Abstract
Blood concentrations of cyclosporine were determined in adult and pediatric patients following orthotopic liver transplantation to quantitate cyclosporine blood clearance and oral absorption. Seventeen bioavailability studies were performed following transplantation surgery in nine children and seven adults. The intravenous cyclosporine study was performed following an average dose of 2.1 mg/kg. The patients were again studied when they received the same intravenous dose plus an oral dose of cyclosporine of 8.6 mg/kg or an oral dose alone. Blood samples were collected and analyzed for cyclosporine using high-performance liquid chromatography. Cyclosporine blood clearance ranged from 29 to 203 mL/min (1.9–21.5 mL/min/kg) in children and from 253 to 680 mL/min (3.2–7.6 mL/min/kg) in adults. The mean cyclosporine clearance value was 9.3 mL/min/kg in the pediatric patients and 5.5 mL/min/kg in the adults. Cyclosporine bioavailability was less than 5% in six studies on five pediatric patients in the immediate postoperative period. The bioavailability varied from 8% to 60% in adult liver transplant patients (mean, 27%). We conclude that: (1) cyclosporine clearance is highly variable between patients, (2) pediatric patients clear the drug more rapidly than adults and therefore need a higher cyclosporine dose on a body weight basis, (3) cyclosporine is poorly and variably absorbed in liver transplant patients, and (4) cyclosporine blood concentration monitoring is essential following orthotopic liver transplantation.
Orthotopic liver transplantation is now considered a therapeutic alternative for children with diseases such as biliary atresia and alpha1-antitrypsin deficiency and for adults with primary biliary cirrhosis and sclerosing cholangitis.1–5 Therapy with cyclosporine and low-dose steroids constitutes a major advance in the treatment of patients undergoing orthotopic liver transplantation.6
Liver transplantation presents special problems in maintaining adequate pharmacologic immunosuppression. The absorption, metabolism, and excretion of pharmacologic agents can be expected to be significantly altered by the transplantation procedure. Aggressive drug therapy is often needed to achieve adequate immunosuppression.
Adequate blood concentrations of cyclosporine are maintained following orthotopic liver transplantation by prolonged intravenous (IV) and combined IV and oral therapy. This cyclosporine dosing regimen is different than that used following renal or heart transplantation and prompted us to investigate the clearance and bioavailability of cyclosporine following orthotopic liver transplantation.
PATIENTS AND METHODS
All patients who underwent orthotopic liver transplantation were considered candidates for the study. Seventeen studies were performed after patient (seven adults) or parental (nine children) consent was obtained. Fifteen patients received the same intravenous cyclosporine dosage every eight hours for at least 24 hours before the start of the study.
Protocol
On the first study day, the patients received their prescribed IV cyclosporine dosage infused over two hours by Harvard infusion pump (Bard Medical Systems, North Reading, MA) or an IV drip set. In the ten pediatric studies, heparinized blood samples were obtained before the start of the infusion, at the end of the infusion, and at four, six, and eight hours following the start of the infusion. Hourly blood samples were collected during the dosing interval in adults. On the second study day, ten children and two adults received the same IV dose of cyclosporine at the same time of the day, but also received an oral cyclosporine dose concurrent with the start of the infusion. Five adult patients received only oral cyclosporine q12h on the second day. The oral cyclosporine liquid was diluted with juice or chocolate milk, mixed, and administered as quickly as possible. The blood sampling on the second day followed the same schedule as that of the first day, with the exception that the adult patients were sampled hourly for 12 hours.
Concurrent medications administered with cyclosporine, included methylprednisolone or prednisone, cefotaxime and ampicillin, captropril, hydralazine, furosemide, and nystatin.
