There have been 9 reported attempts of orthotopic homotransplantation of the human liver—7 in Denver16,21,22 and one each in Boston11 and Paris.5 Survival time was 0 to 23 days. There were multiple reasons for death in each case, but in most an important factor was either the failure to obtain good immediate liver function or the inability to subsequently maintain such function.
On July 23 and 31, Sept. 5, and Oct. 8, 1967, 4 more orthotopic liver transplantations were performed in our institutions. The recipients are at the time of this writing 94, 86, 49, and 14 days postoperative. This report is concerned with the first 3 of these patients and with the improvements in therapy which were brought to these cases. In addition, attention will be directed to a number of serious and previously unrecognized problems which were encountered. The diagnosis and treatment of these complications will be presented, as well as some thoughts about how they might be prevented in future cases.
OBSERVATIONS
Case material
The 3 recipients (hereafter called Patients 1 to 3) were 19, 20½, and 13 months old and weighed 10.8, 8.7, and 9.4 kilograms. All were girls. The diagnosis of nonresectable hepatic-cell carcinoma was made in Patient 1 at an abdominal exploration when she was 13 months old. During the next 5 months, the tumor had grown inexorably despite liver irradiation with a total of 975 r and systemic therapy with actinomycin-D, cyclophosphamide, methotrexate, and 5-fluorouracil. Liver function was normal.
Patients 2 and 3 had extrahepatic biliary atresia. Bilirubin levels were 24.1 and 16.1 mg. percent, respectively. Total serum protein concentrations were 7.9 and 7.3 Gm. percent. Other details of preoperative liver function are show in Figures 2 to 4, 6, and 7.
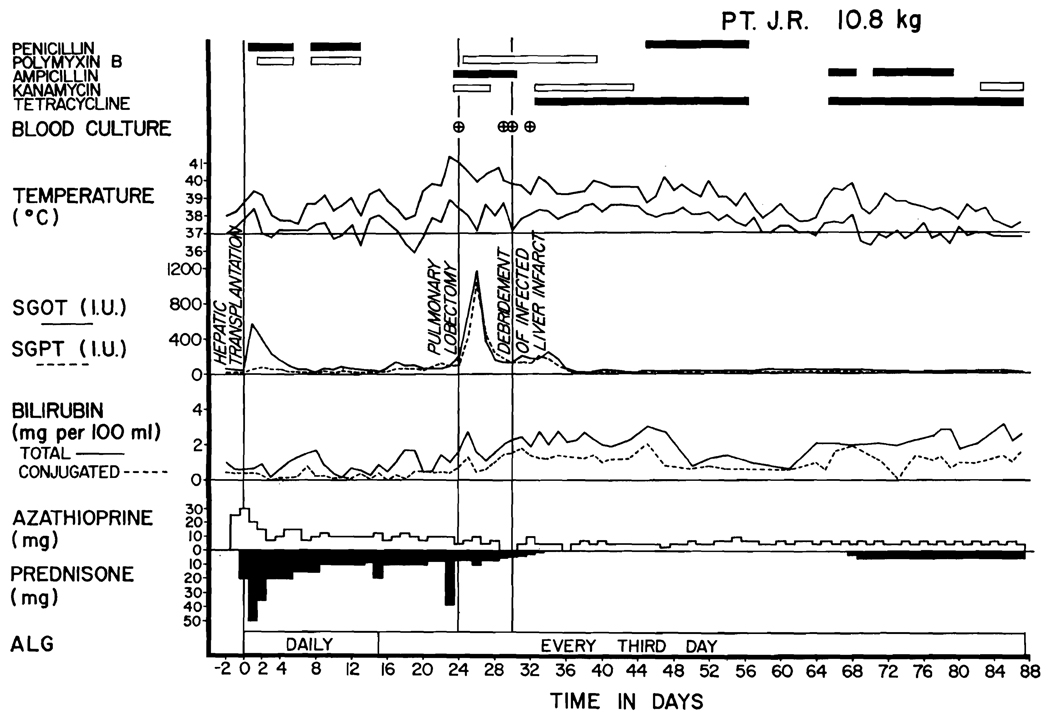
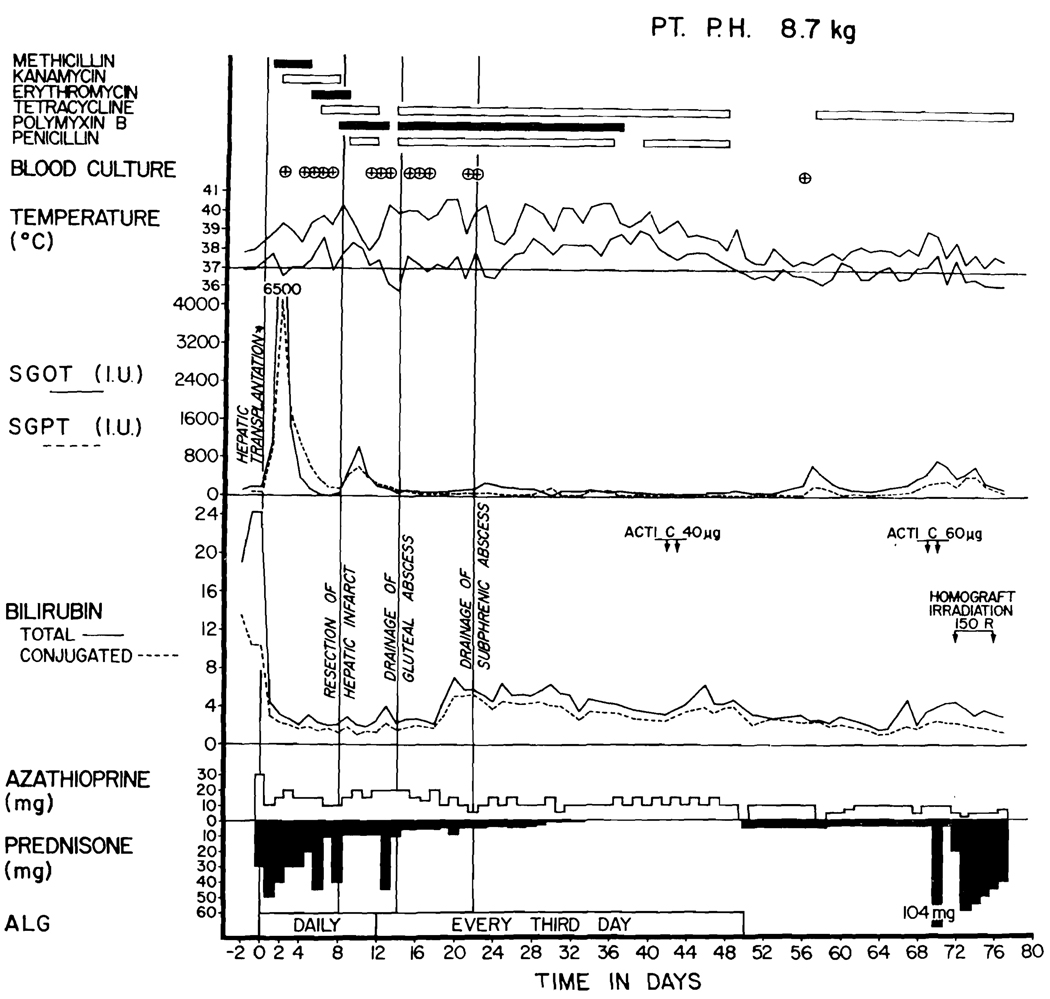
Fig. 2.
