Abstract
“Ca2+ buffers,” a class of cytosolic Ca2+-binding proteins, act as modulators of short-lived intracellular Ca2+ signals; they affect both the temporal and spatial aspects of these transient increases in [Ca2+]i. Examples of Ca2+ buffers include parvalbumins (α and β isoforms), calbindin-D9k, calbindin-D28k, and calretinin. Besides their proven Ca2+ buffer function, some might additionally have Ca2+ sensor functions. Ca2+ buffers have to be viewed as one of the components implicated in the precise regulation of Ca2+ signaling and Ca2+ homeostasis. Each cell is equipped with proteins, including Ca2+ channels, transporters, and pumps that, together with the Ca2+ buffers, shape the intracellular Ca2+ signals. All of these molecules are not only functionally coupled, but their expression is likely to be regulated in a Ca2+-dependent manner to maintain normal Ca2+ signaling, even in the absence or malfunctioning of one of the components.
Calcium-binding proteins, such as parvalbumin, modulate spatiotemporal aspects of calcium signals. They are integral components of a system of mutually dependent pumps, channels, and other proteins that preserves calcium homeostasis.
DEFINITION OF A CYTOSOLIC Ca2+ BUFFER
Molecules serving as chelators for Ca2+ ions must contain negatively charged groups arranged in such a way as to fulfill the necessary geometrical constraints for chemical coordination. Proteins with appropriately spaced acidic side-chain residues (e.g., glutamate, aspartate) and/or backbone carbonyl groups provide the “cage” in which a Ca2+ ion may fit in. Evolutionarily well-conserved protein families differing in the way the Ca2+ ions are bound include the annexins, the C2-domain proteins, the EF-hand proteins, the pentraxins, the vitamin-K-dependent proteins, and the intraorganellar low-affinity, high-capacity Ca2+-binding proteins; for more details on the structural diversity of EF-hand motifs, see Gifford et al. (2007), and for other Ca2+-binding sites, see Bindreither and Lackner (2009). However, the term Ca2+ buffer is applied only to a small subset of cytosolic proteins of the EF-hand family, including parvalbumins (PV; alpha and beta isoforms), calbindin-D9k (CB-D9k), calbindin-D28k (CB-D28k), and calretinin (CR). The majority of EF-hand proteins belong to the group of “Ca2+ sensors”; that is, binding of Ca2+ ions induces a conformational change, which permits them to interact with specific targets in a Ca2+-regulated manner. The prototypical examples of Ca2+ sensors are calmodulin (Chin and Means 2000) and proteins of the S100 family. However, if present at sufficiently high concentrations, Ca2+ sensors may also function as Ca2+ buffers. Importantly, cytosolic Ca2+ buffers do not act as buffers in analogy to chemical buffers such as pH buffers. The latter serve to clamp the pH to a predetermined value in such a way that the addition of an acid or a base elicits only a minor change in the pH of the solution. Therefore, the buffering capacity is highest when the pH is close to the pK value of the corresponding acid/base pair. The situation is fundamentally different for the cytosolic Ca2+ buffers. Under basal conditions, [Ca2+]i is in the order of 20–100 nM. Yet, the dissociation constants for Ca2+ (KD,Ca) of most Ca2+ buffers are almost one order of magnitude larger, ≈200 nM–1.5 µM (Table 1). Thus, in a resting cell, Ca2+ buffers are at large in their Ca2+-free state, ready to bind Ca2+ ions whenever [Ca2+]i increases. As a result of their specific properties, Ca2+ buffers act in different ways to modulate the spatiotemporal aspects of cytosolic Ca2+ signals. How a given Ca2+ buffer affects intracellular Ca2+ signals depends on several parameters, including the intracellular concentration (Intracellular Concentration), the affinity for Ca2+ and other metal ions (Metal-binding Affinities), the kinetics of Ca2+ binding and release (Metal-binding Kinetics), and the intracellular mobility (Mobility and Interaction with Ligands). When measuring the total Ca2+-buffering capacity (κS) of a cell, often the distinction is made between mobile and immobile buffers (Zhou and Neher 1993). The latter ones are defined as molecules capable of binding cytosolic Ca2+ that are not washed out when, for example, the plasma membrane is patched with a pipette. Very little is known about the molecular identity of immobile Ca2+ buffers, except their relatively low Ca2+ affinity; presumably, they are made up of cytosol-exposed stretches of membrane proteins and/or membrane-associated proteins with a rather low affinity for Ca2+ ions. Additionally, negatively charged phospholipid headgroups of the inner leaflet of the plasma membrane serve as “weak” Ca2+ chelators (McLaughlin et al. 1981). Thus, the slowly mobile or immobile buffers, together with mobile Ca2+ buffers, are responsible for the rather slow diffusion of Ca2+ ions inside a cell (Mobility and Interaction with Ligands).
Table 1.
Properties of selected Ca2+-binding proteins (adapted from Schwaller 2009).
| α PV | β PV (OM) | CB-D9k | CB-D28k | CR | |
|---|---|---|---|---|---|
| Ca2+-binding sites (functional) | 3 (2) | 3 (2) | 2 (2) | 6 (4) | 6 (5) |
| Ca2+-specific/ mixed Ca2+/Mg2+ sites | 0/2 | 1/1 | 2/0 | 4/0a | 5/0 |
| KD,Ca (nM) | 4–9b | Mixed: 42–45d | KD1 ≈ 200–500e KD2 ≈ 60–300 | high aff. (h)f KD1 ≈ 180–240 | KD(T) 28 µMg KD(R) 68 KD(app) 1.4 µM |
| Ca2+-specific: 590–780d | medium aff. (m) KD2 ≈ 410–510 | EF5: 36 µM | |||
| KD,Mg | ≈ 30 µMb | 160–250 µMd | 714 µMa | 4.5 mMh | |
| KD,Ca(app) (nM) at [Mg2+] of 0.5–1 mM | 150–250c | 230–310c | |||
| kon,Ca (µM−1s−1) | 6b | up to 1000i | h sites ≈ 12f | T sites: 1.8g | |
| m sites ≈ 82 | R sites: 310 | ||||
| kon,Mg (µM−1s−1) | 0.1–1c | site EF5: 7.3 | |||
| cooperativity | nok | ? yesl | yesm | yes | yes |
| nH ≈ 1.1–1.2a | nH ≈ 1.3–1.9g | ||||
| DCabuffer (µm2s−1) | 37–43n | >100o | ≈ 25p | ||
| ∼12 | ≈ 25 |
aAlthough considered as Ca2+-specific, at physiological [Mg2+]i, the apparent Ca2+ affinity is approximately two-fold lower (Berggard et al. 2002a).
cKD,Ca and kon for PV are [Mg2+] dependent; calculated values are estimates at [Mg2+]i 0.6–0.9 mM.
d(Cox et al. 1990).
fCB-D28k has high-affinity (h) and medium-affinity (m) sites, the stochiometry h/m is either 2/2 or 3/1 (Nagerl et al. 2000).
gFor details, see text and Faas et al. (2007).
iThe value represents the diffusion limit, assuming a maximal Ca2+ diffusion rate of ≈ 200 µm2s−1 (Martin et al. 1990).
kPV has 2 essentially identical Ca2+-binding sites with nH close to 1.
m(Akke et al. 1991).
nThe diffusion coefficients DCabuffer for PV in muscle myoplasm (37 µm2s−1) (Maughan and Godt 1999) and Purkinje cell dendrites of 43 µm2s−1; (Schmidt et al. 2003a); smaller values are measured in PC soma and axons (≈12 µm2s−1); (Schmidt et al. 2007a).
oCB-D28k’s mobility in PC dendrites is 26 µm2s−1 (Schmidt et al. 2005), clearly slower than in water (Gabso et al. 1997), and also slower than PV in PC dendrites.
pEstimation based on the similar size of CB-D28k and CR.
