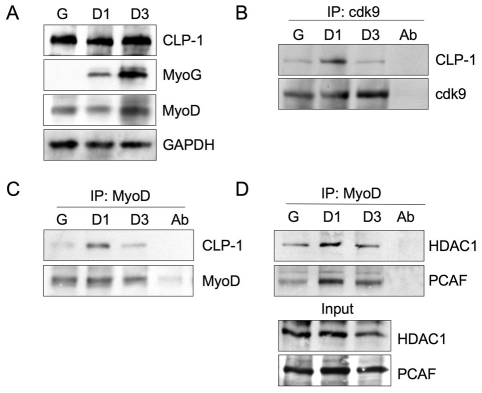
To investigate the role of CLP-1 in skeletal muscle cell differentiation, we first examined its expression in C2C12 skeletal muscle cells, which represent a highly suitable model for analysis of myogenic differentiation. In high serum (10% FBS) growth medium, C2C12 myoblasts proliferate until they reach confluency. Differentiation into multinucleated myotubes is triggered by switching to low serum (2% horse serum) differentiation medium. As shown in Fig. 1A, CLP-1 was present in C2C12 cells in growth medium (growth stage G) and in differentiation medium at 24 hours (differentiation stage D1) and 72 hours (differentiation stage D3). Myogenin, a known marker for differentiation, is expressed At D1 and D3 but not in G. MyoD is expressed in both myoblasts and myotubes, as expected.
Fig. 1.
Association of CLP-1 with cdk9 and MyoD is dynamic in C2C12 cell differentiation. Whole-cell lysates were collected from growth medium (G) and differentiation medium at 24 hours (D1) and at 72 hours (D3). (A) Equal amounts of protein were subjected to SDS-PAGE followed by immunoblotting. Myogenin (MyoG) served as a marker of differentiation. GAPDH served as a loading control. (B) Immunoprecipitation (IP) of C2C12 cell lysates with anti-cdk9 antibody, and western blotting with anti-CLP-1 antibody. Western blot with anti-cdk9 antibody served as a control of total immunoprecipitated protein. (C) IP of C2C12 cells with anti-MyoD antibody and western blotting with anti-CLP-1 antibody. Western blot with anti-MyoD antibody served as a control of total immunoprecipitated protein. (D) IP using C2C12 cell lysates with anti-MyoD antibody and western blotting with anti-HDAC1 and anti-PCAF antibodies. Direct western blots of HDAC1 and PCAF served as input controls. In all cases, IP with antibody alone served as a control for immunoreactivity (Ab). Data shown represent one of three separate experiments.
Next, CLP-1 association to the P-TEFb complex was analyzed by immunoprecipitation with anti-cdk9 antibody followed by western blotting with anti-CLP-1 antibody (Fig. 1B). There was a prominent level of association of CLP-1 with P-TEFb in early differentiation (D1), whereas in both proliferative cells (G) and the terminally differentiated cells (D3) it was negligible. Thus, it appeared that there was a clear shift in P-TEFb equilibrium to the CLP-1 bound state in C2C12 cell differentiation, which suggests that CLP-1-mediated regulation of P-TEFb activity might have a role in the transition of C2C12 cells from growth to differentiation.
Because the kinase activity of P-TEFb is influenced by the association of CLP-1, one might envision that association of CLP-1 to the P-TEFb/MyoD complex will regulate MyoD-mediated transcriptional activity. We coimmunoprecipitated with MyoD antibody using lysates from proliferating C2C12 myoblasts and from cells induced to differentiate for 24 hours and 72 hours. We observed that there was association between MyoD and CLP-1 in the differentiation stage D1, whereas it was markedly reduced in G and D3 (Fig. 1C).
HDACs are implicated in the regulation of skeletal myogenesis by their interaction with MyoD and in regulating MyoD-mediated gene transcription (Mal et al., 2001). HDAC1 binding to MyoD is implicated in blocking the function of MyoD in initiating the myogenic program. To examine whether MyoD association with HDAC1 occurs in growth and/or differentiation conditions, coimmunoprecipitations were performed on proliferating C2C12 cells and on differentiating C2C12 cells at D1 and D3 (Fig. 1D). The results show that MyoD binds to HDAC1 and PCAF in both growth and differentiation conditions, albeit at a relatively lower level in growth conditions. The association of MyoD with HATs and HDACs suggests that both activation and suppression mechanisms coexist, perhaps in directing distinct promoters toward differentiation.
Association of CLP-1 with HDACs in C2C12 cell differentiation
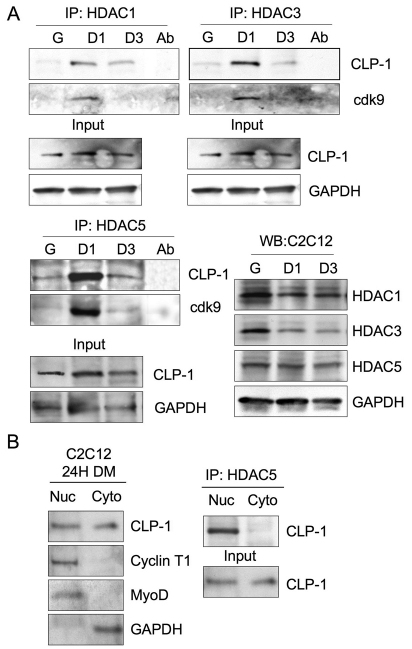
We then examined the interaction of CLP-1 with HDACs in C2C12 cells using immunoprecipitation with antibodies against HDAC1, HDAC3 and HDAC5, and western blotting for the presence of CLP-1 and cdk9. As seen in Fig. 2A, CLP-1 associates with class I HDAC1 and HDAC3 and with class II HDAC5, preferentially at the D1 stage of differentiation. HEXIM1 was previously reported to bind directly to HDAC3 in HeLa cells (Fu et al., 2007). Also, upon western blotting with anti-cdk9 antibody, we observed that association of cdk9 is highest in D1 (Fig. 2A). The pattern of CLP-1 association with HDACs was distinct from HDAC protein expression, as seen by direct western blotting (Fig. 2A, bottom right panel). It is known that class II HDACs, which includes HDAC5, translocate from the nucleus to the cytoplasm, whereas Class I HDACs are restricted to the nucleus. To ascertain whether CLP-1 association to HDAC5 is nuclear or cytoplasmic, we fractionated C2C12 cells and immunoprecipitated with anti-HDAC5 antibody. Western blotting with CLP-1 antibody showed that CLP-1 was present in both the nuclear and cytosolic fractions, whereas cyclin T1 and MyoD were present in nuclei only, as expected (Fig. 2B). Coimmunoprecipitation analysis showed that the CLP-1/HDAC5 complex was localized only in the nuclear fraction (Fig. 2B).
