Abstract
The management of human epidermal growth factor receptor 2-positive (ErbB2+) breast cancer is challenging; patients with ErbB2+ breast tumors have more aggressive disease and a poor prognosis. The increasing incidence of breast cancer in Asia and the limitations of existing treatments pose additional challenges. In this review, we summarize the preclinical and clinical evidence that indicates how lapatinib, a novel inhibitor that targets the human epidermal growth factor receptor (ErbB1) and ErbB2 may help clinicians address four particularly challenging issues in the management of ErbB2+ breast cancer. These issues are: (i) trastuzumab therapy failure, (ii) development of central nervous system metastases, (iii) minimizing toxicity and (iv) selecting the most appropriate partners (chemotherapy and non-chemotherapy) for combination therapy with lapatinib. Lapatinib, in combination with chemotherapeutic agents, such as capecitabine, provides clinical benefits to patients with ErbB2+ breast cancer, including patients who develop progressive disease on trastuzumab. Lapatinib, in combination with non-chemotherapeutic agents, such as letrozole, may also provide a chemotherapy-free treatment option for postmenopausal patients with estrogen receptor-positive/ErbB2+ metastatic breast cancer. Encouraging results have also emerged regarding the synergistic effects of lapatinib in combination with other agents for the treatment of ErbB2+ breast cancer. Promising findings have also been reported for the use of lapatinib to prevent and treat central nervous system metastases. Collectively, these results indicate that the judicious use of lapatinib, an effective oral therapy with a manageable toxicity profile, can enhance the management of patients with ErbB2+ breast cancer.
Keywords: breast cancer, ErbB1, ErbB2, lapatinib, tyrosine kinase inhibitor, review
INTRODUCTION
The incidence of breast cancer is increasing in Japan and many other Asian countries (1). Among Asian women with breast cancer, an estimated 20% (as determined by immunohistochemistry grading of 3+) to 28% (as determined by positive fluorescence in situ hybridization) of breast tumors are human epidermal growth factor receptor 2-positive (ErbB2+, HER2+) (Dr Y. Tan, personal communication, December 2009). ErbB2+ breast cancer is of clinical concern, given that these tumors are correlated with more aggressive disease and a poor prognosis (2–4). Clinical studies have clearly shown, however, that patients with ErbB2+ breast cancer can achieve meaningful clinical benefits from anti-erbB2 therapy (3).
Lapatinib (GW572016) is a unique, orally bioavailable, small-molecule dual tyrosine kinase inhibitor developed by GlaxoSmithKline that targets tumor cells overexpressing both human epidermal growth factor receptor (EGFR; ErbB1) and ErbB2 tyrosine kinases (5). Lapatinib inhibition of ErbB1 and ErbB2 kinase activity prevents the activation of downstream cellular signals that promote tumor cell survival and proliferation (6–8) (Fig. 1). Using a rational drug design approach, more than 3200 quinazoline and quinazoline-like compounds with potential tyrosine kinase activity were screened and assayed. Lapatinib was eventually selected from these compounds as it was a selective and potent inhibitor of ErbB1 and ErbB2 that had predictable oral bioavailability and acceptable in vivo toxicity in the targeted patient population (9). First-in-human studies with lapatinib were initiated in 2001; in 2007 lapatinib was approved in the USA for use in combination with capecitabine for the treatment of ErbB2+ advanced or metastatic breast cancer in patients who had received previous treatment including an anthracycline, a taxane and trastuzumab (10) (Fig. 2). Additional approvals for this indication have been granted in 90 more countries, including Japan. The clinical development of lapatinib is continuing with attention focused on ErbB2+ breast cancer as well as other cancers that overexpress ErbB2.
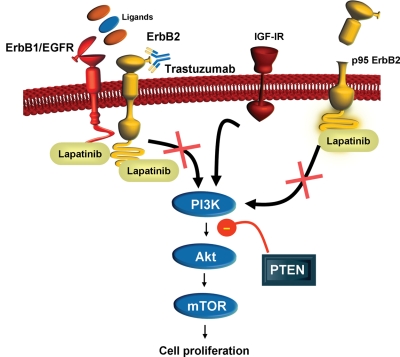
Figure 1.
ErbB2 cellular signaling pathways and lapatinib mechanism of action. ErbB2 (HER2) is a transmembrane tyrosine kinase activated by dimerization with itself or other ErbB proteins (i.e. ErbB1, ErbB3). Binding of ErbB1 ligands to ErbB1 stimulates heterodimerization with ErbB2 and activation of downstream signaling pathways, including PI3K, Akt protein kinase and mTOR, resulting in an increase in cell proliferation. The PTEN protein has tumor suppressor activity in this signaling pathway and loss of PTEN, as well as upregulation of IGF-1R signaling, is associated with trastuzumab resistance. Lapatinib blocks the activation of the ErbB2 signaling pathway by inhibiting the intracellular tyrosine kinase of ErbB1 and ErbB2 and may circumvent trastuzumab resistance associated with upregulation of IGF-1R signaling. Lapatinib also binds to the p95 truncated variant of ErbB2 (p95 ErbB2) and inhibits cell proliferation in trastuzumab-resistant cells expressing p95 ErbB2. ErbB1, human epidermal growth factor receptor 1 (EGFR); ErbB2, human epidermal growth factor receptor 2 (ErbB2); IGF-1R, insulin-like growth factor-1 receptor; PI3K, phosphatidylinositol-3-kinase; PTEN, Phosphatase and tensin homolog deleted on chromosome 10; mTOR, mammalian target of rapamycin.
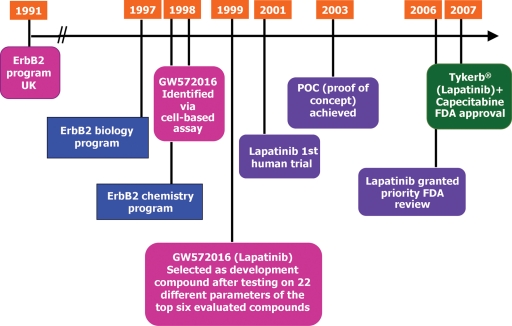
Figure 2.
Timeline and history of the preclinical and clinical development of lapatinib. Preclinical development was initiated in 1991 and the first-in-human lapatinib clinical study was conducted in 2001. The proof of concept (POC) milestone to establish a registration indication for lapatinib was achieved in 2003. Lapatinib received registration approval from the US Food and Drug Administration (FDA) in 2007 for use in combination with capecitabine for the treatment of ErbB2+ advanced or metastatic breast cancer in patients who had received previous treatment including an anthracycline, a taxane and trastuzumab.
Although lapatinib provides a new treatment option for the management of ErbB2+ breast cancer, clinicians and patients still face a number of clinical challenges, including: (i) managing trastuzumab failure; (ii) preventing and managing central nervous system (CNS) metastases; (iii) minimizing toxicity; and (iv) selecting the most appropriate partner for combination therapy with lapatinib.
The aim of our review is to provide clinicians in Asia with insight into how lapatinib may help address the clinical challenges associated with ErbB2+ breast cancer. For each challenge, we will summarize relevant preclinical and clinical evidence and provide our perspective on what this evidence means to the practicing clinician.
Managing Trastuzumab Failure: Role for Lapatinib?
Trastuzumab has advanced the management of patients with ErbB2+ metastatic breast cancer; however, ∼66–88% of patients treated with trastuzumab as a single agent and 20–50% of those treated with trastuzumab in combination therapy do not respond to trastuzumab (i.e. de novo or primary resistance) (11,12). Further, many patients with metastatic breast cancer, who initially respond to trastuzumab, develop resistance (i.e. acquired or secondary resistance) and the majority of these patients develop progressive disease within 1 year of commencing treatment (13–16). Accumulating preclinical and clinical evidence suggests that de novo and acquired trastuzumab resistance in ErbB2+ breast cancer may occur via several different molecular mechanisms (3,11,17). Clinical data also indicate, however, that patients may benefit from continued ErbB2 suppression with trastuzumab therapy after tumor progression on trastuzumab (18–20). Alternatively, evidence also exists that suggests that other anti-erbB2 therapies, such as lapatinib, may provide benefit in patients with ErbB2+ breast cancers that do not respond to trastuzumab therapy (19,21).
Preclinical Evidence: Trastuzumab Failure and Lapatinib
The potential for lapatinib to inhibit ErbB2-driven tumor cell growth in trastuzumab-resistant breast cancers has been investigated in various preclinical studies, including studies on trastuzumab failure associated with (i) transactivation of ErbB2 by other tyrosine kinases such as insulin-like growth factor-1 receptor (IGF-1R); (ii) expression of p95 ErbB2, a truncated form of ErbB2 lacking the extracellular trastuzumab-binding domain; and (iii) increase in phosphatidylinositol-3-kinase (PI3K)/Akt signaling due to loss of phosphatase and tensin homolog deleted on chromosome 10 (PTEN) expression or PI3K catalytic subunit alpha (PI3KCA) mutation (Fig. 1).
A number of in vitro studies have clearly shown that ErbB2+ breast cancer cells, rendered trastuzumab-resistant by long-term exposure to trastuzumab, remain responsive to lapatinib (22,23). Trastuzumab failure may be mediated, at least in part, by upregulation of IGF-1R. For example, preclinical studies have shown that IGF-1R interaction with ErbB2 is increased in trastuzumab-resistant breast cancer cells (24,25). Encouragingly, lapatinib was shown to block ErbB2 and IGF-1R crosstalk and inhibit cell growth in a trastuzumab-resistant breast cancer cell line (23).
Results from preclinical studies also suggest that lapatinib may be effective in treating p95 ErbB2+ trastuzumab-resistant breast cancers. Owing to the absence of a trastuzumab-binding domain on p95 ErbB2, breast tumor cell lines and tumor xenografts expressing this truncated variant of ErbB2 appear to be resistant to trastuzumab. In vitro and in vivo studies have demonstrated that lapatinib can effectively inhibit the growth of trastuzumab-resistant breast cancer cell lines and tumor xenografts that express p95 ErbB2, presumably because lapatinib targets the intracellular tyrosine kinase component of ErbB2 (26).
