Cooperative functions of Chk1 and Chk2 reduce tumour susceptibility in vivo
Niida et al provide the first conclusive evidence that the DNA-damage checkpoint kinases Chk1 and Chk2 are bona fide tumour suppressors, also giving insight into the unique and redundant functions of these two factors in vivo.
Keywords: apoptosis, cancer, checkpoint, DNA damage, senescence
Abstract
Although the linkage of Chk1 and Chk2 to important cancer signalling suggests that these kinases have functions as tumour suppressors, neither Chk1+/− nor Chk2−/− mice show a predisposition to cancer under unperturbed conditions. We show here that Chk1+/−Chk2−/− and Chk1+/−Chk2+/− mice have a progressive cancer-prone phenotype. Deletion of a single Chk1 allele compromises G2/M checkpoint function that is not further affected by Chk2 depletion, whereas Chk1 and Chk2 cooperatively affect G1/S and intra-S phase checkpoints. Either or both of the kinases are required for DNA repair depending on the type of DNA damage. Mouse embryonic fibroblasts from the double-mutant mice showed a higher level of p53 with spontaneous DNA damage under unperturbed conditions, but failed to phosphorylate p53 at S23 and further induce p53 expression upon additional DNA damage. Neither Chk1 nor Chk2 is apparently essential for p53- or Rb-dependent oncogene-induced senescence. Our results suggest that the double Chk mutation leads to a high level of spontaneous DNA damage, but fails to eliminate cells with damaged DNA, which may ultimately increase cancer susceptibility independently of senescence.
Introduction
Aberrant regulation of DNA-damage response in multicellular organisms is thought to lead to genomic instability and cancer development (Bartkova et al, 2005; Gorgoulis et al, 2005). Signals initiated by DNA-damage sensors are rapidly transduced to ATM/ATR kinases that in turn phosphorylate a large number of substrates, including checkpoint kinases (Chks), Chk1 and Chk2. In cell culture-based systems, Chk1 and Chk2 behave similarly and appear to regulate activities involving the Cdc25 family of phosphatases (Sanchez et al, 1997; Matsuoka et al, 1998; Kaneko et al, 1999; Tominaga et al, 1999), p53 (Shieh et al, 2000) and DNA-repair factors (Lee et al, 2000; Sorensen et al, 2005). In knockout mice, however, the respective phenotypes are very different (Hirao et al, 2000; Liu et al, 2000; Takai et al, 2000, 2002), suggesting that these kinases regulate distinct pathways in vivo. In the light of the relationship between these checkpoint kinases and important cancer signalling pathways (Bartkova et al, 2005; Gorgoulis et al, 2005) and the genetic alterations of Chk1 and Chk2 observed in sporadic (Bertoni et al, 1999) and familial tumours (Bell et al, 1999; Bartek and Lukas, 2003), the lack of an overt tumour-prone phenotype in Chk1 and Chk2 knockout mice was somewhat unanticipated (Hirao et al, 2000; Liu et al, 2000; Takai et al, 2000, 2002). Interestingly, tumour incidence was increased in Chk1+/−, WNT-1 transgenic mice (Liu et al, 2000), Chk2−/−Brca1−/− (McPherson et al, 2004), Chk2−/−Brca1Δ11/Δ11 (Cao et al, 2006), Chk2−/−NBS1ΔB/ΔB and Chk2−/−Mre11ATLD1/ATLD1 mice (Stracker et al, 2008). This may be explained by the redundancy found in biochemical studies, which show that both kinases can phosphorylate the same sites on the same substrates, at least in vitro, and that either Chk1 or Chk2 is sufficient to mediate anti-tumour signalling. Alternatively, Chk1 and Chk2 may function in non-redundant DNA-damage response and either response is sufficient to prevent tumour formation in vivo.
In this respect, Lam et al (2004) showed that Chk1 haplo-insufficiency could potentially have a tumour suppressor function. Inactivation of one Chk1 allele showed inappropriate entry into S phase, accumulation of spontaneous DNA damage during DNA replication and a failure to restrain mitotic entry in the presence of a deregulated S phase. Nevertheless, inactivation of one Chk1 allele per se did not lead to cancer predisposition. Thus, apparent cancer-related phenotypes in Chk1+/− cells might be suppressed in in vivo tumourigenesis through other DNA-damage responses. To address this critical question, a more exhaustive tumourigenesis study performed in double-mutant mice would be necessary. This study clearly shows that Chk1 and Chk2 are bona fide and cooperatively haplo-insufficient tumour suppressors in vivo that regulate cell cycle checkpoints and apoptosis, but not premature senescence. Mice with the combined loss of two anti-tumour barriers are not able to eliminate cells with a high level of DNA damage and this may be sufficient for the predisposition to spontaneous tumourigenesis.
Results
Tumourigenesis in Chk1/Chk2 double-mutant mice
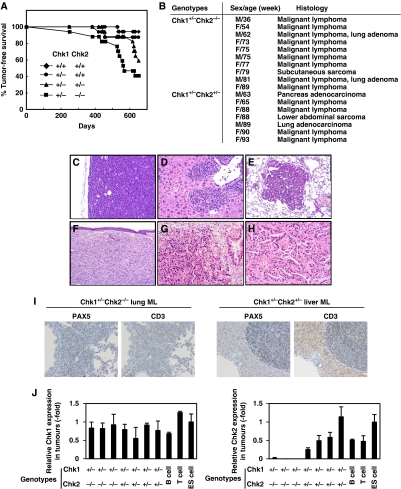
We generated Chk1+/−Chk2−/− mice to conduct a systematic evaluation of the checkpoint kinase function in vivo because at least one Chk1 allele is essential for survival and proliferation of both embryonic (Liu et al, 2000; Takai et al, 2000) and somatic cells (Shimada et al, 2008). Exhaustive characterization of mice bearing single or combined germline Chk1 and Chk2 deletions revealed that a significantly higher percentage of mice developed aggressive malignant lymphomas, sarcomas or lung adenomas in Chk1+/−Chk2−/− mice (Figure 1A and B; HR=19.2, 95% CI=2.5–147.4, P=0.004). Unexpectedly, Chk1+/−Chk2+/− mice also showed a predisposition to cancer, bearing lymphomas, sarcomas or carcinomas (Figure 1C–H) (HR=9.3, 95% CI=1.2–74.3, P=0.035), although tumour production occurred later than in Chk1+/−Chk2−/− mice. Examination of Chk1+/−Chk2+/+ and Chk1+/+Chk2−/− mice revealed no apparent cancer-prone phenotypes during this experimental period (Figure 1A and data not shown). We examined the lineage of lymphomas observed in Chk1+/−Chk2+/− and Chk1+/−Chk2−/− mice by immunostaining using antibodies to PAX5 as a B-cell marker and CD3 as a T-cell marker. Those lymphomas were positive for PAX5 and negative for CD3, indicating that they were from the B-cell lineage. The typical staining of those lymphomas is shown in Figure 1I.
Figure 1.
Development of spontaneous tumours in mice bearing germline deletions of Chk1 and Chk2. (A) Kaplan–Meier analysis of tumour-free survival of wild type (diamonds, n=17), Chk1+/−Chk2+/+ (circles, n=15), Chk1+/−Chk2+/− (triangles, n=17) and Chk1+/−Chk2−/− (squares, n=17) mice. The animals were monitored for up to 22 months or until they succumbed to cancer. All tumour cases were identified based on the results of pathological analysis. The statistical significance of the survival curves was assessed using the log-rank test. (B) Table summarizing the cancer types observed in Chk1+/−Chk2−/− and Chk1+/−Chk2+/− mice, together with the sexes and ages of the mice; 59% of Chk1+/−Chk2−/−, 41% of Chk1+/−Chk2+/−, 13% of Chk1+/−Chk2+/+ and 6% of Chk1+/+Chk2+/+ mice succumbed to cancer during this experimental period. (C–H) Images of haematoxylin and eosin histology of six representative tumours found in Chk1+/−Chk2+/− and Chk1+/−Chk2−/− mice. A malignant lymphoma in a lymph node of a Chk1+/−Chk2−/− mouse, × 200 (C); a malignant lymphoma in the liver of a Chk1+/−Chk2−/− mouse, × 200 (D); a lung adenoma in a Chk1+/−Chk2−/− mouse, × 100 (E); a subcutaneous sarcoma in Chk1+/−Chk2−/− mice, × 100 (F); a pancreatic adenocarcinoma, × 200 (G) and a lung adenocarcinoma, × 200 (H). (I) Representative immunohistochemical staining of a malignant lymphoma invading the lung of a Chk1+/−Chk2−/− mouse (left) and liver of a Chk1+/−Chk2+/− mouse (right) with PAX5 used as a B-cell marker and CD3 as a T-cell marker, × 100. PAX5 was positive in the nucleus of invading atypical small round cells and CD3 was negative in the membrane of these cells. (J) Expression of Chk1 (left panel) and Chk2 (right panel) transcripts in tumours from Chk1+/−Chk2+/− and Chk1+/−Chk2−/− mice. Expression levels of Chk1 and Chk2 transcripts were measured by real-time PCR using RNAs prepared from paraffin-embedded tumour tissues and RNAs from B cells, T cells and ES cells as controls. Normalization was performed relative to the level of GAPDH transcripts and the data are presented as relative expressions to those in ES cells.