Fig. 2.
CLP-1 associates with HDACs at the onset of C2C12 cell differentiation. (A) Immunoprecipitation (IP) of C2C12 cell lysates from growth (G) and differentiation medium at 24 hours (D1) and at 72 hours (D3) with antibodies specific for HDAC1, HDAC3 and HDAC5, and western blot with anti-CLP-1 and anti-cdk9 antibodies. IP with antibody alone served as a control for immunoreactivity (Ab). Western blot with anti-CLP-1 and anti-GAPDH antibodies served as input controls. Data represent one of three separate experiments for each antibody. Bottom right panel: western blot of total cell lysates with anti-HDAC1, anti-HDAC3 and anti-HDAC5 antibodies. Western blot with GAPDH served as input control. (B) Left panel: nuclear (Nuc) and cytoplasmic (Cyto) fractions of C2C12 cells in differentiation medium at 24 hours were subjected to direct western blotting with antibodies specific for CLP-1, cyclin T1, MyoD and GAPDH. Right panel: lysates were subjected to IP with anti-HDAC5 antibody and western blotting with anti-CLP-1 antibody. Direct western blot of CLP-1 served as an input control.
CLP-1 associates with MyoD and HDAC on the cyclin D1 promoter
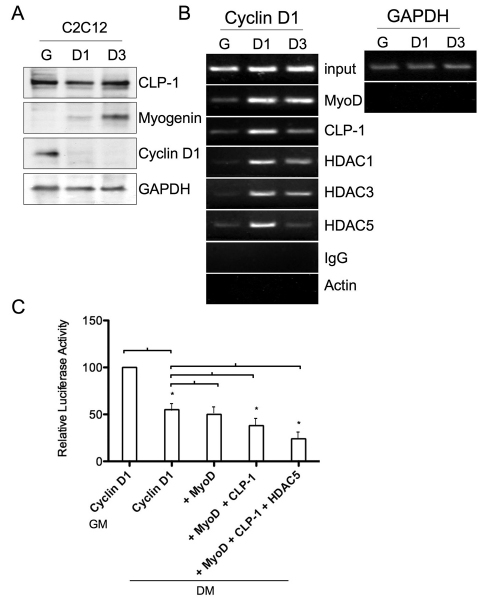
In order to gain mechanistic insights into how CLP-1 regulates skeletal muscle differentiation, we hypothesized that CLP-1 associates with MyoD and HDAC and is involved in downregulation of cell-cycle genes, for example cyclin D1, to allow expression of differentiation-specific genes. We examined association of this inhibitory complex to the cyclin D1 promoter because cyclin D1 is a cell cycle regulatory protein whose levels were reported to change in response to HEXIM1 (Ogba et al., 2008). Cyclin D1 protein in C2C12 cells is expressed only under growth conditions, as shown by western blotting (Fig. 3A). Chromatin immunoprecipitation (ChIP) assay revealed that the inhibitory complex was associated with the promoter of the MyoD-target cyclin D1 gene. DNA was amplified using primers flanking two putative E-box sequences, the MyoD target-site, within the cyclin D1 promoter (insert size 188 bp). Complexes containing CLP-1/MyoD/HDAC were associated with the cyclin D1 promoter at MyoD-target binding sites, preferentially in differentiating muscle cells (Fig. 3B). MyoD association was maximal in D1 and D3. CLP-1, HDAC1 and HDAC3 had little association in G as compared with D1. The association of HDAC5 was reduced in G and D3. These results are consistent with our immunoprecipitation experimental data above.
Fig. 3.
Analysis of the cyclin D1 promoter in C2C12 cell differentiation. (A) Analysis of C2C12 cells in growth medium (G), and differentiation medium at 24 hours (D1) and 72 hours (D3) by western blotting with antibodies specific for CLP-1, myogenin and cyclin D1. GAPDH served as a loading control. (B) ChIP was performed on C2C12 cells using antibodies specific for MyoD, CLP-1, HDAC1, HDAC3 and HDAC5. Nonspecific IgG and anti-actin antibody served as negative controls. Precipitated DNA was amplified by PCR for regions of the cyclin D1 gene corresponding to the 5′ upstream promoter region encompassing two E-box sequences. Input DNA represents 10% of total chromatin used in each reaction. Primers specific to GAPDH were used before (input) and after immunoprecipitation as a control to monitor immunoprecipitation specificity. Data represent one of three separate experiments. (C) Luciferase assay in C2C12 cells in growth media (GM) and for 24 hours in differentiation media (DM). Cells were co-transfected with cyclin D1-luciferase plasmid along with expression plasmids encoding MyoD, CLP-1 and HDAC5. Renilla luciferase expression was used for normalization. Luciferase activities for cyclin-D1 in differentiation media is expressed relative to the mean value derived from cells co-transfected with cyclin D1-luciferase and Renilla luciferase in growth media. Luciferase activity for + MyoD, + MyoD + CLP-1 and + MyoD + CLP-1, + HDAC5 in differentiation media are expressed relative to the mean value derived from cells co-transfected with cyclin D1-luciferase and Renilla luciferase in differentiation media. Experiments were repeated three times. Bars show mean + s.e.m., *P<0.05.