Trastuzumab resistance may also be mediated in some ErbB2+ breast tumors by an increase in PI3K/Akt signaling associated with either the loss or inactivation of PTEN expression or PI3KCA mutation (17,27). Presence of PTEN is associated with tumor suppressor activity (17). Loss of PTEN appears to counteract the anti-tumor effects of trastuzumab by promoting PI3K/Akt activation, which, in turn, stimulates tumor cell growth (17). In vitro studies in PTEN-deficient ErbB2+ breast tumor cell lines showed that tumor cells remained responsive to lapatinib and that lapatinib sensitivity appeared to be PTEN-independent (28). Transfection of ErbB2-overexpressing cell lines with mutant PI3KCA or wild-type PI3KCA resulted in trastuzumab resistance, suggesting that activation of the PI3K signaling pathway by PI3KCA mutation appeared to mediate resistance (27). Further, oncogenic mutations of PI3KCA, identified in several different ErbB2+ human breast cancer cell lines, are associated with trastuzumab resistance in vitro (29). Contrary to earlier preclinical findings that showed that lapatinib sensitivity was PTEN-independent, a recent in vitro study has shown that hyperactivation of the PI3K pathway by either loss-of-function mutations in PTEN or PI3KCA mutation may also confer resistance to lapatinib in breast cancer cell lines (30). Another recent in vitro study found that isolated clones of ErbB2+ breast cancer cell lines with acquired resistance to lapatinib were also cross-resistant to trastuzumab and exhibited increased expression of AXL, a receptor tyrosine kinase (31). This finding suggests that upregulation of AXL may be a novel mechanism involved in the development of lapatinib and trastuzumab resistance. Additional preclinical studies are required to determine the role of PI3K activation and AXL upregulation in modulating lapatinib and trastuzumab resistance.
Lapatinib has yet to be investigated in other molecular mechanisms of trastuzumab resistance, such as MUC4-mediated resistance. Preclinical studies have shown that the overexpression of the membrane-bound mucin glycoprotein, MUC4, in a trastuzumab-resistant human cell line, interferes with the binding of trastuzumab to ErbB2 (32). Tumors that overexpress MUC4 may potentially promote tumorigenesis by activating ErbB2, suppressing apoptosis and inhibiting immune recognition of tumor cells (11,33).
Collectively, the results from these and other preclinical studies provided a strong scientific rationale for the conduct of clinical studies with lapatinib in patients with trastuzumab-resistant ErbB2+ breast cancer.
Clinical Evidence: Trastuzumab Failure and Lapatinib
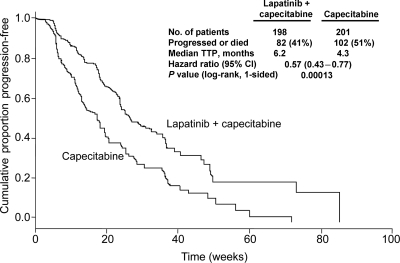
Clinical evidence from a recent systematic review of observational studies (18) and a randomized clinical trial (20) suggest that patients with breast tumors that progress on trastuzumab treatment may still benefit from continued ErbB2 suppression with trastuzumab (19). However, accumulating clinical data also indicates that treatment with other anti-erbB2 therapies, such as lapatinib, may also improve clinical outcomes in this patient population (19,34). Several clinical trials have been undertaken to examine the effect of lapatinib in patients with trastuzumab-resistant ErbB2+ breast cancer (19,35,36). The pivotal EGF100151 study (Table 1) (36), was a Phase III, randomized, controlled trial of 399 patients with ErbB2+ locally advanced or metastatic progressive disease. Patients were randomized to lapatinib plus capecitabine or to capecitabine alone. Treatment with lapatinib plus capecitabine significantly increased time to progression (TTP), compared with capecitabine alone (6.2 versus 4.3 months, respectively; hazard ratio [HR; 95% CI] = 0.57; 0.43–0.77; P < 0.001; Fig. 3). Significant differences in the overall response rate (ORR: 24 versus 14%; odds ratio [OR, 95% CI] = 1.9, 1.1–3.4; P = 0.017) and clinical benefit rate (CBR: 29 versus 17%; [OR, 95% CI] = 2.0, 1.2–3.3; P = 0.008) were observed (36). An exploratory subgroup analysis was also completed to assess the effect of the extent of pretreatment on TTP and overall survival (OS) (34,37). Among patients pretreated with fewer than three regimens, both TTP and OS were significantly greater for those treated with lapatinib plus capecitabine compared with capecitabine alone (TTP: 49.4 versus 19.7 weeks, respectively, [HR, 95% CI] = 0.37, 0.18–0.77; P = 0.006; OS: 87.3 versus 55.1 weeks, respectively, [HR, 95% CI] = 0.51, 0.30–0.86; P = 0.009). Among patients pretreated with more than three regimens, TTP, but not OS, was significantly greater for those treated with lapatinib plus capecitabine compared with capecitabine alone (TTP: 25.4 versus 18.6 weeks, respectively, [HR, 95% CI] = 0.59, 0.43–0.82; P = 0.001; OS: 71.4 versus 66.6 weeks, respectively, [HR, 95% CI] = 0.95, 0.76–1.21; P = 0.7) (34,37). These findings indicate that lapatinib plus capecitabine was superior to capecitabine alone in patients whose disease had progressed on trastuzumab and that less heavily pretreated patients had the greatest benefit in terms of improved TTP and OS compared with more heavily pretreated patients. The results from the EGF100151 trial facilitated registration approval for the use of lapatinib in combination with capecitabine to treat patients with ErbB2+ breast cancer whose disease has progressed after treatment with trastuzumab-based regimens.
Table 1.
Phase III trials of lapatinib plus chemotherapy or non-chemotherapy agents for locally advanced or metastatic breast cancer
| Reference | Patient population | Therapy | N | Outcomes |
|---|---|---|---|---|
| Lapatinib plus chemotherapy agents | ||||
| Cameron et al. (36) (EGF100151) | ErbB2+, LABC or MBC | Lapatinib + capecitabine versus capecitabine | 399 | TTP: 6.2 versus 4.3 months; HR (95% CI): 0.57 (0.43–0.77); P < 0.001 |
| CBR: 29 versus 17%; OR (95% CI): 2.0 (1.2–3.3); P = 0.008 | ||||
| ORR: 24 versus 14%; OR (95% CI): 1.9 (1.1–3.4); P = 0.017 | ||||
| OS: 15.6 versus 15.3 months; HR (95% CI): 0.78 (0.55–1.12); P = 0.177 | ||||
| Di Leo et al. (74). (EGF30001) | First-line MBC | Lapatinib + paclitaxel versus placebo + paclitaxel | 579 | ErbB2+ subgroup (n = 86) |
| TTP: 36.4 versus 25.1 weeks; HR (95% CI): 0.53 (0.31–0.89); P = 0.005 | ||||
| CBR: 69.4 versus 40.5%; OR (95% CI): 3.5 (1.3–9.7); P = 0.011 | ||||
| ORR: 63.3 versus 37.8%; OR (95% CI): 3.0 (1.1–8.5); P = 0.023 | ||||
| OS: 104.6 versus 82.4 weeks; HR (95% CI): 0.74 (0.4–1.4); P = 0.365 | ||||
| Lapatinib plus non-chemotherapy agents | ||||
| Johnston et al. (66) (EGF30008) | First-line MBC | Lapatinib + letrozole versus placebo + letrozole | 1286 | Primary population: ErbB2+ (n = 219) |
| PFS: 8.2 versus 3.0 months; HR (95% CI): 0.71 (0.53–0.96); P = 0.019 | ||||
| CBR: 48 versus 29%, OR (95% CI): 0.4 (0.2–0.8); P = 0.003 | ||||
| ORR: 28 versus 15%, OR (95% CI): 0.4 (0.2–0.9); P = 0.021 | ||||
| OS: 33.3 versus 32.3 months; HR (95% CI): 0.74 (0.5–1.1); P = 0.113 | ||||
| O'Shaughnessy et al. (42); Blackwell et al. (35) (EGF104900) | ErbB2+, MBC | Lapatinib + trastuzumab versus lapatinib | 296 | PFS: 12.0 versus 8.1 weeks; HR (95% CI): 0.73 (0.57–0.93); P = 0.008 |
| CBR: 24.7 versus 12.4%, OR (95% CI): 2.2 (1.2–4.5); P = 0.020 | ||||
| ORR: 10.3 versus 6.9%, OR (95% CI): 1.5 (0.6–3.9); P = 0.46 | ||||
| OS: 60.7 versus 41.4 weeks; HR (95% CI): 0.74 (0.57–0.97); P = 0.026 | ||||
CBR, clinical benefit rate; CI, confidence interval; ErbB2+, human epidermal growth factor receptor 2-positive; HR, hazard ratio; LABC, locally advanced breast cancer; MBC, metastatic breast cancer; OR, odds ratio; ORR, overall response rate; OS, overall survival PFS, progression-free survival; TTP, time to progression.
Figure 3.
Time to progression (TTP) in patients with ErbB2+ breast cancer treated with lapatinib plus capecitabine compared with capecitabine alone (EGF100151 study). Data include the intent-to-treat population of patients with ErbB2+, trastuzumab-resistant, locally advanced or metastatic breast cancer. Five patients with competing risk were censored. Figure adapted and reprinted from the publication by Cameron et al. (36) with kind permission from Springer Science + Business media.
Lapatinib, as monotherapy, has been investigated in several clinical studies in patients with trastuzumab-naïve or trastuzumab-refractory ErbB2+ locally advanced or metastatic breast cancer (38–41). Clinical findings in these studies suggest that lapatinib monotherapy had anti-tumor activity in both trastuzumab-naive and trastuzumab-refractory patient populations and that the treatment was well-tolerated (38–41). Lapatinib, in combination with trastuzumab, was also assessed in a randomized clinical study of 296 patients with trastuzumab-refractory ErbB2+ metastatic breast cancer (35,42). In this study (EGF104900 study; Table 1), lapatinib plus trastuzumab significantly improved median OS, compared with lapatinib alone (60.7 versus 41.4 weeks; [HR, 95% CI = 0.74, 0.57–0.97; P = 0.026) in patients heavily pretreated with trastuzumab (35). These clinical benefits reinforce the merit of continued ErbB2 suppression and dual blockade of ErbB2 after disease progression.