Course of Patient 1. Note the high rise in SGOT and SGPT at the time the septic liver infarction was diagnosed. The septicemia, indicated by encircled crosses, was with E. coli or Aerobacter-Klebsiella. The patient’s present condition is excellent.
Fig. 4.
Course of Patient 3. Note the normal SGOT and SGPT levels which evolved after the injury. These rose again with the development of a septic infarct. All the positive blood cultures had Aerobacter-Klebsiella; the first one also contained E. coli.
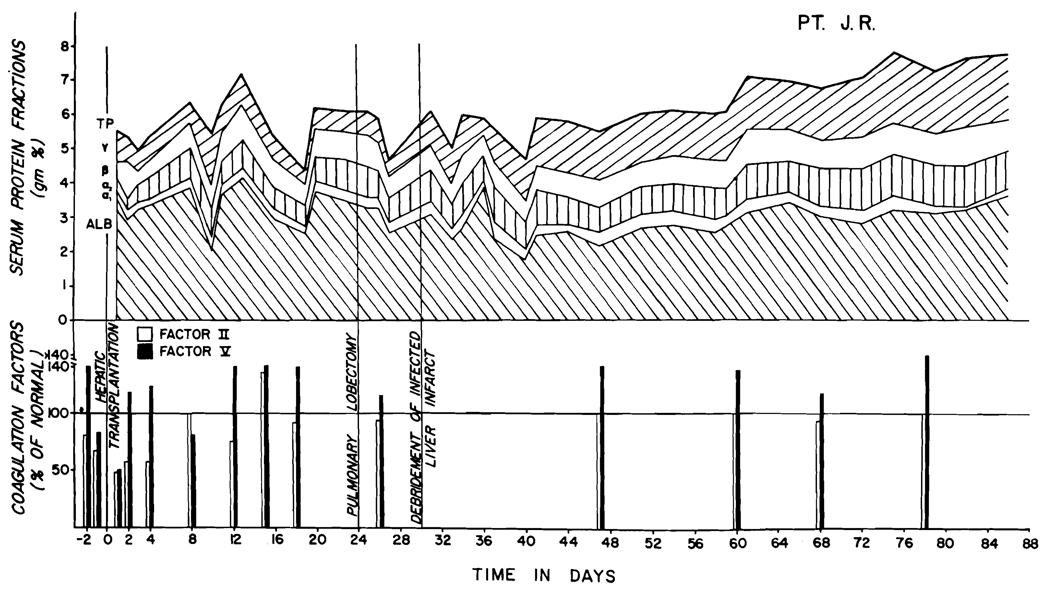
Fig. 6.
Serum proteins after liver homotransplantation in Patient 1. There was a significant increase in gamma globulins beginning one month after operation. Note the maintenance of normal coagulation factors II and V.
Fig. 7.
Serum proteins in Patient 2. Extreme late hypergammaglobulinemia is evident. Note the subnormal clotting factors at all times after the third postoperative week. The defects of protein metabolism in this patient correlated with the instability of other measures of liver function which are illustrated in Fig. 3.
The homograft donors
The donors for Patients 1 to 3 were 18 months, 4 years, and 18 months old and weighed 6.4, 13.0, and 8.2 kilograms. The first was a boy and the second and third were girls. The red-cell types of the 3 donors were the same as those of the recipients, namely O, O, and A.
The condition of the donors was hopeless at the time of admission to the hospital because of brain damage and apnea. One was a microcephalic who had been resuscitated from a postaspiration cardiac arrest at a domiciliary center. At autopsy he had widespread pneumonitis. The second child had a severe central nervous system injury after resuscitation from drowning. Postmortem examination revealed widespread micro-thrombi in all visceral organs; presumably the transplanted liver was similarly afflicted. The third donor had become apneic after a penicillin injection given in a physician’s office and was thought to have had an anaphylactic reaction. At autopsy, the spinal cord had widespread degeneration of anterior horn cells such as that found in acute poliomyelitis or a Coxsackie infection.
The donors were all maintained on mechanical ventilators from the time of admission until death 3, 5, and 8 days later. In each instance, electroencephalograms were isoelectric. All the children had multiple cardiac arrests which were promptly treated. In each case, the patients were pronounced dead after a final cardiac arrest by representatives of the service responsible for their care. However, cardiac massage and ventilatory support were continued in transit to the operating room and during the abdominal skin preparation. Cardiac activity intermittently returned during this time of massage in all 3 patients, and in one (Donor 2) a sustained and strong heart beat was restored almost an hour after death had been pronounced. The family was informed of the unexpected development and, with their permission, the initial stages of the hepatectomy were performed with an intact circulation. By the time hepatectomy was started, the temperature of the donors had fallen to 29 to 32° C.
Liver preservation
The livers were chilled in situ by perfusion through the inframesocolic superior mesenteric vein with 1,000 to 2,000 ml. of balanced electrolyte solution, to which low molecular weight dextran and heparin had been added. Egress for the fluid was through a venotomy in the lower inferior vena cava. Long segments of the portal vein and infrahepatic vena cava were retained with the specimen, as well as the full length of the hepatic artery in continuity with the celiac axis and the attached aorta; at the time of vascular anastomoses, the vessels were recut. After excision, the livers for Patients 1 and 3 were kept for 146 and 122 minutes with a preservation system that included hypothermia, hyperbaric oxygenation at 4 atmospheres, and low flow perfusion with diluted homologous blood. The flow rates used and other details of technique were exactly the same as reported earlier in dog experiments.2
The times from donor death to revascularization of the homografts in the recipients were 5 hours 11 minutes, 4 hours 42 minutes, and 4 hours 31 minutes in Patients 1 to 3 respectively. In none was there a warm ischemic interval of more than a few minutes without some circulation, since the aforementioned resuscitative measures were continued without interruption until cold perfusion of the liver could be started.
