Abstract
The effect of prednisolone on renal handling of sodium (Na) was studied in rats under three experimental conditions: 1) hydropenia, 2) water diuresis, and 3) distal tubular blockade (DTB). Prednisolone, 0.25 mg/100 g per hr, was infused directly into left renal artery and urine was collected separately from each kidney. Predominantly unilateral increases in urine flow (V) and Na excretion were noticed in all experiments during prednisolone infusion. In the hydropenic rats the maximal increments on the infused side were, for V (mean ± SD), from 9.3 ± 1.5 to 21.4 ± 0.8 μl/min (P < 0.001); for CNa/CIn, from 0.28 ± 0.11 to 2.97 ± 0.71 % (P < 0.005); and for , from 2.93 ± 2.26 to 5.32 ± 1.92% (P < 0.05). In the rats with water diuresis, the maximal increases were, for V/CIn, from 5.87 ± 1.97 to 10.1 ± 6.0% (P < 0.005); for CH2O/CIn, from 4.09 ± 0.68 to 6.00 ± 0.44% (P < 0.0005); and for CNa/CIn, from 0.22 ± 0.07 to 0.70 ± 0.38% (P < 0.01). In DTB-rats the maximal increases were for V from 48.6 ± 9.0 to 72.7 ± 14.1 μl/min (P < 0.0005) and for CNa/CIn from 9.42 ± 2.97 to 20.23 ± 7.34% (P < 0.005). In the contralateral kidney these changes were less pronounced. These observations suggest that prednisolone depresses directly Na reabsorption. The association of natriuresis with augmented and CH2O/CIn during hydropenia and water diuresis, respectively, and the increases in V and CNa/CIn during DTB, all are consistent with inhibition of Na reabsorption in the proximal tubule.
Keywords: natriuresis, proximal tubule, hydropenia, water diuresis, distal tubular blockade
The effect of glucocorticoids on renal handling of sodium has not been well defined as yet. Although sodium-retaining action has been well demonstrated in numerous studies (15, 20, 22, 25, 29), under certain conditions glucocorticoids have been shown to increase urinary excretion of sodium (3, 6, 14, 34). The natriuretic response was interpreted by some workers as the consequence of enhanced glomerular filtration rate which was associated with the administration of glucocorticoids (11, 14, 20, 24). Acute increase in the excreted fractions of filtered sodium despite concomitant decrease in glomerular filtration rate has been recently observed in humans immediately after large intravenous doses of prednisolone (27). Similar observations were reported earlier by other workers, demonstrating an increase in sodium excretion after the administration of glucocorticoids even without noticeable changes in glomerular filtration rate (6).
In the absence of altered glomerular filtration rate, the natriuretic response to glucocorticoids could be accounted for by two possible tubular mechanisms: 1) direct interference of the hormone with tubular reabsorption of sodium, and 2) indirect effect mediated by an increase in extracellular fluid volume. The regulation of sodium and water distribution between the intracellular and the extracellular compartments has been attributed to glucocorticoids (23, 32, 33). A shift of sodium and water into the extracellular space following the administration of the hormone could lead to extracellular fluid volume expansion with a resulting decrease in tubular reabsorption of sodium and an increase of sodium excretion in the urine (5).
The present study was designed to evaluate the acute effect of prednisolone on renal handling of sodium in the rat. In addition, attempts were made 1) define the site in the nephron at which the steroid may exert its action, and 2) to determine whether such an action is mediated by direct or indirect mechanism(s).
METHODS
White female Sprague-Dawley rats (200–300 g) and Brattleboro rats (Carworth, Inc., New City, N.Y.) with hereditary diabetes insipidus (250–300 g) fed Purina pellet chow diet with tap water ad libitum were studied. Acute clearance studies were performed under three experimental conditions: 1) hydropenia with saline infusion, 2) water diuresis, and 3) distal tubular blockade.
Clearance Studies
The clearance studies were performed in all animals at the same part of the day between 8:00 AM and 4:00 PM. Following the induction of anesthesia with intramuscular injection of sodium pentobarbital (40 mg/kg body wt), the animals were placed on heated operating boards and a tracheostomy tube was inserted. The femoral artery and vein were exposed through an inguinal incision and PE-20 tubings (Clay-Adams, Inc., Parsippany, N. J.) were inserted into each vessel. The arterial line was used for the collection of blood samples while the venous line was extended to a syringe mounted on a variable-speed continuous infusion pump (Harvard Apparatus Co., Inc., Millis, Mass.). Both ureters were exposed through a suprapubic incision and catheterized individually with PE-10 tubings for divided urine collections. The abdominal aorta was exposed retro-peritoneally through a left longitudinal paravertebral incision. The origin of the left renal artery was identified and a 6-0 stitch was tied into the adventitia of the aorta 2 mm from the origin of the renal artery leaving two long, free ends. The wall of the aorta was punctured medially to the 6-0 stitch with a 27-gauge needle after the blood flow in the aorta was arrested by pulling a 0 thread which had been earlier passed around it between the origins of both renal arteries. The needle was withdrawn and a tapered, pear-shaped tip of PE-10 catheter was introduced in a quick fashion into the left renal artery through the puncture site. The line was extended and connected with an adapter to a syringe mounted on a calibrated slow-speed infusion pump (Cobe pump, Cobe Laboratories, Inc., Denver, Colo.) delivering 1 ml of normal saline per hour. The preparation of the catheter and the technique of renal artery catheterization were previously described in great detail by Beuzeville (1). After the catheterization had been completed, the left kidney and its artery remained exposed and were carefully observed for another 5 min to ascertain that the vessel was patent and pulsatile and that the color and the consistency of the kidney remained the same as before the manipulation.
After the closure of all incision sites, a priming dose of inulin, 5 mg/100 g body wt, was given intravenously. The priming injection was followed by a sustaining infusion delivering 0.17 mg/min per 100 g body wt of inulin in normal saline at the rate of 0.025 ml/min per 100 g body wt (the volumes and the composition of the intravenous solutions differed in each experimental group and are given in more detail in the forthcoming sections). Following an equilibration period, divided urine collections were started. The urine was collected from each ureter individually into a graduated tube at 20- to 30-min intervals. The control clearance periods were begun only after the flow rate had stabilized and successive urine collections on both sides showed comparable volumes. Blood samples of 0.5 ml were obtained at the midpoints of all clearance periods. These samples were spun immediately and the red cells were suspended in freshly prepared plasma from similar rats (in a volume equal to that of the removed plasma) and were transfused back to the animal, to avoid blood loss.
Following 2–3 control clearances, prednisolone (Hydeltrasol, Merck Sharp and Dohme, West Point, Pa.) in a dose of 0.25 mg/100 g body was infused directly into the left renal artery over a 1-hr period. Additional 2–3 clearances were obtained after the discontinuation of prednisolone infusion. During the control collections before and after prednisolone infusion, the intrarenal arterial infusion delivered normal saline at the rate of 1 ml/hr.