Consistent with preclinical findings, clinical studies have shown that truncation of the extracellular domain of ErbB2 (p95 ErbB2), loss of PTEN expression, or PI3KCA mutations in ErbB2+ breast cancer is associated with a poor response to trastuzumab and may be markers for trastuzumab failure (17,26,43). Further support for a role for lapatinib in the management of patients with trastuzumab failure comes from a clinical study of patients with ErbB2+ breast tumors expressing low PTEN or PI3KCA mutations (43). This study showed that low PTEN expression or PI3KCA mutation was correlated with trastuzumab, but not lapatinib, resistance (43). This clinical finding is discordant with recent preclinical evidence that suggests that loss-of-function mutations in PTEN or PI3KCA mutations could confer lapatinib resistance in ErbB2+ human breast cancer cell lines (30). The lack of a validated clinical test to identify patients with low PTEN tumors and relatively low patient numbers may potentially have limited the findings in the clinical study. Further clinical studies using a validated measure of PTEN expression in ErbB2+ breast tumors are required to better establish a potential correlation between low PTEN and resistance to lapatinib (30).
Given the promising findings from preclinical studies, the role of concomitant inhibition of the IGF-1R and ErbB2 signaling pathways is currently being investigated in a Phase II study in patients with trastuzumab-resistant locally advanced or metastatic ErbB2+ breast cancer (44). Patients will be treated with lapatinib plus capecitabine with or without the anti-IGF-1R monoclonal antibody, cixutumumab (IMC-A12). The primary endpoint will be progression-free survival (PFS) (45). This study should provide timely and critical insight into whether lapatinib plus capecitabine can overcome IGF-1R-mediated trastuzumab failure.
On the basis of the results from preclinical and clinical studies, lapatinib, may have an important role in improving the management of ErbB2+ trastuzumab-resistant progressive disease.
Preventing and Managing CNS Metastases in ErbB2+ Breast Cancer
Preventing and managing CNS metastases has emerged as an increasingly important clinical challenge for clinicians treating patients with ErbB2+ breast cancer. Approximately 25–50% of trastuzumab-treated patients will develop CNS metastases (46,47). Currently, those who develop CNS metastases have few effective treatment options available. Systemic chemotherapy, surgery (including stereotactic radiosurgery), whole brain radiotherapy and continued trastuzumab therapy provide some improvement in OS; however, the median time from the diagnosis of CNS metastases to death is only 4–15 months (46–48). On the basis of comparisons with historical controls (i.e. patients treated in the pretrastuzumab era), there has been an apparent increase in the incidence of CNS metastases in trastuzumab-treated patients with ErbB2+ breast cancer (46,47,49). Several hypotheses have been suggested for the observed increase in CNS metastases in this patient population, including:
ErbB2+ tumors appear to have a more aggressive phenotype and are more likely to metastasize to the CNS (49–51);
The availability of trastuzumab therapy has resulted in better control of systemic disease, which has increased survival, but paradoxically, has also increased the opportunity for CNS metastases to develop (46); and
The blood-brain barrier (BBB) may create a ‘sanctuary’ site in the CNS by preventing systemic anti-cancer agents from entering the CNS, thus, allowing ErbB2+ tumors to colonize and grow (46,52)
Trastuzumab's large molecular size prevents the antibody from crossing the BBB and inhibiting the growth of ErbB2+ CNS tumors. In patients treated with trastuzumab, the ratio of trastuzumab levels in serum to trastuzumab levels in cerebrospinal fluid was 420:1. After whole brain radiotherapy, this ratio was reduced to 76:1, suggesting that the BBB was still an effective barrier to trastuzumab, even though the barrier was somewhat impaired by radiotherapy (53).
Although systemic disease appears to be responsible for the lower survival rates in patients with ErbB2+ breast cancer in the pretrastuzumab era, the use of trastuzumab has altered the clinical course of the disease (46,47). Thus, with improved systemic control, the treatment of CNS disease is now a clinically relevant issue that requires effective proactive management.
Lapatinib is a logical candidate to assess in clinical studies for the treatment and prevention of CNS metastases in patients with ErbB2+ breast cancer because of its potent anti-erbB2 activity and its small molecular size. Preclinical and clinical studies indicate that lapatinib can penetrate the BBB and exert an anti-tumor effect in the CNS.
Preclinical Evidence: CNS Metastases in ErbB2+ Breast Cancer and Lapatinib
The recent development of an in vivo mouse model of ErbB2+ brain metastases has helped researchers gain new insights into the cellular and molecular mechanisms involved in CNS metastases (51). Further, this model has proven to be a valuable tool to assess novel therapies that may inhibit the colonization and growth of ErbB2+ tumor cells within the brain. To develop this model, a brain-seeking derivate of a human breast cancer cell line overexpressing ErbB1 (MDA-MB-231) was transfected with an ErbB2-expressing vector (231-BR-HER2) or with an empty control vector (231-BR-vector) (51). After intracardiac injection of 231-R-ER2 or 231-BR-vector cells into BALB/c nude mice, metastatic brain lesions were shown to form 20 to 25 days later. Compared with 231-BR-vector control cells overexpressing only ErbB1, 231-BR-HER2 cells overexpressing ErbB1/ErbB2 showed a 2.5- to 3.0-fold increase in colonization (i.e. large metastases: >300 microns in any single dimension) in the brain. These findings suggest that ErbB2 expression plays an important role in promoting the growth of these cells and the development of brain metastases (51).
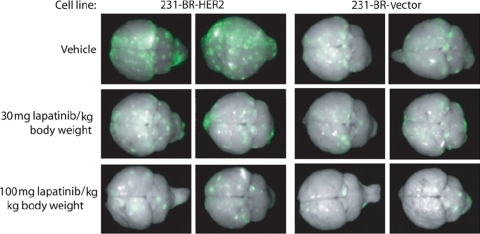
Administration of 30 or 100 mg/kg lapatinib 5 days after injection of cells in this mouse model significantly decreased the total number of large metastases detected in the brains of mice injected with 231-BR-HER2 cells by 50–53% (P < 0.001) (Fig. 4) (54). Further, lapatinib also decreased the number of large metastases in the ErbB1-overexpressing control cells, but only at the highest dose tested. In vitro, lapatinib was shown to inhibit cell proliferation and migration, as well as block the phosphorylation of ErbB1 and ErbB1/ErbB2 in 231-BR-vector control and 231-BR-HER2 brain-seeking breast cancer cell lines, respectively (54). Taken together, these results indicate that lapatinib may prevent the proliferation of ErbB2+ breast cancer cells in the brain.
Figure 4.
Lapatinib inhibition of metastatic colonization of mouse brain by ErbB2-positive human breast cancer cells in a mouse model of brain metastases. Human breast cancer cells expressing ErbB1/ErbB2 (231-BR-HER2) or ErbB1 (231-BR-vector) and enhanced green fluorescent protein (EGFP) were administered by intracardiac injection into the left ventricle of BALB/c nude mice. Five days after injection mice were administered lapatinib (30 or 100 mg/kg body weight) or vehicle twice-daily for 24 days by oral gavage. Brains were dissected at necropsy and imaged to detect EGFP expression in metastases derived from the injected 231-BR cells. Representative dorsal whole brain images from two mice in each treatment group are shown. Image reprinted from the publication entitled “Effect of lapatinib on the outgrowth of metastatic breast cancer cells to the brain” by Gril et al. (54) with permission from Oxford University Press. ErbB1, human epidermal growth factor receptor 1; ErbB2, human epidermal growth factor receptor 2.
Clinical Evidence: CNS Metastases in ErbB2+ Breast Cancer and Lapatinib
A potential role for lapatinib in reducing CNS metastases was first apparent from an exploratory analysis of data from a Phase III study of lapatinib plus capecitabine versus capecitabine alone in patients with advanced ErbB2+ breast cancer (EGF100151) (36,55). This analysis showed that lapatinib plus capecitabine treatment was associated with a lower rate of CNS tumor progression, compared with capecitabine alone (2% [n = 4] versus 6% [n = 13], respectively; P = 0.045 (36).
This finding raised interest in the results from an exploratory analysis of data from a Phase II pilot study of lapatinib monotherapy in 39 patients with ErbB2+ breast cancer who had CNS metastases (56). This analysis showed that lapatinib treatment was associated with a decrease in tumor volume in some patients. Of the 34 patients analyzed, 3 (9%) patients achieved at least a 50% reduction in CNS tumor volume and 7 (21%) patients achieved at least a 10–30% reduction in CNS tumor volume (56). A larger Phase II study (EGF105084) was conducted to investigate the effects of lapatinib monotherapy on CNS tumor volume in 242 patients with ErbB2+ breast cancer whose CNS tumors had progressed after trastuzumab therapy and cranial radiotherapy (57). Of the 200 patients in this study with available data, 19 (8%) patients had at least a 50% reduction in tumor volume and 50 (21%) patients had at least a 20% reduction in tumor volume (57).
Given the findings from the two Phase II studies and the results from the large Phase III lapatinib plus capecitabine registration trial (EGF100151), an extension to the EGF105084 study was deemed appropriate. In the extension phase, patients with ErbB2+ breast cancer whose CNS disease had progressed on lapatinib monotherapy were treated with lapatinib plus capecitabine (57). Findings from this study indicate that lapatinib plus capecitabine treatment was associated with a reduction in the volume of brain metastases. Of the 50 patients who entered the extension phase, 10 (20%, 95% exact CI: 3.0–33.7) patients had an objective CNS response. Further, 11 (22%) patients had at least a 50% reduction in tumor volume and 20 (40%) patients had at least a 20% reduction in tumor volume (57).
More recently, lapatinib plus capecitabine was evaluated in a lapatinib expanded access program (LEAP) and a French Authorisation Temporaire d'Utilisation (ATU) program for ErbB2+ breast cancer patients with CNS metastases (58). These programs provided patients with an opportunity to receive lapatinib after regulatory approval, but before the agent was commercially available. Preliminary analyses of the LEAP/ATU data also suggest that lapatinib plus capecitabine had anti-tumor activity in patients with CNS metastases. Of the 138 patients with progressive disease, 3 (2%) had a complete CNS response and 22 (16%) had a partial CNS response (58). Several other clinical trials are now underway to assess the role of lapatinib in preventing or treating CNS metastases in patients with ErbB2+ breast cancer (Table 2); the results of these studies are eagerly awaited.