Method of homotransplantation
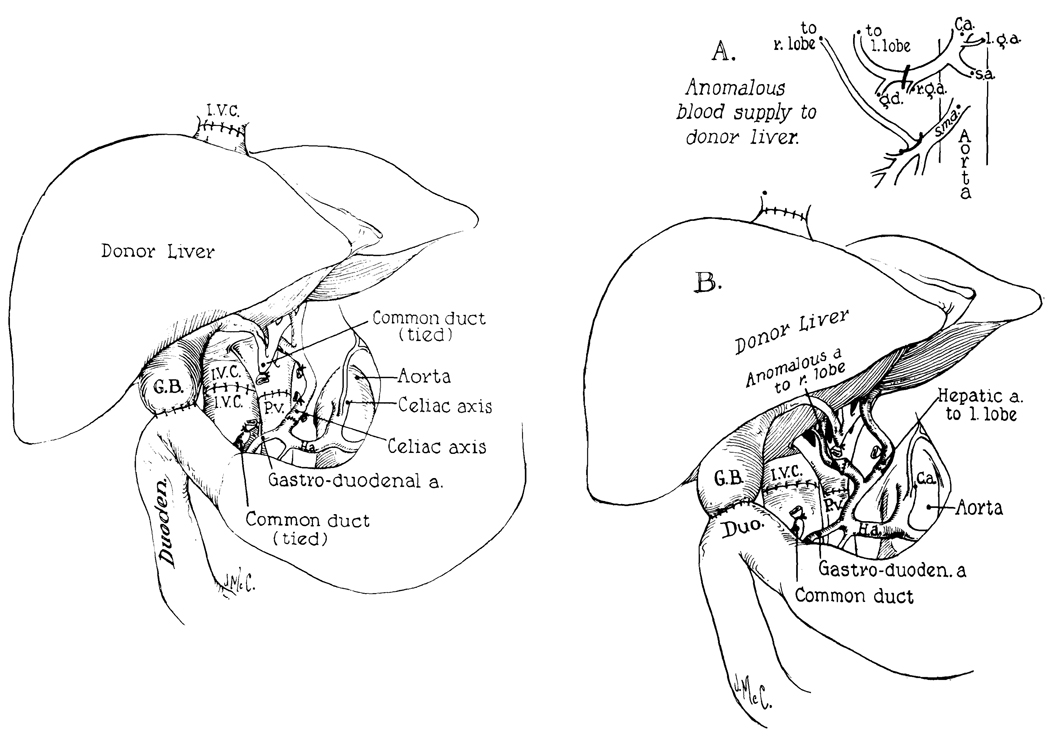
The basic surgical techniques,22 carried out through a bilateral transverse abdominal incision, are summarized in Fig. 1, left. The host hepatic artery was connected in all 3 cases at a site distal to the right gastric and gastroduodenal branches. In Patient 2 a right hepatic artery originated from the donor’s superior mesenteric artery, as well as a left trunk from the celiac axis. The 2 vessels were individually anastomosed with 7-0 silk to the main branches of the recipient proper hepatic artery (Fig. 1, right). In all 3 patients internal biliary drainage was provided with a cholecystoduodenostomy (Fig. 1).
Fig. 1.
Technique of orthotopic liver transplantation. Left, method used in Patients 1 and 3. Note that the celiac axis (or alternatively the common hepatic artery) of the homograft is anastomosed to the proper hepatic artery of the recipient patient. With this distal location in the recipient vessel the requisite dissection is considerably simplified. Right, reconstruction used in Patient 2. The right hepatic arterial branch of the homograft originated from the superior mesenteric artery and the left branch from the celiac axis (A). The vessels were individually attached to the 2 terminal branches of the host proper hepatic artery (B). In all 3 cases internal biliary drainage was with a cholecystoduodenostomy.
During the anhepatic phase, the inferior vena caval and splanchnic venous beds, which were occluded for 40, 89, and 50 minutes in Patients 1 to 3 respectively, were not decompressed with an external bypass. Patient 1 did not have gross evidence of portal hypertension or prominent venous collaterals. With crossclamping of the 2 great veins her blood pressure fell from 90 to 70 mm. Hg; the intestines became slightly dusky. After restoration of venous flow, the changes were immediately reversed. Patient 2 had moderate portal hypertension and many venous collaterals, and in Patient 3 these abnormalities were even more pronounced. The venous occlusion caused a drop in blood pressure from 130 to 110 mm. Hg in Patient 2, but did not result in any detectable alteration in Patient 3.
The excised livers weighed 1,185, 698, and 655 grams. The enlarged spleens were also removed after the homotransplantation was completed. The total operating times for the 3 cases were 5½, 6½, and 4½ hours. The measured blood losses were 460, 600, and 560 ml. There were no unusual problems with bleeding. Patients 1 and 2 were administered 0.1 Gm. per kilogram epsilon amino caproic acid (EACA) at the time of homograft revascularization.
Intraoperative metabolic management
During the anhepatic phase and for 30 minutes following revascularization, each patient was given intravenous glucose at the rate of 1.0 Gm. per kilogram per hour. At all other times 10 percent glucose solutions were used.
Metabolic acidosis characteristically developed during operation as summarized in Table I for the 3 reported cases, as well as for the subsequently treated fourth patient. In Patients 2 and 4, metabolic acidosis was already established prior to the anhepatic phase. In Patients 1, 2, and 4, revascularization of the homograft was apparently followed by release of acid metabolites, since administration of large quantities of bicarbonate at this time caused only modest rises in base excess. The same situation probably obtained in Patient 3, since the base excess decreased during the operation despite the administration of 27 mEq. of sodium bicarbonate.
Table I.
Acid-base changes before, during, and after liver homotransplantation, as well as the therapy with sodium bicarbonate which was used to counteract or prevent acidosis
| Patient No. | Preoper- ative |
Intraoperative | Postoperative | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Before removal of host liver |
5–10 min. before revasc. of graft |
5–10 min. after revasc. of graft |
4–4½ hr. after revasc. of graft |
||||||
| 1 | |||||||||
| pH (units) | 7.45 | — | 6.99 | 7.14 | 7.36 | ||||
| Base excess (mEq./L.) | +4.0 | — | −21 | −16 | −6.5 | ||||
| pCO2 (mm. Hg) | 27 | — | 46 | 37 | 31 | ||||
| Dose intravenous NaHCO3 (mEq.) | 4 | 15 | 39 | ||||||
| 2 | |||||||||
| pH (units) | 7.47 | 7.40 | 7.35 | 7.38 | 7.45 | ||||
| Base excess (mEq./L.) | −4.5 | −11.8 | −9.0 | −8.6 | 0.5 | ||||
| pCO2 (mm. Hg) | 25 | 18 | 28 | 25 | 35 | ||||
| Dose intravenous NaHCO3 (mEq.) | 29 | 18 | 9 | ||||||
| 3 | |||||||||
| pH (units) | 7.41 | — | — | — | 7.31 | ||||
| Base excess (mEq./L.) | −4.0 | — | — | — | −11.0 | ||||
| pCO2 (mm. Hg) | 31 | — | — | — | 32 | ||||
| Dose intravenous NaHCO3 (mEq.) | 27 | ||||||||
| 4 | |||||||||
| pH (units) | 7.37 | 7.35 | 7.41 | 7.63 | 7.38 | ||||
| Base excess (mEq./L.) | −5.9 | −10.7 | −2.0 | 4.5 | −1.0 | ||||
| pCO2 (mm. Hg) | 32 | 24 | 35 | 23 | 40 | ||||
| Dose intravenous NaHCO3 (mEq.) | 22 | 18 | |||||||
Histocompatibility studies
Dr. Paul Terasaki of Los Angeles studied the lymphocyte antigens of the donors and recipients. The results are shown in Table II. There were 0, 1, and 2 incompatibilites of the recently defined major human antigen system,4, 25 as well as a variable number of incompatibilities as detected with less well-classified antisera. Preformed cytotoxic antibodies were not found in the recipients.
Table II.