IMPORTANT PARAMETERS TO CHARACTERIZE Ca2+ BUFFERS
Intracellular Concentration
The difficulty in obtaining reliable values for Ca2+-buffer concentrations is linked to the fact that those cells that strongly express Ca2+ buffers are frequently only a subset of cells (often with complex morphologies) within composite tissues; for example, in a subset of neurons in the brain, in specific segments of the kidney nephron, etc. The concentration of PV is as high as 1.5 mM in the superfast swimbladder muscle of toadfish (Tikunov and Rome 2009) and approximately 1 mM in mouse fast-twitch muscle, while it is lower in other muscles and highly correlated with the speed of muscle relaxation (Heizmann et al. 1982). Within different neuron subpopulations, PV is on average one order of magnitude lower (50–150 µM): 80 µM in mouse (Schmidt et al. 2003b) and 120 µM in rat (Hackney et al. 2005) Purkinje cells; 150 µM in mouse cerebellar interneurons (Collin et al. 2005), and 100–300 µM in inner and outer hair cells from inner ear (Hackney et al. 2005). The concentration of mammalian β PV called oncomodulin (OM) is particularly high in rat cochlear outer hair cells (2–3 mM) (Hackney et al. 2005). High concentrations of CR (1.2 mM) are also present in tall saccular hair cells of the frog (Edmonds et al. 2000). In other cells, calretinin concentration is much lower: approximately 20 µM and 35 µM in rat inner and outer hair cells, respectively (Hackney et al. 2005), and 30–40 µM in mouse cerebellar granule cells (Gall et al. 2003). The concentration of CB-D28k is 150–360 µM in Purkinje cells (for a review, see Schwaller et al. 2002), and 40–50 µM in mature hippocampal dentate gyrus granule cells, CA3 interneurons, and in CA1 pyramidal cells (Muller et al. 2005). In cells expressing various mobile and immobile Ca2+ buffers of unknown identities and Ca2+-binding properties, the concept of the Ca2+-binding ratio of endogenous buffers has proven to be useful. The ratio of buffer-bound Ca2+ changes over free Ca2+ changes (κS ∼ [Ca2+ buffer]/KD,Ca) serves as a measure to compare the Ca2+-buffering capacity of different cell types. According to the single compartment and linear approximation model (Neher 1998), motor neurons with low cytosolic Ca2+ buffer expression also have a very low Ca2+-buffering capacity (κS < 50) (Lips and Keller 1998). Hippocampal principal neurons and PV-expressing interneurons have κS values in the order of 60 and 150, respectively (Lee et al. 2000b). The highest Ca2+-buffering capacity (κS of 900–2000) is seen in cerebellar Purkinje cells expressing high levels of CB-D28k and PV (Fierro and Llano 1996).
Metal-binding Affinities
Structurally, different types of EF-hand domains exist: the canonical EF-hand domain, comprising a Ca2+-binding loop of 12 amino acids, in which Ca2+ ions are mostly coordinated via oxygen atoms of carboxyl side chain groups, and several non-canonical ones (Gifford et al. 2007). Typical for proteins of the S100 family, including CB-D9k (Marenholz et al. 2004), is the presence of a noncanonical loop, termed pseudo (Ψ) EF-hand, consisting of a loop of 14 amino acids with an inside-out conformation compared to the canonical loop; that is, Ca2+-coordination is preferentially provided by backbone carbonyl groups (Nelson et al. 2002). Functionally, two types of EF-hand Ca2+-binding sites are discernable due to their different selectivity and affinity for Ca2+ and Mg2+ ions (Celio et al. 1996). The Ca2+-specific sites display affinities for Ca2+ (KCa) in the order of 10−3–10−7 M and significantly lower ones for Mg2+ (KMg = 10−1–10−2 M). The proteins CB-D28k and CR have 4 and 5 functional Ca2+-binding sites of this type, respectively. The mixed Ca2+/Mg2+ sites bind Ca2+ with high and Mg2+ with moderate affinity in a competitive manner (dissociation constants: KCa = 10−7–10−9 M; KMg = 10−3–10−5 M) (Table 1). PV is the prototypical example of a protein with two mixed sites. Based on PV’s affinities for Ca2+ and Mg2+ and with [Ca2+]i (of approximately 50 nM) and [Mg2+]i (0.5–1 mM) inside a cell under basal conditions, PV’s two Ca2+/Mg2+ sites are, to a large degree, occupied by Mg2+. In most proteins, EF-hand domains are paired; two helix-Ca2+-binding loop-helix regions are connected by a short stretch of 5–10 amino acid residues and form a functional unit. Aside from providing structural stability of EF-hand domains, due to the close apposition also of the Ca2+-binding loops in such a tandem domain (Fig. 1), binding of a Ca2+ ion to one loop may allosterically affect the other, both with respect to affinity and binding kinetics (Faas et al. 2007; Nelson et al. 2002). As a result of this building principle, the majority of EF-hand proteins have an even number of Ca2+-binding domains (2 in CB-D9k, 6 in CB-D28k and CR); the uneven number (3) for PV (alpha and beta) is, together with the group of penta-EF-hand Ca2+-binding proteins (Maki et al. 2002), rather the exception.
Figure 1.
3D-structures of selected EF-hand Ca2+ buffers. (A) Consensus sequence of the canonical EF-hand Ca2+-binding loop of 12 amino acids. Amino acids X, Y, Z, and –Z provide side-chain oxygen ligands, * provides the backbone carbonyl oxygen, and at –X, a water molecule is hydrogen-bonded to a loop residue. Amino acids most often present at a given position are shown below, and shaded residues are the most conserved ones (Marsden et al. 1990). At positions X and –Z, Asp (D) and Glu (E) are generally present, respectively. The seven oxygen ligands coordinating the Ca2+ ion are located at the seven corners of a pentagonal bipyramid, and the Ca2+ ion (not shown) is in the center (right). (B) Solution structure of Ca2+-bound human α PV; PDB: 1RJV. Both the CD domain (green) and EF domain (yellow/red) bind one Ca2+ ion each (green spheres) in canonical Ca2+-binding loops of 12 amino acids. The orthogonally oriented helices E and F (gray-shaded) are connected by the Ca2+-binding loop. Both Ca2+-binding sites in PV are of the Ca2+/Mg2+ mixed type. The N-terminal AB domain (blue) is necessary for protein stability. (C) NMR solution structure of bovine CB-D9k; PDB: 1B1G. The shown structure takes into account the Ca2+ ions and explicit solvent molecules. The N-terminal domain EF1 is a pseudo (Ψ) EF-hand with a larger loop of 14 amino acids, while the second domain (EF2) has a canonical Ca2+-binding loop of 12 amino acids. In both loops, the Glu residue at the position –Z with the 2 carboxyl oxygen atoms (red) serves as a bidentate ligand representing two corners of the pentagonal bipyramid. This residue, most often Glu (rarely Asp), is a critical determinant for the Ca2+ affinity of the entire loop; Ca2+ ions are shown as green spheres. The two Ca2+-binding loops are in close proximity and stabilized via short β-type interactions (gray-shaded area). (D) 3D NMR structure of CB-D28k; PDB: 2G9B. CB-D28k has a relatively compact structure comprising three Ca2+-binding units, each unit consisting of a pair of EF-hands. Ca2+-binding is restricted to the Ca2+-binding loop 1 in the N-terminal unit (blue), to both loops 3 and 4 in the middle unit (green), and to loop 5 in the C-terminal pair (yellow/red). EF-hands 2 and 6 are nonfunctional, with respect to Ca2+-binding. The Ca2+-binding loops flanked by two almost perpendicular alpha-helical regions are numbered from 1 to 6. Images B–D were generated with PDB ProteinWorkshop 1.50 (Moreland et al. 2005).