To examine regulation of cyclin D1 expression by CLP-1, we performed transient transfection assays in C2C12 cells under growth and differentiation conditions. There was a drop in cyclin D1 promoter activity in differentiation conditions compared to growth conditions (Fig. 3C). Co-transfection with MyoD in differentiation medium did not appear to have any effect on cyclin D1 expression. However, co-transfection of MyoD and CLP-1 caused inhibition of cyclin D1 expression (Fig. 3C). Co-transfection with MyoD, CLP-1 and HDAC5 further reduced Cyclin D1 reporter activity. MyoD has been reported to act as a transcriptional activator as well as inhibitor (Bergstrom et al., 2002). In C2C12 skeletal muscle, MyoD inhibited the cyclin D1 promoter, and there was a synergistic effect when negative regulators CLP-1 and HDAC5 were added.
Knock down of CLP-1 inhibits C2C12 cell differentiation
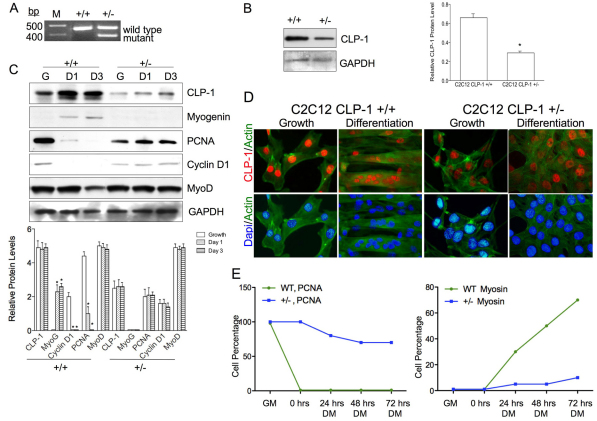
To further investigate the putative function of CLP-1 in early myogenic differentiation, we chose to knock down CLP-1 in C2C12 cells by homologous recombination. C2C12 cells were first transfected with a CLP-1 replacement gene-targeting vector (Huang et al., 2004) and then selected for neomycin resistance. Single clones were examined for expression of the recombinant allele by PCR (Fig. 4A). Individual clones were also assayed for CLP-1 protein level by western blot (Fig. 4B). The CLP-1 protein level was lower in CLP-1 heterozygote (+/−) C2C12 cells than in wild-type (+/+) C2C12 cells, as expected. When C2C12 CLP-1 +/− cells were challenged to differentiate by switching to low-serum medium, the cells were unable to differentiate, as indicated by the absence of myogenin protein in D1 and D3 (Fig. 4C). The CLP-1 +/− cells continued to proliferate, as indicated by the presence of proliferating cell nuclear antigen (PCNA) and cyclin D1 in differentiation conditions (Fig. 4C). The analysis was quantified using densitometry and the results depicted graphically (Fig. 4C, bottom panel). To confirm the differentiation deficiency, we performed immunofluorescence analysis on cells in growth and differentiation medium (Fig. 4D). Under differentiation conditions, wild-type C2C12 cells fused into multinucleated myotubes. By contrast, when challenged to differentiate in low-serum medium, C2C12 CLP-1 +/− cells remained mononucleated and maintained a nondifferentiated phenotype. We also assessed the proliferation and differentiation rate of these cells through immunofluorescence with anti-PCNA and anti-myosin heavy chain antibodies under growth conditions and after differentiation for 24, 48, and 72 hours. Stained cells were counted and compared to total cells, and the results depicted graphically (Fig. 4E). In wild-type cells, PCNA was only expressed under growth conditions, whereas in CLP-1 +/− C2C12 cells, it was also expressed in differentiation media. Also, in differentiation conditions wild-type cells begin to form tubes, expressing myosin heavy chain at 24 hours in differentiation media, whereas heterozygote cells did not express myosin heavy chain except for a minor expression at 72 hours.
Fig. 4.
C2C12 CLP-1 +/− cells are differentiation deficient. (A) PCR using DNA from C2C12 CLP-1 +/+ cells and one CLP-1 +/− clone. Primers generate a 457 base pairs product for the CLP-1 gene and a 383 base pairs product for the mutated allele. M denotes DNA size ladder. Data are shown for one representative clone. (B) Western blot of C2C12 CLP-1 +/+ cells and C2C12 CLP-1 +/− cells in growth medium probed with anti-CLP-1 antibody. GAPDH served as a loading control. Western blot is shown for one representative clone. The analysis was performed on three independently isolated C2C12 CLP-1 +/− cell clones, and means are depicted graphically + s.e.m.; GAPDH was used for normalization (*P<0.05). (C) Western blot of lysates from C2C12 CLP-1 +/+ and +/− cell cultures in growth medium (G) and differentiation medium at 24 hours (D1) and at 72 hours (D3), probed with anti-CLP-1, anti-myogenin, anti-PCNA, anti-cyclin D1 and anti-MyoD antibodies. GAPDH served as a loading control. The analysis was performed three times, and means are depicted graphically + s.e.m.; GAPDH was used for normalization (*P<0.05, **P<0.01). (D) Immunofluorescence of C2C12 CLP-1 +/+ and C2C12 CLP-1 +/− cells in growth and differentiation medium using actin stain (green) and co-stained with anti-CLP-1 (red) or DAPI nuclear stain (blue). This analysis was performed on three independently isolated C2C12 CLP-1 +/− cell cultures. (E) Graphical analysis of immunofluorescence of C2C12 CLP-1 +/+ (WT) and +/− cells in growth media (GM) and differentiation media (DM) for 0, 24, 48 and 72 hours. Antibodies to PCNA and myosin heavy chain were used, and DAPI was used as a nuclear stain. At each time point, cells were counted (n=500) and the ratio of cells expressing PCNA or myosin heavy chain versus total cells is depicted graphically (n=3).