Assay
Blood samples were refrigerated at 4°C until assayed (<7 days). An extraction and a high-performance liquid chromatographic assay similar to that of Sawchuk and Cartier was used.7 The assays were performed using whole blood, and cyclosporin D was the internal standard. The drug was initially extracted into diethyl ether. After drying, the residue was taken up in methanol, combined with hydrochloric acid, and washed twice with hexane. After neutralization, the drug and internal standard were reextracted into ether, dried, and reconstituted with the mobile phase before injection. The samples were injected onto a 15-cm C-18 column (Supelco, Bellefonte, PA) maintained at 70°C. The mobile phase was 68% acetonitrile in water with a flow rate of 1.5 mL/min. Ultraviolet detection was achieved at 214 nm with a Model 441 detector (Waters, Milford, MA). All samples and standards were analyzed in duplicate. The retention times for cyclosporine and cyclosporin D were 8.3 and 11.2 minutes, respectively, The minimum detectable level of this assay is 25 ng/mL using one milliliter of 50–2,000 ng/ml. The interday coefficient of variation of the assay was 8.0% at 250 ng/mL (N = 35), 6.4% at 500 ng/mL (N = 19), and 7.7% at 1,000 ng/mL (N = 23).
Data Analysis
The short dosing interval precluded calculation of a half-life or elimination rate constant. All studies were carried out at steady-state or pseudo-steady-state conditions. The area under the curve (AUC) for the blood concentration versus time curve was calculated by the trapezoidal rule over one dosing interval. Drug clearance was calculated as the IV cyclosporine dose divided by the AUC (IV) and bioavailability percentage (F) was calculated as:
Rank correlation of subgroups were compared using the Wilcoxon two-tailed analysis assuming a level of significance of P ≤ .05.
RESULTS
The characteristics of the patients at the time of study are listed in Table I. Patient 7 was studied twice after two separate transplant operations. Nine of the studies were performed after the first orthotopic liver transplantation, seven after the second, and one after the third operation. Nine of the studies were started on the second or third day postoperatively.
TABLE I.
Patient Characteristics
| Patient | Age | Weight (kg) | Original Diagnosis | Postoperative Day on Which Study Was Performed |
||
|---|---|---|---|---|---|---|
| IV | IV + PO | PO | ||||
| 1 | 34 mo | 11.4 | Biliary atresia | 2 | 3 | — |
| 2 | 67 mo | 16.8 | α1-antitrypsin deficiency | 8 | 9 | — |
| 3 | 52 mo | 12.3 | Biliary atresia | 3 | 4 | — |
| 4 | 39 mo | 15.5 | Biliary atresia | 3 | 4 | — |
| 5 | 11 mo | 8.2 | Biliary atresia | 2 | 3 | — |
| 6 | 51 mo | 17.9 | Biliary atresia | 3 | 4 | — |
| 7a | 36 mo | 15.3 | Hepatitis | 3 | 4 | — |
| 7b | 37 mo | 14.4 | Hepatitis | 2 | 3 | — |
| 8 | 47 mo | 14.3 | Biliary atresia | 2 | 3 | — |
| 9 | 7 mo | 7.0 | Biliary atresia | 2 | 3 | — |
| 10 | 47 yr | 52 | Primary biliary cirrhosis | 3 | — | 43 |
| 11 | 21 yr | 40 | Tyrosinemia, hepatoma | 2 | 24 | — |
| 12 | 42 yr | 88 | Post–necrotic cirrhosis | 18 | — | 29 |
| 13 | 26 yr | 70 | Hepatitis, cirrhosis | 5 | — | 56 |
| 14 | 51 yr | 55 | Primary biliary cirrhosis | 2 | — | 49 |
| 15 | 27 yr | 96 | Post–necrotic cirrhosis | 2. | 21 | — |
| 16 | 36 yr | 61 | Chronic active hepatitis | 29 | — | 30 |
IV = intravenous; PO = oral.