Metal-binding Kinetics
The majority of Ca2+ buffers have dissociation constants (KD,Ca) in the low micromolar range (Table 1). Thus, in a resting cell (e.g., muscle fibers, neurons), Ca2+ buffers are mostly in the Ca2+-free form. It is the kinetics of various Ca2+ buffers that strongly affect the spatiotemporal aspects of Ca2+ signals, in particular, in excitable cells (Schmidt et al. 2007b; Schmidt and Eilers 2009; Schwaller 2009). [Ca2+]i transients last for tens of milliseconds to several hundred milliseconds and, thus, are differently modulated by kinetically distinct Ca2+ buffers. Their on-rates for Ca2+ binding (kon) vary by more than two orders of magnitude. Typical fast buffers with Ca2+-specific sites, including CB-D9k and troponin C, have kon rates >108 M−1s−1 comparable to the on-rate of the synthetic buffer BAPTA. At the other end of the kinetic scale, on-rates for the slow-onset buffer PV are ≈3 × 106 M−1s−1 under physiological conditions with [Mg2+]i of ≈0.5–1.0 mM. PV’s slow on-rate is the result of PV’s Mg2+/Ca2+ binding sites, where the rate for Ca2+-binding is determined by the slow Mg2+ off-rate (Lee et al. 2000c) (Table 1). Of importance, in a nonphysiological setting, that is, in the absence of Mg2+, the on-rate of Ca2+ binding to PV is very rapid (1.08 × 108 M−1s−1), almost as fast as the “fast” buffers (Lee et al. 2000c).
Since all endogenous Ca2+ buffers have more than one Ca2+-binding site, a given protein may have sites with different affinities and kinetics; results for CB-D28k and CR are summarized here. CB-D28k contains two types of binding sites differing in KD,Ca and kon: One type is a high-affinity site (KD,Ca ≈ 200 nM) that binds Ca2+ with a kon comparable to that of EGTA (≈1 × 107 M−1 s−1), and the second type binds Ca2+ with intermediate affinity (KD,Ca ≈ 400–500 nM), but with an approximately 8-fold faster kon (Table 1). The ratio between high:intermediate affinity sites is either 3:1 or 2:2. The experimental data could be equally well modeled with either stochiometry for the two types of binding sites (Nagerl et al. 2000). For determining CR’s kinetic Ca2+-binding properties in vitro, cooperativity was included in the mathematical model (Table 1). CR contains four 4 high-affinity, cooperative binding sites (Schwaller et al. 1997; Stevens and Rogers 1997) organized into two indistinguishable pairs, probably 1 & 2 and 3 & 4 (Faas et al. 2007) and one independent low-affinity Ca2+-binding site (EF5) with an intrinsic dissociation constant (K’D) of ≈0.5 mM (Schwaller et al. 1997). Within cooperative pairs, the two binding sites influence each other in an allosteric manner. In a pair, initially both binding sites are in a T- (tense) state, with a low affinity and slow binding rate. When the first Ca2+ ion is bound to a pair, the unoccupied site changes to an R- (relaxed) state, characterized by a high affinity and a fast binding rate. This leads to a gradual increase in the Ca2+ association rate (kon) as [Ca2+]i increases from a concentration in a resting neuron (≈50 nM) to approximately 1–10 µM after opening of Ca2+ channels (Schwaller 2009). Therefore, the kinetics of Ca2+ buffering of CR powerfully depends on the prevailing Ca2+ concentration prior to a perturbation, resulting in non-linear Ca2+ buffering by CR (for more details, see Fig. 3 in Schwaller 2009 and Faas et al. 2007).
Mobility and Interaction with Ligands
In an aqueous solution, the mobility defined as the diffusion coefficient (D) of a molecule is approximately proportional to the hydrodynamic radius (i.e., approximately proportional to the relative molecular mass; Mr). For relatively large molecules, such as dextrans and globular proteins, D should be proportional to the inverse cubic root of Mr. In Purkinje cells, PV is freely mobile, but D can vary considerably in different compartments: ≈12 µm2/s in axons, somata, and nuclei (Schmidt et al. 2007a) versus ≈43 µm2/s in dendrites (Schmidt et al. 2003a), most likely as the result of different cytoplasmic properties (e.g., tortuosity; i.e., diffusion in a porous medium). The latter value is very similar to PV’s mobility in frog myoplasm, 43 and 32 µm2/s for transverse and longitudinal diffusion, respectively (Maughan and Godt 1999), but clearly smaller than in an aqueous solution: 140 µm2/s (Feher 1984). As expected from the larger Mr of CB-D28k (≈29 kDa) compared to PV (≈12 kDa), D in Purkinje cell dendrites is smaller: 26 µm2/s (Schmidt et al. 2005). How the presence of a Ca2+ buffer affects the spatiotemporal aspects of a cytosolic Ca2+ transient not only depends on the mobility of the Ca2+ buffer, but also on the mobility of the free Ca2+ ions. In the cytosol isolated from Xenopus laevis oocytes, the diffusion coefficient D of inositol 1,4,5-trisphophate (InsP3) is much bigger than that of Ca2+ ions in a solution with [Ca2+]i of 90 nM: 283 µm2/s versus 13 µm2/s, respectively (Allbritton et al. 1992). Even when [Ca2+]i is increased to 1 µM, presumably saturating slowly mobile and immobile buffers, DCa only reaches a value of 65 µm2/s. This indicates that the slow diffusion of Ca2+ ions in a resting cell ([Ca2+]i ≈ 50 nM) is caused by slowly mobile or immobile buffers “acting like velcro” for Ca2+ ions, limiting the effective range of an unbuffered free Ca2+ ion to ≈0.1 µm. Thus, the range of Ca2+ can be increased by buffered diffusion, that is, mobile Ca2+ buffers acting as shuttles transporting Ca2+ through the “mesh of immobile buffers” (Schmidt et al. 2007b).