Analysis of CLP-1-associated proteins in CLP-1 −/− mouse embryos
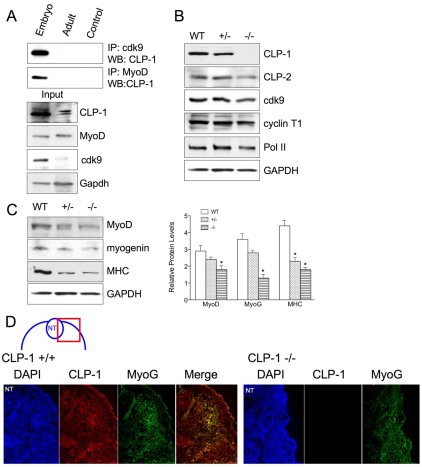
Because the above data on association of CLP-1 with MyoD and HDAC were obtained in in vitro cultured C2C12 cells, we addressed the question whether such functional association exists in vivo, in the animal. We used embryonic day 12 (E12) embryos and adult wild-type skeletal muscle for western blotting. In adult muscle, CLP-1 and cdk9 levels were very low compared to embryonic tissue (Fig. 5A, input). Likewise, CLP-1 association with cdk9 and MyoD was not observed in adult muscle (Fig. 5A). Embryos at day 12 contain myoblasts as well as myotubes, whereas in the adult there are only myotubes in which CLP-1 cooperation with cdk9 and MyoD is no longer necessary. Next, we examined CLP-1 wild type, +/− and −/− E12 embryos by western blot for expression of the components of P-TEFb and MRFs. CLP-1 was not expressed in −/− embryos, as expected, whereas CLP-2 (a homolog of CLP-1) was expressed (Fig. 5B). Cdk9, cyclin T1 and Pol II levels remained steady (Fig. 5B). Skeletal muscle marker MyoD was down in CLP-1 +/− and −/− embryos, and the differentiation markers myogenin and myosin heavy chain were also decreased markedly (Fig. 5C). Next, immunohistochemical analysis of E12 embryos was performed with anti-CLP-1 and anti-myogenin antibodies (Fig. 5D). Embryos were sectioned coronally, and the neural tube (Fig. 5D top left) was used for orientation and localization of the myotome. In wild-type embryos, myogenin was clearly localized adjacent to the neural tube and this colocalized with CLP-1, which is ubiquitously expressed. In CLP-1 −/− embryos, myogenin is still expressed, yet the expression is at a lower level. These findings suggest that the absence of CLP-1 in the knockout mice might lead to reduced myogenesis, which might partly be responsible for embryonic lethality. It was also clear that CLP-2 does not compensate for the loss of CLP-1 because CLP-1 −/− mice die at E17 or E18, despite CLP-2 expression.
Fig. 5.
Analysis of expression of P-TEFb and MRFs in mouse skeletal muscle. (A) Immunoprecipitation (IP) of skeletal muscle lysates from wild-type E12 embryo and adult, with anti-cdk9 and anti-MyoD antibodies and western blotting with anti-CLP-1 antibody. IP with antibody alone served as a control for immunoreactivity. Western blot of total protein served as an input control. GAPDH served as a loading control. (B) Western blotting of skeletal muscle tissue lysates from E12 mouse embryos wild-type (WT), heterozygote (+/−) and homozygote (−/−) for the CLP-1 allele with anti-CLP-1, anti-CLP-2, anti-cdk9, anti-cyclin T1 and anti-RNA polymerase II (Pol II) antibodies. GAPDH served as a loading control. (C) Western blotting of skeletal muscle tissue lysates with anti-MyoD, anti-myogenin and anti-myosin heavy chain (MHC) antibodies. GAPDH served as a loading control. Data represent one of three separate experiments with one embryo per experiment. Relative MyoD, myogenin and MHC levels are depicted graphically as means + s.e.m. GAPDH was used for normalization (*P<0.05). (D) Immunohistochemical analysis of coronal section of CLP-1 +/+ and CLP-1 −/− E12 mice. Sections were stained with DAPI (blue), anti-CLP-1 antibody (red) and anti-myogenin antibody (green). Merge of CLP-1 and myogenin is depicted in the fourth panel (yellow). In diagram above, NT denotes location of the neural tube, and red square denotes relative localization of section. Images were captured at 10× magnification.
Discussion
Mechanistically, eukaryotic cell development and its maintenance are attributed to a critical balance between transcriptional activators and inhibitors and their effect on specific genes. At the transcriptional level, it is evident that the P-TEFb complex plays a role in expression of tissue and developmental stage specific genes. Current data support the notion that the active recruitment of P-TEFb is crucial for the expression of these genes, but it is not known how P-TEFb is specifically recruited and what cellular factors cooperate with P-TEFb. A great deal of information is available on the molecular composition of the P-TEFb complex and its role in transcription elongation. However, there is limited knowledge on the role of P-TEFb in skeletal muscle gene control, and the involvement of CLP-1 is totally unknown.
