Abstract
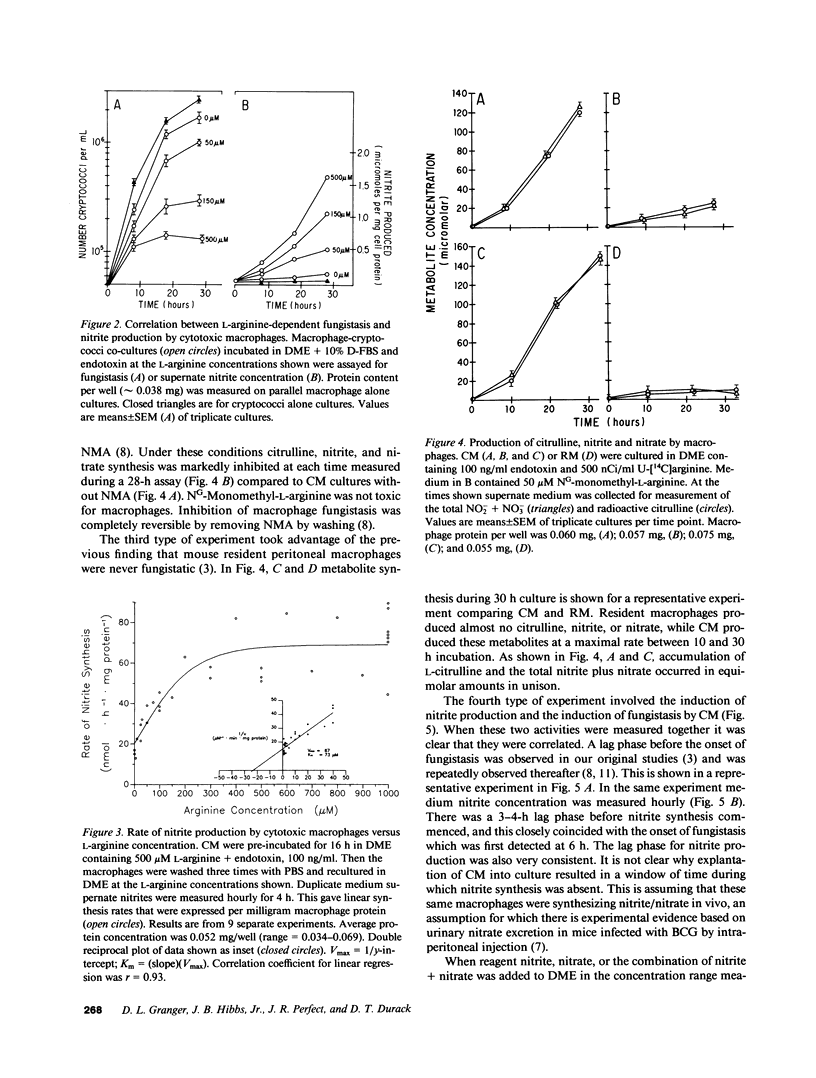
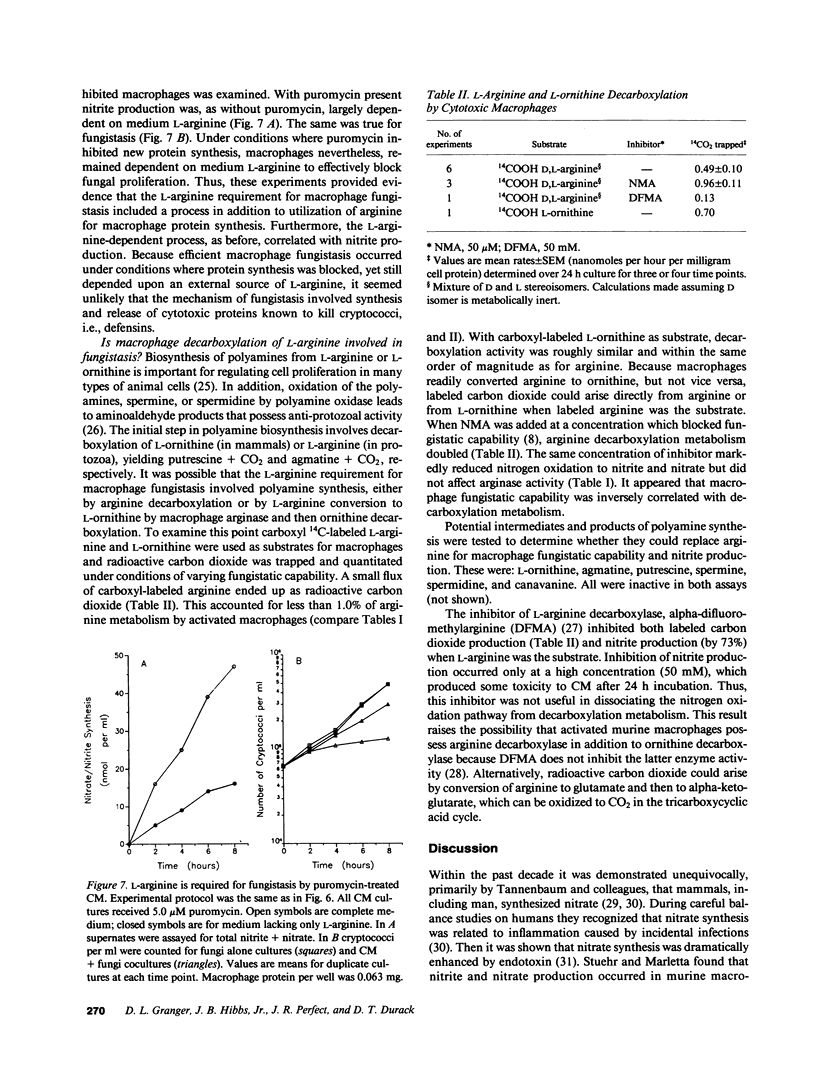
L-arginine is required for the fungistatic action of murine macrophages in vitro. To further investigate this requirement, L-arginine metabolism by macrophages was measured under conditions where fungistasis either succeeded or failed. Macrophage fungistasis correlated with metabolism of L-arginine to citrulline, nitrite, and nitrate. The metabolic rate was dependent on extracellular L-arginine concentration, reaching a maximum of 67 nmol nitrite/h per mg protein. It accounted for one-third of arginine consumed by fungistatic macrophages. Equimolar amounts of citrulline and total nitrite plus nitrate accumulated in medium. This was consistent with the hypothesis that one of the equivalent guanidino nitrogens of L-arginine was oxidized to both nitrite and nitrate leaving L-citrulline as the amino acid reaction product. The analogue, NG-mono-methyl-L-arginine, selectively inhibited nitrogen oxidation and it was shown previously that it inhibited fungistatic capability. Resident macrophages were not fungistatic and their nitrogen oxidation was low. Once macrophages began producing nitrite/nitrate, protein synthesis was not required during the next 8 h for either fungistasis or nitrogen oxidation. Two-thirds of L-arginine consumption was due to macrophage arginase yielding L-ornithine and urea, which accumulated in medium. This activity was dissociated from macrophage fungistasis. Nitrogen oxidation metabolism by macrophages is linked to a mechanism that inhibits proliferation of fungi. This may involve synthesis of an intermediate compound(s) that has antimicrobial properties.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker M. D., Mohammed H. Y., Veening H. Reversed-phase ion-pairing liquid chromatographic separation and fluorometric detection of guanidino compounds. Anal Chem. 1981 Sep;53(11):1658–1662. doi: 10.1021/ac00234a025. [DOI] [PubMed] [Google Scholar]
- Bartholomew B. A rapid method for the assay of nitrate in urine using the nitrate reductase enzyme of Escherichia coli. Food Chem Toxicol. 1984 Jul;22(7):541–543. doi: 10.1016/0278-6915(84)90224-2. [DOI] [PubMed] [Google Scholar]
- Bitonti A. J., Casara P. J., McCann P. P., Bey P. Catalytic irreversible inhibition of bacterial and plant arginine decarboxylase activities by novel substrate and product analogues. Biochem J. 1987 Feb 15;242(1):69–74. doi: 10.1042/bj2420069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummer E., Morozumi P. A., Philpott D. E., Stevens D. A. Virulence of fungi: correlation of virulence of Blastomyces dermatitidis in vivo with escape from macrophage inhibition of replication in vitro. Infect Immun. 1981 May;32(2):864–871. doi: 10.1128/iai.32.2.864-871.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole J. A., Wimpenny J. W. Metabolic pathways for nitrate reduction in Escherichia coli. Biochim Biophys Acta. 1968 Jul 16;162(1):39–48. doi: 10.1016/0005-2728(68)90212-0. [DOI] [PubMed] [Google Scholar]
