Autocatalytic differentiation of epigenetic modifications within the Arabidopsis genome
The mechanisms by which heterochromatic modifications are excluded from active genes are not well understood. This study demonstrates that H3K9 demethylation by IBM1 depends on the transcription of target genes, preventing them from accumulating silent epigenetic marks.
Keywords: chromatin, DNA methylation, histone demethylase, IBM1, transposon
Abstract
In diverse eukaryotes, constitutively silent sequences, such as transposons and repeats, are marked by methylation at histone H3 lysine 9 (H3K9me). Although selective H3K9me is critical for maintaining genome integrity, mechanisms to exclude H3K9me from active genes remain largely unexplored. Here, we show in Arabidopsis that the exclusion depends on a histone demethylase gene, IBM1 (increase in BONSAI methylation). Loss-of-function ibm1 mutation results in ectopic H3K9me and non-CG methylation in thousands of genes. The ibm1-induced genic H3K9me depends on both histone methylase KYP/SUVH4 and DNA methylase CMT3, suggesting interdependence of two epigenetic marks—H3K9me and non-CG methylation. Notably, IBM1 enhances loss of H3K9me in transcriptionally de-repressed sequences. Furthermore, disruption of transcription in genes induces ectopic non-CG methylation, which mimics the loss of IBM1 function. We propose that active chromatin is stabilized by an autocatalytic loop of transcription and H3K9 demethylation. This process counteracts a similarly autocatalytic accumulation of silent epigenetic marks, H3K9me and non-CG methylation.
Introduction
Chromatin modifications, such as methylation of histone H3 at lysine 9 (H3K9me) and DNA methylation at cytosine residues (Bender, 2004; Chan et al, 2005; Grewal and Elgin, 2007), are important for constitutively silencing transposons (Yoder et al, 1997; Walsh et al, 1998; Miura et al, 2001; Lippman et al, 2004; Ding et al, 2007; Mirouze et al, 2009; Tsukahara et al, 2009). On the other hand, these modifications are normally excluded from active genes to ensure their appropriate expression. Mechanisms for the exclusion, however, remain largely unexplored.
In Arabidopsis, methylation in genes is found only at CG sites, although transposons are methylated at all contexts of cytosines, CG, CHG, and CHH (H can be A, C, or T) (Zhang et al, 2006; Zilberman et al, 2007; Cokus et al, 2008; Lister et al, 2008). The CHG methylation in transposons is associated with dimethylation of H3K9 (H3K9me2) (Bernatavichute et al, 2008). H3K9me2 is catalysed by multiple histone methyltransferases, KRYPTONITE/SUVH4 (hereafter KYP), SUVH5, and SUVH6 (Jackson et al, 2002; Malagnac et al, 2002; Ebbs et al, 2005; Ebbs and Bender, 2006). These histone methyltransferases act upstream of DNA methyltransferase CHROMOMETHYLASE3 (CMT3), which catalyses DNA methylation at CHG sites (Bartee et al, 2001; Lindroth et al, 2001; Chan et al, 2005).
In addition to the positive regulators of DNA methylation, negative regulators have been recently identified (Agius et al, 2006; Gehring et al, 2006; Saze et al, 2008). One of the negative regulators of DNA methylation is IBM1 (INCREASE IN BONSAI METHYLATION 1); loss-of-function mutation in the IBM1 gene induces ectopic DNA methylation at non-CG sites (mainly at CHG sites) in thousands of genes, whereas transposons are unaffected (Saze et al, 2008; Miura et al, 2009). Interestingly, the ibm1-induced genic DNA methylation occurs across each individual transcriptional unit (Miura et al, 2009). It is not known how a transcriptional unit can be recognized by the machinery that excludes the transposon-specific modification. The IBM1 protein has a jumonji-C (jmjC) domain, and it is a member of the JHDM2/KDM3 family, which contains H3K9 demethylases (Klose et al, 2006; Lu et al, 2008; Sun and Zhou, 2008; Liu et al, 2010).
To understand the mechanisms that establish differential epigenetic modifications between active genes and silent transposons, we examined genome-wide pattern of H3K9me2 using mutants for IBM1, KYP, and CMT3. The ibm1 mutation induced accumulation of H3K9me2 in thousands of genes, which parallels the increases in DNA methylation. Although IBM1 generally affected H3K9me2 of genes, transposons could also be affected, once they were activated by kyp or cmt3 mutation, suggesting that transcription is an important prerequisite for the IBM1 function. Furthermore, blockage of transcription by T-DNA insertion in the target genes of IBM1 caused ectopic CHG methylation in the body of these genes, which mimics the effect of the loss of IBM1 function. These results lead us to propose that transcription is critical for IBM1 to remove H3K9me2, which mediates stabilization of active chromatin by the autocatalytic loop.
Results
IBM1 is necessary for excluding H3K9me2 from genes
To understand how genomic H3K9me2 patterns are generated, we examined the function of the putative H3K9 demethylase, IBM1, as well as the H3K9 methylase KYP and the CHG methylase CMT3. First, we examined demethylase activity of IBM1 in vivo by overexpressing FLAG-tagged IBM1 in Nicotiana benthamiana leaf. Double immunostaining of nuclei with antibodies against FLAG and methylated histone at different residues shows the histone methylation status at each residue in FLAG-IBM1-overexpressing cells. In the FLAG-stained cells, H3K9me2 was hardly detectable and the H3K9me1 signal was also much reduced compared with that of the cells without FLAG labelling (Figure 1A; Supplementary Figure S1A). The effects on H3K9me2 and H3K9me1 levels were abolished when two amino-acid residues are substituted (H679A and D681A; referred to as FLAG-IBM1m) in the conserved Fe(II) binding site of the IBM1 protein (Figure 1B; Supplementary Figure S1B).
Figure 1.