In this study, we have examined the role of CLP-1 in skeletal muscle cell differentiation using C2C12 mouse myoblast cells. We noted that CLP-1 association with P-TEFb is dynamic and is maximal during the early phase of differentiation, implying that CLP-1 is functionally connected with the transition phase of myoblasts to myotubes. A recent report also examined HEXIM1 association with P-TEFb in C2C12 cells and found that HEXIM1 dissociated from P-TEFb 30 minutes after switching to low-serum medium and the association was shown to be restored at 2 hours (Nojima et al., 2008). However, myogenin is known to be expressed only after 12 hours in low-serum medium (Simone et al., 2002). We therefore used 24 hours as the early phase D1, and 72 hours as the fully established phase D3 of C2C12 cell differentiation.
By reducing CLP-1 expression in C2C12 cells, we saw an arrest in the transition of skeletal myoblast to myotubes, implying that CLP-1, and possibly its association with components of P-TEFb, are obligatory to this transition. Because the CLP-1 knockout mice died at E17 to E18, CLP-2 expression does not substitute CLP-1 function in mice, despite high homology between the C-termini of the two proteins. Although the CLP-1 heterozygosity in C2C12 cells was sufficient to arrest differentiation in cell culture, the CLP-1 +/− mice survive. One might speculate that during embryonic development some compensatory molecule that is not available in the cell line acts as a substitute for CLP-1. Furthermore, embryonic growth undergoes defined phases of development, whereas the C2C12 cells are immediately challenged to differentiate and therefore possibly bypass the regulatory subprograms of gene expression. During early embryonic stages CLP-1 +/− embryos appear smaller than their wild-type litter mates, but after birth they appear phenotypically similar. There might very well be a skeletal muscle deficiency, as evidenced by reduced skeletal muscle markers during development, a phenomenon that needs to be examined in the future.
In this report, we also showed that MyoD and HDACs associated with CLP-1 maximally at 24 hours. This would suggest that perhaps both CLP-1 and HDACs are recruited by MyoD to offer an environment optimal for repression of proliferative genes and allowing the transition of myoblasts to myotubes. At this junction, one would expect that MyoD must also associate with HAT proteins in a separate protein complex to facilitate the promotion of muscle-specific gene expression. In support of this notion, Mal and Harter (Mal and Harter, 2003) have shown that once myoblasts undergo differentiation, MyoD actively engages in recruitment of HAT proteins at skeletal muscle promoters. In our present study, we noted that MyoD associates with HDAC1 and PCAF in the differentiation stage. The role of MyoD as a repressor or activator can be dictated by its association with interacting proteins (HDAC, HAT). Recent reports have examined the effect of HDAC and HAT association with the P-TEFb complex. It was shown that cdk9 is acetylated, but whether this acetylation enhances or inhibits the kinase activity is not settled. Fu and colleagues (Fu et al., 2007) showed that cdk9 was deacetylated by HDAC3, which reduced the association of cdk9 with activator Brd4 and promoted the interaction of P-TEFb with HEXIM1. Sabo and colleagues (Sabo et al., 2008) showed that cdk9 acetylation by PCAF reduced kinase function and P-TEFb transcriptional activity. The cyclin T1 component of P-TEFb can also be acetylated, which results in a decrease in HEXIM1-bound P-TEFb and an increase in cdk9 kinase activity (Cho et al., 2009). In our study, we observed that CLP-1 associates with HDAC1, HDAC3 and HDAC5 in skeletal muscle and that this association is specifically enhanced in the early differentiation phase. CLP-1 associated with HDAC in skeletal muscle might act to counter the activity of acetylated cyclin T1 or cdk9. We speculate that inactive P-TEFb bound by CLP-1 is actively recruited to silence specific gene promoters.
In summary, we have presented evidence that CLP-1 interacts with MyoD, and hypothesize that CLP-1 associated with MyoD is required for downregulation of proliferative genes and to steer the myoblast-to-myotube transition. HEXIM1 expression has been reported to correlate with estrogen receptor-inducible cyclin D1 protein expression and binding to the E2-responsive region on the cyclin D1 promoter (Ogba et al., 2008). Using ChIP assay, we localized CLP-1 and its associated proteins, MyoD and HDACs, on the cyclin D1 promoter in C2C12 cells, which occurs preferentially in the differentiation stage. On the basis of our results, we speculate that MyoD guides CLP-1 to MyoD-target DNA, which is in complex with HDAC proteins. We believe that both suppression and activation of MyoD activity are likely to co-exist in C2C12 cells at the onset of differentiation, to act on distinct promoters. The signaling that triggers specific interaction of these molecules is not known. Future studies to characterize the fundamental regulatory events mediated by CLP-1 would provide further insights into the physiological role of CLP-1 in skeletal muscle cells. Our data collectively highlight a distinct function of CLP-1 in cell-cycle exit, suggesting that regulation of P-TEFb activity by CLP-1 is likely to play a pivotal role in targeting skeletal muscle cells toward differentiation.