- Currie G. A. Activated macrophages kill tumour cells by releasing arginase. Nature. 1978 Jun 29;273(5665):758–759. doi: 10.1038/273758a0. [DOI] [PubMed] [Google Scholar]
- Ding A. H., Nathan C. F., Stuehr D. J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988 Oct 1;141(7):2407–2412. [PubMed] [Google Scholar]
- Drapier J. C., Hibbs J. B., Jr Murine cytotoxic activated macrophages inhibit aconitase in tumor cells. Inhibition involves the iron-sulfur prosthetic group and is reversible. J Clin Invest. 1986 Sep;78(3):790–797. doi: 10.1172/JCI112642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz T., Selsted M. E., Szklarek D., Harwig S. S., Daher K., Bainton D. F., Lehrer R. I. Defensins. Natural peptide antibiotics of human neutrophils. J Clin Invest. 1985 Oct;76(4):1427–1435. doi: 10.1172/JCI112120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger D. L., Hibbs J. B., Jr, Perfect J. R., Durack D. T. Specific amino acid (L-arginine) requirement for the microbiostatic activity of murine macrophages. J Clin Invest. 1988 Apr;81(4):1129–1136. doi: 10.1172/JCI113427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger D. L., Lehninger A. L. Sites of inhibition of mitochondrial electron transport in macrophage-injured neoplastic cells. J Cell Biol. 1982 Nov;95(2 Pt 1):527–535. doi: 10.1083/jcb.95.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger D. L., Perfect J. R., Durack D. T. Macrophage-mediated fungistasis in vitro: requirements for intracellular and extracellular cytotoxicity. J Immunol. 1986 Jan;136(2):672–680. [PubMed] [Google Scholar]
- Granger D. L., Perfect J. R., Durack D. T. Macrophage-mediated fungistasis: requirement for a macromolecular component in serum. J Immunol. 1986 Jul 15;137(2):693–701. [PubMed] [Google Scholar]
- Granger D. L., Perfect J. R., Durack D. T. Virulence of Cryptococcus neoformans. Regulation of capsule synthesis by carbon dioxide. J Clin Invest. 1985 Aug;76(2):508–516. doi: 10.1172/JCI112000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger D. L., Taintor R. R., Cook J. L., Hibbs J. B., Jr Injury of neoplastic cells by murine macrophages leads to inhibition of mitochondrial respiration. J Clin Invest. 1980 Feb;65(2):357–370. doi: 10.1172/JCI109679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L. C., Ruiz de Luzuriaga K., Wagner D. A., Rand W., Istfan N., Young V. R., Tannenbaum S. R. Nitrate biosynthesis in man. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7764–7768. doi: 10.1073/pnas.78.12.7764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L. C., Tannenbaum S. R., Goldman P. Nitrate synthesis in the germfree and conventional rat. Science. 1981 Apr 3;212(4490):56–58. doi: 10.1126/science.6451927. [DOI] [PubMed] [Google Scholar]