Table 2.
Ongoing clinical trials to assess the role of lapatinib in preventing or treating central nervous system metastases in patients with early or advanced/metastatic ErbB2+ breast cancer
| Studya | Patient population | Study design and treatment regimen | Phase | N | Efficacy endpoints |
|---|---|---|---|---|---|
| NCT00374322 (EGF105485, TEACH) | Early BC adjuvant | Double-blind, RCT, lapatinib versus placebo | III | 3000 | 1°: DFS |
| No trastuzumab | 2°: OS, CNS RFI | ||||
| NCT00490139 (EGF106708, BIG 2-06, ALTTO) | BC, adjuvant | Open label, RCT, lapatinib versus trastuzumab versus trastuzumab followed by lapatinib versus lapatinib + trastuzumab | III | 8000 | 1°: DFS |
| 2°: OS, TTR, TTDR, Incidence of CNS metastases | |||||
| NCT00553358 (EGF106903, BIG 1-06, NeoALTTO) | BC, neoadjuvant | Open label, RCT, lapatinib versus trastuzumab versus lapatinib + trastuzumab | III | 450 | 1°: DFS |
| 2°: OS, TTR, TTDR, Incidence of CNS metastases | |||||
| NCT00667251 (EGF108919, COMPLETE) | Stage IV MBC | Open label, RCT, lapatinib + paclitaxel or docetaxel versus trastuzumab + paclitaxel or docetaxel | III | 600 | 1°: PFS |
| 2°: ORR, OS, CBR, Incidence of CNS metastases | |||||
| NCT00820222 (EGF111438, CEREBREL) | Stage IV MBC | Open label, RCT, lapatinib + capecitabine versus trastuzumab + capecitabine | III | 650 | 1°: Incidence of CNS metastases as first site of progression |
aStudy identification codes for trials registered in the National Institutes of Health Clinical Trials Registry (http://clinicaltrials.gov, accessed 5 November 2009). BC, breast cancer; 1°, primary endpoint; 2°, secondary endpoint(s); CBR, clinical benefit rate; CNS, central nervous system; CNS RFI, central nervous system recurrence-free intervals; DFS; disease-free survival; MBC, metastatic breast cancer; OS, overall survival; PFS, progression-free survival; RCT, randomized controlled trial; TTDR, time to distant recurrence; TTR, time to recurrence.
In summary, preclinical and clinical studies have yielded promising results regarding the role that lapatinib may have in preventing and managing CNS metastases in patients with ErbB2+ breast cancer.
Minimizing Toxicity: The Promise of Chemotherapy-free Regimens
Minimizing the adverse outcomes and toxicity associated with the use of chemotherapeutic treatments is a challenge for both clinicians and patients. These adverse outcomes increase the cost and complexity of care and reduce the patient's quality of life (59). With advances in our understanding of the pathophysiology of ErbB2+ breast cancer, we are now able to consider whether ErbB2+ breast cancer could be managed with chemotherapy-free regimens such as lapatinib plus trastuzumab (as previously described) or lapatinib plus letrozole. This is an exciting possibility for clinician and patient alike. Preclinical and clinical evidence indicate that this possibility may be achieved for selected patients through the use of therapies that target more than one growth signaling receptor. The combined use of other targeted therapies, such as lapatinib and anti-estrogens, could not only yield clinical benefits, but could also help overcome the problem of endocrine therapy resistance.
Preclinical Evidence: Chemotherapy-free Regimens and Lapatinib
Preclinical studies support the rationale for pursuing chemotherapy-free treatments for breast cancer; these studies have shown that lapatinib can have additive or synergistic inhibitory effects when combined with anti-estrogen therapies. Results from in vitro studies on breast cancer cell lines demonstrate that lapatinib and tamoxifen can cause a faster and more profound inhibition of cell cycle progression than tamoxifen alone (60). The synergistic effects of lapatinib and tamoxifen treatment were reflected in a greater increase in p27 and a greater decrease in cyclin D1 and cyclin E-cdk2 activity, relative to the effect of either drug alone (60). Results from in vitro studies with lapatinib plus fulvestrant have shown that these agents can additively or synergistically inhibit the growth of breast cancer cell lines (31). Lapatinib plus fulvestrant have been shown to promote G1-S blockade and increase apoptosis in an additive manner (61). Together, lapatinib and fulvestrant decreased the expression levels of Bcl-2 and survivin and increased the expression levels of p21 and p27 (61). Lapatinib plus fulvestrant have also been shown to synergistically inhibit the growth of a number of breast cancer cell lines through the downregulation of cell signaling proteins, such as p-PDK1, ERK1/2 and p-ERK (62).
As ErbB2+ tumors have an increased resistance to endocrine therapy, compared with ErbB2-negative (ErbB2–) tumors (31,63), much attention has focused on whether anti-ErbB2 therapies might restore or enhance sensitivity to endocrine therapies. The molecular crosstalk between the estrogen receptor (ER) and the ErbB1/ErbB2 signaling pathways may contribute to endocrine resistance (64) (Fig. 5). Therefore, treatments that interfere with the ErbB1/ErbB2 signaling pathway, such as lapatinib, have the potential to modify ER and ErbB crosstalk and subsequently restore sensitivity to endocrine therapy. Results from preclinical studies support this hypothesis. Lapatinib and tamoxifen effectively inhibited the growth of tamoxifen-resistant breast cancer xenograft tumors in vivo; both the rate and volume of tumor growth were reduced with combined treatment (60). Lapatinib in combination with estrogen deprivation also effectively blocked the growth of lapatinib-resistant ErbB2+ breast cancer cell colonies (31).
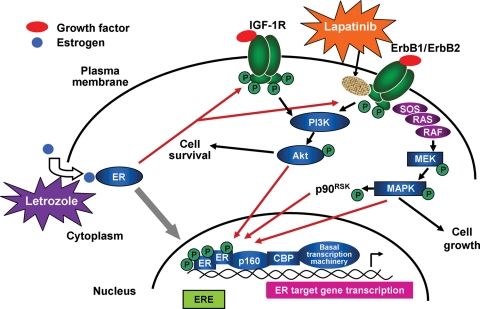
Figure 5.
Molecular crosstalk between the ER and ErbB1/ErbB2 cellular signaling pathways in endocrine-resistant ErbB2-positive breast cancer cells. Estrogen bound to the ER activates estrogen-regulated genes via a classical signaling pathway. ErbB1/ErbB2 stimulation by growth factors results in activation of the PI3K/Akt and MAPK signaling pathways, leading to tumor cell growth. Long-term tamoxifen therapy may promote endocrine resistance via bidirectional crosstalk between the ER and growth factor receptor (i.e. IGF-1R or ErbB1/ErbB2) signaling pathway components. Bidirectional activation of these pathways promotes ER phosphorylation and ER target gene transcription as well as ErbB1/ErbB2/MAPK-mediated signaling and IGF-1R-mediated PI3K/Akt growth signaling pathways. Modulation of these pathways by combined use of lapatinib and anti-estrogen therapy (e.g. letrozole) may overcome endocrine resistance. CBP, cAMP response element binding protein (CREB)-binding protein; ER, estrogen receptor; ErbB1, human epidermal growth factor receptor 1; ErbB2, human epidermal growth factor receptor 2; mitogen-activated protein kinase; MEK, mitogen-activated protein kinase kinase; P, phosphate; p90RSK, p90 ribosomal S6 kinase; p160, p160 steroid receptor co-activator protein(s); PI3K, phosphatidylinositol-3-kinase; PTEN, phosphatase and tensin homologue deleted on chromosome 10; RAF, murine leukemia viral oncogene homologue 1; SOS, son-of-seven less guanine nucleotide exchange factor. Figure adapted from the publication by Johnston (64) (Fig. 1) with permission from the American Association for Cancer Research.
Collectively, the results from in vitro and in vivo preclinical studies have provided strong justification for clinical trials on the efficacy and safety of chemotherapy-free regimens, such as anti-estrogens plus lapatinib, for treating ErbB2+ breast cancer.
Clinical Evidence: Chemotherapy-free Regimens and Lapatinib
Currently, treatment guidelines do not recommend the use of targeted treatment regimens for the management of ER-positive (ER+)/ErbB2+ breast cancer, except for patients with visceral crisis (65). The results from a number of completed (Table 1) and ongoing (Table 3) clinical trials may justify changes to treatment guidelines and clinical practice. For example, recent results from the EGF30008 clinical trial (66) (Table 1) support the use of a first-line chemotherapy-free treatment regimen for postmenopausal women with ER+/ErbB2+ metastatic breast cancer. In this Phase III, randomized, double-blind, placebo-controlled trial, trastuzumab-naïve patients with either ErbB2+ or ErbB2− metastatic breast cancer (N = 1286) received either lapatinib plus letrozole or letrozole plus placebo. The primary endpoint was PFS (as assessed by the investigator) in the ER+/ErbB2+ population (n = 219). In this primary outcome population, treatment with lapatinib plus letrozole significantly increased PFS, compared with letrozole plus placebo (8.2 versus 3.0 months, respectively; [HR, 95% CI] = 0.71, 0.53–0.96; P = 0.019). Significant differences were also apparent in this population for the ORR (28 versus 15%, P = 0.021) and CBR (48 versus 29%, P = 0.003). There was no significant difference in OS between the two regimens (33.3 versus 32.3 months, P = 0.113); however, at the time of publication of these data, <50% of the OS events had been recorded. In the intent-to-treat (ITT) population, there was a modest, but significant, increase in PFS (11.9 versus 10.8 months [HR, 95% CI] = 0.86, 0.76–0.98; P = 0.026) (66). Exploratory analyses examining the effect of early (more than 6 months before study entry) versus recent (<6 months before study entry) tamoxifen discontinuation on clinical outcomes were also completed for the ER+/ErbB2− population of patients. These analyses showed a trend toward improved PFS and CBR in the lapatinib plus letrozole arm, compared with the letrozole plus placebo arm, for those patients who had ceased tamoxifen <6 months before study entry (PFS: 8.3 versus 3.1 months, respectively, P = 0.117; CBR: 44 versus 32%, respectively). This trend was not observed in the subpopulation of patients who had ceased tamoxifen more than 6 months before study entry (PFS: 14.7 versus 15.0 months, P = 0.522; CBR: 62 versus 64%). Although the difference did not reach statistical significance, these findings suggest a potential benefit for combination treatment with lapatinib plus letrozole for patients with ER+/ErbB2− breast cancer who develop tamoxifen resistance early during adjuvant treatment with tamoxifen (66). Results from the safety analyses of the ITT population in the EGF30008 trial showed that adverse events were similar and manageable between the two treatment regimens. The most common adverse events were diarrhea, rash, nausea, arthralgia and fatigue (66). Treatment guidelines for the management of lapatinib-associated toxicities (primarily diarrhea and rash) are now available (67–71). As clinical experience with lapatinib has increased, clinicians are now able to manage these toxicities more effectively in their routine clinical practice.