Materials and Methods
Antibodies
Polyclonal rabbit anti-CLP-1 antibody was generated to the peptide HRQQERAPLSKFGD (Proteintech Group). Cdk9 (C-20, D-7), cyclin T1 (H-245), GAPDH (FL-335), HDAC1 (H-11), HDAC3 (B-12), HDAC5 (H-714), Pol II (N-20), myogenin (F5D), MyoD (5.8A, C-20), HEXIM2 (M-90), PCAF (E-8), PCNA (PC10) and actin (C-2) antibodies were from Santa Cruz Biotechnology. Anti-myosin heavy chain (MF20) antibody was from Developmental Studies Hybridoma Bank. Anti-cyclin D1 antibody was from Abcam. HDAC antibody sampler kit was from Cell Signaling Technology. ChIP Grade antibodies anti-HEXIM1 and anti-MyoD (C-20x) were from Abcam and Santa Cruz Biotechnology, respectively.
Cell culture
C2C12 mouse myoblasts obtained from ATCC were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS and penicillin/streptomycin/amphotericin b (growth medium). To induce differentiation, growth medium was substituted with differentiation medium (DMEM supplemented with 2% horse serum and penicillin/streptomycin/amphotericin b). Cells were incubated at 37°C with 5% CO2.
Stable transfections
CLP-1 allele replacement vector DNA (pKO-HR-CLP-1neo) (Huang et al., 2004) was transfected into C2C12 cells at 10% confluency using Fugene 6 (Roche) transfection reagent. Neomycin (G418) antibiotic was used to isolate resistant clones. C2C12 cells heterozygous for the targeted CLP-1 allele were determined by PCR. DNA was isolated and genotype determined by PCR using previously described primers (Huang et al., 2004).
Coimmunoprecipitation
Cells were lysed in buffer A (Michels et al., 2004) supplemented with 1 mM dithiothreitol, 10 mM NaF, 1 mM Na3VO4, 1 mM PMSF, protease inhibitor cocktail (Sigma) and RNasin (Promega). Lysate was subjected to “freeze” on dry ice, and “thaw” at 37°C, followed by centrifugation at 16,000 g. Equal concentrations of lysates were incubated with antibodies overnight at 4°C with rotation. For protein capture, protein A/G plus agarose beads (Santa Cruz Biotechnology) were added at 4°C for 2 hours with rotation. After extensive washing with buffer A, bound proteins were eluted by boiling in 1× SDS loading buffer.
Cell fractionation
Cells were lysed in cell fractionation buffer A (10 mM HEPES pH 7.4, 15 mM KCl, 2 mM MgCl2, 0.1 mM EDTA) supplemented with 1 mM dithiothreitol, 1 mM PMSF, 10 mM NaF, 1 mM Na3VO4, protease inhibitor cocktail (Sigma), and RNasin (Promega) with homogenization using a dounce homogenizer and monitored microscopically with trypan blue. Samples were centrifuged (1500 g) at 4°C for 5 minutes and the supernatant collected as cytoplasmic extract. The pellet was incubated with cell fractionation buffer B (10 mM HEPES, 150 mM NaCl, 2 mM MgCl2, 0.5 mM EDTA, 0.5% NP-40) supplemented with 1 mM dithiothreitol, 1 mM PMSF, 10 mM NaF, 1 mM Na3VO4, protease inhibitor cocktail (Sigma) and RNasin (Promega). Samples were subjected to “freeze” on dry ice, and “thaw” at 37°C, with vortexing. After centrifugation (16,000 g) at 4°C for 10 minutes, the supernatant was collected as nuclear extract.
Whole-cell lysis
Cells were lysed in RIPA buffer (50 mM Tris pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% Triton-X 100) supplemented with 1 mM dithiothreitol, 1 mM PMSF, 10 mM NaF, 1 mM Na3VO4 and protease inhibitor cocktail (Sigma).
Western blot analysis
Proteins were separated by SDS-PAGE and transferred onto nitrocellulose membrane in electroblotting buffer (20 mM Tris, 150 mM glycine, 20% methanol) for 1 hour. The membranes were blocked in TBS-T with 5% nonfat dry milk and probed with primary antibody in TBS overnight, followed by incubation with horse-radish-peroxidase-conjugated secondary antibody for detection with enhanced chemiluminescence reagent.
Immunofluorescence analysis
C2C12 cells were seeded in six-well tissue culture dishes containing sterile glass coverslips. Cells were fixed with 4% paraformaldehyde in PBS for 15 minutes at room temperature. Cells were permeabilized in PBS containing 0.1% Triton X-100. After blocking in 5% FBS in PBS, cells were incubated with primary antibody at 4°C overnight, followed by washes in PBS and incubation with secondary antibody (1:200) conjugated to either Alexa Fluor 488 or Alexa Fluor 594 (Invitrogen) for 1 hour. For actin staining, cells were incubated for 20 minutes with 2 units of Alexa Fluor 594/phalloidin (Invitrogen). Cell nuclei were stained with 4,6-diamidino-2-phenylindole (DAPI). Imaging was captured at 40× magnification using a Zeiss Axiokop microscope, Axio CamMRc camera and Axio vision software.
Chromatin immunoprecipitation assay
Proteins were crosslinked to DNA in culture medium using 1% formaldehyde (10 minutes, room temperature). Cells were lysed in 10 mM Tris pH 8.1, 1.5 mM MgCl2, 10 mM KCl and the nuclei sedimented by centrifugation (1500 g) for 5 minutes at 4°C. The pellet was resuspended in nuclear lysis buffer (50 mM Tris-HCl, 10 mM EDTA, 1% SDS). Chromatin was sonicated five times, for 30 seconds each time, generating DNA fragments of 250–1000 base pairs. DNA concentration was quantified, and 10 mg of DNA was used per immunoprecipitation. Lysates were diluted with ChIP dilution buffer (20 mM Tris pH 8.1, 150 mM NaCl, 2 mM EDTA, 0.01% SDS, 1% Triton X-100). Antibodies were added, and after rotation overnight at 4°C, the immune complexes were collected by the addition of protein G agarose beads/salmon sperm DNA (Millipore). After extensive washes, immune complexes were eluted (1% SDS, 0.1 M NaHCO3) and crosslinking was reversed by the addition of 190 mM NaCl overnight at 65°C. DNA was purified using a PCR purification kit (Qiagen) and amplified by PCR. Sequence of PCR primers: cyclin D1 5′-TATCCTGGAAGGGCGACTAA-3′ and 5′-AATTCCAGCAACAGCTCAAGA-3′; GAPDH primer control 5′-CGGTGCGTGCCCAGTTG-3′ and 5′-GCGACGCAAAAGAAGATG-3′.