Antigen incompatibilities in the 3 cases of liver transplantation. The relationship of the Terasaki antigen groups to the nomenclature used by other investigators has been summarized elsewhere25
| Patient No. | Antigen groups | No. of sera mismatched |
|||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3–4 | 5 | 6 | 7 | ||
| 1 | − | − | − | − | − | − | 4/60 |
| 2 | − | + | − | − | − | − | 14/60 |
| 3 | − | − | − | ± | − | + | 8/60 |
Immunosuppression
Prednisone and azathioprine were given by the dose schedule shown in Figs. 2 to 4. All 3 children were also treated with heterologous antilymphocyte globulin (ALG) daily for the first 2 postoperative weeks and then every third day. The individual intramuscular doses of the equine globulin were 1.0 to 1.5 ml. The ALG17, 19 had a leukoagglutinating titer of 1:8,000 to 16,000 and a protein concentration of 5.0 to 9.0 Gm. percent. In Patient 2, injections were stopped after 7 weeks (Fig. 3) because the patient had developed a very high precipitin titer against horse protein and was having transient facial edema with each dose.
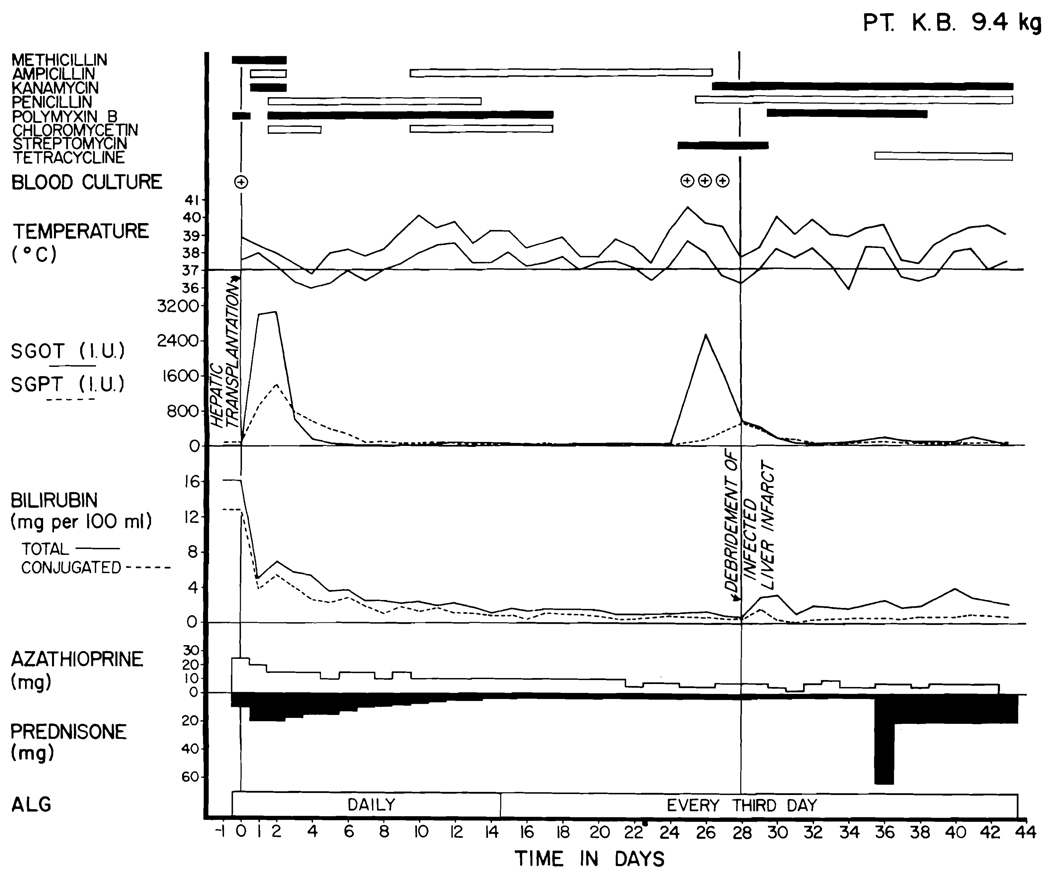
Fig. 3.
Course of Patient 2. Note the rapid clearing of the pre-existing hyperbilirubinemia. The hepatic infarction which occurred early was probably on a technical basis. The positive blood cultures were all Bacteroides fragilis. Note the instability of liver function throughout the entire postoperative period, which makes longterm prognosis extremely guarded.
Respiratory complications
During the early postoperative period, special measures were taken to insure adequate oxygenation. The 3 recipient patients were maintained on respirators for 2 to 11 hours. Subsequently, intensive endotracheal suctioning was continued for 1 or 2 days. In spite of these precautions, transient lower lobe pneumonitis developed in each patient.
Before operation, all 3 patients had high diaphragms due to hepatomegaly and splenomegaly, but there was no evidence of diaphragmatic denervation. Afterward, each was shown to have paralysis of the right hemi-diaphragm, which was thought to have been due to crushing of the phrenic nerve by the upper vena caval vascular clamp during the transplantation. As a consequence, Patient 1 had persistent atelectasis of the right upper lobe; right upper lobectomy was performed 24 days after transplantation from which recovery was uncomplicated. Phrenic nerve function returned in Patients 1 to 3 after 10, 7, and 2 weeks.
Preoperatively, all 3 patients had evidence of the venous-to-arterial intrapulmonary shunts which are common in children with advanced liver disease.13, 14 The patients did not have clinical cyanosis, and their arterial oxygen saturation was 85 to 88 percent while breathing room air. However, these values were not significantly increased when breathing pure oxygen. The magnitude of calculated shunting3 was approximately 50 percent of the cardiac output in each case. After transplantation, the shunting immediately decreased to 5 to 15 percent in Patients 1 and 2, and in Patient 3 a similar improvement was noted in the course of a 10 day period.
Liver sepsis
All 3 patients developed life-threatening infections in the homografts coincident with gram-negative septicemia. In Patient 1, a liver scan 17 days after transplantation was normal (Fig. 5, A). However, 23 days after operation she developed hypotension and a fever of 41° C. Blood cultures then and 12 hours later contained Escherichia coli. A repeat liver scan showed a large, filling defect in the right lobe (Fig. 5, B). The child was treated for the next week with intravenous kanamycin, polymyxin, and ampicillin (Fig. 2), but in this interval Aerobacter-Klebsiella was found in 2 other blood cultures.
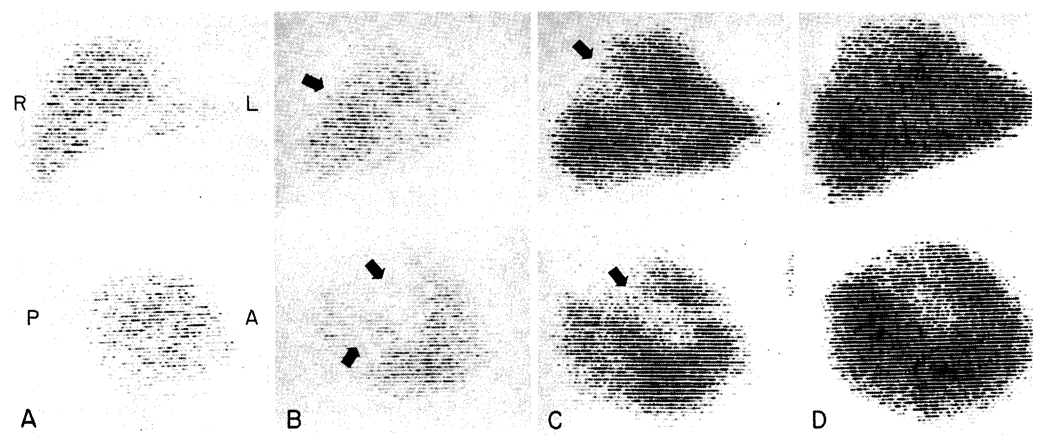
Fig. 5.