On completion of the experiment, the left kidney was exposed again and examined as at the beginning of the experiment. In all animals both kidneys were biopsied and hematoxylin-eosin stained histological sections were evaluated.
All plasma and urine specimens were analyzed for inulin, sodium, and osmolality. Inulin was determined by modifying Galli’s methodology (12) to handle micro amounts of plasma and urine. One hundred microliters of plasma were diluted with 0.9 ml of water. The proteins were precipitated by adding 0.9 ml of cadmium sulphate (1.2 g/100 ml) with 100 μl of 1.1 N NaOH. Urine inulin was determined in 1:100 dilutions of the original samples. Sodium was determined with an Instrumentation Laboratory flame photometer, model 143. Osmolality was measured with an Advanced Osmometer. From these determinations, urinary excretion rates and clearances were calculated. Solute-free water clearance ( ) was determined by subtracting osmolal clearance (Cosm) from minute volume (V), CH2O = V − Cosm; and solute-free water reabsorption ( ) was determined by subtracting minute volume from osmolal clearance, . The fractional solute-free water clearance and solute-free water reabsorption were determined by factoring the respective clearances by inulin clearance.
Experimental Groups
Group 1: animals with hydropenia and saline infusion
Water was withheld for 18 hr prior to the experiment. The animals received initially aqueous vasopressin (Pitressin: Parke, Davis & Company, Detroit) intravenously, 2.2 mU/100 g body wt, followed with a continuous infusion of 2.4 mU/100 g per hr. The sustaining infusion throughout the experiment delivered normal saline at the rate of 1.5 ml/100 g per hr. The same protocol was used for an additional control group of animals which did not receive prednisolone infusion.
Group 2: animals with water diuresis
Brattleboro rats with hereditary diabetes insipidus were studied. Prior to the experiment the animals received a water load, 7.5 ml of tap water per 100 g body wt with an orogastric tube. The sustaining infusion consisted of 0.4 % NaCl solution given at a rate of 6 ml/100 g per hr.
Group 3: animals with distal tubular blockage
These animals received throughout the whole experiment continuous infusion of ethacrynic acid (Edecrin, Merck Sharp and Dohme, West Point, Pa.) 3.5 mg/100 g per hr combined with chlorothiazide (Diuril) 2 mg/100 g per hr. These doses were established after a series of preliminary experiments in which the combined diuretic effect was tested with varying proportions of both agents. The sustaining infusion delivered normal saline (with KCl 5 mEq/liter) at a rate of 3 ml/100 g per hr, which provided an adequate replacement for the urine output. The same protocol was applied for an additional control group of animals to which prednisolone was not given.
The analysis of variations associated with prednisolone infusion is based on the comparison of the observations during prednisolone infusion with those during the preceding control periods. The determination of significant difference between the control and the experimental observations was made with the use of the paired Student t test.
RESULTS
Only animals with kidneys that appeared normal on histological examination were included in the results.
Group 1
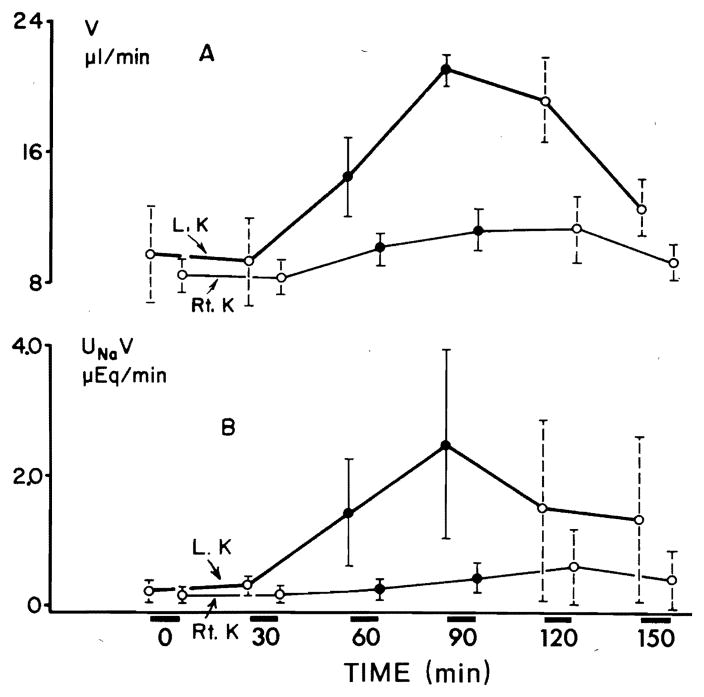
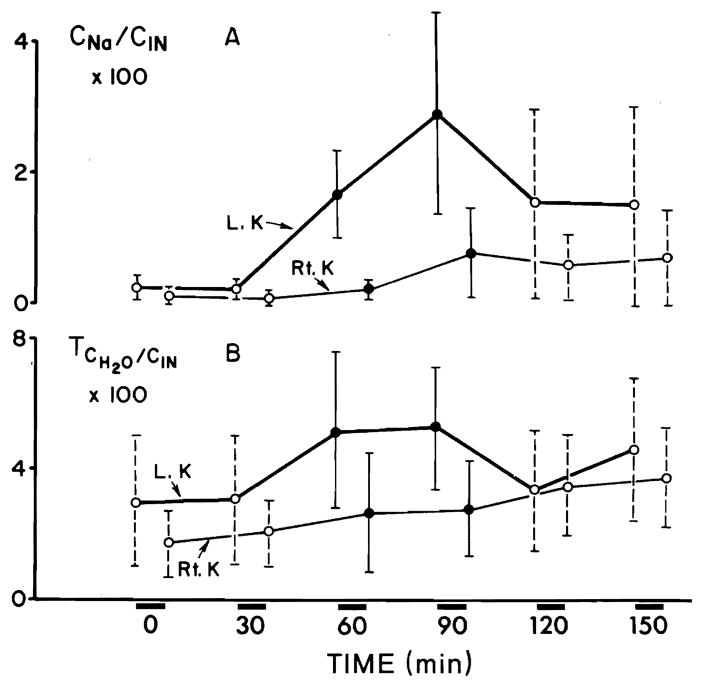
Figure 1 illustrates a predominantly unilateral diuretic response to prednisolone in six hydropenic animals. The urine flow (V) showed a significant increase on both sides (P < 0.001) within the first 30 min of infusion. The maximal increment in V on the left side amounted to 12.0 μl/min and on the right side to 2.7 μl/min. V decreased after the discontinuation of prednisolone; however, it still remained significantly greater than its control rates (Fig. 1A). Sodium excretion rate (UNaV) rose significantly (P < 0.005) on the left side within the first 30 min, with a maximal increment of 2.2 μEq/min. UNaV on the right side did not show significant changes (Fig. 1B). The percent of filtered sodium excreted (CNa/CIn × 100) increased significantly (P < 0.005) on the left side within the first 30 min of prednisolone infusion with a maximal increment of 2.6 % (Fig. 2A). No significant changes in CNa/CIn × 100 were noticed on the right side. The fractional solute-free water reabsorption ( ) increased significantly (P < 0.005) on the left side in the first 30 min of prednisolone infusion (Fig. 2B). On the right side a significant (P < 0.01) rise in was noticed after 60 min. The maximal mean increase in on the left side was 2.3 % and on the contralateral side 1.8 %. During two clearance periods following the discontinuation of prednisolone infusion, was still significantly elevated above the control values. No significant changes in all above parameters were recorded in six rats which served as a control group without prednisolone infusion.