We then examined whether Chk1 and Chk2 function as typical or haplo-insufficient tumour suppressors. Quantitative real-time PCR using cDNAs from tumour sections revealed that Chk1 was still expressed in tumours of Chk1+/−Chk2+/− and Chk1+/−Chk2−/− mice and Chk2 was also expressed in those of Chk1+/−Chk2+/− mice (Figure 1J). These results suggest that both Chk1 and Chk2 are haplo-insufficient tumour suppressors. Aside from their striking cancer susceptibility, Chk1+/−Chk2−/− mice were indistinguishable from their wild-type siblings. Chk1−/−Chk2−/− mice, however, died at an early embryonic stage (Supplementary Table SI), indicating that Chk2 depletion failed to rescue embryonic lethality in Chk1−/− mice and suggesting that in mice a single Chk1 allele is sufficient for normal embryonic development or post-natal life.
Aberrant cell cycle checkpoints in Chk1/Chk2 double-mutant cells
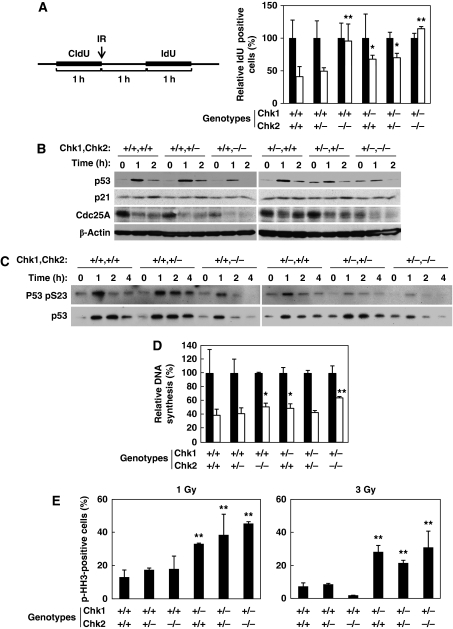
In order to characterize the tumourigenicity observed in Chk1+/−Chk2+/− and Chk1+/−Chk2−/− mice, we generated primary mouse embryonic fibroblasts (MEFs) from littermates obtained from double-heterozygote breeders. Our strategy to assess the immediate G1/S phase checkpoints after exposure to ionizing radiation (IR) involved staggered CIdU/IdU labelling. The labelling strategy is shown in Figure 2A (left panel). In this assay, single-labelled IdU cells are ones that entered S phase after IR, so this allowed us to evaluate initiation of the G1/S checkpoint without any effect from the intra-S phase checkpoint. Although irradiation reduced the proportion of IdU single-positive cells to 40% in Chk1+/+Chk2+/+ cells, those of Chk1+/−Chk2+/+ and Chk1+/−Chk2+/− cells were only reduced to 67% (P<0.05) and 70% (P<0.05), respectively (Figure 2A). No reduction in S phase entry was observed in Chk1+/+Chk2−/− and Chk1+/−Chk2−/− cells. Thus, a synergistic effect on this checkpoint was observed by combined deletion of a single Chk1 allele in Chk2−/− cells, indicating that initiation of IR-induced G1/S arrest is independently regulated by Chk1 and Chk2.
Figure 2.
Chk1 and Chk2 regulate non-redundant DNA-damage checkpoints. (A) Synergistic regulation of G1/S checkpoint by Chk1 and Chk2. Diagram of our strategy to assess the G1/S checkpoint after IR irradiation. Primary MEFs with the indicated genotypes are labelled with CIdU for 1 h and then IR irradiated (white bars) or mock irradiated (black bars). After washing out the CIdU, the cells were incubated in fresh medium for 1 h and then incubated with IdU for 1 h. In order to record the number of cells newly entering S phase after IR irradiation, single IdU-positive cells were counted (at least 300 cells) and the results were represented as a percentage of the total cells. Data are means±s.d. of at least three independent experiments. Statistical significance compared with Chk1+/+Chk2+/+ MEFs was assessed by Student's t-test (*P<0.05, **P<0.01). (B) Accumulation of p53 and reduction of Cdc25A upon IR treatment. Primary MEFs with the indicated genotypes were irradiated with IR (4 Gy) and harvested at 0, 1 and 2 h after irradiation. Cell lysates were subjected to immunoblotting using anti-p53 (upper panels), anti-p21 (2nd panels), anti-Cdc25A (3rd panels) and anti-β-actin (bottom panels) antibodies. (C) Phosphorylation of p53 at S23 after IR treatment. Primary MEFs with the indicated genotypes were irradiated with IR (4 Gy) and harvested at 0, 1, 2 and 4 h after irradiation. Cell lysates were immunoprecipiated with anti-p53 antibodies and the precipitates were subjected to immunoblotting using an anti-phospho-p53 at S23 antibody (upper panels) and an anti-p53 antibody (lower panels). (D) Radio-resistant DNA synthesis was examined as described in Materials and methods. The rate of DNA synthesis was determined by the radioactivity of [3H] divided by that of [14C]. The relative DNA synthesis is represented as a percentage of DNA synthesis relative to that observed in cells without DNA damage. Data are means±s.d. of at least three independent experiments. Statistical significance compared with Chk1+/+Chk2+/+ MEFs was assessed by Student's t-test (*P<0.05, **P<0.01). (E) Defective G2/M checkpoint in mice lacking a single Chk1 allele. Primary MEFs from mice with the indicated genotypes were treated with two distinct doses of IR (1 Gy: left panel and 3 Gy: right panel). The mitotic index was determined as the percentage of mitotic cells (pH3 Ser10 positive) relative to the total cells at 0.5 h after irradiation. The mitotic index was then calculated as a percentage relative to the non-irradiated cells. Data are means±s.d. of at least three independent experiments. Statistical significance compared with Chk1+/+Chk2+/+ MEFs was assessed by Student's t-test (**P<0.01).
Given that initiation of the G1/S checkpoint is regulated by p53 and Cdc25A (Bartek and Lukas, 2001), we examined changes in the expression of p53 and Cdc25A after IR. The level of p53 was increased as early as 1 h and then decreased at 2 h after IR in Chk1+/+Chk2+/+ and Chk1+/+Chk2+/− cells (Figure 2B). This increase in p53 protein level was reduced in Chk1+/+Chk2−/− and Chk1+/−Chk2+/− cells and was not observed at all in Chk1+/−Chk2−/− cells. Surprisingly, the level of p53 was significantly higher in the absence of DNA damage in Chk1+/−Chk2+/− and Chk1+/−Chk2−/− cells. Consistent with these effects on p53 level, induction of p21 expression was not detectable in Chk1+/+Chk2−/− or Chk1+/−Chk2−/− cells. In contrast to p53, the level of Cdc25A was very low in the absence of DNA damage in Chk2−/− cells and the abrupt reduction of Cdc25A after DNA damage was significantly impaired by the single deletion of one Chk1 allele. These results suggest that Chk1 and Chk2 cooperatively regulate the initiation of the G1/S checkpoint through regulation of Cdc25A protein level. Our results also show that Chk2 is required to maintain a proper level of Cdc25A in the absence of DNA damage, but not after DNA damage, although the molecular mechanism remains to be clarified.
P53 protein level is regulated at multiple levels, transcriptionally, translationally and post-translationally (Appella and Anderson, 2001). One of these controls is Chk1- and Chk2-dependent phosphorylation of p53 at S23 in mice (corresponding to S20 in human) (Shieh et al, 2000) and subsequent p53 stabilization by prevention of its interaction with the ubiquitin ligase Mdm2 (Chehab et al, 1999). The level of p53 phosphorylation at S23 was increased as early as 1 h and then decreased at 4 h after IR in Chk1+/+Chk2+/+ and Chk1+/+Chk2+/− cells, whereas the increase was slightly less in Chk1+/+Chk2−/− and Chk1+/−Chk2+/+ cells and was only a little in Chk1+/−Chk2+/− and Chk1+/−Chk2−/− cells (Figure 2C). These results suggest that Chk1 and Chk2 independently phosphorylate p53 at S23 after DNA damage and cooperatively regulate its stability.
With respect to the intra-S phase checkpoints, we found that IR reduced incorporation of radio-labelled thymidine to 39% in Chk1+/+Chk2+/+ cells as compared with untreated cells (Figure 2D). This reduction was significantly impaired in Chk1+/+Chk2−/− cells (51%, P<0.05), Chk1+/−Chk2+/+ cells (49%, P<0.05) and Chk1+/−Chk2−/− cells (65%, P<0.01). Therefore, these results show that the intra-S phase checkpoint in response to double-stranded breaks (DSBs) was regulated by both Chk1 and Chk2.