Parvalbumins
Structural Aspects of Parvalbumins
The first prototypical structure of an EF-hand domain was determined in PV (Kretsinger and Nockolds 1973), an atypical EF-hand protein; the protein (Mr ≈ 12 kDa; human gene symbol: PVALB) has an uneven number (3) of EF-hand domains, and the Ca2+ -binding sites are Ca2+/Mg2+ mixed sites (Table 1). While the two C-terminal domains CD and EF are functional metal-binding sites, the N-terminal AB site is necessary for the PV’s stability (Fig. 1) (Cox et al. (1999). The CD and EF domains form a pair consisting of two helix-loop-helix regions linked by a short stretch of 5–10 amino acid residues. In both sites, the Ca2+ ion in the center of the loop is coordinated by seven ligands sitting in the corners of a pentagonal bipyramid (Swain et al. 1989). Results based on solution structures of α PV and β PV (Babini et al. 2004) in the Ca2+-loaded and the apo (metal-free) form are summarized: (I) PV’s Ca2+-loaded EF-hand domains and the linker region connecting the CD and EF domains are rather rigid structures; also the N- and C-termini of PV have a low intrinsic mobility (Baldellon et al. 1998); (II) Differences in the structure of apo- and Ca2+-loaded forms of rat PV are small, mostly confined to the loop region. Thus, Ca2+ binding does not require major structural rearrangements (Henzl and Tanner 2008); (III) The first two points also hold true for the Ca2+/Mg2+ (EF) site in rat β PV, while the noncanonical CD site undergoes significant structural alterations, when Ca2+ is removed from β PV (Henzl and Tanner 2007). Thus, the global rigidity of α PV favors this molecule to serve as a “simple” Ca2+ buffer, while the Ca2+-dependent conformational changes in β PV may provide β PV also with a Ca2+ sensor function.
Functional Aspects of Parvalbumin and Oncomodulin
Cells with high PV expression levels include a subset of mostly GABA-ergic neurons, fast-twitch muscle fibers, and epithelial cells in the early distal convoluted tubule (DCT1) in nephrons of the kidney. PV’s slow-onset Ca2+-binding properties affect Ca2+ transients in a particular way: The rate in rise in [Ca2+]i is hardly affected, but the initial rate of [Ca2+]i decay is increased. In the later phase of the decay, the unbinding of Ca2+ ions from PV prolongs the late phase of the [Ca2+]i decay. Thus, PV’s hallmark is the conversion of a monoexponential [Ca2+]i decay into a biexponential one (Collin et al. 2005; Lee et al. 2000c). In fast-twitch muscles, this increases muscle relaxation of an electrically induced muscle twitch, while barely affecting the contraction phase. In PV knockout mice (PV−/−), twitch half-relaxation rates in fast muscles are slower than in the PV-expressing wild-type (WT) muscles (Schwaller et al. 1999). Conversely, the overexpression of PV by injection of Pvalb cDNA into the rat slow-twitch muscle, soleus, significantly increases the speed of relaxation, without affecting the contraction (Muntener et al. 1995). Pvalb gene delivery in rat heart in vivo increases the rate of heart relaxation in normal hearts and in an animal model of slowed cardiac muscle relaxation (Szatkowski et al. 2001). Thus, PV or genetically “tuned” PV variants are discussed as potential tools to enhance cardiac diastolic function (Rodenbaugh et al. 2007; Wang and Metzger 2008). An often-neglected aspect is the role of Ca2+ buffers in acting as transient Ca2+ sources, prolonging the [Ca2+]i decay. In fast-twitch muscles subjected to long tetanic contractions, PV saturates with Ca2+ and consequently slows down relaxation (Raymackers et al. 2000). The slow decay component mediated by Ca2+-bound PV also leads to a robust, PV-dependent, delayed transmitter release at cerebellar interneuron–interneuron synapses subsequent to presynaptic bursts of action potentials (Collin et al. 2005).
The PV-mediated, biexponential [Ca2+]i decay is observed in: (I) PV-injected chromaffin cells (Lee et al. 2000c); (II) PV-containing hippocampal interneurons (Lee et al. 2000b); (III) PV-expressing molecular layer interneurons (MLI) in the cerebellum (Collin et al. 2005); (IV) presynaptic terminals of the calyx of Held (Muller et al. 2007); and (V) in Purkinje cell dendrites (Schmidt et al. 2007a). PV’s acceleration of the early phase of [Ca2+]i decay limits or slows down the buildup of residual [Ca2+]i in presynaptic terminals, thus affecting short-term plasticity. The effect is most pronounced at timepoints, when [Ca2+]i decay curves in the presence or absence of PV show the largest differences. PV’s effect on decreasing/preventing paired-pulse facilitation at synapses between MLI and Purkinje cells is most pronounced at ≈33 Hz (Caillard et al. 2000). Also in the presynaptic terminals of the calyx of Held, PV accelerates the decay of spatially averaged [Ca2+]i and paired-pulse facilitation (Muller et al. 2007). In hippocampal PV-interneurons, differences in paired-pulse modulation between WT and PV−/− mice are apparent only when trains at 33, 50, and 100 Hz are delivered (Vreugdenhil et al. 2003), likely due to differences in components of the Ca2+ signaling toolkit and/or more efficient presynaptic Ca2+ extrusion mechanisms. The largest relative effect of PV in preventing facilitation is seen at approximately 33 Hz, within the range of gamma frequency (30–80 Hz) oscillations. As a result, the power of kainite-induced gamma oscillations in area CA3 in vitro is approximately 3-fold higher in PV−/− versus WT tissue. This can be explained by an increased facilitation of GABA release at persistent high frequencies. In accordance with the hypothesis that changes in the inhibitory activity of PV neurons in the neocortex—often critically involved in strong perisomatic inhibition—may be a major mechanism underlying epileptic seizures (Mihaly et al. 1997). PV−/− mice have a lower threshold for pentylenetetrazole (PTZ)-induced seizures (Schwaller et al. 2004). The subpopulation of PV-immunoreactive (PV-ir) neurons is critically involved in controlling the output of principal neurons (Freund 2003); moreover, PV is not only a marker for these GABA-ergic interneurons, but contributes to controlling the network activity. The absence of PV in the cerebellar circuitry leads to the emergence of 160-Hz oscillations in vivo sustained by synchronous, rhythmic-firing Purkinje cells aligned along the parallel fiber axis (Servais et al. 2005). Also, PV−/− Purkinje cell-firing properties are different from WT ones: The complex spike duration and the spike pause are decreased, and the simple spike-firing rate is increased. These differences in firing properties, together with the oscillations, are the likely cause for the mild locomotor phenotype, that is, a slight impairment of motor coordination/motor learning (Farre-Castany et al. 2007).
Much less is known about OM’s specific function. OM is present in the organ of Corti (Thalmann et al. 1995), more precisely in cochlear outer hair cells (Sakaguchi et al. 1998) in gerbil, rat, and mouse. An extracellular role for OM in retinal ganglion cell regeneration (Yin et al. 2006) was reported, but see also Hauk et al. (2008) and Schwaller (2009). An up-regulation of OM occurs in PV−/− mice in a sparse subpopulation of neurons in the thalamus and in the dentate gyrus, as well as in partly varicose axons in the diencephalon (Csillik et al. 2010). The functional significance of ectopic OM expression and the exact identity of neurons expressing OM in PV−/− mice remain to be shown.