FIG. 1.
Effect of prednisolone on urine flow (V) (A) and on urinary excretion of sodium (UNaV) (B) from left (L) and right (R) kidneys during hydropenia. Results are presented as means ± SD for whole group of animals. Open circles are control collections before and after prednisolone infusion, whereas closed circles arc collections during infusion. Duration of each collection period was 30 min.
FIG. 2.
Effect of prednisolone on fractional excretion of sodium (CNa/CIn × 100) (A) and on fractional solute-free water reabsorption ( ) (B) during hydropenia.
The variations in glomerular filtration rate (CIn) during all clearance periods were not significant. Table 1 illustrates a representative experiment with a hydropenic animal. In this, as in other experiments, the equilibration period before the urine flow reached stable levels was 5 hr. This long waiting time before control collections could be started was due to large variations in successive urine volumes. These variations could be due to the extensive surgery with marked operative trauma, which could affect the extracellular fluid volume and other unknown factors regulating urine flow.
TABLE 1.
Representative study of effect of prednisolone on renal handling of sodium in a hydropenic rat with divided urine collections from left (L) and right (R) kidneys
| Time, min | Body Wt 260 g |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| V, μl/min |
CIn, μl/min |
UNaV, μEq/min |
CNa/CIn × 100 |
Uosm, mOsm/kg H2O |
Cosm, μl/min |
|
||||||||
| L | R | L | R | L | R | L | R | L | R | L | R | L | R | |
| 0 | Prime with inulin 5 mg/100 g body wt and aqueous vasopressin 2.2 mU/100 g body wt and continue sustaining infusion delivering inulin 10 mg/100 g per hr and vasopressin 2.4 mμ/100 g per hr in normal saline given at rate of 1.5 ml/100 g per hr | |||||||||||||
| 0–300 | Equilibration period | |||||||||||||
| 300–330 | 10.0 | 10.0 | 667 | 667 | 0.32 | 0.27 | 0.33 | 0.27 | 1,360 | 990 | 46.5 | 30.3 | 5.7 | 3.1 |
| 330–360 | 10.0 | 10.0 | 633 | 600 | 0.26 | 0.21 | 0.29 | 0.24 | 1,325 | 825 | 46.0 | 25.9 | 5.7 | 2.7 |
| Begin infusion of prednisolone 0.25 mg/100 g per hr into left renal artery | ||||||||||||||
| 360–390 | 15.0 | 10.0 | 633 | 633 | 1.62 | 0.27 | 1.80 | 0.28 | 1,200 | 890 | 56.0 | 28.0 | 6.7 | 2.8 |
| 390–420 | 21.7 | 11.7 | 633 | 667 | 5.10 | 1.36 | 5.50 | 1.40 | 1,010 | 1,055 | 70.0 | 39.3 | 7.6 | 4.1 |
| Discontinue prednisolone infusion | ||||||||||||||
| 420–450 | 25.0 | 13.3 | 596 | 596 | 3.88 | 1.54 | 4.48 | 1.78 | 1,050 | 850 | 81.0 | 37.0 | 8.9 | 4.0 |
| 450–480 | 15.0 | 10.0 | 561 | 571 | 4.42 | 0.88 | 5.41 | 2.50 | 1,200 | 1,155 | 59.0 | 38.4 | 7.8 | 3.2 |
Group 2
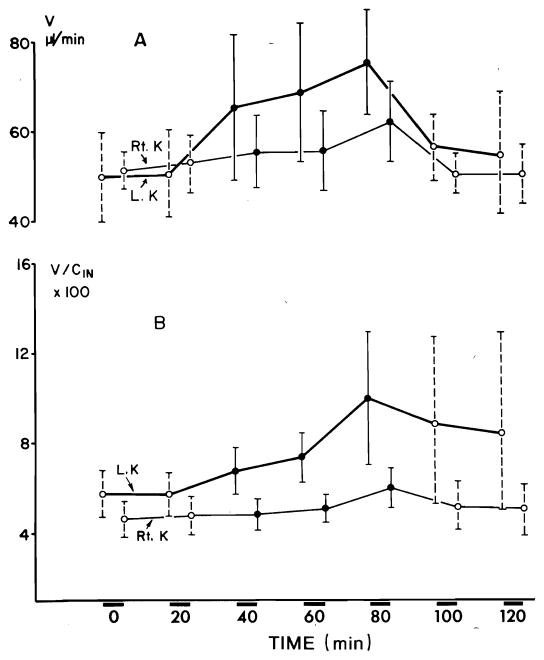
In six Brattleboro rats undergoing water diuresis, V increased significantly (P < 0.005) on the left side within the first 20 min of prednisolone infusion (Fig. 3A). On the right side a significant (P < 0.01) increase in V was noticed after 40 min. V returned to control level immediately after the discontinuation of prednisolone infusion. The maximal increase in V on the left side was 24.6 μl/min and on the contralateral side 8.8 μl/min. The variations in fractional urine flow (V/CIn × 100) followed a trend similar to that of V (as one would expect in the absence of significant changes in GFR). The maximal increase in V/CIn × 100 on the left side was 4.1 % and on the contralateral side 1.2 % (Fig. 3B). Fractional solute-free water clearance (CH2O/CIn × 100) increased significantly (P < 0.0005) on the left side within the first 20 min of prednisolone infusion and on the right side a significant (P < 0.025) increase was noticed after 20 min (Fig. 4A). The maximal increment in CH2O/CIn × 100 on the left side was 1.9 % and on the right side 1.1 %. CNa/CIn × 100 increased significantly (P < 0.01) on the left side within the first 20 min, no significant change was noticed on the contralateral side (Fig. 4B). The maximal increment in CNa/CIn × 100 on the left side was 0.48 %. Glomerular filtration did not alter significantly throughout the experiment. Table 2 presents results of a typical experiment with an animal undergoing water diuresis.
FIG. 3.
A: effect of prednisolone on urine flow (V) during water diuresis. Each collection period lasted 20 min. B: effect of prednisolone on fractional urine flow (V/CIn × 100) during water diuresis.