IBM1 downregulates H3K9me2 at genes. (A, B) Overexpression of FLAG-IBM1 in tobacco leaf cells induced reduction in H3K9me2 and H3K9me1. Nuclei transfected with FLAG-IBM1 (A) or a mutated construct, FLAG-IBM1m (B), were mixed with control nuclei without the transfection (see Materials and methods for details). The transfected nuclei and control nuclei could be distinguished by immunostaining with anti-FLAG (shown in red). Histone methylation was visualized by immunostaining with rabbit polyclonal methylation-specific antibodies (green). Nuclei were visualized by DAPI staining (blue). Arrows indicate nuclei transfected with FLAG-IBM1 or FLAG-IBM1m. Quantification of these signals, together with those from other samples, is shown in Supplementary Figure S1A and B. (C, D) Representative examples of H3K9me2 analysed by tilling array for a gene-rich region (C, position 20 829 000–20 934 000 on chromosome 1) and a transposon-rich region (D, positions 16 494 000–16 592 000 on chromosome 1). Profile of DNA methylation (Miura et al, 2009) is also shown. Blue and red boxes indicate genes and transposons, respectively. They are oriented 5′–3′ on the top and 3′–5′ on the bottom. Each vertical bar represents the log2 ratio of the immunoprecipitated DNA to input control for the DNA methylation (DNAme) and H3K9me2, respectively. Each brown bar represents difference of signals for each comparison. (E) H3K9me2 level in each gene (blue dot) and transposon (red dot), compared between wild type and ibm1. (F) Comparison between the effect of ibm1 mutation on DNA methylation (DNAme) and H3K9me2 in each gene (blue dot) and transposon (red dot). The changes in DNA methylation and H3K9me2 levels were not proportional, most likely because each gene differs in the CG methylation level, which is not affected by the ibm1 mutation (Miura et al, 2009).
H3K9me3 and the three types of methylation at each of other sites, H3K4, H3K27, and H3K36, were indistinguishable between cells with or without the FLAG-IBM1 signal (Supplementary Figure S1C–F). Among the 12 modifications examined, H3K9me2 and H3K9me1 were the only modifications affected by the FLAG-IBM1. These results support the prediction that IBM1 is a histone demethylase specific for H3K9me2 and H3K9me1.
We then examined H3K9me2 genome-wide in wild-type Columbia-0 (Col-0) and ibm1 mutant samples by chromatin immunoprecipitation (ChIP) coupled with DNA microarray analysis (ChIP-chip). In order to minimize cross-reacting signals from other modifications, we used a monoclonal antibody specific for H3K9me2 (Kimura et al, 2008). In wild type, H3K9me2 level was high in transposons and repeats, but it was very low in annotated expressed genes (Figure 1C and D; Supplementary Figure S2). The ibm1 mutation caused ectopic H3K9me2 accumulation in transcribed regions of a large number of genes (Figure 1C–E). Essentially the same set of genes was affected in two biological replicates (Supplementary Figures S3). On the other hand, transposons were unaffected by the ibm1 mutation (Figure 1D and E). The ibm1-induced genic H3K9me2 is accompanied by DNA methylation changes (Figure 1F).
As is the case in the ibm1-induced DNA methylation in genes, the ibm1-induced H3K9me2 showed specific characteristics in relation to the transcription units (Miura et al, 2009); long genes and constitutively transcribed genes were affected severely (Supplementary Figure S4A–E), and central region of a transcription unit showed more signal than the 5′ and 3′ regions (Supplementary Figure S5A–C).
The kyp and cmt3 mutations partially abolish H3K9me2 in transposons
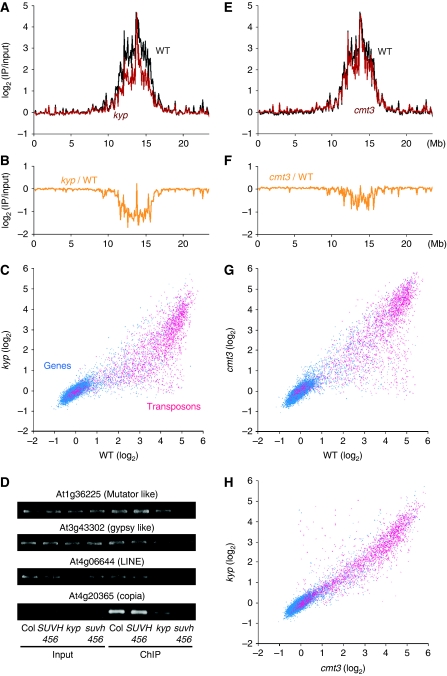
In addition to the negative regulator of H3K9me2, we examined the contribution of positive regulators, KYP and CMT3. Compared with wild-type, kyp mutant samples showed reduced H3K9me2 levels in many transposons both in pericentromeric regions and arm regions, although significant levels of H3K9me2 still remained (Figure 2A–C; Supplementary Figure S5D). Other methyltransferase(s) such as SUVH5 and SUVH6 may contribute to the remaining H3K9me2 in transposons and repeats, as has been suggested previously (Ebbs and Bender, 2006). Consistent with the proposed functional redundancy, the H3K9me2 levels were reduced in the triple mutant for KYP, SUVH5, and SUVH6 (suvh456) compared with the single mutant kyp (Figure 2D).
Figure 2.
Effects of kyp and cmt3 mutations on H3K9me2 level. (A, B) Global view of H3K9me2 patterns along the chromosome 3, compared between wild type and kyp. Average signals in each 100 kb regions (A) or the differences of the signals between wild type and kyp (B) were plotted. (C) H3K9me2 levels in genes (blue dots) and transposons (red dots), compared between wild type and kyp. (D) H3K9me2 levels in wild type (Col), wild-type sibling of suvh456 (SUVH456), kyp, and suvh456 triple mutant examined with ChIP-PCR. Amplifications from input control and ChIP DNA are shown. (E, F) Global view of H3K9me2 patterns in wild type and cmt3 (E) or the difference in H3K9me2 between wild type and cmt3 (F). (G, H) H3K9me2 levels in genes (blue dots) and transposons (red dots), compared between wild type and cmt3 (G) or cmt3 and kyp (H).
The cmt3 mutant also showed some reduction of H3K9me2 in heterochromatic regions, although the effect was generally less than that seen in kyp samples (Figure 2E–H; Supplementary Figure S5D). The dependence of H3K9me2 on CMT3 function suggests a function for DNA methylation in maintaining H3K9me2 in transposons. We then examined the relationships between the levels of DNA methylation at different contexts and the H3K9me2 levels in wild-type, kyp, and cmt3 samples. Consistent with previous report (Bernatavichute et al, 2008), the frequency of methylated CHG per length ([mCHG]) was positively correlated with H3K9me2 level in transposons (Supplementary Figure S6A). The kyp and cmt3 mutant samples showed a greater decrease in H3K9me2 at transposons with higher [mCHG] (Supplementary Figure S6C, E, G, and H), suggesting that CHG methylation is indeed required for a high level of H3K9me2 at transposons. Interestingly, the decreases of H3K9me2 in kyp and cmt3 mutants were more pronounced at transposons with lower [mCG] than those with higher [mCG] (Supplementary Figure S6B, D, F, I, and J). The results suggest that an additional pathway, which depends on CG methylation, may also be involved in the H3K9me2 of transposons (Soppe et al, 2002; Tariq et al, 2003).