Table 3.
Ongoing clinical trials of lapatinib combination therapy for early or advanced/metastatic breast cancer
| Studya | Patient population | Study design and treatment regimen | Phase | N |
|---|---|---|---|---|
| Lapatinib plus chemotherapy agents | ||||
| NCT00753207 | Relapsed stage III/IV BCb | Open label, dose escalation to MTD, lapatinib + epirubicin | I | 24 |
| NCT00513058 | ErbB2+ relapsed stage III/IV BC | Open label, dose escalation to MTD, lapatinib + vinorelbine | I | 60 |
| NCT00614978 (LAPTEM) | ErbB2+ relapsed brain metastases in BC | Open label, dose escalation to MTD, lapatinib + temozolamide | I | 18 |
| NCT00477464 (109749) | Japanese ErbB2+ trastuzumab-failed MBC | Open label, single-arm, lapatinib + capecitabine | II | 50 |
| NCT00313599 | ErbB2+ relapsed stage III/IV solid tumor | Open label, dose escalation to MTD, lapatinib + Nab-paclitaxel | I | 22 |
| NCT00709761 | ErbB2+ second-line MBC | Open label, single-arm, lapatinib + Nab-paclitaxel | II | 60 |
| NCT00331630 | ErbB2+ BC, neoadjuvant | Open label, pilot study, lapatinib + Nab-paclitaxel | II | 30 |
| NCT00756470 | ErbB2+ inflammatory BC, neoadjuvant | Open label, single-arm, lapatinib + paclitaxel then lapatinib + fluorouracil + epirubicin + cyclophosphamide | II | 60 |
| NCT00404066 | ErbB2+ BC, neoadjuvant | Open label, single-arm, doxorubicin + cyclophosphamide then lapatinib + docetaxel | II | 72 |
| Lapatinib plus chemotherapy and non-chemotherapy agents | ||||
| NCT00632489 | Relapsed stage III/IV solid tumorb | Open label, dose escalation to MTD in three arms, lapatinib + LBH589 versus LBH589 + capecitabine versus lapatinib + LBH589 + capecitabine | I | 55 |
| NCT00820872 | ErbB2+ BC, adjuvant | Open label, single-arm, lapatinib + docetaxel + carboplatin + trastuzumab | II | 33 |
| NCT00841828 | ErbB2+ BC, neoadjuvant | Open label, RCT, lapatinib + epirubicin + cyclophosphamide + docetaxel versus trastuzumab + epirubicin + cyclophosphamide + docetaxel | II | 102 |
| NCT00769470 | ErbB2+ BC, neoadjuvant | Open label, RCT, lapatinib + carboplatin + docetaxel versus trastuzumab + carboplatin + docetaxel versus lapatinib + trastuzumab + carboplatin + docetaxel | II | 140 |
| NCT00684983 (45) | ErbB2+ first-line or relapsed MBC | Open label, RCT, lapatinib + capecitabine + IMC-A12 versus lapatinib + capecitabine | II | 154 |
| NCT00770809 (CALGB 40 601) | ErbB2+ BC, neoadjuvant | Open label, RCT, lapatinib + paclitaxel versus trastuzumab + paclitaxel versus lapatinib + trastuzumab + paclitaxel | III | 400 |
| NCT00667251 (EGF108919, COMPLETE) | ErbB2+ stage IV MBC | Open label, RCT, lapatinib + paclitaxel or docetaxel versus trastuzumab + paclitaxel or docetaxel | III | 600 |
| NCT00820222 (EGF111438, CEREBREL) | ErbB2+ stage IV MBC | Open label, RCT, lapatinib + capecitabine versus trastuzumab + capecitabine | III | 650 |
| NCT00567554 (GepaQuinto) | ErbB2+ BC, neoadjuvant | Open label, RCT, lapatinib + epirubicin + cyclophosphamide + docetaxel versus trastuzumab + epirubicin + cyclophosphamide + docetaxel versus bevacizumab + epirubicin + cyclophosphamide + docetaxel versus epirubicin + cyclophosphamide + docetaxel versus paclitaxel | III | 2547 |
| Lapatinib plus non-chemotherapy agents | ||||
| NCT00352443 | Relapsed stage III/IV solid tumorb | Open label, dose escalation to MTD, lapatinib + everolimus | I | 48 |
| NCT00499681 | ErbB2+ BC, neoadjuvant | Double-blind, RCT, lapatinib + letrozole versus placebo + letrozole | II | 36 |
| NCT00118157 | Tamoxifen-resistant MBCb | Open label, single-arm, lapatinib + tamoxifen | II | 41 |
| NCT00548184 | ErbB2+ BC, neoadjuvant | Double-blind RCT, lapatinib + trastuzumab + endocrine therapy versus lapatinib + trastuzumab | II | 64 |
| NCT00390455 (CALGB 40 302) | First-line or relapsed advanced BCb | Open label, RCT, lapatinib + fulvestrant versus placebo + fulvestrant | III | 324 |
| NCT00688194 | Aromatase inhibitor-relapsed MBCb | Double-blind, RCT, lapatinib + fulvestrant versus placebo + fulvestrant versus lapatinib + aromatase inhibitor + fulvestrant versus aromatase inhibitor + fulvestrant | III | 396 |
| NCT00553358 (EGF106903, BIG 1-06, NeoALTTO) | ErbB2+ BC, neoadjuvant | Open label, RCT, lapatinib versus trastuzumab versus lapatinib + trastuzumab; addition of paclitaxel for all treatment arms after 6 weeks | III | 450 |
| NCT00486668 (NSABP B-41) | ErbB2+ BC, neoadjuvant | Open label, RCT, lapatinib + AC + paclitaxel versus trastuzumab + AC + paclitaxel versus lapatinib + trastuzumab + AC + paclitaxel | III | 522 |
| NCT00490139 (EGF106708, BIG 2-06, ALTTO) | ErbB2+ BC, adjuvant | Open label, RCT, lapatinib versus trastuzumab versus trastuzumab then lapatinib versus lapatinib + trastuzumab | III | 8000 |
AC, doxorubicin + cyclophosphamide; BC, breast cancer; ErbB2+, human epidermal growth factor receptor 2-positive; MBC, metastatic breast cancer; MTD, maximum tolerated dose; RCT, randomized controlled trial.
aStudy identification codes for trials registered in the National Institutes of Health Clinical Trials Registry (http://clinicaltrials.gov, accessed 5 November 2009).
bPatient population ErbB2 status unknown.
The efficacy and safety results from this major clinical trial indicate that concurrent inhibition of ER and ErbB2 could indeed provide a new, oral, chemotherapy-free treatment regimen for patients with ER+/ErbB2+ metastatic breast cancer. Clinical acumen would still be required, however, to determine the most appropriate treatment strategy for each patient. Clinicians would need to take patient-related factors into account, such as the relative resistance to endocrine therapy, age, symptom status, rate of disease progression, tumor burden and extent of visceral disease.
Selecting the Most Appropriate Partners for Combination Therapy with Lapatinib
In an ideal world, clinicians would be able to review evidence from head-to-head comparator trials in different patient populations to help them select the most appropriate combination treatment regimen for each particular patient. In the real world, clinicians have to take several factors into account when deciding on which combinations of chemotherapeutic and non-chemotherapeutic agents are most appropriate for a particular patient. These factors might include synergy between agents, non-overlapping toxicity profiles, non-cross-resistant mechanisms of action, previous treatment exposure, generalizability of clinical data and affordability. These factors will likely also influence a clinician's choice of lapatinib-containing combination therapies that have been shown to be of clinical benefit in specific patient populations.
Preclinical Evidence: Combination Therapy with Lapatinib
Given lapatinib's targeted mechanism of action on ErbB1/ErbB2, preclinical studies have also been conducted to investigate the efficacy of lapatinib when partnered with either chemotherapy or other targeted non-chemotherapy agents. In the ErbB2+ BT474 mouse xenograft model, combinations of lapatinib and various chemotherapy agents (e.g. paclitaxel, docetaxel and vinorelbine) have resulted in significantly greater tumor growth inhibition than that achieved with chemotherapy agents alone (72). In addition, synergy between the lapatinib derivative, GW282974X and the capecitabine metabolite, 5′-deoxy-5-flurouridine, has been demonstrated in vitro (73). Preclinical studies have also shown the benefits of partnering lapatinib with non-chemotherapy agents that target pathways different to the ErbB2 pathway. As described in previous sections, lapatinib has been shown to act synergistically with endocrine treatments, such as tamoxifen and fulvestrant (60–62). Targeting the same pathway, but in different ways has also proven beneficial. Lapatinib, which targets both the ErbB1 and ErbB2 intracellular tyrosine kinase domain, has shown synergy in vitro with trastuzumab, which targets the ErbB2 extracellular domain, in the ErbB2-overexpressing MDA-MB-361 breast cancer cell line (22). The positive results from these preclinical studies provided the scientific justification for investigating lapatinib combination therapy in clinical trials.