FIG. 4.

Effect of prednisolone on fractional solute-free water excretion (CH2O/CIn × 100) (A) and on fractional sodium excretion (CNa/CIn × 100) (B) during water diuresis.
TABLE 2.
Representative study of effect of prednisolone on renal handling of sodium in a Brattleboro rat undergoing water diuresis with individual urine collections from left (L) and right (R) kidney
| Time, min | Body Wt 250 g |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| V, μl/min |
CIn, μl/min |
UNaV, μEq/min |
CNa/CIn × 100 |
Uosm, mOsm/kg H2O |
Cosm, μl/min |
CH2O/CIn × 100 |
||||||||
| L | R | L | R | L | R | L | R | L | R | L | R | L | R | |
| 0 | Prime with inulin 5 mg/100 g and continue sustaining infusion delivering inulin 10 mg/100 g per hr in 0.4% NaCl at rate of 3 ml/100 g per hr | |||||||||||||
| 0–300 | Equilibration period | |||||||||||||
| 300–320 | 43 | 55 | 690 | 740 | 0.21 | 0.22 | 0.20 | 0.22 | 62 | 75 | 11.3 | 17.5 | 4.6 | 5.0 |
| 320–340 | 40 | 50 | 710 | 700 | 0.26 | 0.16 | 0.26 | 0.14 | 64 | 76 | 10.8 | 16.2 | 4.2 | 4.8 |
| 340–360 | 45 | 55 | 775 | 730 | 0.23 | 0.16 | 0.20 | 0.14 | 60 | 78 | 11.4 | 18.1 | 5.3 | 5.0 |
| Begin infusion of prednisolone 0.25 mg/100 g per hr into left renal artery | ||||||||||||||
| 360–380 | 55 | 55 | 660 | 715 | 0.44 | 0.22 | 0.48 | 0.17 | 74 | 75 | 17.5 | 17.7 | 5.7 | 5.0 |
| 380–420 | 63 | 57 | 661 | 730 | 0.66 | 0.28 | 0.73 | 0.26 | 93 | 76 | 25.3 | 17.9 | 5.8 | 4.8 |
| 420–440 | 70 | 63 | 702 | 760 | 0.91 | 0.60 | 1.00 | 0.50 | 102 | 80 | 31.0 | 20.0 | 5.6 | 5.1 |
| Discontinue prednisolone infusion | ||||||||||||||
| 440–460 | 79 | 68 | 710 | 710 | 1.00 | 0.62 | 1.10 | 0.65 | 104 | 92 | 35.0 | 25.0 | 6.2 | 6.0 |
| 460–480 | 68 | 59 | 700 | 740 | 0.81 | 0.65 | 0.82 | 0.64 | 96 | 89 | 30.0 | 25.2 | 5.4 | 5.1 |
Group 3
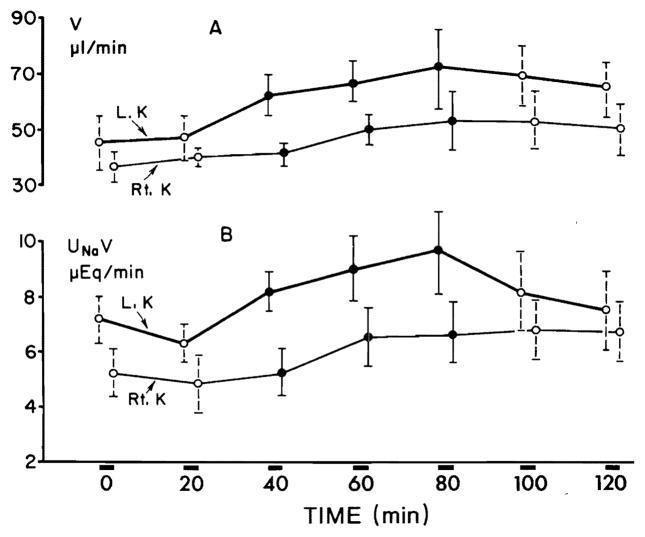
In six animals with distal tubular blockade, V on the left side showed a significant (P < 0.0005) increment within the first 20 min of prednisolone infusion, whereas the response on the right side was delayed by 20 min (Fig. 5A). The maximal mean increment in V on the left side was 24.0 μl/min and on the right side 15.3 μl/min. V remained significantly elevated above the control rate during two clearance periods following the discontinuation of prednisolone. UNaV increased significantly on the infused side within the first 20 min (P < 0.005), the response on the contralateral side was delayed by 20 min (Fig. 5B). The maximal mean increment in UNaV on the left side was 3.1 μEq/min and on the right side was 1.8 μEq/min. CNa/CIn × 100 on the left side increased (Fig. 6) significantly (P < 0.05) during the first 20 min of prednisolone infusion and on the right side after a delay of 20 min. CNa/CIn × 100 remained elevated significantly during the clearance periods following the discontinuation of prednisolone infusion. The maximal mean increment of CNa/CIn × 100 on the left side was 10.8 % and on the right side 9.5 %. Serum sodium and potassium concentrations remained stable throughout the study. Glomerular filtration rate showed no significant variation during all clearance periods. Table 3 presents the results of a representative experiment with distal tubular blockade. No significant changes in any of the excretory functions could be noticed in six rats which served as control group.
FIG. 5.
Effect of prednisolone on urine flow (V) (A) and on sodium excretion (UNaV) (B) during distal tubular blockade. Each collection period lasted 20 min.
FIG. 6.
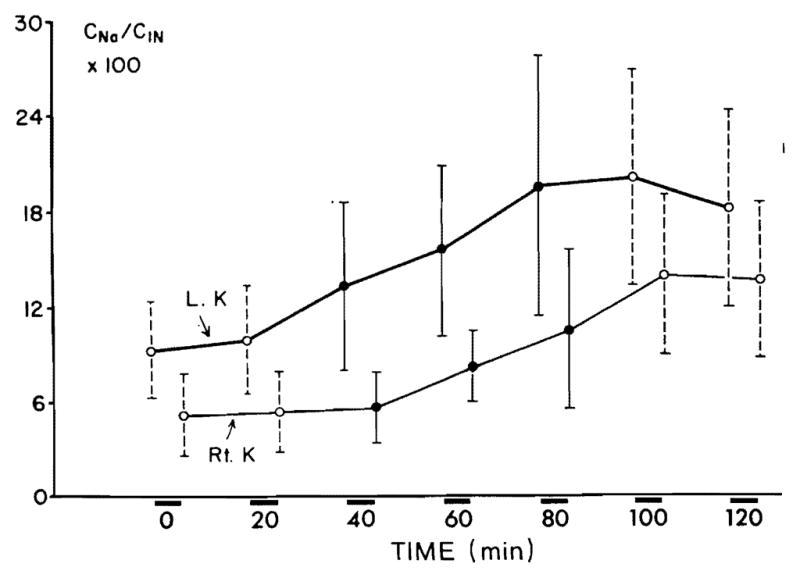
Effect of prednisolone on fractional sodium excretion (CNa/CIn × 100) during distal tubular blockade.