The kyp and cmt3 mutations fully suppress the ibm1-induced H3K9me2
Although the kyp mutation induced only a partial reduction of H3K9me2 in transposons (Figure 2A–C), this mutation suppressed the developmental phenotypes induced by the ibm1 mutation (Saze et al, 2008), which are probably due to ectopic H3K9me2 and/or CHG methylation in genic regions. We therefore examined the effects of the ibm1 mutation in the kyp mutant background. Notably, in the kyp mutant background, ibm1-induced genic H3K9me2 was lost almost completely (Figure 3A and B; Supplementary Figure S7). This result is in striking contrast to the effect of the kyp mutation on H3K9me2 in transposons, which is only partial (Figure 2A–C). Taken together, the results suggest that different H3K9 methyltransferases function in genes and transposons: only KYP in genes and KYP and others in transposons.
Figure 3.
The ibm1-induced genic H3K9me2 depends on KYP and CMT3. (A) and (D) are from the data shown in Figure 1E. Effect of the ibm1 mutation on H3K9me2 levels was also examined in the background of kyp (B, E) or cmt3 (C, F) mutant. Genes (A–C) and transposons (D–F) are separately shown.
Surprisingly, the cmt3 mutation also suppressed the ibm1-induced genic H3K9me2 almost completely (Figure 3C; Supplementary Figure S7). This result suggests that CMT3-mediated CHG methylation is required for the increase of H3K9me2 at genes in the ibm1 mutant. On the other hand, the impact of the cmt3 mutation on H3K9me2 in transposons was only subtle (Figure 2E–G).
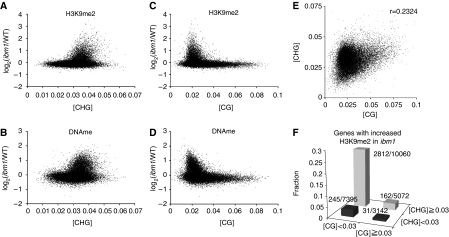
To test whether CHG methylation, rather than an unidentified function of CMT3, mediates its effect on H3K9me2 induction in ibm1, we calculated the proportion of CHG sites (total number of CHG sites in transcribed region per length of annotation unit; hereafter [CHG]) for all annotated transcription units and examined the relationship of the [CHG] with the effect of the ibm1 mutation on DNA methylation and H3K9me2. [CHG] strongly correlated with the degree of response to the ibm1 mutation not only in DNA methylation, but also in H3K9me2 (Figure 4A and B). Together with the result of ibm1 cmt3 double mutant, these results suggest that CMT3-mediated CHG methylation is required for the induction of genic H3K9me2 in ibm1.
Figure 4.
[CHG] and [CG] correlate with the effect of ibm1 on H3K9me2. (A, B) Relationships between [CHG] and the ibm1-induced changes in H3K9me2 (A) or DNA methylation (B) level in each gene. (C, D) Relationships between [CG] and the ibm1-induced changes in H3K9me2 (C) or DNA methylation (D) level in each gene. The effects of [CHG] and [CG] are independent of the effect of gene length (Supplementary Figure S8A–D). (E) Positive correlation between [CHG] and [CG]. (F) The proportion of genes with increased H3K9me2 in ibm1 (Supplementary Figure S3A), within four gene groups with high and low [CHG] and [CG]. Actual gene numbers (ibm1 affected/all) in each group are also indicated.
Unexpectedly, we also found that [CG] of genes negatively correlated with the induction of H3K9me2 and DNA methylation in the ibm1 mutant (Figure 4C and D). Despite the opposite effects, [CHG] and [CG] show a weak positive correlation (Figure 4E), suggesting that [CHG] and [CG] independently affect H3K9me2 and DNA methylation. Genes with higher [CHG] and lower [CG] were most strongly affected by the ibm1 mutation (Figure 4F).
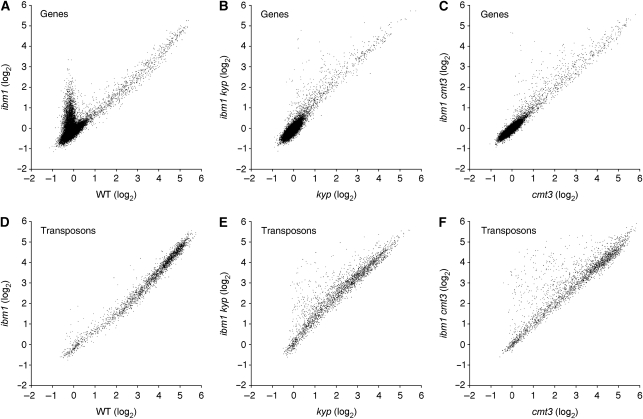
IBM1 affects transposons de-repressed in kyp or cmt3 mutant backgrounds
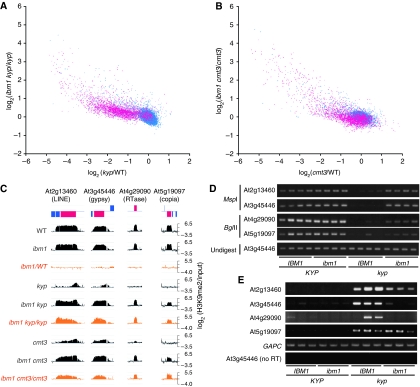
In kyp or cmt3 mutant backgrounds, the ibm1 mutation did not affect H3K9me2 in genes (Figure 3A–C). However, a significant number of transposons, which were not affected by the ibm1 single mutation (Figure 3D), responded to the ibm1 mutation in those backgrounds (Figure 3E and F). These transposons showed a decrease of H3K9me2 in kyp and cmt3 single mutants that was recovered in the ibm1 kyp and ibm1 cmt3 double mutants (Figure 5A–C). Not only H3K9me2, but also CHG methylation was recovered in the ibm1 kyp double mutant for these transposons (Figure 5D). These results suggest that a subset of transposons can be targets of IBM1 in a kyp or cmt3 background.
Figure 5.