We next examined IR-induced G2 checkpoint function. In contrast to the G1 checkpoint, loss of a single Chk1 allele resulted in an impaired IR-induced G2 arrest at both low (1 Gy) and high (3 Gy) doses of irradiation (Figure 2E), whereas null depletion of Chk2 had no effect in this regard. Consistent with our observations, recent reports have shown that Chk2 is dispensable for the degradation of Cdc25A and initiation of G2/M arrest after DNA damage in human (Jallepalli et al, 2003; Jin et al, 2008), mouse (Takai et al, 2002) and Drosophila cells (Varmark et al, 2010). Thus, our results suggest that IR-induced G2 arrest is mainly regulated by Chk1, although maintenance of G2 arrest might be affected by Chk2 depletion as reported earlier (Hirao et al, 2000; Yu et al, 2001).
Aberrant DNA-damage-induced apoptosis and DNA-repair activity in Chk1/Chk2 double-mutant cells
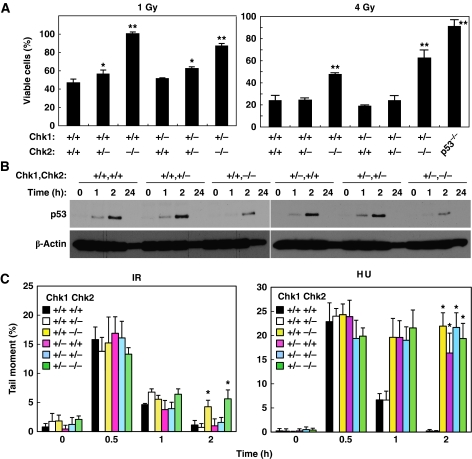
Chk2 has been reported to regulate DNA-damage-induced apoptosis (Hirao et al, 2002; Takai et al, 2002). Therefore, it is possible that Chk1 and Chk2 may cooperatively regulate p53-dependent apoptosis. Thymocytes from Chk-depleted mice were irradiated and their survival was assessed. Depletion of two Chk2 alleles, but not a single Chk1 allele, resulted in impaired induction of thymocyte apoptosis after IR treatment at a high dose (4 Gy) (Figure 3A, right panel). Intriguingly, deletion of a single Chk2 allele also resulted in a partial impairment of apoptosis at a low dose (1 Gy) of IR (Figure 3A, left panel), suggesting that Chk2 is haplo-insufficient for induction of apoptosis.
Figure 3.
Chk1 and Chk2 have a non-redundant function in DNA-damage-induced apoptosis and DNA repair. (A) Chk2 is haplo-insufficient for IR-induced apoptosis of thymocytes. Thymocytes from mice with the indicated genotypes were exposed to 1 Gy (left panel) or 4 Gy (right panel) irradiation. Sub G1 population was determined by FACS and viable cells were calculated as a percentage of non-sub G1 cells relative to the total cell number. Data are means±s.d. of at least three independent experiments. Statistical significance compared with Chk1+/+Chk2+/+ MEFs was assessed by Student's t-test (*P<0.05, **P<0.01) (B) Accumulation of p53 upon IR treatment in thymocytes. Thymocytes from mice with the indicated genotypes were irradiated with IR (4 Gy) and harvested at 0, 1, 2 and 24 h after irradiation. Cells were subjected to immunoblotting using anti-p53 and anti-β-actin antibodies. (C) Chk1 and Chk2 regulate the efficiency of DNA repair. Primary MEFs of the indicated genotypes were treated with IR (left panel) or hydroxyurea (HU: right panel) and subjected to an alkaline-comet assay at the indicated times. Tail moments were determined using TriTek Comet Score Freeware. Data are means±s.d. of counting at least 50 cells per sample in three independent experiments. Statistical significance compared with Chk1+/+Chk2+/+ MEFs was assessed by Student's t-test (*P<0.005).
We then sought to determine whether impaired induction of apoptosis in Chk2-depleted thymocytes was due to reduced induction of p53 protein upon DNA damage. Induction of p53 was observed as early as 1 h and peaked at 2 h after IR in Chk1+/+Chk2+/+ thymocytes (Figure 3B). This induction was severely impaired in Chk1+/+Chk2−/− and Chk1+/− Chk2−/− thymocytes. It should be noted that, unlike MEFs, thymocytes from Chk1+/−Chk2+/− and Chk1+/−Chk2−/− mice did not contain a higher level of p53 under unperturbed conditions when compared with Chk1+/+Chk2+/+ mice. These results suggest that Chk2 regulates IR-induced apoptosis in thymocytes at least in part through induction of p53 expression.
Chk1 and Chk2 are reported to be involved in DNA repair through phosphorylation of Rad51 (Sorensen et al, 2005) and BRCA1 (Lee et al, 2000), respectively. Therefore, the cooperative effect of Chk1 and Chk2 in suppressing tumourigenesis may be explained by their ability to ensure proper DNA repair. To address this question, we analysed DNA repair in double Chk1- and Chk2-depleted primary MEFs using an alkaline-comet assay. Deletion of two Chk2 alleles resulted in a significant aberration of IR-induced DNA repair, although loss of a single Chk1 allele had no effect (Figure 3C). In contrast, loss of a single Chk1 allele, as well as deletion of two Chk2 alleles, caused aberrant DNA repair when DNA damage was induced by hydroxyurea. This indicated that DNA damage-induced activation of DNA repair requires Chk1 or Chk2, but which is required depends on the type of DNA damage.
Spontaneous DNA damage in proliferating Chk1+/− Chk2+/− and Chk1+/−Chk2−/− MEFs under unperturbed condition
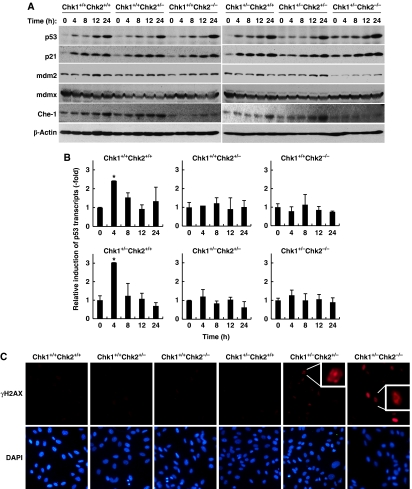
The increase in the level of p53 observed in proliferative Chk1+/−Chk2+/− and Chk1+/−Chk2−/− MEFs under unperturbed conditions (Figure 2B) raises the possibility that Chk1 and Chk2 directly regulate factors that determine p53 stabilization or prevent accumulation of spontaneous DNA damage that eventually increases p53 protein levels. In Chk1+/+Chk2+/+ MEFs, IR induced a biphasic increase in p53, showing a rapid induction of expression within 4 h and a slow induction at 24 h (Figures 2B and 4A). A similar IR-induced biphasic accumulation of p53 was also reported in normal human embryonic cells (Ghosh et al, 2000). As seen in Figure 2B, an increased level of p53 was detected in Chk1+/−Chk2+/− and Chk1+/−Chk2−/− MEFs under unperturbed conditions (time 0). The level of p21 was also higher in these cells under unperturbed conditions. Surprisingly, the level of Mdm2 was very low in Chk1+/−Chk2−/− MEFs, although the level of p53, a transcriptional activator of Mdm2, was high during this experimental period, suggesting that Chk1 and Chk2 may cooperatively stabilize Mdm2 through an unknown mechanism. In contrast to apparent impairment of the rapid accumulation of p53 observed in Chk1+/−Chk2+/− and Chk1+/−Chk2−/− MEFs, a slow induction was observed in all other MEFs tested. Recently, it was reported that Che-1, an RNA polymerase II-binding protein, was phosphorylated by ATM/ATR and Chk1 after DNA damage (Bruno et al, 2006). The phosphorylation of Che-1 resulted in its accumulation and recruitment to the p53 promoter to activate p53 gene transcription. We examined the induction of Che-1 as well as changes in p53 transcript level after DNA damage. However, in contrast to the previous report (Bruno et al, 2006), the accumulation of Che-1 was only impaired upon Chk2 deletion (Chk1+/+Chk2−/− and Chk1+/−Chk2−/− MEFs) when compared with what is observed in Chk1+/+Chk2+/+ MEFs (Figure 4A). Deletion of a single Chk1 allele did appear to slightly affect accumulation of Che-1, consistent with the previous observation that Chk1 as well as Chk2 could phosphorylate Che-1 in vitro. P53 transcription was increased as early as 4 h after DNA damage and then decreased in Chk1+/+Chk2+/+ and Chk1+/−Chk2+/+ MEFs, whereas it did not vary in MEFs in which either 1 or 2 copies of Chk2 were deleted (Figure 4B). These results show that a single Chk2 allele is not sufficient for recruitment of Che-1 to the p53 promoter and, therefore, Chk2 is haplo-insufficient for Che-1-dependent transcriptional activation of p53. However, this transcriptional activation was apparently not involved in the slow induction of p53 expression after DNA damage.
Figure 4.