Clinical Evidence: Combination Therapy with Lapatinib
The encouraging results from preclinical studies with lapatinib combination therapy are being complemented by positive efficacy and safety results from completed (Table 1) and ongoing (Table 3) clinical trials. In addition to trials using lapatinib plus capecitabine combination therapy, clinical trials of lapatinib and other chemotherapy agents have also had positive results. For example, lapatinib plus paclitaxel combination therapy in patients with ErbB2+ breast cancer resulted in a significant increase in TTP, compared with paclitaxel alone (EGF30001 study; Table 1). The most common adverse events (e.g. alopecia, rash and diarrhea) were expected and manageable (74). The availability of a large number of other effective chemotherapeutic agents for metastatic breast cancer and the lack of overlapping toxicities has allowed the development of ongoing clinical trials that combine lapatinib with other chemotherapy agents, such as docetaxel, doxorubicin, epirubicin, vinorelbine and temozolamide (Table 3). Promising efficacy and safety results have also been achieved in clinical trials of lapatinib and non-chemotherapy agents. Significant increases in PFS have been achieved when lapatinib has been partnered with letrozole (EGF30008) (66) or with trastuzumab (EGF104900) (Table 1) (35,42); there were no unexpected adverse events with either regimen and each regimen was well-tolerated. Interest in the potential role of vascular endothelial growth factor (VEGF) in ErbB2+ breast cancer has also led to clinical trials of lapatinib and non-chemotherapy agents that target VEGF or the VEGF receptor. A combination of lapatinib plus the VEGF receptor inhibitor, pazopanib, was associated with a significant increase in the proportion of patients who were progression-free at 12 weeks, compared with the proportion of patients treated with lapatinib alone (VEG20007) (75). Encouraging results were also obtained for PFS at 12 weeks in a single-arm clinical trial of lapatinib plus the anti-VEGF antibody, bevacizumab (EGF103890) (76). These combination regimens were well-tolerated and adverse events were consistent with expectations.
CONCLUSION
The management of patients with ErbB2+ breast cancer presents a number of challenges for clinicians in Asia, especially given the increasing incidence of breast cancer in Asia and the adverse clinical consequences of ErbB2+ breast cancer. Of particular clinical concern are challenges such as trastuzumab therapy failure, the development of CNS metastases, chemotherapy-related toxicity and selecting the most appropriate partners for combination therapy. Preclinical and clinical evidence suggests that lapatinib may help address these clinical challenges. Preclinical and clinical studies have shown that lapatinib is effective in inhibiting the growth of ErbB2+ tumors, including trastuzumab-resistant tumors. Notably, lapatinib plus capecitabine is approved for the treatment of patients with ErbB2+ locally advanced or metastatic breast cancer who develop progressive disease after treatment with trastuzumab-based regimens. Clinical studies have also shown that lapatinib, in combination with hormonal agents (e.g. letrozole), may provide a chemotherapy-free treatment option for postmenopausal patients with ER+/ErbB2+ metastatic breast cancer. More recently, promising results have emerged on the use of lapatinib to prevent and treat CNS metastases and on the synergy that may be achieved when lapatinib is combined with chemotherapeutic and non-chemotherapeutic agents for the treatment of ErbB2+ breast cancer. The number and nature of ongoing studies with lapatinib highlight the strong international interest in gaining further insight into how lapatinib may enhance the future management of ErbB2+ breast cancer. Nevertheless, considering the existing evidence base and our own clinical experience, we believe that lapatinib is a clinically effective and well-tolerated targeted oral therapy that clinicians in Asia, and around the world, can use judiciously to enhance their current management of patients with ErbB2+ breast cancer.
Funding
To enhance reader access to this peer-reviewed article, the authors opted for an open access publication. The open access and color printing publication charges for this article were funded by GlaxoSmithKline Asia Pacific.
Conflict of interest statement
Arlene Chan has received payments as an advisory board member from Roche and for speaking from Roche and GlaxoSmithKline; Junichi Kurebayashi has received payments for speaking from GlaxoSmithKline; Brunilde Gril has received grant support from the US Department of Defense Breast Cancer Research Program; Li Liu is an employee of and has shares in GlaxoSmithKline; Yen-Shen Lu has received grant support and payments for speaking from GlaxoSmithKline; Hanlim Moon is an employee of and has shares in GlaxoSmithKline; Charles Vogel has received grant support and payments for consulting and speaking from Genentech and grant support and payments for consulting, speaking and participation as a clinical trial investigator and advisory board member from GlaxoSmithKline.
Acknowledgements
The authors take full responsibility for the content of the manuscript, but wish to acknowledge Dr Patricia S. Steeg (National Cancer Institute, National Institutes of Health) as well as scientific and medical personnel at GlaxoSmithKline for reviewing this manuscript. The authors also acknowledge the independent medical writing assistance provided by Julie Ely, PhD and Karen Woolley, PhD of ProScribe Medical Communications (www.proscribe.com.au), funded from an unrestricted financial grant from GlaxoSmithKline Asia Pacific. ProScribe's services complied with international guidelines for Good Publication Practice 2.
References
- 1.Hirabayashi Y, Zhang M. Comparison of time trends in breast cancer incidence (1973–2002) in Asia, from cancer incidence in five continents, Vols IV–IX. Jpn J Clin Oncol. 2009;39:411–2. doi: 10.1093/jjco/hyp054. doi:10.1093/jjco/hyp054. [DOI] [PubMed] [Google Scholar]
- 2.Pegram MD, Pauletti G, Slamon DJ. HER-2/neu as a predictive marker of response to breast cancer therapy. Breast Cancer Res Treat. 1998;52:65–77. doi: 10.1023/a:1006111117877. doi:10.1023/A:1006111117877. [DOI] [PubMed] [Google Scholar]
- 3.Ross JS, Slodkowska EA, Symmans WF, Pusztai L, Ravdin PM, Hortobagyi GN. The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist. 2009;14:320–68. doi: 10.1634/theoncologist.2008-0230. doi:10.1634/theoncologist.2008-0230. [DOI] [PubMed] [Google Scholar]
- 4.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–82. doi: 10.1126/science.3798106. doi:10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 5.Rusnak DW, Lackey K, Affleck K, Wood ER, Alligood KJ, Rhodes N, et al. The effects of the novel, reversible epidermal growth factor receptor/ErbB-2 tyrosine kinase inhibitor, GW2016, on the growth of human normal and tumor-derived cell lines in vitro and in vivo. Mol Cancer Ther. 2001;1:85–94. [PubMed] [Google Scholar]
- 6.Medina PJ, Goodin S. Lapatinib: a dual inhibitor of human epidermal growth factor receptor tyrosine kinases. Clin Ther. 2008;30:1426–47. doi: 10.1016/j.clinthera.2008.08.008. doi:10.1016/j.clinthera.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 7.Paul B, Trovato JA, Thompson J. Lapatinib: a dual tyrosine kinase inhibitor for metastatic breast cancer. Am J Health Syst Pharm. 2008;65:1703–10. doi: 10.2146/ajhp070646. doi:10.2146/ajhp070646. [DOI] [PubMed] [Google Scholar]
- 8.Spector NL, Xia W, Burris H, III, Hurwitz H, Dees EC, Dowlati A, et al. Study of the biologic effects of lapatinib, a reversible inhibitor of ErbB1 and ErbB2 tyrosine kinases, on tumor growth and survival pathways in patients with advanced malignancies. J Clin Oncol. 2005;23:2502–12. doi: 10.1200/JCO.2005.12.157. doi:10.1200/JCO.2005.12.157. [DOI] [PubMed] [Google Scholar]
- 9.Lackey KE. Lessons from the drug discovery of lapatinib, a dual ErbB1/2 tyrosine kinase inhibitor. Curr Top Med Chem. 2006;6:435–60. doi: 10.2174/156802606776743156. doi:10.2174/156802606776743156. [DOI] [PubMed] [Google Scholar]
- 10.Lapatinib (Tykerb®) [full prescribing information] Research Triangle Park, NC: GlaxoSmithKline; 2009. August. [Google Scholar]
- 11.Nahta R, Esteva FJ. HER2 therapy: molecular mechanisms of trastuzumab resistance. Breast Cancer Res. 2006;8:215. doi: 10.1186/bcr1612. doi:10.1186/bcr612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valabrega G, Montemurro F, Aglietta M. Trastuzumab: mechanism of action, resistance and future perspectives in HER2-overexpressing breast cancer. Ann Oncol. 2007;18:977–84. doi: 10.1093/annonc/mdl475. doi:10.1093/annonc/mdl475. [DOI] [PubMed] [Google Scholar]
- 13.Burstein HJ, Kuter I, Campos SM, Gelman RS, Tribou L, Parker LM, et al. Clinical activity of trastuzumab and vinorelbine in women with HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2001;19:2722–30. doi: 10.1200/JCO.2001.19.10.2722. [DOI] [PubMed] [Google Scholar]
- 14.Marty M, Cognetti F, Maraninchi D, Snyder R, Mauriac L, Tubiana-Hulin M, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol. 2005;23:4265–74. doi: 10.1200/JCO.2005.04.173. doi:10.1200/JCO.2005.04.173. [DOI] [PubMed] [Google Scholar]