De-repressed transposons can be targets of IBM1. (A) Scatter plot showing the relationship between changes in H3K9me2 from wild type to kyp (x axis) and from kyp to ibm1 kyp (y axis). (B) Scatter plot showing the relationship between changes in H3K9me2 from wild type to cmt3 (x axis) and from cmt3 to ibm1 cmt3 (y axis). Genes are shown in blue and transposons in red. (C) ChIP-chip result of four transposons showing decrease in H3K9me2 in kyp and cmt3, and restoration in ibm1 kyp and ibm1 cmt3 double mutants. (D) DNA methylation at CHG sites analysed by digestion with methylation-sensitive restriction enzymes MspI and BglII and subsequent PCR (details in Materials and methods). Homozygous plants segregating in self-pollinated progeny of a double heterozygote (IBM1/ibm1 KYP/kyp) were used for analyses. Compared with the kyp IBM1 plants with the hypomethylated transposons, the kyp ibm1 plants showed increased signal reflecting robust methylation. The sibling wild-type KYP plants showed high-level methylation irrespective of the genotypes of the IBM1 gene. (E) RT–PCR analysis for the expression of the four transposons in the wild type, ibm1, kyp, and kyp ibm1 double mutant. Total RNA isolated from individual plants was used for each RT–PCR analysis. GAPC gene was used to normalize RNA template amounts. Negative control (no RT) lacked reverse transcriptase. We also examined expression pattern of transposons in kyp and cmt3 mutants genome wide (Supplementary Figure S9). Other transposons de-repressed by kyp or cmt3 mutation also showed increased H3K9me2 in the ibm1 mutant.
One possible mechanism for this observation is that even transposons can be targets of IBM1 when they are transcribed. Indeed, these transposons were transcribed in the kyp or cmt3 single mutant (Figure 5E; Supplementary Figure S9). Even though the transcriptional activation tended to be weak in the absence of IBM1 function (ibm1 kyp in Figure 5E), robust activation could be achieved when IBM1 functions (IBM1 kyp in Figure 5E). IBM1 apparently stabilizes the transcriptionally active state, most likely by active H3K9 demethylation.
T-DNA insertion induces non-CG methylation within IBM1 target genes
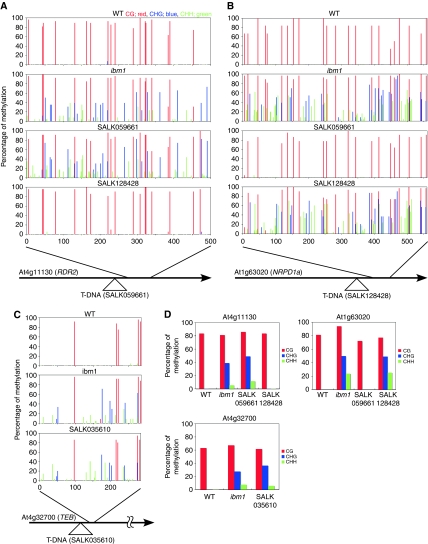
These observations suggest that transcription of the target gene is necessary for IBM1 function. In order to test this prediction directly, we examined the effect of blocking transcription by T-DNA insertion. DNA methylation was examined in T-DNA insertion alleles for three of the IBM1 target genes (At4g11130, At1g63020, and At4g32700). In all of them, non-CG (mostly CHG) methylation was induced, a situation which is analogous to the effects of the ibm1 mutation (Figure 6). This observation also supports the prediction that IBM1 function depends on transcription of the target gene.
Figure 6.
Bisulphite sequencing analysis of cytosine methylation at T-DNA-inserted genes. (A–C) Schematic representations of At4g11130 (A), At1g63020 (B), and At4g32700 (C) loci and cytosine methylation level analysed by bisulphite sequencing. The percentage of methylated cytosines is indicated by vertical bar for each site (red, CG; blue, CHG; green, CHH). T-DNA insertion mutants showed decreases of expressions (Supplementary Figure S10E), and increases of non-CG methylation, as in ibm1 mutant, at downstream regions of T-DNA inserted genes. Mutants for the genes in same pathway (RDR2 and NRPD1a) did not affect the DNA methylation at reciprocal gene. (D) Proportions of methylated cytosines at different contexts in regions shown in (A–C).
In addition to the region downstream of the T-DNA insertion (Figure 6), the upstream regions sometimes, but not always, showed increased non-CG methylation (Supplementary Figure S10), suggesting that function of IBM1 might also be affected by the insertion downstream.
Discussion
Differentiation of active and inactive chromatin by transcription and histone demethylation
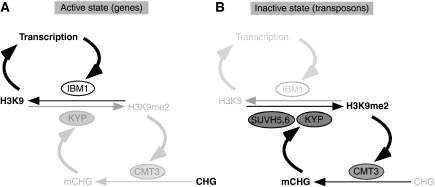
In the absence of IBM1 function, thousands of genes accumulated epigenetic marks of silent chromatin, such as H3K9me2 and cytosine methylation at non-CG sites. The IBM1 protein is necessary for excluding these marks from transcribed genes. Although transposons were generally not affected by the ibm1 mutation (Figure 1; Miura et al, 2009), de-repressed transposons behaved like genes in regard to their response to ibm1 (Figure 5; Supplementary Figure S9). Conversely, loss of transcription by T-DNA insertion in the target gene of IBM1 caused induction of non-CG methylation (Figure 6). These results lead us to propose that IBM1 demethylates H3K9me2 from transcribed genes. This would stabilize the expressed state of active genes by preventing accumulation of H3K9me2 (Figure 7A).
Figure 7.
Proposing model for differential epigenetic modifications between genes and transposons. (A) IBM1 demethylates H3K9 of transcribed genes, which in turn ensure robust transcription, generating the autocatalytic loop. In the absence of IBM1 function, H3K9me2 and mCHG accumulate even in transcribed genes by the self-reinforcing mechanism of KYP and CMT3 (grey arrows). (B) Even in wild type, the self-reinforcing loop of KYP and CMT3 operates in transposons. In transposons, additional backup of H3K9me2 machinery exists that depends on SUVH5 and/or SUVH6. When de-repressed, the autocatalytic loop of transcription and H3K9 demethylation runs even in transposons (grey arrows). The genes and transposons differ in that (i) IBM1 functions only in transcribed genes and that (ii) the KYP alone can function in genes for the methylation of H3K9, but additional SUVH proteins operate in transposons.
How could transcription affect IBM1 function? The IBM1 protein may be recruited to transcriptional machinery, as H3K4 and H3K36 methyltransferases are (Li et al, 2002; Krogan et al, 2003; Ng et al, 2003). If so, this recruitment might account for the long-standing enigmatic observations in the mutant of CG methylase gene MET1. The met1 mutation induces not only global loss of CG methylation and de-repression of transposons, but also ectopic methylation at non-CG sites in a subset of genes (Jacobsen and Meyerowitz, 1997; Cokus et al, 2008; Lister et al, 2008). This might be due to depletion of the IBM1 protein in genes, which is caused by de-repression of transposons and recruitment of IBM1 protein there. Consistent with this interpretation, genes showing ectopic CHG methylation in ibm1 and met1 largely overlap (Miura et al, 2009).