Spontaneous DNA damage and induction of p53 under unperturbed conditions in Chk1+/−Chk2+/− and Chk1+/−Chk2−/− MEFs. (A) Cell lysates from the indicated MEFs were irradiated with IR (4 Gy). Cells were harvested at the indicated times after IR and cell lysates were subjected to immunoblotting with anti-p53 (top), anti-p21 (second), anti-mdm2 (third), anti-mdmX (fourth), anti-Che-1 (fifth) and anti-β-actin (bottom) antibodies. (B) Changes in the p53 transcripts after IR irradiation. Primary MEFs with the indicated genotypes were irradiated with IR (4 Gy) and harvested at the indicated times after irradiation. Total RNA was then extracted and the expression levels of p53 transcript were measured by quantitative real-time PCR. The results were normalized to the level of GAPDH transcripts used as an internal control and data are presented as means±s.d. of at least three independent experiments. Statistical significance compared with cells without IR irradiation (time 0) was assessed by Student's t-test (*P<0.01). (C) Spontaneous DNA damage under unperturbed conditions in Chk1+/−Chk2+/− and Chk1+/−Chk2−/− MEFs. Asynchronized primary MEFs with the indicated genotypes without any genotoxic stress were fixed and subjected to immunofluorescence staining with anti-phospho-H2AX (γH2AX) antibodies (upper panel). Cells were counterstained with DAPI to detect nuclei (lower panel), × 200. Magnified images of cells with γH2AX foci are shown in the white boxes (Chk1+/−CHk2+/− and Chk1+/−Chk2−/− cells).
Given that a significant number of heterozygous lobuloalveolar mammary epithelial cells contained spontaneous DNA damage in Chk1 conditional heterozygotes (Lam et al, 2004), we speculate that this was also the case in primary MEFs from Chk1 heterozygotes. To examine this possibility, MEFs from Chk1/Chk2 double-mutant mice were stained with phospho-H2AX specific for Ser139 (γH2AX) without the addition of any exogenous DNA-damaging agent. A significant number of γH2AX-positive cells were readily detected in Chk1+/−Chk2+/− and Chk1+/−Chk2−/− MEFs, but not in MEFs from other genotypes (Figure 4C). Signals for γH2AX in Chk1+/−Chk2−/− MEFs were much stronger than those in Chk1+/−Chk2+/− MEFs. However, some of the γH2AX signals in Chk1+/−Chk2−/− MEFs as well as most of the signals in Chk1+/−Chk2+/− MEFs showed typical discrete foci, presumably because of the level of spontaneous DNA damage. These results suggest that Chk1 and Chk2 cooperatively prevent the accumulation of cells with spontaneous DNA damage.
Accumulation of DNA damage can easily result in genome instability. We thus examined metaphase chromosome spreads from Chk1/Chk2 double-mutated primary MEFs and CD19-positive B cells. Aberrations including chromosomal breaks and many fusions appeared in Chk1+/−Chk2+/− and Chk1+/−Chk2−/− MEFs (Table I). Although the level of the chromosomal aberrations observed in the activated B cells was relatively low when compared with MEFs, presumably because of the short in vitro culture period (72 h) of B cells, higher levels of chromosomal aberrations were detected in B cells from Chk1+/−Chk2+/− and Chk1+/−Chk2−/− mice (Table II). These results confirm that Chk1+/−Chk2+/− and Chk1+/−Chk2−/− mice are more susceptible to B-cell lymphomas and show that Chk1 and Chk2 synergistically prevent susceptibility to global chromosomal rearrangement.
Table 1. Chromosomal abnormalities in primary MEFs from Chk1/Chk2 double-mutant micea.
| Samples | Metaphases analysed | Chromosomes/metaphase | Total aberrant cells (%) |
|---|---|---|---|
| Chk1+/+Chk2+/+ | 58 | 40.30±0.60 | 2 (3.45%) |
| Chk1+/+Chk2+/− | 62 | 40.37±1.09 | 4 (6.45%) |
| Chk1+/+Chk2−/− | 58 | 42.54±1.38 | 9 (15.52%) |
| Chk1+/−Chk2+/+ | 59 | 39.96±0.66 | 5 (8.47%) |
| Chk1+/−Chk2+/− | 58 | 40.66±0.61 | 11 (18.97%) |
| Chk1+/−Chk2−/− | 59 | 44.12±1.56 | 12 (20.34%) |
| aChromosomes were stained with 4′, 6′-diamidino-2-phenylindole (DAPI) and observed under a Zeiss axioplan imaging 2 microscope. The abnormal chromosomes including fusions and fragments were counted in metaphase chromosome spreads of MEFs with the indicated genotypes. | |||
Table 2. Chromosomal abnormalities in primary B cells from Chk1/Chk2 double-mutant micea.
| Samples | Metaphases analysed | Chromosomes/metaphase | Total aberrant cells (%) |
|---|---|---|---|
| Chk1+/+Chk2+/+ | 65 | 39.97±0.17 | 1 (1.54%) |
| Chk1+/+Chk2+/− | 52 | 39.88±0.61 | 1 (1.92%) |
| Chk1+/+Chk2−/− | 55 | 39.53±1.36 | 3 (5.45%) |
| Chk1+/−Chk2+/+ | 36 | 37.80±3.38 | 2 (5.56%) |
| Chk1+/−Chk2+/− | 37 | 39.02±2.11 | 3 (8.11%) |
| Chk1+/−Chk2−/− | 33 | 39.39±1.03 | 3 (9.09%) |
| aPrimary B cells from Chk1/Chk2 double-mutant mice were isolated using magnetic bead-conjugated antibodies against CD19 and AutoMACS, and stimulated by lipopolysaccharide as described in ‘Materials and methods'. Chromosomes were stained with 4', 6'-diamidino-2-phenylindole (DAPI) and observed under a Zeiss axioplan imaging 2 microscope. The abnormal chromosomes including fusions and fragments were counted in metaphase chromosome spreads of B cells with the indicated genotypes. | |||
Chk1 and Chk2 do not appear to be involved in the induction of premature senescence
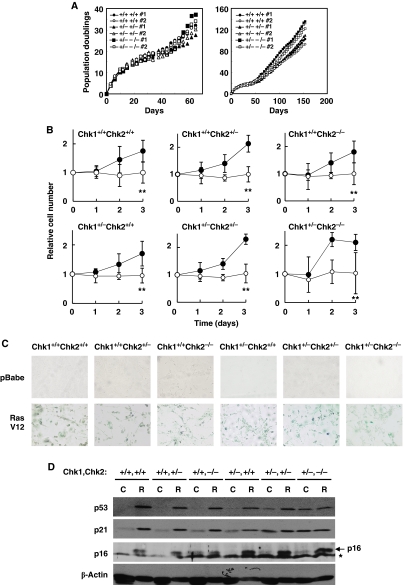
Senescence offers a major protective mechanism against tumour development and is triggered by DNA damage responses (Lowe et al, 2004). In several studies using human diploid cells, activation of Chk1 and Chk2 was proposed to be important for oncogene-induced and replicative senescence (d'Adda di Fagagna et al, 2003; Bartkova et al, 2006; Di Micco et al, 2006; Mallette et al, 2007). Therefore, we hypothesized that the cooperative function of Chk1 and Chk2 in suppression of tumourigenesis might be a means to ensure induction of senescence upon exposure of cells to genotoxic stress. To test this hypothesis, we first examined stress-induced senescence in culture, known as ‘culture shock', in which mammalian cells are exposed to an unrelenting onslaught of mitogenic signals (Sherr and DePinho, 2000). Wild type and other types of Chk1/Chk2-depleted primary MEFs tested were cultured in serum-containing medium and passaged every 3 days after a 3T3 subculture schedule. All types of MEFs had similar doubling times for the first 10 generations. Their growth rate then slowed and eventually reached a non-dividing state even at a subconfluent density (Figure 5A). These cells then recovered their growth capability and became immortal at around 40 days with a doubling time similar to that of the initial culture. Unexpectedly, ectopic expression of oncogenic Ras clearly induced both growth arrest (Figure 5B) and a senescent phenotype as assessed by senescence-associated β-gal staining (Figure 5C) in all types of double Chk1- and Chk2-depleted primary MEFs. Given that homozygous deletion of Chk1 resulted in growth arrest at S phase (Shimada and Nakanishi, 2008) and presented a senescence-like morphology, and knockdown of Chk1 by its siRNA drastically increased the number of SA-β-gal-positive cells (Shimada et al, paper in preparation), our results indicated that both Chk1 and Chk2 are apparently dispensable for oncogene-induced senescence.
Figure 5.
Chk1 and Chk2 are apparently dispensable for culture-induced (culture shock) and oncogene-induced senescence. (A) Growth rates of short-term (left panel) and long-term (right panel) cultures of primary MEFs of the indicated genotypes. (B) Representative growth curves corresponding to primary MEFs with the indicated genotypes infected with control (closed circles) or H-RasV12-expressing (open circles) retroviruses. Statistical significance of relative numbers of cells expressing RasV12 at day 3 compared with those expressing control vector was assessed by Student's t-test (**P<0.01). (C) Photographs of cells stained for SA-β-gal activity 3 days after infection. (D) Induction of p53, p21 and p16 proteins in cells of the indicated genotypes infected with either control (C) or H-RasV12 (R). An asterisk represents non-specific bands.