TABLE 3.
Representative study effect of prednisolone on renal handling of sodium in a rat undergoing distal tubular blockade with individual urine collection from left (L) and right (R) kidneys
| Time, min | Body Wt 200 g |
|||||||
|---|---|---|---|---|---|---|---|---|
| V, μl/min |
CIn, μl/min |
UNaV, μEq/min |
CNa/CIn × 100 |
|||||
| L | R | L | R | L | R | L | R | |
| 0 | Prime with inulin 5 mg/100 g and continue sustaining infusion of inulin 10 mg/100 g/hr with chlorothiazide 4 mg/hr and ethacrynic acid 7 mg/hr with normal saline at rate of 3 ml/100 g per hr | |||||||
| 0–300 | Equilibration | |||||||
| 300–320 | 50 | 40 | 338 | 420 | 6.8 | 6.8 | 13.6 | 11.0 |
| 320–340 | 50 | 40 | 292 | 350 | 6.6 | 6.5 | 15.4 | 12.5 |
| Begin infusion of prednisolone 0.25 mg/100 g per hr into left renal artery | ||||||||
| 340–360 | 70 | 43 | 287 | 370 | 8.8 | 6.2 | 21.3 | 11.5 |
| 360–380 | 75 | 60 | 286 | 343 | 9.8 | 7.8 | 23.4 | 16.0 |
| 380–400 | 83 | 70 | 303 | 370 | 10.4 | 8.8 | 24.1 | 16.8 |
| Discontinue prednisolone infusion | ||||||||
| 400–420 | 70 | 63 | 233 | 305 | 10.0 | 8.4 | 29.9 | 19.5 |
| 420–440 | 70 | 63 | 317 | 380 | 9.1 | 8.3 | 20.2 | 15.3 |
The variations in inulin clearances in all experimental groups during all periods are shown in Table 4. No significant differences could be noticed between successive collections and no significant disparity was seen between the left and right kidneys.
TABLE 4.
Variations in clearances of inulin (CIn) in all experimental groups during control (C) and prednisolone-infusion (P) periods
| Group | Kidney | CIn, μl/min |
||||||
|---|---|---|---|---|---|---|---|---|
| C | C | P | P | P | C | C | ||
| 1 | L | 575 ± 210 | 587 ± 172 | 600 ± 179 | 582 ± 158 | 574 ± 164 | 583 ± 182 | |
| R | 669 ± 129 | 642 ± 92 | 638 ± 148 | 574 ± 200 | 620 ± 193 | 590 ± 187 | ||
| 2 | L | 869 ± 396 | 900 ± 381 | 944 ± 354 | 905 ± 357 | 929 ± 222 | 884 ± 207 | 957 ± 500 |
| R | 957 ± 448 | 924 ± 191 | 934 ± 355 | 902 ± 343 | 889 ± 382 | 889 ± 394 | 955 ± 462 | |
| 3 | L | 526 ± 209 | 500 ± 243 | 594 ± 197 | 546 ± 170 | 511 ± 143 | 501 ± 108 | 603 ± 112 |
| R | 564 ± 253 | 609 ± 289 | 604 ± 236 | 582 ± 182 | 542 ± 197 | 500 ± 129 | 562 ± 81 | |
Values are means ± SD. L = left kidney. R = right kidney.
The average values for serum Na (SNa), V, UNaV, Uosm, Cosm, and CIn for each kidney, each animal, for control, prednisolone, and control periods in the hydropenic and in the water diuresis groups are shown in Table 5A and B, respectively.
TABLE 5.
Averages of serum (S) and urine values for control and prednisolone periods in six hydropenic rats (R) and six rats with diabetes insipidus (RD)
| Control |
Prednisolone |
Control |
Control |
Prednisolone |
Control |
Control |
Prednisolone |
Control |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L | R | L | R | L | R | L | R | L | R | L | R | L | R | L | R | L | R | |
| A) Six hydropenic rats | ||||||||||||||||||
| R1 | R3 | R5 | ||||||||||||||||
| SNa, mEq/liter | 143 | 144 | 144 | 145 | 140 | 145 | 144 | 144 | 145 | |||||||||
| V, μl/min | 10.0 | 10.0 | 18.3 | 10.8 | 20.0 | 11.7 | 6.7 | 8.3 | 16.7 | 10.8 | 14.2 | 10.0 | 11.7 | 8.3 | 19.2 | 10.2 | 16.7 | 10.0 |
| UNaV, μEq/min | 0.29 | 0.24 | 3.36 | 0.81 | 4.15 | 1.21 | 0.19 | 0.23 | 3.17 | 2.15 | 1.34 | 0.77 | 0.17 | 0.11 | 1.48 | 0.15 | 0.13 | 0.15 |
| Uosm, mOsm/kg H2O | 1,340 | 910 | 1,105 | 972 | 1,125 | 1,002 | 1,188 | 1,100 | 907 | 1,180 | 1,157 | 1,440 | 430 | 415 | 450 | 380 | 465 | 380 |
| Cosm, μl/min | 46.0 | 28.2 | 63.0 | 33.2 | 70.0 | 37.7 | 26.5 | 30.5 | 47.5 | 38.2 | 51.4 | 46.0 | 16.1 | 11.0 | 25.8 | 12.4 | 24.5 | 12.0 |
| CIn, μl/min | 650 | 632 | 633 | 632 | 578 | 581 | 656 | 770 | 800 | 800 | 797 | 856 | 403 | 535 | 390 | 426 | 354 | 388 |
| R2 | R4 | R6 | ||||||||||||||||
| SNa, mEq/liter | 140 | 141 | 140 | 135 | 136 | 136 | 140 | 142 | 143 | |||||||||
| V, μl/min | 9.2 | 8.3 | 19.2 | 10.8 | 14.2 | 9.1 | 10.0 | 8.3 | 18.3 | 10.8 | 15.0 | 10.8 | 7.5 | 6.5 | 28.2 | 7.4 | 16.5 | 8.1 |
| UNaV, μEq/min | 0.30 | 0.15 | 0.53 | 0.14 | 0.55 | 0.28 | 0.23 | 0.17 | 1.01 | 0.26 | 0.63 | 0.13 | 0.26 | 0.17 | 1.91 | 0.72 | 1.36 | 0.51 |
| Uosm, mOsm/kg H2O | 903 | 765 | 726 | 780 | 730 | 968 | 507 | 572 | 500 | 524 | 500 | 599 | 1,345 | 1,299 | 881 | 1,850 | 1,190 | 1,820 |
| Cosm, μl/min | 26.1 | 20.5 | 44.5 | 26.8 | 31.3 | 28.3 | 17.0 | 17.2 | 30.2 | 19.0 | 24.1 | 21.6 | 32.4 | 26.0 | 75.4 | 43.2 | 55.0 | 42.7 |
| CIn, μl/min | 568 | 603 | 503 | 466 | 430 | 455 | 511 | 581 | 497 | 494 | 514 | 580 | 749 | 810 | 744 | 800 | 736 | 823 |
| B) Six rats with diabetes insipidus | ||||||||||||||||||
| RD1 | RD3 | RD5 | ||||||||||||||||
| SNa, mEq/liter | 140 | 141 | 140 | 139 | 139 | 140 | 130 | 133 | 133 | |||||||||
| V, μl/min | 43.0 | 53.3 | 62.3 | 58.2 | 73.5 | 63.5 | 47.5 | 43.0 | 93.5 | 45.0 | 47.5 | 47.0 | 60.0 | 55.0 | 77.3 | 64.0 | 52.5 | 47.5 |
| UNaV, μEq/min | 0.23 | 0.15 | 0.67 | 0.36 | 0.50 | 0.63 | 0.59 | 1.19 | 1.03 | 0.82 | 0.22 | 0.37 | 0.60 | 0.96 | 1.17 | 0.96 | 0.67 | 0.71 |
| Uosm, mOsm/kg H2O | 62 | 76 | 89 | 77 | 100 | 90 | 81 | 66 | 91 | 79 | 61 | 105 | 45 | 63 | 59 | 53 | 43 | 62 |
| Cosm, μl/min | 11.1 | 16.9 | 26.6 | 18.5 | 32.5 | 25.1 | 14.0 | 10.5 | 21.0 | 13.0 | 11.0 | 18.5 | 9.5 | 12.2 | 17.0 | 13.0 | 8.5 | 11.0 |