- 15.Montemurro F, Donadio M, Clavarezza M, Redana S, Jacomuzzi ME, Valabrega G, et al. Outcome of patients with HER2-positive advanced breast cancer progressing during trastuzumab-based therapy. Oncologist. 2006;11:318–24. doi: 10.1634/theoncologist.11-4-318. doi:10.1634/theoncologist.11-4-318. [DOI] [PubMed] [Google Scholar]
- 16.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92. doi: 10.1056/NEJM200103153441101. doi:10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 17.Nagata Y, Lan KH, Zhou X, Tan M, Esteva FJ, Sahin AA, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6:117–27. doi: 10.1016/j.ccr.2004.06.022. doi:10.1016/j.ccr.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 18.Fabi A, Metro G, Ferretti G, Giannarelli D, Di Cosimo S, Papaldo P, et al. Do HER-2 positive metastatic breast cancer patients benefit from the use of trastuzumab beyond disease progression? A mono-institutional experience and systematic review of observational studies. Breast. 2008;17:499–505. doi: 10.1016/j.breast.2008.03.006. doi:10.1016/j.breast.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 19.Metro G, Mottolese M, Fabi A. HER-2-positive metastatic breast cancer: trastuzumab and beyond. Expert Opin Pharmacother. 2008;9:2583–601. doi: 10.1517/14656566.9.15.2583. doi:10.1517/14656566.9.15.2583. [DOI] [PubMed] [Google Scholar]
- 20.von Minckwitz G, du Bois A, Schmidt M, Maass N, Cufer T, de Jongh FE, et al. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a German Breast Group 26/Breast International Group 03–05 study. J Clin Oncol. 2009;27:1999–2006. doi: 10.1200/JCO.2008.19.6618. doi:10.1200/JCO.2008.19.6618. [DOI] [PubMed] [Google Scholar]
- 21.Nahta R, Yu D, Hung M-C, Hortobagyi GN, Esteva FJ. Mechanisms of Disease: understanding resistance to HER2-targeted therapy in human breast cancer. Nat Clin Pract Oncol. 2006;3:269–80. doi: 10.1038/ncponc0509. doi:10.1038/ncponc0509. [DOI] [PubMed] [Google Scholar]
- 22.Konecny GE, Pegram MD, Venkatesan N, Finn R, Yang G, Rahmeh M, et al. Activity of the dual kinase inhibitor lapatinib (GW572016) against HER-2-overexpressing and trastuzumab-treated breast cancer cells. Cancer Res. 2006;66:1630–9. doi: 10.1158/0008-5472.CAN-05-1182. doi:10.1158/0008-5472.CAN-05-1182. [DOI] [PubMed] [Google Scholar]
- 23.Nahta R, Yuan LXH, Du Y, Esteva FJ. Lapatinib induces apoptosis in trastuzumab-resistant breast cancer cells: effects on insulin-like growth factor I signaling. Mol Cancer Ther. 2007;6:667–74. doi: 10.1158/1535-7163.MCT-06-0423. doi:10.1158/1535-7163.MCT-06-0423. [DOI] [PubMed] [Google Scholar]
- 24.Lu Y, Zi X, Zhao Y, Mascarenhas D, Pollak M. Insulin-like growth factor-I receptor signaling and resistance to trastuzumab (Herceptin) J Natl Cancer Inst. 2001;93:1852–7. doi: 10.1093/jnci/93.24.1852. doi:10.1093/jnci/93.24.1852. [DOI] [PubMed] [Google Scholar]
- 25.Nahta R, Yuan LXH, Zhang B, Kobayashi R, Esteva FJ. Insulin-like growth factor-I receptor/human epidermal growth factor receptor 2 heterodimerization contributes to trastuzumab resistance of breast cancer cells. Cancer Res. 2005;65:11118–28. doi: 10.1158/0008-5472.CAN-04-3841. doi:10.1158/0008-5472.CAN-04-3841. [DOI] [PubMed] [Google Scholar]
- 26.Scaltriti M, Rojo F, Ocana A, Anido J, Guzman M, Cortes J, et al. Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J Natl Cancer Inst. 2007;99:628–38. doi: 10.1093/jnci/djk134. [DOI] [PubMed] [Google Scholar]
- 27.Berns K, Horlings HM, Hennessy BT, Madiredjo M, Hijmans EM, Beelen K, et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007;12:395–402. doi: 10.1016/j.ccr.2007.08.030. doi:10.1016/j.ccr.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 28.Xia W, Husain I, Liu L, Bacus S, Saini S, Spohn J, et al. Lapatinib antitumor activity is not dependent upon phosphatase and tensin homologue deleted on chromosome 10 in ErbB2-overexpressing breast cancers. Cancer Res. 2007;67:1170–5. doi: 10.1158/0008-5472.CAN-06-2101. doi:10.1158/0008-5472.CAN-06-2101. [DOI] [PubMed] [Google Scholar]
- 29.Kataoka Y, Mukohara T, Shimada H, Saijo N, Hirai M, Minami H. Association between gain-of-function mutations in PIK3CA and resistance to HER2-targeted agents in HER2-amplified breast cancer cell lines. Ann Oncol. 2009 doi: 10.1093/annonc/mdp304. Epub 2009 July 24. doi: 10.1093/annonc/mdp304. [DOI] [PubMed] [Google Scholar]
- 30.Eichhorn PJA, Gili M, Scaltriti M, Serra V, Guzman M, Nijkamp W, et al. Phosphatidylinositol 3-kinase hyperactivation results in lapatinib resistance that is reversed by the mTOR/phosphatidylinositol 3-kinase inhibitor NVP-BEZ235. Cancer Res. 2008;68:9221–30. doi: 10.1158/0008-5472.CAN-08-1740. doi:10.1158/0008-5472.CAN-08-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu L, Greger J, Shi H, Liu Y, Greshock J, Annan R, et al. Novel mechanism of lapatinib resistance in HER2-positive breast tumor cells: activation of AXL. Cancer Res. 2009;69:6871–8. doi: 10.1158/0008-5472.CAN-08-4490. doi:10.1158/0008-5472.CAN-08-4490. [DOI] [PubMed] [Google Scholar]
- 32.Nagy P, Friedlander E, Tanner M, Kapanen AI, Carraway KL, Isola J, et al. Decreased accessibility and lack of activation of ErbB2 in JIMT-1, a Herceptin-resistant, MUC4-expressing breast cancer cellline. Cancer Res. 2005;65:473–82. [PubMed] [Google Scholar]
- 33.Carraway KL, Price-Schiavi SA, Komatsu M, Jepson S, Perez A, Carraway CA. Muc4/sialomucin complex in the mammary gland and breast cancer. J Mammary Gland Biol Neoplasia. 2001;6:323–37. doi: 10.1023/a:1011327708973. doi:10.1023/A:1011327708973. [DOI] [PubMed] [Google Scholar]
- 34.Frampton JE. Lapatinib: a review of its use in the treatment of HER2-overexpressing, trastuzumab-refractory, advanced or metastatic breast cancer. Drugs. 2009;69:2125–48. doi: 10.2165/11203240-000000000-00000. doi:10.2165/11203240-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 35.Blackwell KL, Burstein H, Storniolo AM, Rugo H, Sledge GW, Koehler M, et al. A randomized study of lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol. 2010;28:1124–30. doi: 10.1200/JCO.2008.21.4437. doi:10.1200/JCO.2008.21.4437. [DOI] [PubMed] [Google Scholar]
- 36.Cameron D, Casey M, Press M, Lindquist D, Pienkowski T, Romieu CG, et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: updated efficacy and biomarker analyses. Breast Cancer Res Treat. 2008;112:533–43. doi: 10.1007/s10549-007-9885-0. doi:10.1007/s10549-007-9885-0. [DOI] [PubMed] [Google Scholar]
- 37.Crown J, Casey MA, Cameron D, Newstat B, Stein SH. Lapatinib (L) plus capecitabine (C) in HER2+ metastatic breast cancer (MBC): exploratory analyses by prior therapy. Eur J Cancer Suppl. 2009;7:285. Abstr: P-5082 doi:10.1016/S1359-6349(09)70974-7. [Google Scholar]
- 38.Blackwell KL, Pegram MD, Tan-Chiu E, Schwartzberg LS, Arbushites MC, Maltzman JD, et al. Single-agent lapatinib for HER2-overexpressing advanced or metastatic breast cancer that progressed on first- or second-line trastuzumab-containing regimens. Ann Oncol. 2009;20:1026–31. doi: 10.1093/annonc/mdn759. doi:10.1093/annonc/mdn759. [DOI] [PubMed] [Google Scholar]
- 39.Burstein HJ, Storniolo AM, Franco S, Forster J, Stein S, Rubin S, et al. A phase II study of lapatinib monotherapy in chemotherapy-refractory HER2-positive and HER2-negative advanced or metastatic breast cancer. Ann Oncol. 2008;19:1068–74. doi: 10.1093/annonc/mdm601. doi:10.1093/annonc/mdm601. [DOI] [PubMed] [Google Scholar]
- 40.Gomez HL, Doval DC, Chavez MA, Ang PCS, Aziz Z, Nag S, et al. Efficacy and safety of lapatinib as first-line therapy for ErbB2-amplified locally advanced or metastatic breast cancer. J Clin Oncol. 2008;26:2999–3005. doi: 10.1200/JCO.2007.14.0590. doi:10.1200/JCO.2007.14.0590. [DOI] [PubMed] [Google Scholar]
- 41.Toi M, Iwata H, Fujiwara Y, Ito Y, Nakamura S, Tokuda Y, et al. Lapatinib monotherapy in patients with relapsed, advanced, or metastatic breast cancer: efficacy, safety, and biomarker results from Japanese patients phase II studies. Br J Cancer. 2009;101:1676–82. doi: 10.1038/sj.bjc.6605343. doi:10.1038/sj.bjc.6605343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O'Shaughnessy J, Blackwell KL, Burstein H, Storniolo AM, Sledge G, Baselga J, et al. A randomized study of lapatinib alone or in combination with trastuzumab in heavily pretreated HER2+ metastatic breast cancer progressing on trastuzumab therapy. J Clin Oncol. 2008;26:1015. [cited 2009 Nov 17];Oral presentation available from: http://www.asco.org/ASCOv2/MultiMedia/Virtual+Meeting?vmview=vm_session_presentations_view&confID=55&sessionID=353 . [Google Scholar]
- 43.Migliaccio I, Gutierrez M, Wu M, Wong H, Pavlick A, Hilsenbeck SG, et al. PI3 kinase activation and response to trastuzumab or lapatinib in HER-2 overexpressing locally advanced breast cancer (LABC) Cancer Res. 2009;69 CTRC-AACR San Antonio Breast Cancer Symposium 2008. Abstr: 34. [Google Scholar]
- 44.McKian KP, Haluska P. Cixutumumab. Expert Opin Investig Drugs. 2009;18:1025–33. doi: 10.1517/13543780903055049. doi:10.1517/13543780903055049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.National Cancer Institute. Clinicaltrials.gov [updated 2009 Nov 30; cited 2009 Dec 3] NCT00684983: Capecitabine and lapatinib with or without cixutumumab in treating patients with previously treated HER2-positive stage IIIB, Stage IIIC, or stage IV breast cancer. Available from: http://www.clinicaltrials.gov/ct2/show/NCT00684983 .