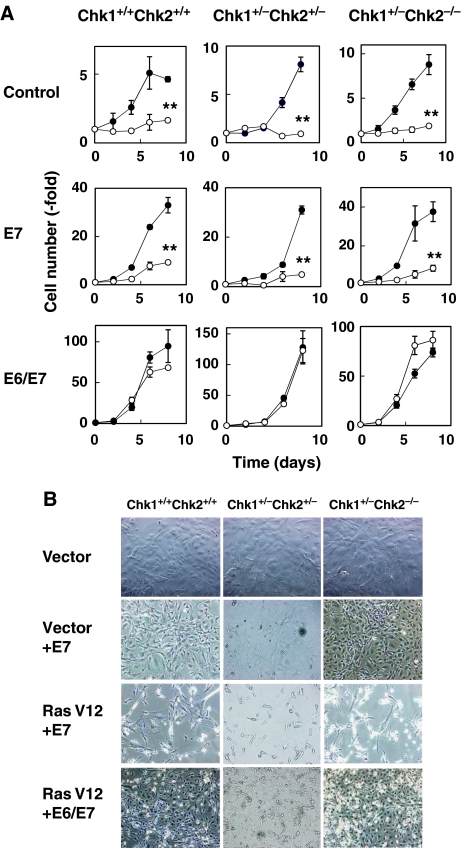
As the induction of senescent phenotypes is regulated by both p16-Rb and p53-dependent mechanisms (Courtois-Cox et al, 2008), we wondered whether senescent phenotypes in Chk-depleted MEFs were induced by either or both pathways. Expression of oncogenic Ras resulted in the clear induction of p16 protein in all types of MEFs (Figure 5D). As described above, the level of p53 was significantly higher in control-transfected Chk1+/−Chk2+/− and Chk1+/−Chk2−/− MEFs than in MEFs of other genotypes. Intriguingly, the levels of p53 and p21 in control-transfected Chk1+/−Chk2−/− MEFs were almost identical to those observed in Ras-transfected senescent cells. These results suggest that senescent phenotypes among Chk1+/−Chk2−/− MEFs might be induced by activation of p16-Rb pathway. To further clarify this point, we introduced papillomavirus E7, which binds to and inactivates Rb (Chellappan et al, 1992), into Chk1+/−Chk2+/− and Chk1+/−Chk2−/− primary MEFs in the presence or absence of oncogenic Ras. Surprisingly, E7 failed to prevent growth arrest and a senescence-like cell morphology in Chk1+/−Chk2+/− and Chk1+/−Chk2−/− primary MEFs expressing oncogenic Ras as well as in Chk1+/+Chk2+/+ MEFs cells (Figure 6A and B). Co-introduction of papillomavirus E6, which targets p53 for degradation (Scheffner et al, 1990), with E7 completely reversed growth arrest and morphological changes in Chk1+/−Chk2+/− and Chk1+/−Chk2−/− primary MEFs expressing oncogenic Ras. These results indicate that senescence is induced in Chk1+/−Chk2+/− and Chk1+/−Chk2−/− cells at least in part by oncogene-induced activation of the p53 pathway even in the absence of p16-Rb pathway. Although the actual contribution of the p16-Rb pathway to senescence induction in Chk1+/−Chk2+/− and Chk1+/−Chk2−/− MEFs is unknown, it should be noted that abrogation of a p53-Arf pathway (Serrano et al, 1997; Palmero et al, 1998), but not loss of p16 (Sharpless et al, 2001), is sufficient to prevent oncogenic Ras-induced senescence in primary MEFs. Taken together, our results indicate that cancer predisposition of Chk1+/−Chk2+/− and Chk1+/−Chk2−/− mice is not due to impaired induction of senescence in response to genotoxic stress.
Figure 6.
Chk1 and Chk2 are apparently dispensable for p53- and Rb-dependent senescence pathways. (A) Growth curve of MEFs with or without RasV12-expressing mock (control), E7 (E7) or both E6 and E7 (E6/E7). The cells were infected with empty (open circles) or Ras-expressing retrovirus (filled circles). Cell numbers were counted at the indicated times after selection. Data are means±s.d. of triplicate experiments. Statistical significance of relative numbers of cells expressing RasV12 at day 8 compared with those expressing control vector was assessed by Student's t-test (**P<0.01). (B) Morphology of cells obtained at 8 days after selection. The MEFs expressing mock (top panel), E7 (second and third panels) and E6/E7 (bottom panel) were infected with empty (top and second panels) or Ras-expressing retrovirus (third and bottom panels).
Discussion
Our results clearly indicate that Chk1 and Chk2 act cooperatively to prevent tumourigenesis by regulating partly redundant, but mainly non-redundant responses to DNA damage or genotoxic stress, including cell cycle arrest, apoptosis and DNA repair. As a result, combined loss of Chk1 and Chk2 causes the accumulation of cells with spontaneous DNA damage under unperturbed conditions leading to genomic instability and then tumour development. Accumulation of cells with DNA damage under unperturbed conditions appears to result from increased DNA damage during S phase because of reduced Chk1 activity and failure to eliminate cells with DNA damage by loss of Chk2 function. This idea is strongly supported by the fact that Chk1 depletion causes severe DNA damage during S phase (Niida et al, 2005; Syljuasen et al, 2005) and that Chk2-deficient MEFs are highly resistant to DNA-damage-induced apoptosis (Figure 3A) (Hirao et al, 2002; Takai et al, 2002). In addition, increased expression of p53 in Chk1/Chk2 double-depleted cells under unperturbed conditions was observed only in proliferative MEFs and not in G0-arrested T cells (Figures 2B, 3B and 4A), further supporting the idea that DNA damage in these cells occurred during S phase. Recently, Zaugg et al (2007) reported that Chk1 and Chk2 functioned in mostly non-redundant DNA-damage response and that the loss of Chk1 activated Chk2 in thymocytes, suggesting the existence of physiological cross-talk between Chk1 and Chk2. Taken together, these results indicate that high tumour susceptibility in Chk1+/−Chk2+/− and Chk1+/−Chk2−/− mice is likely due to the combined impairment of non-redundant DNA-damage response mediated by both Chk1 and Chk2.
Although mutations in CHEK2 (the gene encoding Chk2) do not account for the cancer-predisposing Li–Fraumeni syndrome as originally thought, rare germline mutations have been detected with high incidence in a number of familial cancers and rare somatic mutations have been reported in some tumours, suggesting that CHEK2 is indeed a cancer susceptibility gene (Meijers-Heijboer et al, 2002; Vahteristo et al, 2002; Antoni et al, 2007). However, we and others have shown that complete loss of both Chk2 alleles is not sufficient to increase tumour incidence in mice (Hirao et al, 2002; Takai et al, 2002). In this regard, it should be noted that most of the CHEK2 abnormalities in human tumours are mutations (not complete gene deletion) that generate truncated or mutated forms of the Chk2 protein (Antoni et al, 2007). Thus, these modified forms may impair other tumour suppressive pathways in a dominant-negative manner. Alternatively, it may take a much longer time to generate tumours by loss of Chk2 function, so that they are not observed during the short life span of the mice.
Chk1+/−Chk2+/− and Chk1+/−Chk2−/− mice developed B-cell tumours, but not T-cell lymphomas. Intriguingly, B-cell lymphomas have been observed in mice bearing a p53 S23A/S23A knockin mutation (corresponding to human S20) (MacPherson et al, 2004). It is, therefore, possible that tumourigenicity in the double-mutant mice resulted from impaired phosphorylation of p53 at S23 that is redundantly regulated by both Chk1 and Chk2 in response to DNA damage, as indicated from in vitro studies (Shieh et al, 2000). P53 is activated by numerous types of stress, such as DNA damage, viral infection and metabolic stress, through phosphorylation of S23. CK1 is a p53 S23 kinase activated in response to DNA viral infection (MacLaine et al, 2008), AMPK is another p53 S23 kinase activated in response to elevation of AMP/ATP ratio (Maclaine and Hupp, 2009) and DAPK-1 is a third p53 S23 kinase activated in response to inappropriate oncogene activation (Craig et al, 2007). However, the kinase responsible for S23 phosphorylation in response to IR has not been identified. Unexpectedly, knockdown of either or both Chk1 and Chk2 in human cells failed to abrogate the damage-dependent induction of p53 expression (Ahn et al, 2003), and depletion of both Chk2 alleles in human colon cancer cells does not compromise p53 phosphorylation at S20 (corresponding to mouse S23) (Jallepalli et al, 2003). Although these results cast doubt on the function of Chk1 and Chk2 in the damage-induced phosphorylation of human p53 at S20 (corresponding to mouse S23), our present results clearly show that DNA-damage-induced mouse p53 phosphorylation at S23 was abrogated in primary MEFs from Chk1+/−Chk2−/− mice. One potential explanation for this discrepancy is that our experiments were performed with primary knockout MEFs, whereas other studies used human cancer cells and did not analyse the phosphorylation status of p53 at S20 (corresponding to mouse S23) in Chk1/Chk2 double-knockdown cells. Therefore, although the contribution of Chk1 and Chk2 to the phosphorylation of p53 at S23 in response to DNA damage appears to be somewhat different between human and mouse cells, both enzymes are likely to function as damage-induced p53 S23 kinases in a redundant manner.