Interplay between DNA methylation and H3K9me2
The ibm1-induced genic H3K9me2 required CMT3, the DNA methylase for CHG sites (Figure 3C). How does CHG methylation affect H3K9me2? One proposed mechanism posits the direct recognition of DNA methylation by the histone methyltransferase; SRA domains within KYP and other SUVH proteins were recently shown to have methylcytosine-binding activity (Unoki et al, 2004; Johnson et al, 2007; Woo et al, 2007). Interestingly, the SRA domain of KYP binds preferentially to methylated CHG and CHH than methylated CG in vitro (Johnson et al, 2007). If KYP recognizes only CHG and CHH methylation in vivo, it could explain why the cmt3 mutation alone fully suppresses the ibm1-induced H3K9me2 despite the presence of CG methylation. All observations are consistent with the model that genic H3K9me2 and CHG methylation are established by the self-reinforcing mechanism (Figure 7).
Unlike the ibm1-induced genic H3K9me2, the H3K9me2 in transposons was not much affected by the cmt3 mutation (Figure 2E–G). Cytosine methylation at context(s) other than CHG may also function upstream of H3K9me2. Interestingly, cmt3 mutation affects H3K9me2 at transposons with low CG methylation (Supplementary Figure S6G–J). This observation is consistent with the interpretation that the remaining H3K9me2 in transposons depends on CG methylation. In fact, mutation in the CG methylase gene MET1 induces a reduction of H3K9me in transposon-rich regions (Soppe et al, 2002; Tariq et al, 2003). Furthermore, siRNA-guided DNA methylation also occurs in transposon sequences (Matzke et al, 2009), which can be excluded from genes by DNA demethylases (Penterman et al, 2007; Zhu et al, 2007). Taken together, H3K9me of transposons seems to be targeted by multiple pathways, resulting in robust maintenance of heterochromatin marks.
Effect of [CHG] on the ibm1-induced H3K9me2 could be understood by the contribution of methylated CHG; a puzzling observation is that [CG] had a strong reverse effect (Figure 4C and D). This could be explained, at least in part, by underrepresentation of CG sites by high mutation rate of methylated cytosine (Tran et al, 2005). In fact, [CG] level was lower than that expected from [C] and [G] in genes with high CG methylation rate (Supplementary Figure S8E), and those methylated genes tend to respond more to the ibm1 mutation (Supplementary Figure S8F). In addition to this indirect effect, it would be interesting if unmethylated CG sites directly inhibit H3K9me2 or CHG methylation in genes (Supplementary Figure S8G–I). If such interaction exists, then that would also affect the differential methylation pattern within the genome.
Control of epigenetic marks in the body of transcribed gene
We are proposing that IBM1 negatively regulates accumulation of H3K9me2 in transcribed genes. On the other hand, in the absence of the IBM1 function, constitutively transcribed genes tend to have more H3K9me2 and CHG methylation than genes expressed in a tissue-specific manner or expressed at very low level (Supplementary Figure S4B–E; Miura et al, 2009). Exactly the same trend is known for genic methylation at CG sites in wild-type background (Zhang et al, 2006; Zilberman et al, 2007). The strikingly similar spectra suggest that genic CG methylation might be mechanically connected to the CHG methylation, detected in the absence of IBM function. However, CHG methylation in the ibm1 mutant can be found even in genes lacking CG methylation (Miura et al, 2009). In addition, mutation in the CG methylase gene MET1 did not suppress the ibm1-induced CHG hypermethylation (SI and TK, unpublished), suggesting that CG methylation is not upstream of the genic CHG methylation. On the other hand, it is tempting to speculate the reverse causative relationship; the component(s) of the self-reinforcing loop of H3K9me2 and CHG methylation may trigger the genic CG methylation. Consistent with that model, the genic methylation level at CG sites positively correlated with [CHG] (proportion of CHG sites, irrespective of methylated or not; data not shown). Once induced, CG methylation can be epigenetically inherited over generations by the maintenance methylation (Saze et al, 2003; Takeda and Paszkowski, 2006).
As in Arabidopsis, recent genome-wide analyses of DNA methylation in the human genome revealed that the methylation is found in body of genes with high transcription activity (Lister et al, 2009). Furthermore, in human ES cells and iPS cells, the hypermethylation of transcribed genes was also found at non-CG sites, which disappears during differentiation (Lister et al, 2009). The correlation with the transcription is much stronger for methylation at non-CG sites than at CG sites (Lister et al, 2009). Despite its prevalence in diverse eukaryotes, the underlying mechanisms remain enigmatic for the body methylation of constitutively transcribed genes (Zemach et al, 2010). Arabidopsis would further serve as a model organism to understand mechanisms for the interactions between DNA methylation (CG and non-CG sites) and histone modifications/replacements, which can also be affected by transcription (Zilberman et al, 2008; Bell and Schübeler, 2009).
Materials and methods
Plant materials
Arabidopsis thaliana strain Col-0 was used as ‘wild type'. The isolation of ibm1 mutants was described previously (Saze et al, 2008). We used a presumed null allele ibm1–4. cmt3i11 mutant was originally isolated in Wassilewskija background (Bartee et al, 2001), and we crossed that to Col-0 six times before use. The kyp mutant (SAIL588F05) is in Col-0 background and has T-DNA insertion in SET domain-coding region; suvh456 triple mutant was made from kyp-4 (SALK044606), suvh5-2 (Ebbs and Bender, 2006), and suvh6-1 (Ebbs et al, 2005), which are all in Col-0 background. T-DNA insertion mutants for At4g11130, At1g63020, and At4g32700 are SALK059661, SALK128428, and SALK035610, respectively. The SALK lines (Alonso et al, 2003) were obtained from Arabidopsis Biological Resource Center.