- Green L. C., Wagner D. A., Glogowski J., Skipper P. L., Wishnok J. S., Tannenbaum S. R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982 Oct;126(1):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z. Iron depletion: possible cause of tumor cell cytotoxicity induced by activated macrophages. Biochem Biophys Res Commun. 1984 Sep 17;123(2):716–723. doi: 10.1016/0006-291x(84)90288-2. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z. Macrophage cytotoxicity: role for L-arginine deiminase and imino nitrogen oxidation to nitrite. Science. 1987 Jan 23;235(4787):473–476. doi: 10.1126/science.2432665. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z., Rachlin E. M. Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem Biophys Res Commun. 1988 Nov 30;157(1):87–94. doi: 10.1016/s0006-291x(88)80015-9. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Vavrin Z., Taintor R. R. L-arginine is required for expression of the activated macrophage effector mechanism causing selective metabolic inhibition in target cells. J Immunol. 1987 Jan 15;138(2):550–565. [PubMed] [Google Scholar]
- Iyengar R., Stuehr D. J., Marletta M. A. Macrophage synthesis of nitrite, nitrate, and N-nitrosamines: precursors and role of the respiratory burst. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6369–6373. doi: 10.1073/pnas.84.18.6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierszenbaum F., Wirth J. J., McCann P. P., Sjoerdsma A. Arginine decarboxylase inhibitors reduce the capacity of Trypanosoma cruzi to infect and multiply in mammalian host cells. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4278–4282. doi: 10.1073/pnas.84.12.4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung J. T., Brooks S. B., Jakway J. P., Leonard L. L., Talmage D. W. Suppression of in vitro cytotoxic response by macrophages due to induced arginase. J Exp Med. 1977 Sep 1;146(3):665–672. doi: 10.1084/jem.146.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Ferrari L. G., Patterson-Delafield J., Sorrell T. Fungicidal activity of rabbit alveolar and peritoneal macrophages against Candida albicans. Infect Immun. 1980 Jun;28(3):1001–1008. doi: 10.1128/iai.28.3.1001-1008.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B., Auclair D. J., Lagrange P. H. Immunopotentiation with BCG. I. Immune response to different strains and preparations. J Natl Cancer Inst. 1973 Nov;51(5):1655–1667. doi: 10.1093/jnci/51.5.1655. [DOI] [PubMed] [Google Scholar]