Post-transplantation liver scans in Case 1, performed with technetium-99m sulfide. For each examination an anteroposterior view is shown above and a lateral view below. A, 17 days; there are no defects B, 29 days; large nonvisualizing areas are evident (arrows). C, 32 days; 48 hours after debridement of the affected necrotic areas. D, 78 days; showing extensive regeneration into the previous defects.
Consequently, the right lobe of the liver was explored through a lateral incision in the tenth intercostal space; entry into either the chest or peritoneal cavity was avoided. A thin fluid was found in the right subphrenic space, and a 1.5 cm. necrotic area was found on the lateral surface of the right lobe. The dead liver tissue was traced by debridement into the central portion of the right lobe, from which there were 3 additional communicating tracts. A purulent collection was not found. The dead tissue which was removed had a yellow, cheesy appearance. There was no fibrous reaction around the necrotic tracts. The second day following the surgical procedure there was an additional positive blood culture with Aerobacter-Klebsiella, but in the ensuing 2 months all further cultures have been negative. The multiple sinus tracts (Fig. 5, C) were packed and irrigated daily and have healed almost to the skin edge. Most of the defects within the liver substances have filled in with functional liver tissue (Fig. 5, D).
Patient 2, who received a homograft with a double arterial supply, immediately developed an infarct of the posterior segment of the right liver lobe; 48 hours after operation the serum glutamic oxalacetic transaminase (SGOT) and serum glutamic pyruvic transaminase (SGPT) rose to 6,500 and 4,090 I.U. (upper normal 32 and 36) despite otherwise excellent liver function. On the second, fourth, and sixth postoperative days Bacteroides fragilis, which was sensitive only to tetracycline, was cultured from the blood. A liver scan on the second and seventh postoperative days showed a large defect in the right lobe. Eight days after transplantation, the original incision was reopened and a posterior segmentectomy carried out. The specimen contained a 2 cm. purulent collection surrounded by necrotic tissue. The subphrenic space was drained anteriorly at the time of hepatic resection. Two weeks later it was redrained laterally through the tenth intercostal space, because of evidence of a residual fluid collection and because of several more positive blood cultures of Bacteroides. Subsequent wound management was similar to that in Patient 1. One positive blood culture of Bacteroides was obtained 56 days after operation. In this patient, there has also been evidence of liver regeneration on liver scans.
In the third case, a culture of the recipient duodenum at the time of homotransplantathe completion of the procedure a blood culture revealed both the same organism as well as Aerobacter-Klebsiella. Antibiotic therapy was provided, as shown in Fig. 4. Early convalescence was uncomplicated. Liver scans 2, 10, and 20 days after operation were normal. However, on the twenty-fifth day post operation, the patient developed gram-negative septicemia with Aerobacter-Klebsiella, hypoglycemia (plasma sugar 0 mg. per cent), and respiratory arrest. After resuscitation a liver scan showed a central defect in the right lobe which, within 2 days, had spread to involve almost 25 per cent of the liver. An emergency debridement procedure similar to that in Patient 1 was carried out laterally through the tenth intercostal space. All subsequent blood cultures have been negative.
In each of the cases there was marked improvement in the patient’s condition after the debridement procedures. These were all carried out under heavy antibiotic coverage, as specified in Figs. 2 to 4. The antimicrobial therapy was continued for many weeks afterward, at first solely by the intravenous route and later by oral or intramuscular administration.
Other septic complications
The child with the Bacteroides liver infection also developed a gluteal abscess at the site of ALG injection. This was drained on the fourteenth postoperative day. The fleeting pneumonitis seen in all 3 patients was mentioned earlier. Pathogenic organisms were not identified from their tracheal aspirates.
Liver function
There was good early liver function despite transient acute rises in SGOT and SGPT (Figs. 2 to 4). All 3 of the liver homografts produced bile during operation. Patient 1 was not jaundiced either before or after transplantation. Serum bilirubin cleared from 24.1 and 16.1 mg. percent, respectively, to 3.1 and 6.9 mg. percent in Patients 2 and 3 within 48 hours. Total serum protein concentrations were depressed for a few days but rapidly returned to normal (Figs. 6 and 7); parenteral protein infusions were not used.
In each case, liver function remained adequate until the appearance of the infected hepatic infarctions; then there were high rises in SGOT and SGPT with little or no deterioration of other measures of liver function, except in Patient 3, who also had profound transient hypoglycemia. The enzyme changes were quickly reversible.
Subsequently, changes in liver function evolved much more slowly. Patient 1 has retained excellent biochemical values in spite of small ultimate doses of both azathioprine and prednisone (Fig. 2). Patient 2 developed evidence of indolent rejection with variable elevations in SGOT and SGPT, with low grade jaundice (Fig. 3) and a fall in serum protein concentration, and with the slow development of ascites. She was treated with increased doses of prednisone, actinomycin C, and local homograft irradiation (Fig. 3). Patient 3 has not had late deterioration of hepatic function.
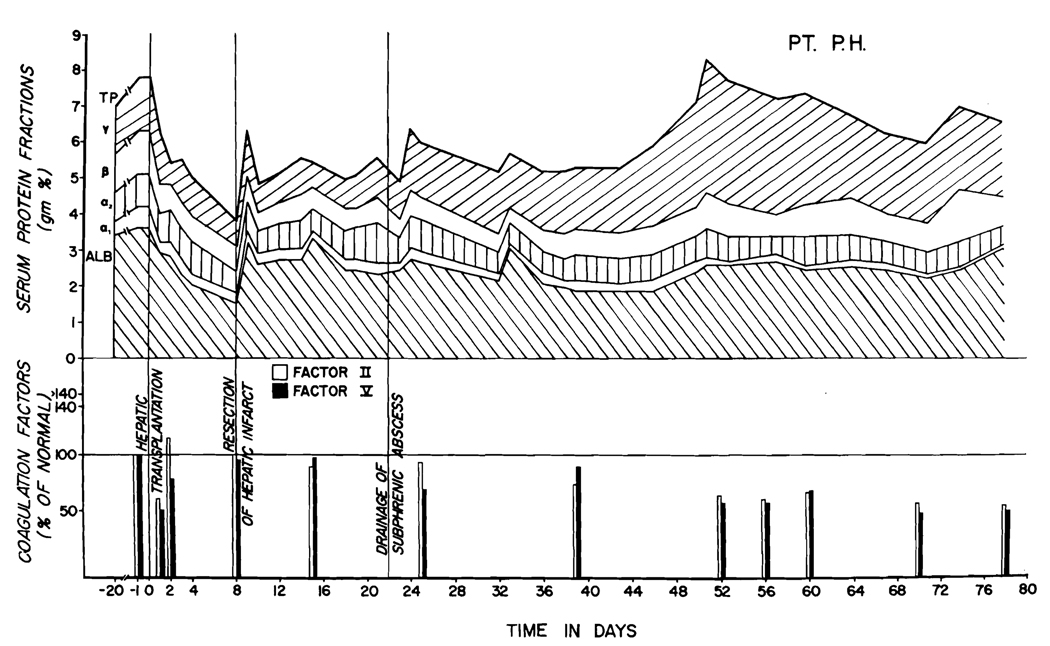
Studies of the total proteins and the protein fractions in the first 2 of the patients are summarized in Figs. 6 and 7. In both, there was a striking and persistent increase in the gamma globulin fraction during the second month. In Patients 1 and 3, the levels of other protein moieties have been well maintained, but in Patient 2 the serum proteins have been slightly depressed.