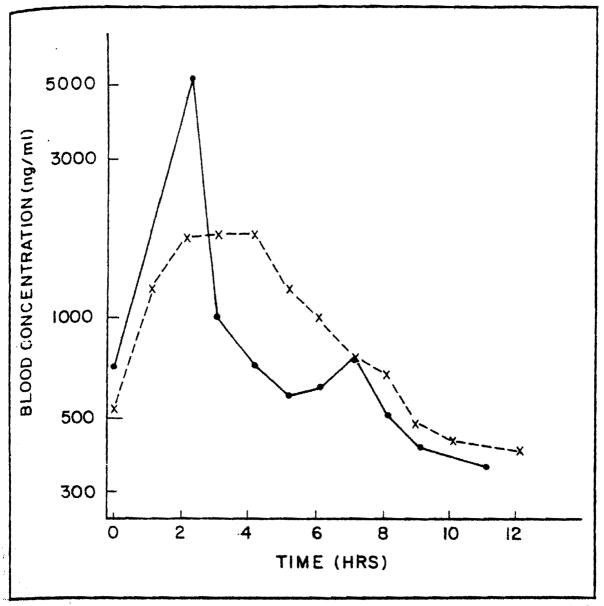
The maximum blood concentration after the IV cyclosporine was observed at the end of the infusion and the blood concentration declined in a biexponential fashion. Figures 1 and 2 present the blood concentration versus time for a representative adult and pediatric patient. Table II lists the estimated pharmacokinetic parameters derived from the blood concentration versus time data. The average cyclosporine dosage was 2.1 mg/kg IV and 8.6 mg/kg orally. The mean trough concentration at eight hours was 298 ng/mL following IV cyclosporine in the pediatric patients. Four of the pediatric patients had a cyclosporine clearance of greater than 10 mL/min/kg. The clearance of cyclosporine in the adult patients ranged from 3.2 to 7.6 mL/min/kg. The mean cyclosporine clearance was 9.3 mL/min/kg in the pediatric population and 5.5 mL/min/kg in the adults.
Figure 1.
Cyclosporine blood concentration versus time in adult study patient 16. Concentrations following an intravenous (●– –●) and an oral (x - -x) dose are presented.
Figure 2.
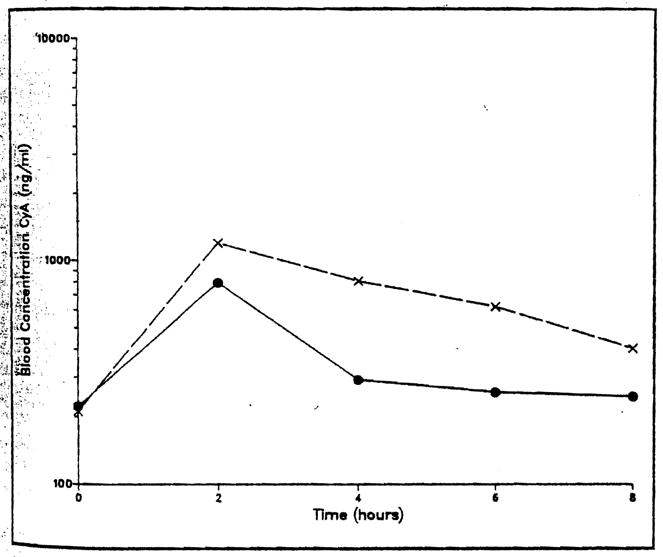
Cyclosporine blood concentration versus time in pediatric study patient 6. Concentrations following an intravenous (●– –●) and an intravenous plus oral (x - - x) dose are presented.
TABLE II.