Structural and Functional Aspects of Calbindin-D9k
CB-D9k (human gene symbol: S100G) is the smallest protein with four alpha-helical regions forming an EF-hand pair consisting of a canonical (EF2) and a noncanonical/pseudo (EF1) EF-hand domain, joined by a linker region of 10 amino acids (Fig. 1). The tandem domain is stabilized by a short beta-type interaction between the two Ca2+-binding loops (Kordel et al. 1993). The Ca2+-binding affinities of individual subdomains are several orders of magnitude lower than for the corresponding sites within the intact protein. Thus, EF-hands organized in tandem domains are the physiological relevant structures (Finn et al. 1992; Nelson et al. 2002). KD,Ca values are almost identical for both sites (Table 1), and the two Ca2+ ions bind with positive cooperativity (Linse et al. 1991). CB-D9k undergoes Ca2+-induced conformational changes; they are, however, less pronounced than in the prototypical sensor calmodulin. This, together with no identified binding partners, indicates that CB-D9k most likely functions as a Ca2+ buffer, rather than a Ca2+ sensor (Skelton et al. 1994).
In a rat kidney, CB-D9k is expressed in the loop of Henle, the distal convoluted tubule, and in intercalated cells of the collecting duct (Bindels et al. 1991a). In a mouse, CB-D9k expression is present in late distal convoluted tubules (Lee et al. 2006) and the connecting tubules. Strong expression of CB-D9k is restricted to the first 2 cm of the duodenum (Huybers et al. 2007). CB-D9k is assumed to be a freely mobile molecule in the cytoplasm of specific epithelial cells of the kidney and duodenum. Its Ca2+-binding properties, the regulation by 1,25(OH)2 vitamin D3 in the intestine, and CB-D9k’s relative electrophoretic mobility led to the name calbindin-D9k (Kallfelz et al. 1967). In other tissues, CB-D9k expression is regulated also in other ways (Choi and Jeung 2008), for example, by estrogen in the uterus (Darwish et al. 1991) or by PTH in mouse primary renal tubular cells (Cao et al. 2002). Suggested functions of CB-D9k include a role in the regulation of Ca2+ transport processes across epithelial cells (Bindels et al. 1991b), but also as a Ca2+ buffer/shuttle optimally tuned for transcellular Ca2+ transport (Choi and Jeung 2008).
Calbindin-D28k
Structural and General Aspects of CB-D28k
CB-D28k (Mr ≈ 29 kDa; human gene symbol: CALB1) has six EF-hand domains, four of which bind Ca2+ with medium/high affinity (Cheung et al. 1993). EF-hand 2 is nonfunctional and under physiological conditions EF6 most likely is as well. The four medium/high affinity sites (Nagerl et al. 2000) are considered Ca2+-specific, albeit low affinity Mg2+ binding (KD,Mg ≈ 700 µM) to the same sites at physiological [Mg2+]i decreases the apparent Ca2+ affinity approximately two-fold; additionally, Mg2+ binding increases the cooperativity of Ca2+ binding (Berggard et al. 2002a). The NMR solution structure of Ca2+-bound rat CB-D28k reveals that it consists of a single, almost globular (ellipsoid) fold with six distinguishable EF-hand domains (Kojetin et al. 2006) (Fig. 1). The on-rates of CB-D28k’s fast binding sites (kon ≈ 8. x 107 M−1s−1) are fast enough to affect the early rising phase of Ca2+ transients; the peak amplitude is significantly decreased in WT Purkinje cells, when compared to Purkinje cells from CB-D28k−/− mice (Airaksinen et al. 1997). The fact that the time to peak is not significantly different in CB-D28k−/− and WT Purkinje cells indicates that the initial rise in [Ca2+]i is principally governed by the properties (density, kinetics) of the Ca2+ channels. In Purkinje cell dendrites, CB-D28k acts as a fast Ca2+ buffer for the first approximately 100 ms, reducing the peak [Ca2+]i amplitude to about one half, while later on prolonging the decay by acting as a Ca2+ source (Schmidt et al. 2003b).
Although principally considered as a freely mobile protein, CB-D28k binds to several identified target proteins including Ran-binding protein (RanBP) M (Lutz et al. 2003); caspase-3 (Bellido et al. 2000); 3′,5′-cyclic nucleotide phosphodiesterase (Reisner et al. 1992); plasma membrane ATPase (Morgan et al. 1986); L-type Ca2+ channel α subunit (CANAC1C) (Christakos et al. 2007); myo-inositol monophosphatase (IMPase) (Berggard et al. 2002b); and in the kidney to TRPV5 (Lambers et al. 2006). In most cases, binding studies were performed in vitro; the physiological implications of these interactions are not clear yet. In dendrites and spines of Purkinje cells, approximately 20% of CB-D28k molecules are temporarily, that is, for several seconds, immobile by their binding to IMPase, a key enzyme of the InsP3-signaling cascade, and the fraction of immobilized CB-D28k increases by climbing fiber stimulation (Schmidt et al. 2005). In summary, the above findings, together with CB-D28k’s Ca2+-dependent conformational changes, indicate additional Ca2+ sensor functions (Berggard et al. 2002a).
Functional Aspects of CB-D28k
Reported functions for CB-D28k include a role in Ca2+ resorption in the kidney (Boros et al. 2009) and modulation of insulin production and secretion in pancreatic beta cells (Reddy et al. 1997; Sooy et al. 1999). Data on a putative neuroprotective role against excitotoxicity have not yet resulted in a consistent picture (for a review, see Schwaller et al. 2002 and Schwaller 2009). Results obtained in CB-D28k−/− mice are summarized. At first glance, these mice show no phenotype related to development, the general morphology of the nervous system, the visual (Wassle et al. 1998) and auditory (Airaksinen et al. 2000) systems, or behavior under standard housing conditions (Airaksinen et al. 1997). CB-D28k−/− mice show a mild—however more severe than PV−/− mice—impairment in motor coordination/motor learning (Airaksinen et al. 1997; Farre-Castany et al. 2007), likely resulting from the 160-Hz oscillations in the cerebellum (Cheron et al. 2004; Servais et al. 2005). The motor coordination phenotype is due to CB-D28k’s absence in Purkinje cells, since this phenotype and alterations in Purkinje cell physiology also occur in mice with Purkinje cell-specific Calb1 ablation (Barski et al. 2003). At the cellular level, short-term plasticity between cortical multipolar bursting cells and pyramidal cells, or at the mossy fiber-CA3 pyramidal cell synapse in the hippocampus, is affected by CB-D28k (Blatow et al. 2003). The rapid saturation of presynaptic CB-D28k transiently decreases the Ca2+ buffering capacity, leading to enhanced facilitation (Blatow et al. 2003) by a mechanism called “facilitation by buffer saturation” (Maeda et al. 1999; Neher 1998). Ca2+ buffers, such as CB-D28k, also affect Ca2+-dependent inactivation (CDI) of voltage-dependent Ca2+ currents (ICa). In dentate gyrus granule cells with low or absent CB-D28k expression resulting from Ammon’s horn sclerosis in humans (AHS) (Nagerl and Mody 1998) or in mice with Calb1 gene ablation (Klapstein et al. 1998), CDI is increased, compared to CB-D28k-expressing granule cells, thereby decreasing the total Ca2+ load. Increased CDI in the absence of CB-D28k may be viewed as a protective/homeostatic mechanism to limit Ca2+ influx in order to augment the resistance against excitotoxicity and to protect the surviving neurons.
Structural and Functional Aspects of Calretinin
Human calretinin (Mr ≈ 31 kDa; gene symbol: CALB2) consists of 271 amino acids and has 6 EF-hand domains, five of which are able to bind Ca2+ ions (Schwaller et al. 1997; Stevens and Rogers 1997). Structural data (NMR) is available only for the N-terminal 100 amino acids of rat CR comprising EF-hand domains 1 and 2 (Palczewska et al. 2001). As in CB-D28k, the two domains form a relatively tight structure. CR’s Ca2+-dependent conformational changes, together with results from other in vitro studies, suggest that CR also may have Ca2+-sensor functions (Billing-Marczak and Kuznicki 1999).