Dr. Noboru Kashiwagi has shown in our laboratories that specific changes in protein synthesis followed these liver homotransplantations. In Patients 1 and 2, the haptoglobins of the recipients were both Smithies15 type 2-1, whereas the haptoglobins in the sera of their donors were 2-2 and 1-1, respectively.
After operation, the serum haptoglobin of each patient completely changed to that of the donor. In addition, Patient 1 had a change in the group specific component of the alpha2, globulin to the genotype of the donor.
Coagulation changes
The early events were similar in all 3 cases. During and in the first few hours after operation there was an increase in fibrinolysins and a decrease in coagulation factors V (accelerator globulin) and VIII (antihemophilic globulin). At that time, the plasma fibrinogen and factor II (prothrombin) had only minor and inconsistent depressions. After 24 hours, factor II was decreased, but within a few days all coagulation measures returned to normal. These have since remained at normal levels (Fig. 6), except late in the course of Patient 2 (Fig. 7).
The platelet counts showed no significant changes during or after the operation, but in the following 2 to 3 weeks marked thrombocytopenia developed in all 3 cases. In the ensuing 1 to 3 months, the platelet counts gradually rose to normal or near normal.
Pathologic studies of the homografts
Because of the necessity for debridement or resection of the infected homografts, valuable tissue became available from 8 to 30 days after transplantation. In Patient 1, the piece of liver removed 30 days postoperatively contained multiple infarcts. Microscopically, each was rimmed by abundant neutrophiles, many eosinophiles, and some lymphoid cells, but no fibrous tissue. The necrotic centers of many, but not all, of the infarcts contained gram negative bacilli. In the surrounding viable hepatic tissue the portal and central vein branches were surrounded and infiltrated by many mononuclear cells. About 40 percent of these cells had pyroninophilic cytoplasm, and several were in mitosis. The branches of the hepatic artery, the sinusoids, and the bile ductules appeared unaltered. The portal lymphatics were not dilated.
The hepatic tissue removed 8 days after transplantation in Patient 2 contained several large infarcts. In the necrotic centers of several of these there were many gram-negative bacilli. In the adjacent surviving liver, the portal and central veins were surrounded by a moderately dense collection of lymphoid cells. About 30 percent of these cells had pyroninophilic cytoplasm. Small branches of the hepatic artery were occluded by organizing thrombus.
In Patient 3, several pieces of hepatic tissue were removed 27 days after transplantation. In these samples there were a few small infarcts; the necrotic centers of some of these contained gram-negative bacilli. The surrounding viable liver showed a normal lobular pattern. There was excess fat in the centrilobular hepatocytes, but elsewhere the liver cells were normal. The portal tracts were densely crowded with small lymphocytes, some plasma cells, large lymphoid cells, and a few eosinophil polymorphonuclear leukocytes. About 10 percent of the mononuclear cells had pyroninophilic cytoplasm. This cellular infiltration was most dense around the portal vein branches, and similar cells were present in the walls of these vessels. The central veins were also surrounded by a dense collection of mononuclear cells. The branches of the hepatic artery, the bile ducts, the sinusoids, and the Kupffer cells were normal.
In none of these hepatic homografts was there evidence of thrombotic or fibrin emboli. In Patients 1 and 3 the infarcts were recent and did not date back to the time of transplantation. As a few of the infarcts were uninfected, it seems improbable that infarction followed sepsis.
DISCUSSION
As mentioned earlier, seven previous clinical attempts at orthotopic liver homotransplantation in our institutions all resulted in early death of the recipients. Several changes in management probably contributed to survival in the 3 more recent cases. A vital factor was the quality of the organs which were used. Every effort was made to maintain some circulation until these livers were cooled. In addition, the preservation system which was used after the organs had been extirpated is an extremely effective one which can uniformly maintain canine livers in excellent condition for at least 8 hours,2 a time limit which far exceeded the needs in the clinical cases.
Even though the homografts provided good function from the outset, a combined pre-and postmortem ischemic injury was reflected in each recipient by transient rises in SGOT and SGPT and by abrupt depressions of the serum proteins, which lasted for several days after transplantation. It is probable that the swiftly developing metabolic acidosis at the time of organ revascularization was on the same basis. The prompt treatment of this complication may be essential, especially in young patients. There have been at least 2 sudden deaths shortly after revascularization of auxiliary liver homografts in children.7, 8 Sudden acidosis has also been described after reimplantation of limbs.10
It is highly likely that some of the earlier attempts at orthotopic transplantation would have succeeded had there not been the development of pulmonary emboli. It is tragic to realize now that the external bypasses, which undoubtedly contributed to the complication in those cases,21, 22, 26 were probably unnecessary, since all the earlier patients had extensive venous collaterals secondary to their liver disease. None of the 3 children in the present report had decompression of the venous pools, which were occluded during the anhepatic phase. The ability to tolerate this insult seemed proportional to the severity of portal hypertension and the richness of venous collaterals, but in none of the cases were there serious adverse consequences from the omission of the bypasses.
In past experience, changes in blood coagulation during and after experimental or clinical liver transplantation presented difficult problems of management. Intraoperatively, there may be an increased fibrinolysis,22, 26 hypofibrinogenemia,22, 26 thrombocytopenia,9 or the appearance of heparinlike substances in the circulation.23 If the transplanted liver provides good immediate function, these changes are transient and may easily be overcorrected by imprudent therapy with clot promoting agents, with the resultant development of a hypercoagulable state.22, 26 In contrast, recipients of badly damaged livers quickly develop an even more complex clotting disorder in which there are rapid falls in all the known factors synthesized by the liver. The latter findings carry a grave if not hopeless prognosis.12
Most of the foregoing problems are avoided first by the use of optimally preserved livers and second by the omission of prosthetic bypasses. If these conditions can be met, iatrogenic manipulation of the clotting process is probably not warranted. Should uncontrolled bleeding develop after revascularization, epsilon-aminocaproic acid (EACA), protamine sulfate, fibrinogen, platelet infusions, fresh blood, and fresh plasma may all be helpful.