| CIn, μl/min | 725 | 723 | 710 | 735 | 705 | 725 | 533 | 581 | 541 | 547 | 532 | 541 | 1,365 | 1,455 | 1,440 | 1,403 | 1,369 | 1,490 |
| RD2 | RD4 | RD6 | ||||||||||||||||
| SNa, mEq/liter | 126 | 123 | 123 | 128 | 126 | 126 | 130 | 128 | 127 | |||||||||
| V, μl/min | 70.0 | 60.0 | 98.3 | 69.0 | 73.5 | 52.5 | 47 | 52.5 | 66.0 | 57.3 | 55.5 | 50.0 | 42.5 | 52.5 | 58.3 | 56.6 | 50.0 | 55.0 |
| UNaV, μEq/min | 0.41 | 0.67 | 0.85 | 0.59 | 0.47 | 0.50 | 0.48 | 0.79 | 1.09 | 0.69 | 0.47 | 0.71 | 0.16 | 0.26 | 0.30 | 0.28 | 0.25 | 0.28 |
| Uosm, mOsm/kg H2O | 62 | 88 | 85 | 90 | 50 | 71 | 65 | 118 | 95 | 130 | 76 | 51 | 95 | 118 | 114 | 130 | 86 | 127 |
| Cosm, μl/min | 17.0 | 20.5 | 32.0 | 23.3 | 13.2 | 14.0 | 11.0 | 23.0 | 24.5 | 29.0 | 15.5 | 9.5 | 14.5 | 23.0 | 25.0 | 27.0 | 16.2 | 26.5 |
| CIn, μl/min | 1,230 | 1,320 | 1,231 | 1,172 | 1,458 | 1,265 | 882 | 940 | 924 | 908 | 920 | 922 | 583 | 625 | 608 | 594 | 537 | 584 |
DISCUSSION
The present study demonstrated a natriuretic response to prednisolone under varying experimental conditions which was not associated with significant changes in glomerular filtration rate. The observed response was characterized by an immediate onset and predominantly unilateral effect manifested by the left infused kidney. The response on the contralateral side was more variable; the natriuresis when present was usually delayed and less striking. These observations are consistent with a direct renal action of prednisolone, however they do not exclude an additional systemic effect which could also affect sodium excretion. The relatively small response of the noninfused side could represent either the dilution of prednisolone during its circulation before reaching the right kidney and/or an indirect action mediated by a systemic natriuretic mechanism. The observed renal response to large doses of prednisolone does not necessarily represent the physiologic effect of glucocorticoids in normal rats.
The present data do not provide evidence as to whether the natriuresis resulted from a direct depression of tubular transport of sodium, or was secondary to altered renal hemodynamics.
The objective of the experimental design using three different groups of animals was to define, with clearance techniques, the site in the nephron at which depression of sodium reabsorption occurred.
Reduced reabsorption of sodium in the proximal tubule by causing the delivery of increased amounts of filtrate to the loop of Henle and the distal convolution would augment CH2O during water diuresis and during water restriction (26). During hydropenia with maximal ADH stimulation, depression of sodium reabsorption in the proximal tubule would increase because of the availability of more osmotically active solute for transport into medulla (26). Since is directly related to the tonicity of medulla, increase in medullary tonicity and in could result from a primary increase in sodium reabsorption in the loop of Henle without appreciable decrease in proximal tubular reabsorption. However, under such circumstances any increase in would be expected to be accompanied by a fall in urine flow and in sodium excretion. The association of an increasing with an increased urine flow and sodium excretion (without significant change in its filtered load) as noticed in the present study in the hydropenic group suggest that the main action of prednisolone was depression of sodium reabsorption in the proximal tubule.
In the absence of antidiuretic hormone it is assumed that the distal nephron is maximally impermeable to water, and therefore that the urine volume is a close approximation of the quantity of tubular fluid escaping reabsorption by the proximal tubule. Thus V/GFR represents the fraction of glomerular filtrate which is delivered to the distal tubule and an increase in V/GFR is representative of a decreased proximal tubular reabsorption of glomerular filtrate (9, 28, 31). The amount of solute-free water (CH2O) generated is an estimate of the quantity of sodium removed by the diluting segment. Changes in CH2O reflect the alterations in sodium reabsorption at the distal water clearing sites and changes in CH2O + CNa provide an estimate of changes in the rate of delivery of sodium to distal sites (9, 30). Decreased sodium reabsorption in the proximal nephron would be expected to enhance CH2O during water diuresis because more sodium would be presented to the diluting sites for reabsorption and also there would be relatively less ADH independent backdiffusion of water from the collecting duct at high rates of urine flow (17). Inhibition of sodium reabsorption in the water impermeable distal tubule would be expected to decrease CH2O and would have little if any effect on urine flow (23, 28, 31). In the present study, the infusion of prednisolone to animals with hereditary diabetes insipidus undergoing water diuresis induced an increase in V/GFR which was associated with an enhanced CNa/GFR and CH2O/GFR. These observations support further the notion that the major acute effect of prednisolone is suppression of sodium reabsorption in the proximal tubule.