A role for CR in neuroprotection against glutamate toxicity was postulated, but evidence is most often indirect or obtained in model systems in vitro (D’Orlando et al. 2001; Lukas and Jones 1994; Pike and Cotman 1995). For more details, see Schwaller 2009. CR−/− mice show impaired long-term potentiation (LTP) in the hippocampus (Gurden et al. 1998; Schurmans et al. 1997). While the effect on hippocampal LTP is indirect, the uniform expression of CR in cerebellar granule cells, together with the stereotypic cerebellar organization, has allowed for a detailed investigation of CR in vivo. In CR−/− granule cells, the excitability is increased, they show faster action potentials, and, under conditions generating repetitive spike discharges, show enhanced increases in frequency with injected currents (Gall et al. 2003). This leads to altered Ca2+ homeostasis in Purkinje cells. The firing properties of Purkinje cells are altered in alert CR−/− mice: The simple spike-firing rate increased the complex spike duration and the spike pause is shorter (Schiffmann et al. 1999). As in PV−/− and CB-D28k−/− mice, alert CR−/− mice show 160 Hz oscillations (Cheron et al. 2004) that appear phenotypically as an impairment of motor coordination. In alert “rescue” mice, where CR in CR−/− mice is selectively re-expressed in granule cells, granule cell excitability, as well as Purkinje cell firing, resembles that in WT mice. As a consequence, neither 160-Hz oscillations, nor motor coordination impairment, are detected in the rescue mice (Bearzatto et al. 2006).
The similarity of the oscillations and motor coordination deficits in mice deficient for either one of the three CaBPs points toward an effect at the cerebellar network level. In all three knockout strains, the oscillations are temporarily reduced by blocking of: (I) gap junctions between interneurons; (II) N-methyl-D-aspartate receptors; or (III) GABAA receptors. This indicates that oscillations emerge via a mechanism that synchronizes assemblies of Purkinje cells (mediated by parallel fiber excitation) and the network of chemically-coupled MLI. In addition, recurrent Purkinje cell collaterals (Orduz and Llano 2007) may be implicated in these oscillations (de Solages et al. 2008).
COMPARISON OF THE PHYSIOLOGICAL EFFECTS BROUGHT ABOUT BY CYTOSOLIC Ca2+ BUFFERS AND IMMOBILE “Ca2+ BUFFERS/STORES”, IN PARTICULAR BY MITOCHONDRIA
Cytosolic Ca2+ buffers may be viewed as transitory Ca2+ sinks/stores, and together with the plasmalemmal extrusion systems, Ca2+ uptake into ER compartments and mitochondria serve as a cell’s Ca2+ “off mechanisms”; Ca2+-loaded buffers, together with release from Ca2+-filled organelles (ER, mitochondria), subserve as the intracellular “on mechanisms” (Berridge et al. 2003). Here, I briefly put side-by-side the role of mitochondria in Ca2+ buffering/sequestration with the role of mobile cytosolic Ca2+ buffers. The comparison is primarily focused on excitable cells, mostly neurons (for a more detailed role on mitochondria and Ca2+ signaling, see Rimessi et al. 2008 and Szabadkai and Duchen 2008). In the large glutamatergic presynaptic terminals of the calyx of Held, mitochondria contribute to increase the rate in [Ca2+]i decay, when peak [Ca2+]i is >2.5 µM (Kim et al. 2005). This mitochondria-mediated increase in [Ca2+]i decay closely resembles the action of the “slow buffer” PV in the same terminals (Muller et al. 2007). At the physiological level, this delayed buffering by PV and mitochondria affects plasticity of synaptic transmission. More importantly, it has an effect on both short-term facilitation and short-term depression. Blocking mitochondrial Ca2+ uptake in the calyx of Held slows down the recovery from synaptic depression (Billups and Forsythe 2002), an effect that can be reverted by the addition of 1 mM EGTA. Slow release of mitochondrial Ca2+, but not from ER stores, leads to the post-tetanic potentiation (PTP) in motor axons contacting the opener muscle of the crayfish Procambarus clarkii leg (Tang and Zucker 1997). In analogy, at synapses between molecular layer interneurons (MLI), release of Ca2+, likely from Ca2+-bound PV, increases delayed transmitter release after an AP train (Collin et al. 2005). Both PV and mitochondria hardly affect basal synaptic transmission and show similar effects with respect to short-term modulation. The increased removal of intracellular Ca2+ by PV prevents the buildup of residual [Ca2+] and thus reduces paired-pulse facilitation at the calyx of Held and in MLI axon terminals; these findings were deduced by comparing PV−/− and WT mice (Collin et al. 2005; Muller et al. 2007). The reduced density of mitochondria in presynaptic axon terminals of synaptophilin knockout mice (snph−/−) affects short-term facilitation in cultured snph−/− hippocampal neurons. While basal synaptic transmission evidenced by single EPSCs and miniature AMPA currents is unaltered, facilitation is increased (Kang et al. 2008). This effect closely resembles the one observed at hippocampal interneuron/CA1 pyramidal neuron synapses in PV−/− mice, where facilitation of IPSCs is augmented at stimulation frequencies >33 Hz (Vreugdenhil et al. 2003).
Both mitochondria and cytosolic Ca2+ buffers have an effect on the spreading of intracellular Ca2+ waves. While in Xenopus laevis oocytes, energized mitochondria promote the propagation of Ca2+ waves (Boitier et al. 1999), mitochondria in astrocytes limit the rate and extent of Ca2+ wave propagation (Jouaville et al. 1995). Also, Ca2+ buffers affect Ca2+ waves. The fast buffer CR promotes the spreading of InsP3-evoked Ca2+ signals in oocytes, while the slow buffer PV shortens the duration of these Ca2+ signals and restricts the global responses to discrete localized events (puffs) (Dargan et al. 2004). In summary: (I) Both mitochondria and cytosolic Ca2+ buffers participate in the shaping of Ca2+ signals in presynaptic terminals and consequently have an effect on short-term modulation of synaptic plasticity, that is, facilitation, potentiation, and depression; (II) They also affect the spreading of Ca2+ waves; the effect depends on the cell type, on the kinetic properties of the cytosolic Ca2+ buffers, and also on ER luminal regulatory mechanisms involving the luminal Ca2+-binding protein calreticulin (Camacho and Lechleiter 1995); (III) In the systems investigated so far, PV and mitochondria mostly behave as slow-onset buffers, rarely affecting the maximal amplitude of Ca2+ signals, but increasing the rate of [Ca2+]i decay. In presynaptic terminals, the time window most strongly affected by the Ca2+ buffering action of PV and mitochondria is ≈10–200 ms after peak [Ca2+]I; (IV) The physiological effect of Ca2+ buffering/sequestering by mitochondria and Ca2+ buffers is dependent on the cell type, morphology of involved compartments (e.g., presynaptic terminal, soma) and, importantly, the contribution of all other components from the Ca2+ signaling toolkit; (V) Evidently, PV and mitochondria cannot completely replace one another with respect to Ca2+ buffering. They are still different with respect to several parameters: mobility, Ca2+ storing capacity, effects of Ca2+ binding/uptake on metabolism, Mg2+ effects, kinetics of Ca2+ binding/release, kinetics of synthesis/degradation, etc. The finding that mitochondria volume and PV expression levels are inversely correlated in several systems is discussed later in this article.