DNA was amplified for 18 cycles (annealing 60°C). Product was visualized on 2% agarose gel by ethidium bromide stain and an 8-bit digital camera.
Transient transfection and luciferase assay
C2C12 cells were plated in 12-well dishes and transiently transfected using Lipofectamine 2000 (Invitrogen) with 2 μg of reporter gene construct, 0.5 μg expression vector and 20 ng of thymidine kinase promoter driven Renilla luciferase vector, which was used to normalize transfection efficiency. Human cyclin D1-luciferase promoter was provided by Richard Pestell (Thomas Jefferson University, Philadelphia, PA) (Albanese et al., 1995). Mouse MyoD expression vector was from Addgene (plasmid 8399) provided by Andrew Lassar (Harvard Medical School, Boston, MA). HDAC5 expression vector was provided by Eric Verdin (University of California, San Francisco, CA) (Fischle et al., 1999). pcDNA6-CLP-1 expression vector was previously created in our laboratory (Huang et al., 2002). At 24 hours after transfection, cells were harvested, lysed, and cell lysates assayed using the Dual-luciferase Reporter Assay system (Promega) and OptoComp I Luminometer (MGM instruments). Statistical analysis was performed using a paired Student's t-test. P values of <0.05 were considered significant.
Animals
CLP-1 knockout mice have been described previously (Huang et al., 2004). All experiments were performed in accordance with the Guidelines of the National Institute of Health. Experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee.
Immunohistochemistry
Embryos were fixed in 4% paraformaldehyde/PBS overnight at 4°C, washed in PBS, and transferred to 30% sucrose overnight at 4°C. Tissue was embedded in M-1 matrix (Thermo Scientific) and 14 μm cryosections were collected. Slides were blocked in 5% FBS in PBS, incubated with primary antibody overnight at 4°C, washed in PBS, and incubated with secondary antibody conjugated to either Alexa Fluor 488 or Alexa Fluor 594 for 1 hour. Cell nuclei were stained with DAPI. Imaging was captured at 10× magnification using a Zeiss Axiokop microscope, Axio CamMRc camera and Axio vision software.
Statistical analysis
For quantitative western blot analysis, films were scanned and the band signal intensities determined using NIH ImageJ software. The densitometry values were expressed as a fold level relative to the control, and standardized to corresponding total GAPDH densitometry values obtained from the same sample. Statistical analyses were performed using a paired Student's t-test. P values of <0.05 were considered significant.
Acknowledgments
This work was supported by NIH grant HL073399 (to M.A.Q.S.). Deposited in PMC for release after 12 months.
References
- Albanese C., Johnson J., Watanabe G., Eklund N., Vu D., Arnold A., Pestell R. G. (1995). Transforming p21ras mutants and c-Ets-2 activate the cyclin D1 promoter through distinguishable regions. J. Biol. Chem. 270, 23589-23597 [DOI] [PubMed] [Google Scholar]
- Bergstrom D. A., Penn B. H., Strand A., Perry R. L., Rudnicki M. A., Tapscott S. J. (2002). Promoter-specific regulation of MyoD binding and signal transduction cooperate to pattern gene expression. Mol. Cell 9, 587-600 [DOI] [PubMed] [Google Scholar]
- Cho S., Schroeder S., Kaehlcke K., Kwon H. S., Pedal A., Herker E., Schnoelzer M., Ott M. (2009). Acetylation of cyclin T1 regulates the equilibrium between active and inactive P-TEFb in cells. EMBO J. 28, 1407-1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinoza-Derout J., Wagner M., Salciccioli L., Lazar J. M., Bhaduri S., Mascareno E., Chaqour B., Siddiqui M. A. (2009). Positive transcription elongation factor b activity in compensatory myocardial hypertrophy is regulated by cardiac lineage protein-1. Circ. Res. 104, 1347-1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischle W., Emiliani S., Hendzel M. J., Nagase T., Nomura N., Voelter W., Verdin E. (1991). A new family of human histone deacetylases related to Saccharomyces cerevisiae HDA1p. J. Biol. Chem. 274, 11713-11720 [DOI] [PubMed] [Google Scholar]
- French B. A., Chow K. L., Olson E. N., Schwartz R. J. (1991). Heterodimers of myogenic helix-loop-helix regulatory factors and E12 bind a complex element governing myogenic induction of the avian cardiac alpha-actin promoter. Mol. Cell. Biol. 11, 2439-2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J., Yoon H. G., Qin J., Wong J. (2007). Regulation of P-TEFb elongation complex activity by CDK9 acetylation. Mol. Cell. Biol. 27, 4641-4651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu T. J., Peng J., Lee G., Price D. H., Flores O. (1999). Cyclin K functions as a CDK9 regulatory subunit and participates in RNA polymerase II transcription. J. Biol. Chem. 274, 34527-34530 [DOI] [PubMed] [Google Scholar]