Chk2 has also been reported to cooperate with other factors involved in DNA-damage response to suppress oncogenic potential. For example, Brca1Δ11/Δ11Chk2−/− mice showed cancer predisposition (Cao et al, 2006). Similarly, Chk2−/− mice with a conditional deletion of Brca1 in the thymus or mammary gland developed tumours in these tissues (McPherson et al, 2004). Very recently, NBS1ΔB/ΔBChk2−/− and Mre11ATLD1/ATLD1Chk2−/− mice have also been reported to develop tumours with latency similar to that observed in Brca1Δ11/Δ11Chk2−/− mice (Stracker et al, 2008). Both the MRN complex and Brca1 have an essential function in sensing DNA double-strand breaks and in transmitting the signal to downstream targets. In contrast, Chk1 and Chk2 are specifically activated depending on the type of DNA damage and thus act in a complementary manner (Bartek and Lukas, 2003). Our results suggest that Chk1-dependent cell cycle arrest might serve as a backup system for DSBs that must be repaired before completion of DNA replication or eliminated by Chk2-dependent apoptosis to prevent severe genomic instability and transformation of normal cells into cancer cells. In this regard, it should be noted that ATM regulates the recruitment of ATR to DSBs, leading to double-strand break-induced Chk1 phosphorylation (Adams et al, 2006; Jazayeri et al, 2006).
Surprisingly, neither Chk1 nor Chk2 was apparently involved in mouse senescence pathways, which may explain why tumour development in Chk1+/−Chk2+/− and Chk1+/−Chk2−/− mice had a late onset. These observations are in clear contrast with those observed in human cells, in which both Chk1 and Chk2 are activated after induction of premature or replicative senescence (d'Adda di Fagagna et al, 2003; Gire et al, 2004; Bartkova et al, 2006; Di Micco et al, 2006; Mallette et al, 2007). In addition, knockdown of Chk2 suppresses oncogene-induced senescence (Di Micco et al, 2006), and ectopic co-expression of dominant-negative forms of Chk1 and Chk2 (d'Adda di Fagagna et al, 2003) or Chk2 knockdown suppress replicative senescence in human cells (Gire et al, 2004). In this regard, mouse cells do not undergo replicative senescence, because they possess far longer telomeres than human cells. Therefore, Chk2 might have a specific function in the induction of replicative senescence that is not observable in mice. We found that complete loss of Chk1 led to a senescence-like permanent growth arrest in MEFs. Intriguingly, constitutive activation of ATR in mouse cells can also induce senescence (Toledo et al, 2008). We and others reported that Chk1 localizes at chromatin under unperturbed conditions and its phosphorylation by ATR releases Chk1 from chromatin (Smits et al, 2006; Niida et al, 2007). Chromatin-bound Chk1 is required for the expression of various cell cycle regulatory genes through phosphorylation of H3-T11 (Shimada et al, 2008). Therefore, constitutive activation of ATR mimics loss of Chk1 bound on chromatin. Taken together, these results indicate that permanent loss of Chk1 from chromatin can drive cells into senescence through epigenetic modifications on cell cycle gene promoters. This appears to be consistent with the apparently dispensable function of Chk1 in the induction of premature senescence.
In conclusion, our results suggest that a combination of partial defects in several tumour-protective barriers might engender a more progressive cancer-prone condition than a severe defect in one mechanism. This is in agreement with the association between genetic variations or mutations in Chk1 and Chk2 genes and the high cancer risk. Therefore, inhibition of Chk1 or Chk2 as an approach to cancer therapy should be undertaken with careful consideration.
Materials and methods
Immunoblotting
For preparation of whole cell extracts, cells were lysed in IP kinase buffer as previously described. The antibodies used for immunoblotting or immunofluorescence were directed against p53 (NCL-p53-505, Novocasta Laboratory), p21 (sc-6246, SantaCruz), p16 (sc-1207, SantaCruz), Cdc25A (sc-7389, SantaCruz), Che-1 (ab39631, Abcam), Mdm2 (sc-965, SantaCruz), Mdmx (sc-28222, SantaCruz), PAX5 (sc-1974, SantaCruz), CD3 (ab49943, Abcam), β-actin (ab2676-100, Abcam), phosphor-Ser-10-histone H3 (06-570, Upstate) and BrdU (B44, BD for IdU; BI1/75, ICR1 for CIdU).
Mice and MEFs
Chk1+/− mice (Takai et al, 2000) were crossed with Chk2+/− mice (Takai et al, 2002) to obtain Chk1+/−Chk2+/− mice. Experimental cohorts were derived from littermates obtained from double-heterozygote breeders. All mice studied had a mixed 129 × C57BL/6 genetic background and were genotyped by PCR. All experiments were performed in compliance with the Nagoya City University Animal Care Committee guidelines. Primary MEFs were derived from E14.5 embryos of double-heterozygote breeders.
Cell cycle, apoptosis analysis and comet assay
The mitotic index was measured as described previously using anti-phosphor-Ser-10-histone H3 (06-570, Upstate) antibodies (Niida et al, 2005). For G1/S checkpoint analysis, primary MEFs were labelled with CIdU (10 μM) for 1 h, then treated with mock or IR (5 Gy) and additionally labelled with IdU (10 μM) for 1 h after incubation with fresh medium for 1 h (Figure 2A). The cells were then fixed in three parts methanol: one part glacial acetic acid at −20°C for 30 min, after which they were treated with 2 N HCl for 30 min. The cells were then washed with PBS, permeabilized with 0.05% Triton X-100 in PBS and blocked with 5% FBS, 0.2% Triton X-100 and 0.1% BSA in PBS. CldU was detected with a 1/100 dilution of anti-BrdU rat monoclonal antibody (BU1/75: ab6326, Abcam), and IdU was detected with a 1/50 dilution of anti-BrdU mouse antibody (clone B44: 347580, BD Biosciences). Clone B44 recognizes both IdU and CldU, but washing for 30 min with a high salt buffer (100 mM Tris, 0.5 M NaCl, 0.5% Tween, pH 8.0) releases it from CldU. A 1/500 dilution of Alexa 594-conjugated goat anti-rat IgG was used to detect BU1/75, and a 1/500 dilution of Alexa 488-conjugated goat anti-mouse IgG was used to detect B44. The cover slips were then mounted onto slides with Mowiol Mounting Medium for Fluorescence with 4′, 6′-diamidino-2-phenylindole (DAPI. For intra-S phase checkpoint analysis, primary MEFs were cultured with medium containing 10 nCi/ml [14C] thymidine (Amersham) for 24 h, washed with PBS and cultured again for 30 min in medium without [14C] thymidine. The cells were then X-irradiated (10 Gy), cultured for 1 h and then pulse labelled with medium containing 2.5 μCi/ml [3H] thymidine for 30 min. The labelled cells were harvested and fixed in ice-cold 70% ethanol overnight. The fixed cells were applied to membranes on the filtration plate, and the membranes were washed with 70% ethanol and then 95% ethanol (Nalgen Nunc; no. 255984). Radioactivity on the membranes was determined with a liquid scintillation counter. Radio-resistant DNA synthesis was determined by the radioactivity of [3H] divided by that of [14C]. For apoptosis analysis, thymocytes were irradiated and cultured for 24 h. Cells were fixed, stained with propidium iodide and analysed by FACS. An alkaline-comet assay was performed using the OxiSelectTMComet Assay kit according to the manufacturer's instructions (CELL BIOLABS INC). DNA was stained with Vista Green DNA Dye.
Isolation of CD19-positive B cells
Splenocytes were obtained by dissection of Chk1/Chk2 double-mutant mice and by manual disruption of the organ. The cell suspension was passed through a BD Falcon cell strainer (REF352340), and centrifuged at 300 g for 10 min at 4°C and suspended in 500 μl MACS running buffer. CD19-positive B cells were selected from single-cell suspensions of splenocytes by labelling the cells with magnetic bead-conjugated antibodies against CD19 (Miltenyi Biotec, 130-052-201), followed by AutoMACS magnetic bead sorting according to the manufacturer's instructions. The CD19-positive cells (2 × 106) were stimulated by 25 μg/ml lipopolysaccharide for 72 h and treated with 0.1 ng/ml colcemid for an additional 2 h. The resultant cells were then subjected to karyotype analysis.
Retroviral-mediated gene transfer
To prepare retroviral particles, PlatE cells (2 × 106) were plated on a 10 cm culture dish, and then transfected with retroviral vectors using FuGene6 (Roche). For infection, primary MEFs at passage 2 were plated at a density of 2 × 105 cells per 10 cm dish and infected by virus from PlatE cells for 24 h. The infection process was repeated four times at 4–12 h intervals. After selection in the presence of 2 μg/ml puromycin for 4 days, growth curves, immunoblotting and senescence analysis of MEFs was carried out. To determine senescence, MEFs were stained for SA-β-gal activity as described previously (Bartkova et al, 2006; Di Micco et al, 2006). For retroviral transfection of E6 and E7 vectors, infected MEFs were selected in medium containing 100 μg/ml hygromycin for 4 days.