In vivo histone demethylation assay
In vivo histone demethylation activity of IBM1 protein was examined by overexpression and subsequent immunostaining (Seward et al, 2007; Lu et al, 2010). Full-length IBM1 genomic-coding sequence with stop codon was cloned into pENTR/D-TOPO vector (Invitrogen) using cx3583: 5′-CACCATGGATTCTGTGGAGGAAGAAG-3′ and cx3585: 5′-CTACATCTTCTCCATTTCTAAT-3′ and then transferred into pEarleyGate202 (pEG202) vector with an N-terminal FLAG tag using LR reaction (Earley et al, 2006). IBM1m has two amino-acid substitutions from IBM1, H679A, and D681A. The mutation construct was performed by Quickchange site-directed mutagenesis kit (Stratagene) using the following primers: cx8060: 5′-AGATTCTGTTACTAAGCTCGCCTGCGCCATGTCTGATGCGGTGAAT-3′ and cx8061: 5′-ATTCACCGCATCAGACATGGCGCAGGCGAGCTTAGTAACAGAATCT-3′. The constructs were transformed into Agrobacterium tumefaciens cells (stain EHA105). Cells were then injected into half of N. benthamiana leaves as described (English et al, 1997). FLAG-tagged IBM1 was overexpressed in half of a 4-week-old tobacco leaf, while using the other half without infiltration as a control. After 72 h post-injection, the whole leaves were harvested for nuclei isolation and immunostaining. Nuclei isolation and immunostaining were carried out as previously described (Houben et al, 2003; Johnson et al, 2002). FLAG M2 antibody from mouse (Sigma) and Histone methylation-specific antibody from rabbit (H3K4me3: Abcam ab8580; H3K4me2: Millipore 07–030; H3K4me1: Millipore 07–436; H3K9me3: Millipore 07–442; H3K9me2: Millipore 07–441; H3K9me1: Millipore 07–450; H3K27me3: gift from Dr Thomas Jenuwein; H3K27me2: Millipore 07–452; H3K27me1: Millipore 07–448; H3K36me3: Abcam ab9050; H3K36me2: Millipore 07–274; H3K36me1: Millipore 07–548) were used for immunoassaying followed by Alexa flour 555-conjugated goat anti-mouse (Invitrogen) and Alexa flour 488-conjugated goat anti-rabbit (Invitrogen). After staining, the slides were mounted by VECTASHIELD mounting medium with DAPI (Vector Laboratory).
ChIP-chip
Leaves of 4-week-old plants were fixed as described previously (Saze et al, 2008). ChIP was performed as described previously (Kimura et al, 2008), using antibody against H3K9me2 (CMA307; Kimura et al, 2008). Non-immunoprecipitated DNA (input DNA) and ChIP samples were amplified, labelled, and hybridized to microarray according to the manufacturer's instruction (protocols for ChIP and amplification, NimbleGen). Input DNA and ChIP DNA were differentially labelled with Cy3 and Cy5, respectively, and competitively hybridized to a microarray chip. We used NimbleGen 2.1M HD2 array covering entire genome of A. thaliana, and 385K array covering entire chromosome 1 and part of chromosome 2. We performed two biological replicate for the H3K9me2 ChIP-chip (two HD2 arrays for wild-type Col-0 and ibm1; one HD2 and one 385K arrays for kyp, ibm1 kyp, cmt3, and ibm1 cmt3). Each feature on the array has a corresponding scaled log2 ratio, which is the ratio of the signals for the ChIP and input samples cohybridized to the array. The log2 ratio is computed and scaled to centre the ratio data around zero. Scaling is performed by subtracting the bi-weight mean for the log2-ratio values for all features on the array from each log2-ratio value. Cytosine methylation profilings shown in Figure 1B and C are on 3 × 385K arrays (Miura et al, 2009), which show minor difference in the probe sets to the HD2 array. The ChIP-chip data, together with the expression results of kyp and cmt3 mutants, are available as SuperSeries GSE23030 in NCBI (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE23030).
Analysis of base composition and DNA methylation
Base compositions, such as [C], [G], [CG], and [CHG], were calculated for each transcription unit. Occurrence of each site was counted for both strands and the number was divided by twice of the length (base) of that region. Frequency of methylated sites, [mCG] and [mCHG], was calculated in a similar manner using the wild-type Col results by Lister et al (2008).
DNA methylation analysis
DNA methylation was analysed using methylation-sensitive restriction enzyme MspI and BglII. A 50 ng of genomic DNA was digested with MspI and PstI, or BglII and EcoRI, in 20 μl reaction mix. After digestion, PCR was performed using 1 μl of the digested sample as a template. Primers used are shown in Supplementary Table S1.
Genomic bisulphite sequencing was performed using BisulFast DNA Modification kit (TOYOBO) from 500 ng of genomic DNA. A one-sixth portion of the bisulphite-treated DNA was used as a template for PCR (50 μl reaction). Purified amplified fragment was cloned into pGEM-T easy vector (Promega) and sequenced.
RT–PCR
Total RNA from leaves was isolated using the RNeasy Plant Mini kit (Qiagen) and was treated with DNase I (TAKARA). cDNA was synthesized using the SuperScript III Reverse Transcriptase (Invitrogen) and random hexamer primer. Primers used in RT–PCR are shown in Supplementary Table S1.
Expression microarray
Total RNA from leaves was isolated and treated with DNase I as described above. Double-stranded cDNA was synthesized using the SuperScript Double-Stranded cDNA Synthesis kit (Invitrogen) and oligo-(dT)20 primer. cDNA was labelled with Cy3, and hybridized to NimbleGen 4 × 72k microarray according to the manufacturer's instruction. Data were normalized by RMA method using Nimble Scan software (NimbleGen).
Supplementary Material
Acknowledgments
We thank Akiko Terui for technical assistance, Judith Bender for Arabidopsis seeds, Ryan Lister and Joe Ecker for the cytosine methylation information in the Arabidopsis genome, Thomas Jenuwein for antibody, and Yasushi Hiromi and Eric Richards for critical comments on the paper. This work was supported by grants from Mitsubishi Foundation, Takeda Science Foundation, Japanese Ministry of Education, Culture, Sports, Science and Technology (19207002 and 19060014) (to TK), National Basic Research Program of China (2009CB941500 and 2005CB522400), and National Natural Science Foundation of China (30930048) (to XC).
Footnotes
The authors declare that they have no conflict of interest.