Cyclosporine Pharmacokinetic Parameters
| Patient | Dose (mg/kg) |
Clearance (mL/min/kg) | Bioavailability (%) | |
|---|---|---|---|---|
| IV | Oral | |||
| 1 | 2.6 | 8.8 | 13.0 | 13 |
| 2 | 1.8 | 8.9 | 12.1 | 18 |
| 3 | 2.4 | 8.1 | 7.1 | <5 |
| 4 | 1.9 | 8.1 | 1.9 | <5 |
| 5 | 3.7 | 18.3 | 8.2 | <5 |
| 6 | 2.0 | 8.4 | 10.4 | 19 |
| 7a | 1.6 | 9.2 | 6.0 | <5 |
| 7b | 2.1 | 10.4 | 6.9 | <5 |
| 8 | 3.1 | 7.0 | 5.6 | <5 |
| 9 | 2.0 | 8.6 | 21.5 | 8 |
| 10 | 1.7 | 9.6 | 4.9 | 10 |
| 11 | 1.8 | 10.0 | 6.3 | 60 |
| 12 | 0.6 | 3.4 | 3.2 | 29 |
| 13 | 2.1 | 3.6 | 5.3 | 15 |
| 14 | 2.0 | 7.3 | 7.6 | 38 |
| 15 | 1.9 | 7.3 | 7.1 | 8 |
| 16 | 2.5 | 7.4 | 4.4 | 31 |
IV = intravenous.
Six studies conducted in the pediatric study population in the immediate postoperative period demonstrated an estimated bioavailability of less than 5% of the orally administered cyclosporine. In these patients, cyclosporine clearance was less than 10 mL/min/kg. The other four children with bioavailability greater than 5% had cyclosporine blood clearances greater than 10 mL/min/kg (P = .01). A wide variation in the extent of absorption in adult patients was observed as indicated by a bioavailability of 8% to 60% (mean ± standard deviation = 27 ± 18%). The two adults with an 8% and 15% bioavailability had a serum bilirubin concentration of greater than 2.0 mg/dL, whereas the five adult patients with greater cyclosporine absorption had a serum bilirubin of less than 2.0 mg/dL. The serum bilirubin concentration was greater than 3.6 mg/dL in all the pediatric patients.
DISCUSSION
The pharmacokinetics of cyclosporine have been investigated in the recipients of allogenic bone marrow grafts and transplanted kidneys and hearts. The harmonic mean cyclosporine blood clearance in kidney transplant recipients is 5.7 mL/min/kg (range, 0.6–23.9 mL/min/kg).8 The plasma clearance of the drug was previously determined to be 12.2 ± 5.6 mL/min/kg in bone marrow transplant patients.9 whereas the blood clearance ranged from 2.1 to 15.1 mL/min/kg in patients receiving heart transplants.10 In each of these patient populations, the clearance values are highly variable.
In the present study, a tenfold variation in the clearance of cyclosporine was observed in pediatric liver transplant patients. The day to day variability in clearance in an individual patient has not been assessed and may have contributed in part to this variation. Pediatric patients had a higher harmonic mean cyclosporine clearance per body weight as compared with adult liver transplant patients, even though they were studied earlier in their course of therapy. The highest cyclosporine clearance value (21.5 mL/min/kg) was noted in the youngest study patient (age, 7 months). The higher cyclosporine clearance in children is consistent with the observations of higher clearance per body weight of other drugs such as theophylline in the pediatric population11 and with our studies of cyclosporine in pediatric kidney and heart transplant patients.12 Pediatric renal transplant patients have the highest cyclosporine blood clearance values, with a harmonic mean clearance of 11.8 mL/min/kg.13 The higher cyclosporine clearance in children is consistent with the higher drug doses on a body weight basis that are frequently necessary to achieve blood concentrations similar to those seen in adults.
Studies in bone marrow and kidney transplant patients have shown considerable variation in the peak plasma levels of cyclosporine following oral administration of the drug.14,15 The bioavailability of cyclosporine has been reported to range from 10% to 50%, with a mean of 34%.16 We have observed an average bioavailability of 27.6% in 41 renal transplant patients in whom a marked variation in the bioavailability of cyclosporine has also been observed (range, <5% to 89%).8
Because of the limitations in the study design imposed by this patient population, the bioavailability parameters calculated are only estimates of the actual bioavailability values. We most likely underestimated bioavailability if hepatic drug clearance had improved on the oral study day, but may have overestimated bioavailability if steady state had not been achieved at the time of the intravenous study. The vehicle used to dilute the drug (juice or milk) will not however affect drug absorption.17 Changes in drug clearance between the intravenous and oral study would also change the bioavailability estimate.