The later management of the patients was probably made safer by the use of heterologous antilymphocyte globulin (ALG) as an adjuvant immunosuppressive drug. When used alone in dogs, this agent can delay or even prevent the rejection of orthotopic liver homografts.19 In human recipients of renal homografts, it has been used in conjunction with azathioprine and prednisone. The therapeutic regimen provided better homograft protection, permitted reduced dosages of azathioprine and prednisone, and seemed to decrease the incidence of septic complications.17, 19 The 3 recipients of the liver homografts were treated with this therapeutic program.
The many complications which have plagued the early course after clinical liver transplantation have been summarized elsewhere.16, 18, 20, 22 Two additional problems were observed in all 3 of the presently reported cases. Each of the 3 children had a temporary paralysis of the right diaphragm. This was evidently caused by accidentally crushing the phrenic nerve with the vascular clamp that was applied to the suprahepatic vena cava. This accident had not occurred in previous adult cases. It was avoided in a fourth, recently treated, child by dissecting free a longer host cuff of vena cava and by applying the vascular clamp at a slightly more inferior level.
A much more serious later complication in all 3 patients was the development of infected hepatic infarction. The initiating factor in Patient 2 was probably technical in nature; there was a double arterial supply of the homograft, and a major ramification of the right branch evidently thrombosed within a few hours.
In Patients 1 and 3, however, the infarctions occurred after 23 and 25 days. Except for acute rises in the SGOT and SGPT, there were minimal abnormalities of liver function. Nevertheless, the tissues removed in the liver debridement procedures had histologic evidence of acute rejection in all 3 homografts. Conceivably, there was subclinical rejection, and the blood flow, which is known to be reduced at these times,6 had fallen below a life-sustaining level in selective portions of the liver. This concept of an immunologically mediated ischemic injury with regional infarction was persuasively stated by Moore and his associates in 1964.11 If it occurred in Patients 1 and 3, it suggests that the patients were not receiving adequate immunosuppression. It could also indicate that the present techniques for diagnosing rejection are too insensitive to be a guide for really incisive treatment, since the dramatic changes in the enzyme studies occurred after extensive necrosis was already complete.
Contrary to the foregoing point of view is the report of Alican and Hardy,1 who found a high incidence of liver abscesses in the early postoperative period after liver autotransplantation. Even the few animals which survived chronically ultimately died from this complication. In their experiments an immunologically induced ischemia was clearly not responsible. Curiously enough, the magnitude of the problem seemed greater in these autotransplantations than with homotransplantation to dogs receiving immunosuppressive agents. Under the latter conditions, the rate of liver abscess formation in the early postoperative period was relatively lower both in our experience20 and in that of Moore and associates11 and Stuart and associates.24 Moreover, none of the more than 25 dogs followed in our laboratories for 4 to 44 months after orthotopic homotransplantation developed a late liver abscess.
Until more information becomes available, it seems most reasonable to consider that hepatic necrosis from a variety of causes can initiate the chain of events which was observed in each of the presently reported cases. The pathologic evidence was that the infarctions came first. At the time of reoperation, they were all of recent origin with little evidence of repair. Apparently the necrotic foci were then invaded by microorganisms from the intestinal tract, via either the portal vein or the choledochoduodenostomy.
Once the complication was established, extensive debridement procedures were necessary. Fortunately, these could be carried out in Patients 1 and 3 without entering the pleural or peritoneal cavities, and in Patient 2 the septic focus was also eventually excluded from the serous cavities. More desirable, of course, would be the prevention of the infections. Possibly, preoperative sterilization of the recipient gastrointestinal tract would be of value. More liberal prophylactic use of some of the potentially toxic antibiotics such as polymyxin and kanamycin may be advisable, since they are highly effective against gram negative organisms.
It is, of course, too early to speculate about the ultimate fate in these 3 cases. The patients have already passed through the period in which all previous attempts at liver transplantation had led to death. The degree to which histoincompatibility will affect the future course is a matter of great interest, since one case had an excellent donor-recipient antigen match, the second had fair compatibility, and the third had poor compatibility. Conceivably, the regenerative abilities of the liver may permit long-term survival, even if chronic rejection causes a slow loss of parenchyma. In our laboratories, dogs have survived for years under these circumstances. Since the debridement procedures, there has already been evidence of extensive parenchymal regeneration in the livers of all 3 patients.
SUMMARY
Orthotopic homotransplantation of cadaver livers was carried out in 3 children, aged 13 to 20½ months, for the treatment of primary liver-cell carcinoma or extrahepatic biliary atresia. The donor-recipient lymphocyte antigen compatibility ranged from good to poor. Immunosuppressive therapy was with azathioprine, prednisone, and heterologous antilymphocyte globulin (ALG).
In all 3 recipients, the right phrenic nerve was paralyzed during operation, probably by crushing it with the vascular clamp that was applied to the suprahepatic inferior vena cava. Normal diaphragmatic movement returned from 2 to 10 weeks later. During the anhepatic phase of the procedure, it was found that decompression of the occluded vena caval and portal venous systems with external bypasses was not necessary.
In the process of transplantation, the homografts sustained from minor to moderate ischemic injury. The anoxia probably contributed to a moderately severe intraoperative metabolic acidosis following revascularization, but the livers provided satisfactory early postoperative function. Subsequently, infected liver infarctions and consequent gram-negative septicemia developed in each patient. In one this was evidently due to a technical complication with resultant thrombosis of part of the hepatic arterial supply, but in the other two the infarctions occurred after more than 3 weeks. In the latter cases, a blood flow reduction caused by clinically inevident rejection or other unknown factors may have been responsible. The septic infarcts were excised or debrided under appropriate antibiotic coverage. The possible etiologic role of rejection was supported by histologic evidence of this process in all 3 of the patients. Regeneration subsequently contributed to a variable filling in of the intrahepatic defects.
Late function has ranged from completely satisfactory to subnormal, as judged by multiple function tests of synthesis and excretion. Only one patient has clinical evidence of liver failure, manifested by persistent jaundice and ascites. All 3 children are alive after 94, 86, and 49 days, as well as a fourth patient, who is now 2 weeks postoperative.
Acknowledgments
This work was supported by United States Public Health Service Grants F05-TW-1154, AM 06283, AM 06344, HE 07735, AM 07772, AI 04152, FR 00051, and FR 00069.
Footnotes
ADDENDUM
Two of the 3 patients in this series are still alive as of January 27, 1968. Patient 1 has now survived for more than 6 months. Liver function remains excellent, but she has developed slow-growing pulmonary metastases. Patient 3 will be 5 months postoperative in 1 week. Patient 2, who had poor liver function at the time the report was submitted, died after 134 days. The immediate cause of death was massive intestinal gangrene.
Two more infants with biliary atresia have received orthotopic homotransplantation. The first died 61 days after transplantation as a consequence of thrombosis of the right hepatic artery and gangrene of the liver lobe. The second child is more than 2 months postoperative and has not had any of the complications described in the text.
Drs. Pierre Daloze of Montreal and Claude Huguet of Paris were important members of the operating teams.
Drs. Anthony Aldrete, Theodore Gingrich, and David LeVine provided the anesthesia.
Three medical students made important contributions to the postoperative care of the patients; Charles Putnam of Northwestern University, Charles Brantigan of Johns Hopkins University, and David Cordes of the University of Illinois.