- 46.Lin NU, Winer EP. Brain metastases: the HER2 paradigm. Clin Cancer Res. 2007;13:1648–55. doi: 10.1158/1078-0432.CCR-06-2478. doi:10.1158/1078-0432.CCR-06-2478. [DOI] [PubMed] [Google Scholar]
- 47.Park YH, Park MJ, Ji SH, Yi SY, Lim DH, Nam DH, et al. Trastuzumab treatment improves brain metastasis outcomes through control and durable prolongation of systemic extracranial disease in HER2-overexpressing breast cancer patients. Br J Cancer. 2009;100:894–900. doi: 10.1038/sj.bjc.6604941. doi:10.1038/sj.bjc.6604941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dawood S, Broglio K, Esteva FJ, Ibrahim NK, Kau SW, Islam R, et al. Defining prognosis for women with breast cancer and CNS metastases by HER2 status. Ann Oncol. 2008;19:1242–8. doi: 10.1093/annonc/mdn036. doi:10.1093/annonc/mdn036. [DOI] [PubMed] [Google Scholar]
- 49.Pestalozzi BC, Zahrieh D, Price KN, Holmberg SB, Lindtner J, Collins J, et al. Identifying breast cancer patients at risk for Central Nervous System (CNS) metastases in trials of the International Breast Cancer Study Group (IBCSG) Ann Oncol. 2006;17:935–44. doi: 10.1093/annonc/mdl064. doi:10.1093/annonc/mdl064. [DOI] [PubMed] [Google Scholar]
- 50.Clayton AJ, Danson S, Jolly S, Ryder WDJ, Burt PA, Stewart AL, et al. Incidence of cerebral metastases in patients treated with trastuzumab for metastatic breast cancer. Br J Cancer. 2004;91:639–43. doi: 10.1038/sj.bjc.6601970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Palmieri D, Bronder JL, Herring JM, Yoneda T, Weil RJ, Stark AM, et al. Her-2 overexpression increases the metastatic outgrowth of breast cancer cells in the brain. Cancer Res. 2007;67:4190–8. doi: 10.1158/0008-5472.CAN-06-3316. doi:10.1158/0008-5472.CAN-06-3316. [DOI] [PubMed] [Google Scholar]
- 52.Pienkowski T, Zielinski CC. Trastuzumab treatment in patients with breast cancer and metastatic CNS disease. Ann Oncol. 2009 doi: 10.1093/annonc/mdp353. Epub 2009 Aug 28. doi: 10.1093/annonc/mdp353. [DOI] [PubMed] [Google Scholar]
- 53.Stemmler J, Schmitt M, Willems A, Bernhard H, Harbeck N, Heinemann V. Brain metastases in HER2-overexpressing metastatic breast cancer: Comparative analysis of trastuzumab levels in serum and cerebrospinal fluid. J Clin Oncol. 2006;24 suppl 18 ASCO Meeting Abstr:1525. [Google Scholar]
- 54.Gril B, Palmieri D, Bronder JL, Herring JM, Vega-Valle E, Feigenbaum L, et al. Effect of lapatinib on the outgrowth of metastatic breast cancer cells to the brain. J Natl Cancer Inst. 2008;100:1092–103. doi: 10.1093/jnci/djn216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–43. doi: 10.1056/NEJMoa064320. doi:10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 56.Lin NU, Carey LA, Liu MC, Younger J, Come SE, Ewend M, et al. Phase II trial of lapatinib for brain metastases in patients with human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2008;26:1993–9. doi: 10.1200/JCO.2007.12.3588. doi:10.1200/JCO.2007.12.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lin NU, Dieras V, Paul D, Lossignol D, Christodoulou C, Stemmler H-J, et al. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin Cancer Res. 2009;15:1452–9. doi: 10.1158/1078-0432.CCR-08-1080. doi:10.1158/1078-0432.CCR-08-1080. [DOI] [PubMed] [Google Scholar]
- 58.Boccardo F, Kaufman B, Baselga J, Dieras V, Link J, Casey MA, et al. Evaluation of lapatinib (Lap) plus capecitabine (Cap) in patients with brain metastases (BM) from HER2+ breast cancer (BC) enrolled in the Lapatinib Expanded Access Program (LEAP) and French Authorisation Temporaire d'Utilisation (ATU) J Clin Oncol. 2008;26 suppl. 15 ASCO Meeting Abstr:1094. [Google Scholar]
- 59.Hassett MJ, O'Malley AJ, Pakes JR, Newhouse JP, Earle CC. Frequency and cost of chemotherapy-related serious adverse effects in a population sample of women with breast cancer. J Natl Cancer Inst. 2006;98:1108–17. doi: 10.1093/jnci/djj305. [DOI] [PubMed] [Google Scholar]
- 60.Chu I, Blackwell K, Chen S, Slingerland J. The dual ErbB1/ErbB2 inhibitor, lapatinib (GW572016), cooperates with tamoxifen to inhibit both cell proliferation- and estrogen-dependent gene expression in antiestrogen-resistant breast cancer. Cancer Res. 2005;65:18–25. [PubMed] [Google Scholar]
- 61.Nomura T. Additive antitimor effects of the HER1/HER2 tyrosine kinase inhibitor, lapatinib with antiestrogen, fulvestrant in breast cancer cells. Kawasaki Med J. 2007;33:277–87. [Google Scholar]
- 62.Emde AM, Maslak K, Liu H, Reles AE, Possinger K, Eucker J. Combination of fulvestrant and lapatinib in non-HER2-overexpressing and adriamycin-resistant breast cancer cell lines. J Clin Oncol. 2007;25 suppl 18 ASCO Meeting Abstr:14050. [Google Scholar]
- 63.De Laurentiis M, Arpino G, Massarelli E, Ruggiero A, Carlomagno C, Ciardiello F, et al. A meta-analysis on the interaction between HER-2 expression and response to endocrine treatment in advanced breast cancer. Clin Cancer Res. 2005;11:4741–8. doi: 10.1158/1078-0432.CCR-04-2569. doi:10.1158/1078-0432.CCR-04-2569. [DOI] [PubMed] [Google Scholar]
- 64.Johnston SR. Combinations of endocrine and biological agents: present status of therapeutic and presurgical investigations. Clin Cancer Res. 2005;11:889s–99s. [PubMed] [Google Scholar]
- 65.The NCCN clinical practice guidelines in oncology™ Breast Cancer (Version 1.2010) © 2009 National Comprehensive Cancer Network, Inc. [cited 2009 Nov 3]. Available from: http://www.nccn.org/professionals/physician_gls/PDF/breast.pdf .
- 66.Johnston S, Pippen J, Jr, Pivot X, Lichinitser M, Sadeghi S, Dieras V, et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J Clin Oncol. 2009;27:5538–46. doi: 10.1200/JCO.2009.23.3734. doi:10.1200/JCO.2009.23.3734. [DOI] [PubMed] [Google Scholar]
- 67.Benson AB, III, Ajani JA, Catalano RB, Engelking C, Kornblau SM, Martenson JA, Jr, et al. Recommended guidelines for the treatment of cancer treatment-induced diarrhea. J Clin Oncol. 2004;22:2918–26. doi: 10.1200/JCO.2004.04.132. doi:10.1200/JCO.2004.04.132. [DOI] [PubMed] [Google Scholar]
- 68.Crown JP, Burris HA, 3rd, Boyle F, Jones S, Koehler M, Newstat BO, et al. Pooled analysis of diarrhea events in patients with cancer treated with lapatinib. Breast Cancer Res Treat. 2008;112:317–25. doi: 10.1007/s10549-007-9860-9. doi:10.1007/s10549-007-9860-9. [DOI] [PubMed] [Google Scholar]
- 69.Lacouture ME, Laabs SM, Koehler M, Sweetman RW, Preston AJ, Di Leo A, et al. Analysis of dermatologic events in patients with cancer treated with lapatinib. Breast Cancer Res Treat. 2009;114:485–93. doi: 10.1007/s10549-008-0020-7. doi:10.1007/s10549-008-0020-7. [DOI] [PubMed] [Google Scholar]
- 70.Moy B, Goss PE. Lapatinib-associated toxicity and practical management recommendations. Oncologist. 2007;12:756–65. doi: 10.1634/theoncologist.12-7-756. doi:10.1634/theoncologist.12-7-756. [DOI] [PubMed] [Google Scholar]
- 71.Snyder RD, Boyle FM, Chan A, Craft PS, Boer RDE, Mainwaring PN, et al. Clinical recommendations for the use of lapatinib ditosylate plus capecitabine for patients with advanced or metastatic HER2-positive breast cancer. Asia Pac J Clin Oncol. 2009;5:4–16. doi:10.1111/j.1743-7563.2009.01194.x. [Google Scholar]
- 72.Mullin RJ, Murray DM, Onori J, Keith BR. Xenograft response to combination therapy with the ErbB1-ErbB2 tyrosine kinase inhibitor GW572016. Proceedings of the 95th Annual Meeting of the American Association for Cancer Research; 27–31 Mar 2004; Orlando, FL, USA. p. 882-c. Abstr: 3823. [Google Scholar]
- 73.Budman DR, Soong R, Calabro A, Tai J, Diasio R. Identification of potentially useful combinations of epidermal growth factor receptor tyrosine kinase antagonists with conventional cytotoxic agents using median effect analysis. Anticancer Drugs. 2006;17:921–8. doi: 10.1097/01.cad.0000224457.36522.60. doi:10.1097/01.cad.0000224457.36522.60. [DOI] [PubMed] [Google Scholar]
- 74.Di Leo A, Gomez HL, Aziz Z, Zvirbule Z, Bines J, Arbushites MC, et al. Phase III, double-blind, randomized study comparing lapatinib plus paclitaxel with placebo plus paclitaxel as first-line treatment for metastatic breast cancer. J Clin Oncol. 2008;26:5544–52. doi: 10.1200/JCO.2008.16.2578. doi:10.1200/JCO.2008.16.2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Slamon D, Gomez HL, Kabbinavar FF, Amit O, Richie M, Pandite L, et al. Randomized study of pazopanib + lapatinib vs. lapatinib alone in patients with HER2-positive advanced or metastatic breast cancer. J Clin Oncol. 2008;26 suppl 15 ASCO Meeting Abstr:1016. [Google Scholar]
- 76.Dickler M, Franco S, Stopeck A, Ma W, Nulsen B, Lyandres J, et al. Final results from a phase II evaluation of lapatinib (L) and bevacizumab (B) in HER2-overexpressing metastatic breast cancer (MBC) Cancer Res. 2009;69 CTRC-AACR San Antonio Breast Cancer Symposium 2008. Abstr: 3133. [Google Scholar]