References
- Agius F, Kapoor A, Zhu JK (2006) Role of the Arabidopsis DNA glycosylase/lyase ROS1 in active DNA demethylation. Proc Natl Acad Sci USA 103: 11796–11801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, Gadrinab C, Heller C, Jeske A, Koesema E, Meyers CC, Parker H, Prednis L, Ansari Y, Choy N, Deen H et al. (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 [DOI] [PubMed] [Google Scholar]
- Bartee L, Malagnac F, Bender J (2001) Arabidopsis cmt3 chromomethylase mutations block non-CG methylation and silencing of an endogenous gene. Genes Dev 15: 1753–1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell O, Schübeler D (2009) Chromatin: sub out the replacement. Curr Biol 19: R545–R547 [DOI] [PubMed] [Google Scholar]
- Bender J (2004) DNA methylation and epigenetics. Annu Rev Plant Biol 55: 41–68 [DOI] [PubMed] [Google Scholar]
- Bernatavichute YV, Zhang X, Cokus S, Pellegrini M, Jacobsen SE (2008) Genome-wide association of histone H3 lysine nine methylation with CHG DNA methylation in Arabidopsis thaliana. PLoS One 3: e3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SW, Henderson IR, Jacobsen SE (2005) Gardening the genome: DNA methylation in Arabidopsis thaliana. Nat Rev Genet 6: 351–360 [DOI] [PubMed] [Google Scholar]
- Cokus SJ, Feng S, Zhang X, Chen Z, Merriman B, Haudenschild CD, Pradhan S, Nelson SF, Pellegrini M, Jacobsen SE (2008) Shotgun bisulphite sequencing of the Arabidopsis genome reveals DNA methylation patterning. Nature 452: 215–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Wang X, Su L, Zhai J, Cao S, Zhang D, Liu C, Bi Y, Qian Q, Cheng Z, Chu C, Cao X (2007) SDG714, a histone H3K9 methyltransferase, is involved in Tos17 DNA methylation and transposition in rice. Plant Cell 19: 9–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley KW, Haag JR, Pontes O, Opper K, Juehne T, Song K, Pikaard CS (2006) Gateway-compatible vectors for plant functional genomics and proteomics. Plant J 45: 616–629 [DOI] [PubMed] [Google Scholar]
- Ebbs ML, Bartee L, Bender J (2005) H3 lysine 9 methylation is maintained on a transcribed inverted repeat by combined action of SUVH6 and SUVH4 methyltransferases. Mol Cell Biol 25: 10507–10515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebbs ML, Bender J (2006) Locus-specific control of DNA methylation by the Arabidopsis SUVH5 histone methyltransferase. Plant Cell 18: 1166–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- English JJ, Davenport GF, Elmayan T, Vaucheret H, Baulcombe DC (1997) Requirement of sense transcription for homology-dependent virus resistance and trans-inactivation. Plant J 12: 597–603 [Google Scholar]
- Gehring M, Huh JH, Hsieh TF, Penterman J, Choi Y, Harada JJ, Goldberg RB, Fischer RL (2006) DEMETER DNA glycosylase establishes MEDEA polycomb gene self-imprinting by allele-specific demethylation. Cell 124: 495–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewal SI, Elgin SC (2007) Transcription and RNA interference in the formation of heterochromatin. Nature 447: 399–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houben A, Demidov D, Gernand D, Meister A, Leach CR, Schubert I (2003) Methylation of histone H3 in euchromatin of plant chromosomes depends on basic nuclear DNA content. Plant J 33: 967–973 [DOI] [PubMed] [Google Scholar]
- Jackson JP, Lindroth AM, Cao X, Jacobsen SE (2002) Control of CpNpG DNA methylation by the KRYPTONITE histone H3 methyltransferase. Nature 416: 556–560 [DOI] [PubMed] [Google Scholar]
- Jacobsen SE, Meyerowitz EM (1997) Hypermethylated SUPERMAN epigenetic alleles in arabidopsis. Science 277: 1100–1103 [DOI] [PubMed] [Google Scholar]
- Johnson L, Cao X, Jacobsen S (2002) Interplay between two epigenetic marks: DNA methylation and histone H3 lysine 9 methylation. Curr Biol 12: 1360–1367 [DOI] [PubMed] [Google Scholar]
- Johnson LM, Bostick M, Zhang X, Kraft E, Henderson I, Callis J, Jacobsen SE (2007) The SRA methyl-cytosine-binding domain links DNA and histone methylation. Curr Biol 17: 379–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura H, Hayashi-Takanaka Y, Goto Y, Takizawa N, Nozaki N (2008) The organization of histone H3 modifications as revealed by a panel of specific monoclonal antibodies. Cell Struct Funct 33: 61–73 [DOI] [PubMed] [Google Scholar]
- Klose RJ, Kallin EM, Zhang Y (2006) JmjC-domain-containing proteins and histone demethylation. Nat Rev Genet 7: 715–727 [DOI] [PubMed] [Google Scholar]
- Krogan NJ, Kim M, Tong A, Golshani A, Cagney G, Canadien V, Richards DP, Beattie BK, Emili A, Boone C, Shilatifard A, Buratowski S, Greenblatt J (2003) Methylation of histone H3 by Set2 in Saccharomyces cerevisiae is linked to transcriptional elongation by RNA polymerase II. Mol Cell Biol 23: 4207–4218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Moazed D, Gygi SP (2002) Association of the histone methyltransferase Set2 with RNA polymerase II plays a role in transcription elongation. J Biol Chem 277: 49383–49388 [DOI] [PubMed] [Google Scholar]
- Lindroth AM, Cao X, Jackson JP, Zilberman D, McCallum CM, Henikoff S, Jacobsen SE (2001) Requirement of CHROMOMETHYLASE3 for maintenance of CpXpG methylation. Science 292: 2077–2080 [DOI] [PubMed] [Google Scholar]
- Lippman Z, Gendrel AV, Black M, Vaughn MW, Dedhia N, McCombie WR, Lavine K, Mittal V, May B, Kasschau KD, Carrington JC, Doerge RW, Colot V, Martienssen R (2004) Role of transposable elements in heterochromatin and epigenetic control. Nature 430: 471–476 [DOI] [PubMed] [Google Scholar]
- Lister R, O'Malley RC, Tonti-Filippini J, Gregory BD, Berry CC, Millar AH, Ecker JR (2008) Highly integrated single-base resolution maps of the epigenome in Arabidopsis. Cell 133: 523–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister R, Pelizzola M, Dowen RH, Hawkins RD, Hon G, Tonti-Filippini J, Nery JR, Lee L, Ye Z, Ngo QM, Edsall L, Antosiewicz-Bourget J, Stewart R, Ruotti V, Millar AH, Thomson JA, Ren B, Ecker JR (2009) Human DNA methylomes at base resolution show widespread epigenomic differences. Nature 462: 315–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Lu F, Cui X, Cao X (2010) Histone methylation in higher plants. Annu Rev Plant Biol 61: 395–420 [DOI] [PubMed] [Google Scholar]