Miss Josephine Ehret did all bacteriologic studies. The coagulation analyses were performed by Dr. Liberto Pechet.
Mr. Paul Taylor, Director of the Research Laboratories, provided invaluable assistance in every stage of the undertaking.
REFERENCES
- 1.Alican F, Hardy JD. Replantation of the liver in dogs. J. S. Res. 1967;7:368. [Google Scholar]
- 2.Brettschneider L, Daloze PM, Huguet C, Groth CG, Kashiwagi N, Hutchison DE, Starzl TE. Successful orthotopic transplantation of liver homografts after 8–25 hours preservation. S. Forum. 1967;18:376. [PMC free article] [PubMed] [Google Scholar]
- 3.Comroe JH, Jr, Forster RE, II, Dubois AB, Briscoe WA, Carlsen E. The lung. Chicago: Year Book Medical Publishers, Inc.; 1962. p. 343. [Google Scholar]
- 4.Dausset J, Rapaport FT, Legrand L. Progress in transplantation. Copenhagen: Ejnar Munksgaards Forlag; 1967. Selection des donneurs par les groupes leucocytaires. [Google Scholar]
- 5.Demirleau, Nourreddine, Vignes, Prawerman, Rciziciner, Larraud, Louvier Tentative d’homogreffe hepatique. Mem. Acad. Chir. (Paris) 1964;90:177. [PubMed] [Google Scholar]
- 6.Groth CG, Porter KA, Otte JB, Daloze PM, Marchioro TL, Brettschneider L, Starzl TE. Studies of blood flow and ultrastructural changes in rejecting and nonrejecting canine orthotopic liver homografts. Surgery. In press. [PMC free article] [PubMed] [Google Scholar]
- 7.Halgrimson CG, Marchioro TL, Faris TD, Porter KA, Peters GN, Starzl TE. Auxiliary liver transplantation: Effect of host portacaval shunt. Arch. Surg. 1966;93:107. doi: 10.1001/archsurg.1966.01330010109014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herman R. Personal communication. Cleveland, Ohio: [Google Scholar]
- 9.Hutchison DE, Genton E, Daloze PM, Huguet C, Brettschneider L, Groth CG, Starzl TE. Platelet changes following clinical and experimental hepatic homotransplantation. Arch. Surg. doi: 10.1001/archsurg.1968.01340010057003. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehl RL, Paul HA, Shorey W, Schneewind J, Beattie EJ., Jr Treatment of “toxemia” after extremity re-plantation. Arch. Surg. 1964;89:871. doi: 10.1001/archsurg.1964.01320050117010. [DOI] [PubMed] [Google Scholar]
- 11.Moore FD, Birtch AG, Dagher F, Veith F, Krisher JA, Order SE, Shucart WA, Dammin GJ, Couch NP. Immunosuppression and vascular insufficiency in liver transplantation. Ann. New York Acad. Sc. 1964;120:729. doi: 10.1111/j.1749-6632.1964.tb34765.x. [DOI] [PubMed] [Google Scholar]
- 12.Pechet L, Groth CG, Daloze PM. Changes in coagulation and fibrinolysis after orthotopic canine liver homotransplantation. Fed. Proc. In press. [PubMed] [Google Scholar]
- 13.Rydell R, Hoffbauer FW. Multiple pulmonary arteriovenous fistulas in juvenile cirrhosis. Am. J. Med. 1956;21:450. doi: 10.1016/0002-9343(56)90043-2. [DOI] [PubMed] [Google Scholar]
- 14.Silverman A, Cooper MD, Moller JH, Good RA. Syndrome of cyanosis, digital clubbing, and hepatic disease in siblings. J. Pediat. 1968;72:70. doi: 10.1016/s0022-3476(68)80402-0. [DOI] [PubMed] [Google Scholar]
- 15.Smithies O. Zone electrophoresis in starch gels and its application to studies of serum proteins. Advances Protein Chem. 1959;14:65. doi: 10.1016/s0065-3233(08)60609-9. [DOI] [PubMed] [Google Scholar]
- 16.Starzl TE, Brettschneider L, Groth CG. Progress in transplantation. Copenhagen: Ejnar Munksgaards Forlag; 1967. Recent developments in liver transplantation. [Google Scholar]
- 17.Starzl TE, Groth CG, Terasaki PI, Putnam CW, Brettschneider L, Marchioro TL. Hcterologous antilymphocyte globulin, histocompatibility matching, and human renal homotransplantation. Surg. Gynec. & Obst. In press. [PMC free article] [PubMed] [Google Scholar]
- 18.Starzl TE, Marchioro TL, Porter KA. Progress in homotransplantation of the liver. In: Welch C, editor. Advances in Surgery. Vol. 2. Chicago: Year Book Medical Publishers, Inc.; 1966. p. 295. [PMC free article] [PubMed] [Google Scholar]
- 19.Starzl TE, Marchioro TL, Porter KA, Iwasaki Y, Cerilli GJ. The use of heterologous antilymphoid agents in canine renal and liver homotransplantation, and in human renal homotransplantation. Surg. Gynec. & Obst. 1967;124:301. [PMC free article] [PubMed] [Google Scholar]
- 20.Starzl TE, Marchioro TL, Porter KA, Taylor PD, Faris TD, Herrmann TJ, Hlad CJ, Waddell WR. Factors determining short- and long-term survival after orthotopic liver homotransplantation in the dog. Surgery. 1965;58:131. [PMC free article] [PubMed] [Google Scholar]
- 21.Starzl TE, Marchioro TL, Rowlands DT, Jr, Kirkpatrick CH, Wilson WEC, Rifkind D, Waddell WR. Immunosuppression after experimental and clinical homotransplantation of the liver. Ann. Surg. 1964;160:411. doi: 10.1097/00000658-196409000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Starzl TE, Marchioro TL, von Kaulla K, Herrmann G, Brittain RS, Waddell WR. Homotransplantation of the liver in humans. Surg. Gynec. & Obst. 1963;117:659. [PMC free article] [PubMed] [Google Scholar]
- 23.Stremple JF, Hussey CV, Ellison EH. Study of clotting factors in liver homotransplantation. Am. J. Surg. 1966;111(1):862. doi: 10.1016/0002-9610(66)90190-5. [DOI] [PubMed] [Google Scholar]
- 24.Stuart FP, Torres E, Hester WJ, Dammin GJ, Moore FD. Orthotopic autotransplantation and allotransplantation of the liver: Functional and structural patterns in the dog. Ann. Surg. 1967;165:325. doi: 10.1097/00000658-196703000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Terasaki PI, Vredevoe DL, Mickey MR. Serotyping for homotransplantation. X. Survival of 196 grafted kidneys subsequent to typing. Transplantation. 1967;5:1057. doi: 10.1097/00007890-196707001-00041. [DOI] [PubMed] [Google Scholar]
- 26.von Kaulla KN, Kaye H, von Kaulla E, Marchioro TL, Starzl TE. Changes in blood coagulation before and after hepatectomy or transplantation in dogs and man. Arch. Surg. 1966;92:71. doi: 10.1001/archsurg.1966.01320190073016. [DOI] [PMC free article] [PubMed] [Google Scholar]