Supplementary Material
Acknowledgments
We thank Drs Nakayama and Nishiyama for analysis of E7.5 embryos, Drs Hayashi and Sakai for anti-Cdc25A antibodies, Dr Yamada-Namikawa, Miss Morimoto, Miss Kojima, Mr Seeni_mohamed and Mr Tang for technical assistance. This work was supported in part by the Ministry of Education, Science, Sports and Culture of Japan through a Grant-in-Aid for Scientific Research on Priority Area (A), a grant for Scientific Research (B), the project for realization of regenerative medicine of MEXT and by the Mitsubishi Foundation, the Naito Memorial Foundation, the Toyoaki Foundation, the Takeda Foundation and the Uehara Foundation awarded to MN. The study was also partially supported by the Intramural Research Program of the NIH, National Cancer Institute.
Author contributions: HN and KM performed the majority of the experiments and data analysis. MS, KO, KO, KS, HF, AKK, BB, PMH, TM IM, TS, NM, MD and EA provided experimental data. HN, MD, NM and EA helped to write the paper. MN conceived of the project, planned and guided the research, and wrote the paper.
Footnotes
The authors declare that they have no conflict of interest.
References
- Adams KE, Medhurst AL, Dart DA, Lakin ND (2006) Recruitment of ATR to sites of ionising radiation-induced DNA damage requires ATM and components of the MRN protein complex. Oncogene 25: 3894–3904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn J, Urist M, Prives C (2003) Questioning the role of checkpoint kinase 2 in the p53 DNA damage response. J Biol Chem 278: 20480–20489 [DOI] [PubMed] [Google Scholar]
- Antoni L, Sodha N, Collins I, Garrett MD (2007) CHK2 kinase: cancer susceptibility and cancer therapy—two sides of the same coin? Nat Rev Cancer 7: 925–936 [DOI] [PubMed] [Google Scholar]
- Appella E, Anderson CW (2001) Post-translational modifications and activation of p53 by genotoxic stresses. Eur J Biochem 268: 2764–2772 [DOI] [PubMed] [Google Scholar]
- Bartek J, Lukas J (2001) Pathways governing G1/S transition and their response to DNA damage. FEBS Lett 490: 117–122 [DOI] [PubMed] [Google Scholar]
- Bartek J, Lukas J (2003) Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell 3: 421–429 [DOI] [PubMed] [Google Scholar]
- Bartkova J, Horejsi Z, Koed K, Kramer A, Tort F, Zieger K, Guldberg P, Sehested M, Nesland JM, Lukas C, Orntoft T, Lukas J, Bartek J (2005) DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature 434: 864–870 [DOI] [PubMed] [Google Scholar]
- Bartkova J, Rezaei N, Liontos M, Karakaidos P, Kletsas D, Issaeva N, Vassiliou LV, Kolettas E, Niforou K, Zoumpourlis VC, Takaoka M, Nakagawa H, Tort F, Fugger K, Johansson F, Sehested M, Andersen CL, Dyrskjot L, Orntoft T, Lukas J et al. (2006) Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature 444: 633–637 [DOI] [PubMed] [Google Scholar]
- Bell DW, Varley JM, Szydlo TE, Kang DH, Wahrer DC, Shannon KE, Lubratovich M, Verselis SJ, Isselbacher KJ, Fraumeni JF, Birch JM, Li FP, Garber JE, Haber DA (1999) Heterozygous germ line hCHK2 mutations in Li-Fraumeni syndrome. Science 286: 2528–2531 [DOI] [PubMed] [Google Scholar]
- Bertoni F, Codegoni AM, Furlan D, Tibiletti MG, Capella C, Broggini M (1999) CHK1 frameshift mutations in genetically unstable colorectal and endometrial cancers. Genes Chromosomes Cancer 26: 176–180 [PubMed] [Google Scholar]
- Bruno T, De Nicola F, Iezzi S, Lecis D, D'Angelo C, Di Padova M, Corbi N, Dimiziani L, Zannini L, Jekimovs C, Scarsella M, Porrello A, Chersi A, Crescenzi M, Leonetti C, Khanna KK, Soddu S, Floridi A, Passananti C, Delia D et al. (2006) Che-1 phosphorylation by ATM/ATR and Chk2 kinases activates p53 transcription and the G2/M checkpoint. Cancer Cell 10: 473–486 [DOI] [PubMed] [Google Scholar]
- Cao L, Kim S, Xiao C, Wang RH, Coumoul X, Wang X, Li WM, Xu XL, De Soto JA, Takai H, Mai S, Elledge SJ, Motoyama N, Deng CX (2006) ATM-Chk2-p53 activation prevents tumorigenesis at an expense of organ homeostasis upon Brca1 deficiency. EMBO J 25: 2167–2177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chehab NH, Malikzay A, Stavridi ES, Halazonetis TD (1999) Phosphorylation of Ser-20 mediates stabilization of human p53 in response to DNA damage. Proc Natl Acad Sci USA 96: 13777–13782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellappan S, Kraus VB, Kroger B, Munger K, Howley PM, Phelps WC, Nevins JR (1992) Adenovirus E1A, simian virus 40 tumor antigen, and human papillomavirus E7 protein share the capacity to disrupt the interaction between transcription factor E2F and the retinoblastoma gene product. Proc Natl Acad Sci USA 89: 4549–4553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtois-Cox S, Jones SL, Cichowski K (2008) Many roads lead to oncogene-induced senescence. Oncogene 27: 2801–2809 [DOI] [PubMed] [Google Scholar]
- Craig AL, Chrystal JA, Fraser JA, Sphyris N, Lin Y, Harrison BJ, Scott MT, Dornreiter I, Hupp TR (2007) The MDM2 ubiquitination signal in the DNA-binding domain of p53 forms a docking site for calcium calmodulin kinase superfamily members. Mol Cell Biol 27: 3542–3555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T, Saretzki G, Carter NP, Jackson SP (2003) A DNA damage checkpoint response in telomere-initiated senescence. Nature 426: 194–198 [DOI] [PubMed] [Google Scholar]
- Di Micco R, Fumagalli M, Cicalese A, Piccinin S, Gasparini P, Luise C, Schurra C, Garre M, Nuciforo PG, Bensimon A, Maestro R, Pelicci PG, d′Adda di Fagagna F (2006) Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature 444: 638–642 [DOI] [PubMed] [Google Scholar]
- Ghosh JC, Izumida Y, Suzuki K, Kodama S, Watanabe M (2000) Dose-dependent biphasic accumulation of TP53 protein in normal human embryo cells after X irradiation. Radiat Res 153: 305–311 [DOI] [PubMed] [Google Scholar]
- Gire V, Roux P, Wynford-Thomas D, Brondello JM, Dulic V (2004) DNA damage checkpoint kinase Chk2 triggers replicative senescence. EMBO J 23: 2554–2563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgoulis VG, Vassiliou LV, Karakaidos P, Zacharatos P, Kotsinas A, Liloglou T, Venere M, Ditullio RA Jr, Kastrinakis NG, Levy B, Kletsas D, Yoneta A, Herlyn M, Kittas C, Halazonetis TD (2005) Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature 434: 907–913 [DOI] [PubMed] [Google Scholar]
- Hirao A, Cheung A, Duncan G, Girard PM, Elia AJ, Wakeham A, Okada H, Sarkissian T, Wong JA, Sakai T, De Stanchina E, Bristow RG, Suda T, Lowe SW, Jeggo PA, Elledge SJ, Mak TW (2002) Chk2 is a tumor suppressor that regulates apoptosis in both an ataxia telangiectasia mutated (ATM)-dependent and an ATM-independent manner. Mol Cell Biol 22: 6521–6532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirao A, Kong YY, Matsuoka S, Wakeham A, Ruland J, Yoshida H, Liu D, Elledge SJ, Mak TW (2000) DNA damage-induced activation of p53 by the checkpoint kinase Chk2. Science 287: 1824–1827 [DOI] [PubMed] [Google Scholar]
- Jallepalli PV, Lengauer C, Vogelstein B, Bunz F (2003) The Chk2 tumor suppressor is not required for p53 responses in human cancer cells. J Biol Chem 278: 20475–20479 [DOI] [PubMed] [Google Scholar]
- Jazayeri A, Falck J, Lukas C, Bartek J, Smith GC, Lukas J, Jackson SP (2006) ATM- and cell cycle-dependent regulation of ATR in response to DNA double-strand breaks. Nat Cell Biol 8: 37–45 [DOI] [PubMed] [Google Scholar]
- Jin J, Ang XL, Ye X, Livingstone M, Harper JW (2008) Differential roles for checkpoint kinases in DNA damage-dependent degradation of the Cdc25A protein phosphatase. J Biol Chem 283: 19322–19328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko YS, Watanabe N, Morisaki H, Akita H, Fujimoto A, Tominaga K, Terasawa M, Tachibana A, Ikeda K, Nakanishi M (1999) Cell-cycle-dependent and ATM-independent expression of human Chk1 kinase. Oncogene 18: 3673–3681 [DOI] [PubMed] [Google Scholar]