In the pediatric orthotopic liver transplantation patients, cyclosporine was very poorly absorbed. The bioavailability ranged from <5% to 18%. The poor absorption of cyclosporine in our pediatric orthotopic liver transplantation recipients was predictable from our previous clinical experience. However, we did not anticipate such a large variation in the absorption in the immediate postoperative period. This observation indicates that not all orthotopic liver transplantation patients require prolonged intravenous therapy. The early initiation of oral cyclosporine in conjunction with the intravenous formulation while monitoring trough blood concentrations is an effective test of drug absorption. This regimen facilitates the early conversion from intravenous to oral therapy with cyclosporine in those patients who adequately absorb the drug.
In contrast with the poor cyclosporine absorption observed in our pediatric population in the immediate postoperative period, adults with improving liver function following orthotopic liver transplantation absorbed 8% to 60% of an oral cyclosporine dose. Cyclosporine bioavailability has been reported to increase three- to fivefold over a three-month time period in renal transplant patients.15 Improving liver function and bile flow in liver transplant recipients may be responsible for improving cyclosporine absorption over time and may permit a dosage reduction.
Increases in the trough blood concentrations of cyclosporine are particularly notable following T-tube damping in orthotopic liver transplantation patients.18 Previous studies have shown this increase is not attributable to increased enterohepatic recycling of cyclosporine following T -tube clamping.19 Increased bile flow into the gut is most likely responsible for the improved cyclosporine absorption.
Cyclosporine absorption did not correlate with serum biochemical parameters such as bilirubin or liver enzyme levels in the immediate postoperative period. Gastric emptying and gut motility were not assessed in this study but may affect bioavailability particularly in the immediate postoperative period. Estimates of hepatic blood flow were likewise not evaluated in this study. Subsequent investigations in adults with liver disease have demonstrated a good correlation between an elevated serum bilirubin concentration and a diminished cyclosporine bioavailability following an oral dose.20
The frequent malabsorption and the marked variability in cyclosporine absorption and metabolism makes individualization of cyclosporine therapy essential in individuals undergoing orthotopic liver transplantation. Cyclosporine clearance on a body weight basis is high in some children and necessitates higher than normal doses of the drug based on body weight. Only a structured program of blood level monitoring can presently provide the essential information on cyclosporine absorption or metabolism following liver transplantation. An adequate monitoring program should include frequent (2–3 times weekly) blood sampling of cyclosporine levels during the early postoperative period and should recognize the limitations of the assay used.21
Acknowledgments
Supported in part by a grant from Sandoz Incorporated, East Hanover, New Jersey and by a PHS grant from NIADDK.
Footnotes
Presented in part at the annual meeting of the American College of Clinical Pharmacy, San Diego, California, June 26, 1984.