In the presence of complete or nearly complete inhibition of sodium reabsorption in the distal nephron by diuretic agents which have minimal or no effect on the reabsorption of sodium in the proximal tubule, the residual reabsorption of water and sodium represents predominantly proximal tubular reabsorption (7, 8, 10). Additional marked changes in urine flow and sodium excretion without significant changes in GFR during distal tubular blockade as observed in the present study in group 3 could be due to direct inhibition of sodium reabsorption in the proximal tubule by prednisolone. The relatively low percent of filtered sodium excreted during the control collections (CNa/CIn 9.45 ± 2.97 μl/min) deserves special consideration.
Micropuncture data in rats indicate that 65 % of glomerular filtrate are reabsorbed in the first 66 % of the proximal tubule (16, 21). These results apply only to the two-thirds of the proximal tubule which are accessible to micropuncture, whereas the fraction of filtrate reabsorbed along the entire length of the proximal tubule remains to be determined. Moreover, the results obtained by micropuncture may represent only the subcapsular but not the deeper nephrons (13). The reabsorption of sodium in the proximal tubule is affected by changes in salt and water balance. In recently reported micropuncture study, the fraction of glomerular filtrate reabsorbed in the accessible portion of the proximal tubule reached 85 % (TF/P 6.5) in salt-depleted rats (4). It is therefore likely that the percent of filtrate reabsorbed along the whole length of the proximal tubule in salt-depleted rats may be dose to 90 %. Under such circumstances only 10 % of glomerular filtrate are available for excretion in the urine during distal tubular blockade. It appears therefore that the validity (or invalidity) of distal tubular blockade may be ascertained only when the fractional reabsorption of sodium in the proximal tubule is known. The relatively low percent of filtered sodium which was excreted in our rats during distal tubular blockade could be accounted for by two possible alternatives: 1) the fractional reabsorption of sodium in the proximal tubule was high possibly due to a state of sodium depletion induced by urinary losses during the long equilibration period. An additional amount of sodium was exchanged for potassium in the distal tubule and was not measured in the final urine in the present study. 2) The distal tubular blockade was incomplete and significant amounts of sodium were reabsorbed in the loop of Henle and in the distal tubule. Another important question pertinent to the experimental use of distal tubular blockade is the reported inhibitory effect of ethacrynic acid on the proximal reabsorption of sodium (8). Earley and Martino (7) expressed the notion that even though ethacrynic acid has a certain effect on sodium reabsorption in the proximal tubule, this part of nephron may still respond to other factors which alter sodium reabsorption at this site.
Although early observations questioned the effectiveness of ethacrynic acid as a diuretic in rats, in which case the natriuresis seen in the animals of group 3 might represent soley the effect of chlorothiazide, recently Deetjen et al. (4) clearly demonstrated that ethacrynic is a highly potent diuretic in rats when given at a dose comparable to that which we used in our study.
Our present findings are in agreement with previously reported observations in which glucocorticoids have been shown to increase acutely free water reabsorption in hydropenic subjects (19, 35) and in hydropenic dogs (18). The absence of an increase in urine flow and sodium excretion in these studies could be due to an avid reabsorption of sodium in the distal nephron resulting from a delayed sodium-retaining effect of the steroid. The fact that our studies were conducted over a shorter time and the clearance periods were of shorter duration as compared with those in the cited studies may explain the differences in the results Moreover, the relatively higher dose of glucocorticoids (per body wt) employed in the present study could decrease the proximal reabsorption to the extent that the distal mechanism was not capable of coping with the excessive amounts of the delivered filtrate leading to an increased urine flow and an increased urinary excretion of sodium.
As demonstrated by the results of the experiments with animals subjected to distal tubular blockade, large doses of glucocorticoids may potentiate strikingly the preexisting effect of distally acting diuretics. This action, if also proved in human subjects, may be of certain value in treating clinical conditions of salt and water retention.
Acknowledgments
This work was supported by Research Grants RR-00051 and RR-00069 from the Veterans Administration.
Footnotes
A part of this paper has been presented in an abstract form (Federation Proc. 30, no. 2299, 1971).
References
- 1.Beuzeville C. Catheterization of renal artery in rats. Proc Soc Exptl Biol Med. 1968;129:932–936. doi: 10.3181/00379727-129-33462. [DOI] [PubMed] [Google Scholar]
- 2.Clapp JR, Nottebohm GA, Robinson RR. Proximal site of action of ethacrynic acid: importance of filtration rate. Am J Physiol. 1971;220:1355–1360. doi: 10.1152/ajplegacy.1971.220.5.1355. [DOI] [PubMed] [Google Scholar]
- 3.Davis AK, Bass AC, Overman RR. Comparative effects of cortisone and DCA on ionic balance and fluid volume of normal and adrenalectomized dogs. Am J Physiol. 1951;166:493–503. doi: 10.1152/ajplegacy.1951.166.3.493. [DOI] [PubMed] [Google Scholar]
- 4.Deetjen P, Büntig WE, Hardt K, Rhode R. The diuretic effect of ethacrynic acid in the rat: a micropuncture study about the relationship of site and mode of action. In: Peters G, editor. Progress in Nephrology. Heidelberg: Springer; 1969. [Google Scholar]
- 5.DeWardener HE, I, Mills H, Clapham WF, Haytet CJ. Studies on the efferent mechanism of the sodium diuresis which follows the administration of intravenous saline in the dog. Clin Sci. 1961;21:249–258. [PubMed] [Google Scholar]
- 6.Dingman JF, Finkenstaedt JT, Laidlaw JC, Renold AE, Jenkins D, Merrill JP, Thorn GW. Influence of intravenously administered adrenal steroids on sodium and water excretion in normal and Addisonian subjects. Metabolism. 1958;7:608–623. [PubMed] [Google Scholar]
- 7.Earley LF, Martino JA. Influence of sodium balance on the ability of diuretics to inhibit tubular reabsorption. Circulation. 1970;42:323–334. doi: 10.1161/01.cir.42.2.323. [DOI] [PubMed] [Google Scholar]