- Lam MH, Liu Q, Elledge SJ, Rosen JM (2004) Chk1 is haploinsufficient for multiple functions critical to tumor suppression. Cancer Cell 6: 45–59 [DOI] [PubMed] [Google Scholar]
- Lee JS, Collins KM, Brown AL, Lee CH, Chung JH (2000) hCds1-mediated phosphorylation of BRCA1 regulates the DNA damage response. Nature 404: 201–204 [DOI] [PubMed] [Google Scholar]
- Liu Q, Guntuku S, Cui XS, Matsuoka S, Cortez D, Tamai K, Luo G, Carattini-Rivera S, DeMayo F, Bradley A, Donehower LA, Elledge SJ (2000) Chk1 is an essential kinase that is regulated by Atr and required for the G(2)/M DNA damage checkpoint. Genes Dev 14: 1448–1459 [PMC free article] [PubMed] [Google Scholar]
- Lowe SW, Cepero E, Evan G (2004) Intrinsic tumour suppression. Nature 432: 307–315 [DOI] [PubMed] [Google Scholar]
- Maclaine NJ, Hupp TR (2009) The regulation of p53 by phosphorylation: a model for how distinct signals integrate into the p53 pathway. Aging (Albany NY) 1: 490–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLaine NJ, Oster B, Bundgaard B, Fraser JA, Buckner C, Lazo PA, Meek DW, Hollsberg P, Hupp TR (2008) A central role for CK1 in catalyzing phosphorylation of the p53 transactivation domain at serine 20 after HHV-6B viral infection. J Biol Chem 283: 28563–28573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson D, Kim J, Kim T, Rhee BK, Van Oostrom CT, DiTullio RA, Venere M, Halazonetis TD, Bronson R, De Vries A, Fleming M, Jacks T (2004) Defective apoptosis and B-cell lymphomas in mice with p53 point mutation at Ser 23. EMBO J 23: 3689–3699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallette FA, Gaumont-Leclerc MF, Ferbeyre G (2007) The DNA damage signaling pathway is a critical mediator of oncogene-induced senescence. Genes Dev 21: 43–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka S, Huang M, Elledge SJ (1998) Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science 282: 1893–1897 [DOI] [PubMed] [Google Scholar]
- McPherson JP, Lemmers B, Hirao A, Hakem A, Abraham J, Migon E, Matysiak-Zablocki E, Tamblyn L, Sanchez-Sweatman O, Khokha R, Squire J, Hande MP, Mak TW, Hakem R (2004) Collaboration of Brca1 and Chk2 in tumorigenesis. Genes Dev 18: 1144–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijers-Heijboer H, van den Ouweland A, Klijn J, Wasielewski M, de Snoo A, Oldenburg R, Hollestelle A, Houben M, Crepin E, van Veghel-Plandsoen M, Elstrodt F, van Duijn C, Bartels C, Meijers C, Schutte M, McGuffog L, Thompson D, Easton D, Sodha N, Seal S et al. (2002) Low-penetrance susceptibility to breast cancer due to CHEK2(*)1100delC in noncarriers of BRCA1 or BRCA2 mutations. Nat Genet 31: 55–59 [DOI] [PubMed] [Google Scholar]
- Niida H, Katsuno Y, Banerjee B, Hande MP, Nakanishi M (2007) Specific role of Chk1 phosphorylations in cell survival and checkpoint activation. Mol Cell Biol 27: 2572–2581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niida H, Tsuge S, Katsuno Y, Konishi A, Takeda N, Nakanishi M (2005) Depletion of Chk1 leads to premature activation of Cdc2-cyclin B and mitotic catastrophe. J Biol Chem 280: 39246–39252 [DOI] [PubMed] [Google Scholar]
- Palmero I, Pantoja C, Serrano M (1998) p19ARF links the tumour suppressor p53 to Ras. Nature 395: 125–126 [DOI] [PubMed] [Google Scholar]
- Sanchez Y, Wong C, Thoma RS, Richman R, Wu Z, Piwnica-Worms H, Elledge SJ (1997) Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25. Science 277: 1497–1501 [DOI] [PubMed] [Google Scholar]
- Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM (1990) The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell 63: 1129–1136 [DOI] [PubMed] [Google Scholar]
- Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW (1997) Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88: 593–602 [DOI] [PubMed] [Google Scholar]
- Sharpless NE, Bardeesy N, Lee KH, Carrasco D, Castrillon DH, Aguirre AJ, Wu EA, Horner JW, DePinho RA (2001) Loss of p16Ink4a with retention of p19Arf predisposes mice to tumorigenesis. Nature 413: 86–91 [DOI] [PubMed] [Google Scholar]
- Sherr CJ, DePinho RA (2000) Cellular senescence: mitotic clock or culture shock? Cell 102: 407–410 [DOI] [PubMed] [Google Scholar]
- Shieh SY, Ahn J, Tamai K, Taya Y, Prives C (2000) The human homologs of checkpoint kinases Chk1 and Cds1 (Chk2) phosphorylate p53 at multiple DNA damage-inducible sites. Genes Dev 14: 289–300 [PMC free article] [PubMed] [Google Scholar]
- Shimada M, Nakanishi M (2008) Checkpoints meet the transcription at a novel histone milestone (H3-T11). Cell Cycle 7: 1555–1559 [DOI] [PubMed] [Google Scholar]
- Shimada M, Niida H, Zineldeen DH, Tagami H, Tanaka M, Saito H, Nakanishi M (2008) Chk1 is a histone H3 threonine 11 kinase that regulates DNA damage-induced transcriptional repression. Cell 132: 221–232 [DOI] [PubMed] [Google Scholar]
- Smits VA, Reaper PM, Jackson SP (2006) Rapid PIKK-dependent release of Chk1 from chromatin promotes the DNA-damage checkpoint response. Curr Biol 16: 150–159 [DOI] [PubMed] [Google Scholar]
- Sorensen CS, Hansen LT, Dziegielewski J, Syljuasen RG, Lundin C, Bartek J, Helleday T (2005) The cell-cycle checkpoint kinase Chk1 is required for mammalian homologous recombination repair. Nat Cell Biol 7: 195–201 [DOI] [PubMed] [Google Scholar]
- Stracker TH, Couto SS, Cordon-Cardo C, Matos T, Petrini JH (2008) Chk2 suppresses the oncogenic potential of DNA replication-associated DNA damage. Mol Cell 31: 21–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syljuasen RG, Sorensen CS, Hansen LT, Fugger K, Lundin C, Johansson F, Helleday T, Sehested M, Lukas J, Bartek J (2005) Inhibition of human Chk1 causes increased initiation of DNA replication, phosphorylation of ATR targets, and DNA breakage. Mol Cell Biol 25: 3553–3562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai H, Naka K, Okada Y, Watanabe M, Harada N, Saito S, Anderson CW, Appella E, Nakanishi M, Suzuki H, Nagashima K, Sawa H, Ikeda K, Motoyama N (2002) Chk2-deficient mice exhibit radioresistance and defective p53-mediated transcription. EMBO J 21: 5195–5205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai H, Tominaga K, Motoyama N, Minamishima YA, Nagahama H, Tsukiyama T, Ikeda K, Nakayama K, Nakanishi M (2000) Aberrant cell cycle checkpoint function and early embryonic death in Chk1(-/-) mice. Genes Dev 14: 1439–1447 [PMC free article] [PubMed] [Google Scholar]
- Toledo LI, Murga M, Gutierrez-Martinez P, Soria R, Fernandez-Capetillo O (2008) ATR signaling can drive cells into senescence in the absence of DNA breaks. Genes Dev 22: 297–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga K, Morisaki H, Kaneko Y, Fujimoto A, Tanaka T, Ohtsubo M, Hirai M, Okayama H, Ikeda K, Nakanishi M (1999) Role of human Cds1 (Chk2) kinase in DNA damage checkpoint and its regulation by p53. J Biol Chem 274: 31463–31467 [DOI] [PubMed] [Google Scholar]
- Vahteristo P, Bartkova J, Eerola H, Syrjakoski K, Ojala S, Kilpivaara O, Tamminen A, Kononen J, Aittomaki K, Heikkila P, Holli K, Blomqvist C, Bartek J, Kallioniemi OP, Nevanlinna H (2002) A CHEK2 genetic variant contributing to a substantial fraction of familial breast cancer. Am J Hum Genet 71: 432–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmark H, Kwak S, Theurkauf WE (2010) A role for Chk2 in DNA damage induced mitotic delays in human colorectal cancer cells. Cell Cycle 9: 312–320 [DOI] [PubMed] [Google Scholar]
- Yu Q, Rose JH, Zhang H, Pommier Y (2001) Antisense inhibition of Chk2/hCds1 expression attenuates DNA damage-induced S and G2 checkpoints and enhances apoptotic activity in HEK-293 cells. FEBS Lett 505: 7–12 [DOI] [PubMed] [Google Scholar]
- Zaugg K, Su YW, Reilly PT, Moolani Y, Cheung CC, Hakem R, Hirao A, Liu Q, Elledge SJ, Mak TW (2007) Cross-talk between Chk1 and Chk2 in double-mutant thymocytes. Proc Natl Acad Sci USA 104: 3805–3810 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.