- Huang F., Wagner M., Siddiqui M. A. (2002). Structure, expression, and functional characterization of the mouse CLP-1 gene. Gene 292, 245-259 [DOI] [PubMed] [Google Scholar]
- Huang F., Wagner M., Siddiqui M. A. (2004). Ablation of the CLP-1 gene leads to down-regulation of the HAND1 gene and abnormality of the left ventricle of the heart and fetal death. Mech. Dev. 121, 559-572 [DOI] [PubMed] [Google Scholar]
- Mal A., Harter M. L. (2003). MyoD is functionally linked to the silencing of a muscle-specific regulatory gene prior to skeletal myogenesis. Proc. Natl. Acad. Sci. USA 100, 1735-1739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mal A., Sturniolo M., Schiltz R. L., Ghosh M. K., Harter M. L. (2001). A role for histone deacetylase HDAC1 in modulating the transcriptional activity of MyoD: inhibition of the myogenic program. EMBO J. 20, 1739-1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinsey T. A., Zhang C. L., Olson E. N. (2001). Control of muscle development by dueling HATs and HDACs. Curr. Opin. Genet. Dev. 11, 497-504 [DOI] [PubMed] [Google Scholar]
- Michels A. A., Fraldi A., Li Q., Adamson T. E., Bonnet F., Nguyen V. T., Sedore S. C., Price J. P., Price D. H., Lania L., et al. (2004). Binding of the 7SK snRNA turns the HEXIM1 protein into a P-TEFb (CDK9/cyclin T) inhibitor. EMBO J. 23, 2608-2619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen V. T., Kiss T., Michels A. A., Bensaude O. (2001). 7SK small nuclear RNA binds to and inhibits the activity of CDK9/cyclin T complexes. Nature 414, 322-325 [DOI] [PubMed] [Google Scholar]
- Nojima M., Huang Y., Tyagi M., Kao H. Y., Fujinaga K. (2008). The positive transcription elongation factor b is an essential cofactor for the activation of transcription by myocyte enhancer factor 2. J. Mol. Biol. 382, 275-287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogba N., Chaplin L. J., Doughman Y. Q., Fujinaga K., Montano M. M. (2008). HEXIM1 regulates 17beta-estradiol/estrogen receptor-alpha-mediated expression of cyclin D1 in mammary cells via modulation of P-TEFb. Cancer Res. 68, 7015-7024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J., Zhu Y., Milton J. T., Price D. H. (1998). Identification of multiple cyclin subunits of human P-TEFb. Genes Dev. 12, 755-762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price D. H. (2000). P-TEFb, a cyclin-dependent kinase controlling elongation by RNA polymerase II. Mol. Cell. Biol. 20, 2629-2634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri P. L., Avantaggiati M. L., Balsano C., Sang N., Graessmann A., Giordano A., Levrero M. (1997). p300 is required for MyoD-dependent cell cycle arrest and muscle-specific gene transcription. EMBO J. 16, 369-383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnicki M. A., Jaenisch R. (1995). The MyoD family of transcription factors and skeletal myogenesis. BioEssays 17, 203-209 [DOI] [PubMed] [Google Scholar]
- Sabo A., Lusic M., Cereseto A., Giacca M. (2008). Acetylation of conserved lysines in the catalytic core of cyclin-dependent kinase 9 inhibits kinase activity and regulates transcription. Mol. Cell. Biol. 28, 2201-2212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabourin L. A., Rudnicki M. A. (2000). The molecular regulation of myogenesis. Clin. Genet. 57, 16-25 [DOI] [PubMed] [Google Scholar]
- Sartorelli V., Puri P. L., Hamamori Y., Ogryzko V., Chung G., Nakatani Y., Wang J. Y., Kedes L. (1999). Acetylation of MyoD directed by PCAF is necessary for the execution of the muscle program. Mol. Cell 4, 725-734 [DOI] [PubMed] [Google Scholar]
- Schulte A., Czudnochowski N., Barboric M., Schonichen A., Blazek D., Peterlin B. M., Geyer M. (2005). Identification of a cyclin T-binding domain in Hexim1 and biochemical analysis of its binding competition with HIV-1 Tat. J. Biol. Chem. 280, 24968-24977 [DOI] [PubMed] [Google Scholar]
- Simone C., Stiegler P., Bagella L., Pucci B., Bellan C., De Falco G., De Luca A., Guanti G., Puri P. L. (2002). Activation of MyoD-dependent transcription by cdk9/cyclin T1. Oncogene 21, 4137-4148 [DOI] [PubMed] [Google Scholar]
- Stockdale F. E. (1992). Myogenic cell lineages. Dev. Biol. 154, 284-298 [DOI] [PubMed] [Google Scholar]
- Yang Z., Zhu Q., Luo K., Zhou Q. (2001). The 7SK small nuclear RNA inhibits the CDK9/cyclin T1 kinase to control transcription. Nature 414, 317-322 [DOI] [PubMed] [Google Scholar]
- Yun K., Wold B. (1996). Skeletal muscle determination and differentiation: story of a core regulatory network and its context. Curr. Opin. Cell Biol. 8, 877-889 [DOI] [PubMed] [Google Scholar]
- Zhou M., Halanski M. A., Radonovich M. F., Kashanchi F., Peng J., Price D. H., Brady J. N. (2000). Tat modifies the activity of CDK9 to phosphorylate serine 5 of the RNA polymerase II carboxyl-terminal domain during human immunodeficiency virus type 1 transcription. Mol. Cell. Biol. 20, 5077-5086 [DOI] [PMC free article] [PubMed] [Google Scholar]