- 8.Earley LE, Martino JA, Friedler RM. Factors affecting sodium reabsorption by the proximal tubule as determined during blockade of distal sodium reabsorption. J Clin Invest. 1966;45:1668–1684. doi: 10.1172/JCI105474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eknoyan G, Suki WN, Rector FC, Jr, Seldin DW. Functional characteristics of the diluting segment of the dog nephron and the effect of extracellular volume expansion on its reabsorptive capacity. J Clin Invest. 1967;46:1178–1188. doi: 10.1172/JCI105611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fillastre JP, Ardaillou R, Isaac R. Influence of extracellular volume expansion on the composition of proximal tubular fluid in man. Clin Sci. 1971;40:479–487. doi: 10.1042/cs0400479. [DOI] [PubMed] [Google Scholar]
- 11.Finkenstaedt JT, Dingman JF, Jenkins D, Laidlaw JC, Merrill JP. The effect of intravenous hydrocortisone and corticosterone on the diurnal rhythm in renal function and electrolyte equilibria in normal and Addisonian subjects. J Clin Invest. 1954;33:933. [Google Scholar]
- 12.Galli A. Colorimetric determination of inulin in blood and in urine. Pathol Biol. 1966;14:991–993. [PubMed] [Google Scholar]
- 13.Garella S, Chazan JA, Cohen JJ. Factors responsible for proximal sodium conservation as assessed by distal tubular blockade. Clin Sci. 1969;37:775–787. [PubMed] [Google Scholar]
- 14.Garrod O, Davies SA, Cahill G., Jr The action of cortisone and desoxycorticosterone acetate on glomerular filtration rate and sodium and water exchange in the adrenalectomized dog. J Clin Invest. 1955;34:761–776. doi: 10.1172/JCI103131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaudino M, Levitt MF. Influence of the adrenal cortex on body water distribution and renal function. J Clin Invest. 1949;28:1487–1497. doi: 10.1172/JCI102214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glabman S, Aynedjian HS, Bank N. Micropuncture study of the effect of acute reductions in glomerular filtration rate on sodium and water reabsorption by the proximal tubules of the rat. J Clin Invest. 1965;44:1410–1416. doi: 10.1172/JCI105246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldberg M, McCurdy DK, Foltz EL, Blumle LW., Jr Effects of ethacrynic acid (a new saluretic agent) on renal diluting and concentrating mechanisms: Evidence for site of action in the loop of Henle. J Clin Invest. 1964;43:201–216. doi: 10.1172/JCI104905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldsmith CH, Beasley HK, Whalley PJ, Rector FC, Jr, Seldin DW. The effect of salt deprivation on the urinary concentrating mechanism in the dog. J Clin Invest. 1961;40:2043–2052. doi: 10.1172/JCI104430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jick H, Synder JG, Moore EW, Morrison RS. The effects of aldosterone and glucocorticoids on free water reabsorption. Clin Sci. 1965;29:25–32. [PubMed] [Google Scholar]
- 20.Laidlaw JC, Dingman JF, Arons WL, Finkenstaedt JT, Thorn GW. Comparison of the metabolic effects of cortisone and hydrocortisone in man. Ann N Y Acad Sci. 1955;61:315–323. doi: 10.1111/j.1749-6632.1955.tb42481.x. [DOI] [PubMed] [Google Scholar]
- 21.Lassiter WE, Gottschalk CW, Mylle M. Micropuncture study of the net transtubular movement of water and urea in non-diuretic mammalian kidney. Am J Physiol. 1961;200:1139–1146. doi: 10.1152/ajplegacy.1961.200.6.1139. [DOI] [PubMed] [Google Scholar]
- 22.Lemann J, Jr, Piering WF, Lennon EJ. Studies on the acute effects of aldosterone and cortisol on the interrelationship between renal sodium calcium and magnesium excretion in normal man. Nephron. 1970;7:117–130. doi: 10.1159/000179814. [DOI] [PubMed] [Google Scholar]
- 23.Levitt MF, Bader ME. Effect of cortisone and ACTH on fluid and electrolyte distribution in man. Am J Med. 1951;11:715–722. doi: 10.1016/0002-9343(51)90022-8. [DOI] [PubMed] [Google Scholar]
- 24.Liddle GW, Pechet MM, Bartter FC. Enhancement of biological activities of corticosteroids by substitution of halogen atoms in. g-alpha position. Science. 1954;120:496–499. doi: 10.1126/science.120.3117.496. [DOI] [PubMed] [Google Scholar]
- 25.Mills JN, Thomas S, Williamson KS. The acute effect of hydrocortisone, deoxycorticosterone and aldosterone upon the excretion of sodium potassium and acid by the human kidney. J Physiol, London. 1960;151:312–331. doi: 10.1113/jphysiol.1960.sp006440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orloff J, Wagner HN, Jr, Davidson DG. The effect of variations in solute excretion and vasopressin dosage on the excretion of water in the dog. J Clin Invest. 1958;37:458–464. doi: 10.1172/JCI103625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Popovtzer MM, Pinggera WF, Robinette J, Holmes JH, Halgrimson CG, Starzl TE. Acute renal response to large doses of intravenous prednisolone in kidney homograft recipients and in normal subjects. J Lab Clin Med. 1971;78:39–52. [PMC free article] [PubMed] [Google Scholar]
- 28.Rector FC, Jr, Van Giessen G, Kill F, Seldin DW. Influence of expansion of extracellular volume on tubular reabsorption of sodium independent of changes in glomerular filtration rate and aldosterone activity. J Clin Invest. 1964;43:341–348. doi: 10.1172/JCI104919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roberts KE, Randall HT. The effect of adrenal steroids on renal mechanisms of electrolyte excretion. Ann N Y Acad Sci. 1955;61:306–314. doi: 10.1111/j.1749-6632.1955.tb42480.x. [DOI] [PubMed] [Google Scholar]
- 30.Stein RM, Abramson RG, Kahn T, Levitt MF. Effects of hypotonic saline loading in hydrated dog: Evidence for a saline-induced limit to distal tubular sodium transport. J Clin Invest. 1967;46:1205–1214. doi: 10.1172/JCI105614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suki W, Rector FC, Jr, Seldin DW. The site of action of furosemide and other sulfonamide diuretics in the dog. J Clin Invest. 1965;44:1458–1469. doi: 10.1172/JCI105252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swingle WW, Parkins WM, Taylor AR, Hays HW. Relation of serum sodium and chloride levels to alterations of body water in the intact and adrenalectomized dog, and the influence of adrenal cortical hormone upon fluid distribution. Am J Physiol. 1936;116:438–445. [Google Scholar]
- 33.Swingle WW, DaVanzo JP, Glenister D, Cross-field HC, Wagle G. Role of gluco- and mineralocorticoids in salt and water metabolism of adrenalectomized dogs. Am J Physiol. 1959;196:283–286. doi: 10.1152/ajplegacy.1959.196.2.283. [DOI] [PubMed] [Google Scholar]
- 34.Thorn GW, Engel LL, Lewis RA. The effect of 17-hydroxycorticosterone and related adrenal cortical steroids on sodium and chloride excretion. Science. 1941;94:348–349. doi: 10.1126/science.94.2441.348. [DOI] [PubMed] [Google Scholar]
- 35.Yunis SL, Bercovitch DD, Stein RM, Levitt MF, Goldstein MH. Renal tubular effects of hydrocortisone and aldosterone in normal hydropenic man: Comment on sites of action. J Clin Invest. 1964;43:1668–1676. doi: 10.1172/JCI105042. [DOI] [PMC free article] [PubMed] [Google Scholar]