- Lu F, Cui X, Zhang S, Liu C, Cao X (2010) JMJ14 is an H3K4 demethylase regulating flowering time in Arabidopsis. Cell Res 20: 387–390 [DOI] [PubMed] [Google Scholar]
- Lu F, Li G, Cui X, Liu C, Wang XJ, Cao X (2008) Comparative analysis of JmjC domain-containing proteins reveals the potential histone demethylases in Arabidopsis and rice. J Integr Plant Biol 50: 886–896 [DOI] [PubMed] [Google Scholar]
- Malagnac F, Bartee L, Bender J (2002) An Arabidopsis SET domain protein required for maintenance but not establishment of DNA methylation. EMBO J 21: 6842–6852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzke M, Kanno T, Daxinger L, Huettel B, Matzke AJ (2009) RNA-mediated chromatin-based silencing in plants. Curr Opin Cell Biol 21: 367–376 [DOI] [PubMed] [Google Scholar]
- Mirouze M, Reinders J, Bucher E, Nishimura T, Schneeberger K, Ossowski S, Cao J, Weigel D, Paszkowski J, Mathieu O (2009) Selective epigenetic control of retrotransposition in Arabidopsis. Nature 461: 427–430 [DOI] [PubMed] [Google Scholar]
- Miura A, Nakamura M, Inagaki S, Kobayashi A, Saze H, Kakutani T (2009) An Arabidopsis jmjC domain protein protects transcribed genes from DNA methylation at CHG sites. EMBO J 28: 1078–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura A, Yonebayashi S, Watanabe K, Toyama T, Shimada H, Kakutani T (2001) Mobilization of transposons by a mutation abolishing full DNA methylation in Arabidopsis. Nature 411: 212–214 [DOI] [PubMed] [Google Scholar]
- Ng HH, Robert F, Young RA, Struhl K (2003) Targeted recruitment of Set1 histone methylase by elongating Pol II provides a localized mark and memory of recent transcriptional activity. Mol Cell 11: 709–719 [DOI] [PubMed] [Google Scholar]
- Penterman J, Uzawa R, Fischer RL (2007) Genetic interactions between DNA demethylation and methylation in Arabidopsis. Plant Physiol 145: 1549–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saze H, Scheid OM, Paszkowski J (2003) Maintenance of CpG methylation is essential for epigenetic inheritance during plant gametogenesis. Nat Genet 34: 65–69 [DOI] [PubMed] [Google Scholar]
- Saze H, Shiraishi A, Miura A, Kakutani T (2008) Control of genic DNA methylation by a jmjC domain-containing protein in Arabidopsis thaliana. Science 319: 462–465 [DOI] [PubMed] [Google Scholar]
- Seward DJ, Gabrielle C, Kim S, Schonewald M, Zhang L, Tripet B, Bentley D (2007) Demethylation of trimethylated histone H3 Lys4 in vivo by JARID1 JmjC proteins. Nat Struct Mol Biol 14: 240–242 [DOI] [PubMed] [Google Scholar]
- Soppe WJ, Jasencakova Z, Houben A, Kakutani T, Meister A, Huang MS, Jacobsen SE, Schubert I, Fransz PF (2002) DNA methylation controls histone H3 lysine 9 methylation and heterochromatin assembly in Arabidopsis. EMBO J 21: 6549–6559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Zhou DX (2008) Rice jmjC domain-containing gene JMJ706 encodes H3K9 demethylase required for floral organ development. Proc Natl Acad Sci USA 105: 13679–13684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda S, Paszkowski J (2006) DNA methylation and epigenetic inheritance during plant gametogenesis. Chromosoma 115: 27–35 [DOI] [PubMed] [Google Scholar]
- Tariq M, Saze H, Probst AV, Lichota J, Habu Y, Paszkowski J (2003) Erasure of CpG methylation in Arabidopsis alters patterns of histone H3 methylation in heterochromatin. Proc Natl Acad Sci USA 100: 8823–8827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran RK, Henikoff JG, Zilberman D, Ditt RF, Jacobsen SE, Henikoff S (2005) DNA methylation profiling identifies CG methylation clusters in Arabidopsis genes. Curr Biol 15: 154–159 [DOI] [PubMed] [Google Scholar]
- Tsukahara S, Kobayashi A, Kawabe A, Mathieu O, Miura A, Kakutani T (2009) Bursts of retrotransposition reproduced in Arabidopsis. Nature 461: 423–426 [DOI] [PubMed] [Google Scholar]
- Unoki M, Nishidate T, Nakamura Y (2004) ICBP90, an E2F-1 target, recruits HDAC1 and binds to methyl-CpG through its SRA domain. Oncogene 23: 7601–7610 [DOI] [PubMed] [Google Scholar]
- Walsh CP, Chaillet JR, Bestor TH (1998) Transcription of IAP endogenous retroviruses is constrained by cytosine methylation. Nat Genet 20: 116–117 [DOI] [PubMed] [Google Scholar]
- Woo HR, Pontes O, Pikaard CS, Richards EJ (2007) VIM1, a methylcytosine-binding protein required for centromeric heterochromatinization. Genes Dev 21: 267–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder JA, Walsh CP, Bestor TH (1997) Cytosine methylation and the ecology of intragenomic parasites. Trends Genet 13: 335–340 [DOI] [PubMed] [Google Scholar]
- Zemach A, McDaniel IE, Silva P, Zilberman D (2010) Genome-wide evolutionary analysis of eukaryotic DNA methylation. Science 328: 916–919 [DOI] [PubMed] [Google Scholar]
- Zhang X, Yazaki J, Sundaresan A, Cokus S, Chan SW, Chen H, Henderson IR, Shinn P, Pellegrini M, Jacobsen SE, Ecker JR (2006) Genome-wide high-resolution mapping and functional analysis of DNA methylation in Arabidopsis. Cell 126: 1189–1201 [DOI] [PubMed] [Google Scholar]
- Zhu J, Kapoor A, Sridhar VV, Agius F, Zhu JK (2007) The DNA glycosylase/lyase ROS1 functions in pruning DNA methylation patterns in Arabidopsis. Curr Biol 17: 54–59 [DOI] [PubMed] [Google Scholar]
- Zilberman D, Coleman-Derr D, Ballinger T, Henikoff S (2008) Histone H2A.Z and DNA methylation are mutually antagonistic chromatin marks. Nature 456: 125–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberman D, Gehring M, Tran RK, Ballinger T, Henikoff S (2007) Genome-wide analysis of Arabidopsis thaliana DNA methylation uncovers an interdependence between methylation and transcription. Nat Genet 39: 61–69 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.