THE Ca2+ HOMEOSTASOME
How can a simple change in [Ca2+]i observed in, for example, muscle contraction, neurotransmission, or cell cycle regulation be used by cells to elicit the correct downstream events, as diverse as membrane fusion of neurosecretory vesicles with the plasma membrane or activation/repression of genes? The obvious parameters are the amplitude of the Ca2+ signal and the duration or the frequency at which these signals are generated. Subtler regulations comprise the cell morphology, where Ca2+ signals are restricted to certain regions: dendrites, soma, or axon terminals of nerve cells. Finally, molecules implicated in Ca2+ signaling may be spatially restricted; for example, Ca2+ channel subunits in active zones (Bucurenciu et al. 2008). To achieve the necessary precision of Ca2+ signals, cells require an accurately tunable system for regulating [Ca2+]i. Opening of plasma membrane Ca2+ channels or Ca2+ release from internal stores results in an initial increase in [Ca2+]i. The shape of the Ca2+ signal in the cytosol, both with respect to space and time, is then modulated by immobile and, if present, by mobile Ca2+ buffers. Finally, extrusion systems such as plasma membrane Ca2+ pumps (PMCA), the Na+/Ca2+ exchanger (NCX), and organellar uptake by the ER and/or mitochondria restore the initial situation with respect to [Ca2+]i. All of the above components are part of a cell’s “Ca2+-signaling toolkit” that is able to regulate the expression of its own components necessary for accurate and cell-specific Ca2+ signaling (Berridge et al. 2003; Schwaller 2009). One of the gene regulators is Ca2+ itself, and effects are mediated by Ca2+/calmodulin-dependent kinases (CaMK) and Ca2+-regulated phosphatases (e.g., calcineurin). As an example, long-term survival of cultured cerebellar granule cells is dependent on accurate Ca2+ signals necessitating temporal changes in the transcription of Ca2+-signaling toolkit components: IP3R and PMCAs 2 and 3 are up-regulated, while a PMCA4 splice variant and plasma membrane NCX2 are down-regulated in a calcineurin-dependent manner (Carafoli et al. 1999). Such adaptative/homeostatic mechanisms are also induced if Ca2+-signaling toolkit components are functionally compromised (e.g., in genetic diseases) or purposely eliminated in knockout mice. The network of molecules implicated in Ca2+ signaling, homeostasis, and its own regulation is termed the Ca2+ homeostasome (Schwaller 2007, 2009).
Results on the modulation of the Ca2+ homeostasome brought about by altered expression of CaBPs (e.g., in transgenic mice) are summarized. The most surprising finding is that in essentially all CaBP knockout mice and in a given cell type, the deleted CaBP is not compensated by up-regulating expression of one of the more than 240 other EF-hand family members. That is, in the subset of identified “PV-ergic” neurons (e.g., Purkinje cells, stellate, and basket cells in the cerebellum), none of the other Ca2+ buffers (CB-D28k, CB-D9k, or CR) are expressed in the above-mentioned neuron subtypes of PV−/− mice (Schwaller et al. 2004). The same holds true for CR-immunoreactive or CB-D28k-immunoreactive neurons in the respective knockout strains, CB-D28k−/− and CR−/− (Airaksinen et al. 1997; Schiffmann et al. 1999). The most notable exception is the up-regulation of CB-D9k in epithelial kidney cells in CB-D28k−/− mice (Zheng et al. 2004). Two plausible explanations for the absence of compensation/homeostatic mechanisms at the level of other Ca2+ buffers are presented: (I) Neurons once committed to express a certain Ca2+ buffer have permanently inactivated/repressed the promoter for other Ca2+ buffers; (II) The specific properties (affinities, kinetics, cooperativity, mobility) of any other Ca2+ buffer would not be adequate to restore “normal” Ca2+ signaling (Schwaller 2009). Thus, if not at the level of other Ca2+ buffers, how do cells cope with the absence of a particular Ca2+ buffer? Also, does the absence or impairment of other components of the Ca2+-signaling toolkit affect the expression of Ca2+ buffers?
Purkinje cells are characterized by extensive Ca2+ signaling and high expression levels of PV and CB-D28k and, thus, are well-suited to address these questions. The two Ca2+ buffers are present in the soma, axon, dendrites, and spines, indicating that they are principally mobile proteins. While PV is freely mobile in all compartments (Schmidt et al. 2007a), a fraction of CB-D28k molecules is immobilized in dendrites and spines by its binding to IMPase (Schmidt et al. 2005). The most striking alterations in the absence of PV are the morphological changes observed in the soma. The volume of mitochondria, Ca2+ sequestrating organelles that also serve as transient Ca2+ stores (Billups and Forsythe 2002; Murchison and Griffith 2000), is increased by about 40% selectively in a narrow compartment underneath the plasma membrane (Chen et al. 2006). Concomitantly, the subplasmalemmal smooth ER compartment is decreased (Fig. 2). These changes in the soma don’t occur in CB-D28k−/− Purkinje cells. In the latter, subtle changes in the spine morphology are evident: Spines are longer and spine head volume is increased (Vecellio et al. 2000). In spiny pyramidal cells, spine heads are considered as separate biochemical compartments with negligible Ca2+ diffusion via the spine neck (Sabatini et al. 2002). However, modeling studies in Purkinje cell spines have revealed that Ca2+ buffers are not only involved in modulating the shape of Ca2+ transients within the spines, but together with the spine neck geometry also define the amount of Ca2+ ions that may reach the parental dendrite and lead to activation of Ca2+-/CaM-dependent signaling cascades (Schmidt and Eilers 2009). Of the two principal Purkinje cell Ca2+ buffers, mostly CB-D28k is involved in spino-dendritic coupling by buffered Ca2+ diffusion, while the contribution of PV is minute. This is a likely explanation for the unaltered PV−/− Purkinje cell spine morphology. The absence of CB-D28k and PV not only affects Purkinje cell morphology, but also components directly involved in Ca2+ signaling. Cav2.1 (P/Q type) channels are the major voltage-operated Ca2+ channels of mature Purkinje cells (>90% of the whole-cell voltage-gated Ca2+ current) and regulate Ca2+ signaling and excitability of these cells. These channels are regulated by Ca2+-dependent feedback mechanisms consisting of both Ca2+-dependent facilitation (CDF) and inactivation (CDI). While the former process is essentially mediated by the Ca2+ sensor calmodulin (CaM) (Lee et al. 2000a), CDI is modulated by synthetic Ca2+ buffers (EGTA, BAPTA) and by the Ca2+ buffers PV and CB-D28k in vitro (Kreiner and Lee 2005). Of note, PV and CB-D28k affect Cav2.1 channel function differently than the synthetic buffers EGTA and BAPTA, often presumed to serve as close substitutes for endogenous Ca2+ buffers. CDI of Cav2.1 channel is assumed to depend on intracellular Ca2+ microdomains around Ca2+ channels. These microdomains are expected to be differently affected by various Ca2+ buffers, which in turn specifically influence the inactivation properties of the Ca2+ channels. Contrary to the expectation based on in vitro experiments, CDI in Purkinje cells of double knockout (CB-D28k−/−PV−/−) mice is not increased. However, P-type currents recorded in these cells exhibit increased voltage-dependent inactivation as the result of a decreased expression of the auxiliary Cavβ2a subunit compared to WT neurons (Kreiner et al. 2010) (Fig. 2). This, together with the observation that spontaneous action potentials are not different in CB-D28k−/−PV−/− and WT Purkinje cells, indicates that increased inactivation due to molecular switching of Cav2.1 beta subunits may preserve normal activity-dependent Ca2+ signals in the absence of PV and CB-D28k.