References
- 1.Starzl TE, Iwatsuki S, Van Thiel DH, et al. Evolution of liver transplantation. Hepatology. 1982;2:614–636. doi: 10.1002/hep.1840020516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calne RY. Recent advances in clinical transplantation of the liver and pancreas. Transplant Proc. 1983;15:1263–1268. [Google Scholar]
- 3.Calne RY, Williams R, Lindop M, et al. Improved survival after orthotopic liver grafting. Br Med J. 1981;283:115–118. doi: 10.1136/bmj.283.6284.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malatack JJ, Zitelli BJ, Gartner JC, et al. Pediatric liver transplantation under therapy with cyclosporin A and steroids. Transplant Proc. 1983;15:1292–1296. [PMC free article] [PubMed] [Google Scholar]
- 5.Starzl TE, Iwatsuki S, Van Thiel DH, et al. Report of Colorado-Pittsburgh liver transplantation studies. Transplant Proc. 1983;15:2582–2585. [Google Scholar]
- 6.Starzl TE, Klintmalm GBG, Porter KA, et al. Liver transplantation with the use of cyclosporin A and prednisone. N Engl J Med. 1981;305:266–269. doi: 10.1056/NEJM198107303050507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sawchuk RJ, Cartier LL. Liquid chromatographic determination of cyclosporin A in blood and plasma. Clin Chem. 1981;27:1368–1371. [PubMed] [Google Scholar]
- 8.Ptachcinski RJ, Venkataramanan R, Rosenthal JT, et al. Cyclosporine kinetics in renal transplantation. Clin Pharmacol Ther. 1985;38:296–303. doi: 10.1038/clpt.1985.174. [DOI] [PubMed] [Google Scholar]
- 9.Kennedy MS, Yee GC, Deeg HJ, et al. Pharmacokinetics and toxicity of cyclosporine in marrow transplant patients. Transplant Proc. 1983;15:2416–2418. [Google Scholar]
- 10.Venkataramanan R, Burckart GJ, Ptachcinski RJ, et al. Cyclosporine pharmacokinetics in heart transplant patients. Transplant Proc. in press. [PMC free article] [PubMed] [Google Scholar]
- 11.Miles MV. Pediatric pharmacokinetics. In: Mungall DR, editor. Applied Clinical Pharmacokinetics. New York: Raven; 1983. pp. 367–388. [Google Scholar]
- 12.Burckart GJ, Venkataramanan R, Starzl TE, et al. Cyclosporine clearance in children following organ transplantation (abstract) J Clin Pharmacol. 1984;24:412. [Google Scholar]
- 13.Ptachcinski RJ, Burckart GJ, Rosenthal JT, et al. Cyclosporine pharmacokinetics in children following cadaveric renal transplantation. Transplant Proc. in press. [Google Scholar]
- 14.Beveridge T, Gratwohl A, Michot F, et al. Cyclosporin A: pharmacokinetics after a single dose in man and serum levels after multiple dosing in recipients of allogenic bone marrow grafts. Curr Ther Res. 1981;30:5–17. [Google Scholar]
- 15.Kahan BD, Ried M, Newburger J. Pharmacokinetics of cyclosporine in human renal transplantation. Transplant Proc. 1983;15:446–453. [Google Scholar]
- 16.Wood AJ, Maurer G, Niederberger W, et al. Cyclosporine pharmacokinetics, metabolism, and drug interactions. Transplant Proc. 1983;15:2409–2412. [Google Scholar]
- 17.Holt DW, Marsden JT, Johnston A. Effects of liquid dispersants on the oral absorption of cyclosporine. Transplant Proc. in press. [Google Scholar]
- 18.Ptachcinski RJ, Venkataramanan R, Burckart GJ. Clinical pharmacokinetics of cyclosporine. Clin Pharmacokinetics. 1986;11:107–132. doi: 10.2165/00003088-198611020-00002. [DOI] [PubMed] [Google Scholar]
- 19.Venkataramanan R, Starzl TE, Yang S, et al. Biliary excretion of cyclosporine in liver transplant patients. Transplant Proc. 1985;17:286–289. [PMC free article] [PubMed] [Google Scholar]
- 20.Venkataramanan R, Ptachcinski RJ, Burckart GJ, et al. Cyclosporine bioavailability in liver disease (abstract) Drug Intell Clin Pharm. 1985;19:451. doi: 10.1177/106002808501900202. [DOI] [PubMed] [Google Scholar]
- 21.Burckart G, Starzl T, Williams L, et al. Cyclosporine monitoring and pharmacokinetics in pediatric liver transplant patients. Transplant Proc. 1985;17:1172–1175. [PMC free article] [PubMed] [Google Scholar]