- Marletta M. A., Yoon P. S., Iyengar R., Leaf C. D., Wishnok J. S. Macrophage oxidation of L-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry. 1988 Nov 29;27(24):8706–8711. doi: 10.1021/bi00424a003. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Rzepczyk C. M., Saul A. J., Ferrante A. Polyamine oxidase-mediated intraerythrocytic killing of Plasmodium falciparum: evidence against the role of reactive oxygen metabolites. Infect Immun. 1984 Jan;43(1):238–244. doi: 10.1128/iai.43.1.238-244.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salerno J. C., Ohnishi T. Tetranuclear and binuclear iron-sulfur clusters in succinate dehydrogenase: a method of iron quantitation by formation of paramagnetic complexes. Biochem Biophys Res Commun. 1976 Dec 6;73(3):833–840. doi: 10.1016/0006-291x(76)90884-6. [DOI] [PubMed] [Google Scholar]
- Sjoerdsma A., Schechter P. J. Chemotherapeutic implications of polyamine biosynthesis inhibition. Clin Pharmacol Ther. 1984 Mar;35(3):287–300. doi: 10.1038/clpt.1984.33. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Gross S. S., Sakuma I., Levi R., Nathan C. F. Activated murine macrophages secrete a metabolite of arginine with the bioactivity of endothelium-derived relaxing factor and the chemical reactivity of nitric oxide. J Exp Med. 1989 Mar 1;169(3):1011–1020. doi: 10.1084/jem.169.3.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuehr D. J., Marletta M. A. Induction of nitrite/nitrate synthesis in murine macrophages by BCG infection, lymphokines, or interferon-gamma. J Immunol. 1987 Jul 15;139(2):518–525. [PubMed] [Google Scholar]
- Stuehr D. J., Marletta M. A. Mammalian nitrate biosynthesis: mouse macrophages produce nitrite and nitrate in response to Escherichia coli lipopolysaccharide. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7738–7742. doi: 10.1073/pnas.82.22.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D. A., Young V. R., Tannenbaum S. R. Mammalian nitrate biosynthesis: incorporation of 15NH3 into nitrate is enhanced by endotoxin treatment. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4518–4521. doi: 10.1073/pnas.80.14.4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton M., Granger D. L., Durack D. T. Mitochondrial iron loss from leukemia cells injured by macrophages. A possible mechanism for electron transport chain defects. J Immunol. 1988 Aug 15;141(4):1311–1317. [PubMed] [Google Scholar]
- Wu-Hsieh B., Howard D. H. Inhibition of growth of Histoplasma capsulatum by lymphokine-stimulated macrophages. J Immunol. 1984 May;132(5):2593–2597. [PubMed] [Google Scholar]