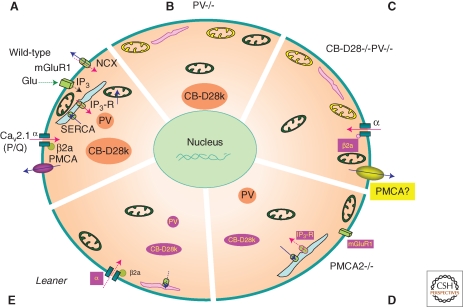
Figure 2.
Homeostatic/adaptive changes in the soma of Purkinje cells (PC) caused by malfunctioning or elimination of Ca2+-signaling toolkit component(s); regulation by the Ca2+ homeostasome. (A) A detailed situation is depicted for wild-type mice. Increases in [Ca2+]i (red arrows) result from influx via CaV2.1 (P/Q) channels or release from internal stores (light blue) via the IP3 receptor. IP3 is generated by the activation of metabotropic glutamate receptors (mGluR). Ca2+ removal systems (blue arrows) include PMCA and NCX in the plasma membrane, SERCA pumps, and mitochondria (green). Identified Ca2+-signaling toolkit components including organelles, which are up- or down-regulated, are marked in yellow and magenta, respectively. (B) In PV−/−, PC subplasmalemmal mitochondria are increased, while ER volume directly underneath the plasma membrane is decreased. (C) In addition to the changes observed in PV−/−, in PC lacking both, PV and CB-D28k the auxiliary Cavβ2a subunit of CaV2.1 (P/Q), is decreased, leading to increased voltage-dependent inactivation of P-type currents. Model studies indicate an up-regulation of Ca2+ extrusion systems, possibly PMCA. (D) In PMCA2−/− PC, expression of mGluR1 and of IP3 receptor type 1 (IP3R1), responsible for the Ca2+ release from ER stores, is decreased. Also, the cytosolic Ca2+ buffering capacity mediated by CB-D28k is decreased. (E) In leaner mice PC that are characterized by strongly attenuated Cav2.1 Ca2+ channel function, the rapid Ca2+ buffering/sequestering capacity is reduced: PV and CB-D28k are down-regulated and (subplasmalemmal) ER is decreased/impaired, leading to reduced Ca2+ uptake.
A cross talk between the regulation of a Ca2+ channel and Ca2+ buffer expression in Purkinje cells is also observed in leaner mice that have a mutation in the pore-forming alpha subunit of Cav2.1. The strongly-reduced Cav2.1 Ca2+ channel function leads to adaptive changes consisting of a diminished rapid Ca2+ buffering/sequestering capacity of Purkinje cells (Dove et al. 2000). The Ca2+ buffering capacity is less than 50% compared to Purkinje cells from WT mice, due to reduced PV and CB-D28k expression levels (Fig. 2). In addition, reduced Ca2+ uptake by the (likely subplasmalemmal) ER further contributes to the reduced buffering ability of leaner mice Purkinje cells (Murchison et al. 2002). Also, impairment of proteins responsible for Ca2+ extrusion in Purkinje cells activates the Ca2+ homeostasome. In Purkinje cells of mice with a mutation in PMCA2 characterized by a reduced Ca2+ extrusion, the rise in [Ca2+]i during high K+-induced depolarization is decreased. This is indicative of a Cav channel down-regulation likely to regulate [Ca2+]i toward normal homeostasis (Ueno et al. 2002). PMCA2−/− mice have decreased expression levels of CB-D28k (Hu et al. 2006), metabotropic glutamate receptor 1 (mGluR1), and of InsP3 receptor type 1 (IP3R1), responsible for the Ca2+ release from ER stores (Kurnellas et al. 2007) (Fig. 2). Again, this reduction in mGluR1-mediated [Ca2+]i elevation may be viewed as an adaptive mechanism to cope with the reduced Ca2+ extrusion. The authors suggest that “the decrease in the expression of mGluR1 and its downstream effectors and perturbations in the mGluR1-signaling complex in the absence of PMCA2 may cumulatively result in aberrant mGluR signaling in Purkinje neurons, leading to cerebellar deficits in the PMCA2-null mouse.” However, the severely distorted PMCA2−/− Purkinje cell morphology (smaller cell body, distorted dendritic tree) may also be a likely cause for the ataxic phenotype (Empson et al. 2007). In addition to the identified changes occurring in Purkinje cells of CB-D28k−/−PV−/− mice, an up-regulation of Ca2+ extrusion/uptake mechanisms was hypothesized, since the decay of dendritic Ca2+ signals could be accurately fitted only when applying a two-fold higher Ca2+ extrusion rate, compared to the rates sufficient to model the Ca2+ transients in PV−/− and WT Purkinje cells (Schmidt et al. 2003b). Currently, no experimental data is available to account for the increased dendritic [Ca2+]i decay in CB-D28k−/−PV−/− Purkinje cells; putative candidates are PMCA isoforms, NCX isoforms, or increased uptake into stores.
What are the evidences that the changes discussed above are not “simple” compensation mechanisms, but may be considered as “truly homeostatic” mechanisms? One argument is the generality of the mechanism and a second one the reciprocality as exemplified for the relationship between PV content and mitochondrial volume. In the absence of PV in PV−/− mice, an up-regulation of mitochondria occurs not only in PV-ergic Purkinje cells (Chen et al. 2006) or cerebellar stellate and basket cells (B Schwaller, unpubl.), but is also seen in PV−/− fast-twitch muscle fibers (Chen et al. 2001). The latter are characterized by high PV expression levels in WT mice. Vice versa, in transgenic mice ectopically expressing PV in striatal neurons (Van Den Bosch et al. 2002), a neuron subpopulation normally not expressing PV, the mitochondrial volume is decreased by almost 50% (Maetzler et al. 2004). This reduction accounts for the heightened excitotoxic injury provoked by a local injection of ibotenic acid. A last example of reciprocality: elimination of PV and CB-D28k from Purkinje cells alters Cav2.1 channel function (Kreiner et al. 2010), while a reduced Ca2+ influx due to a mutation in the Cav2.1 channel down-regulates PV and CB-D28k (Dove et al. 2000). The elucidation of the pathways and molecular mechanisms responsible for the regulation of the Ca2+ homeostasome remains an exciting topic for future research.
ACKNOWLEDGMENTS
I would like to thank Thomas Henzi and Walter-Vincent Blum, University of Fribourg, for helpful comments.
Footnotes
Editors: Martin D. Bootman, Michael J. Berridge, James W. Putney, and H. Llewelyn Roderick
Additional Perspectives on Calcium Signaling available at www.cshperspectives.org
REFERENCES
- Airaksinen L, Virkkala J, Aarnisalo A, Meyer M, Ylikoski J, Airaksinen MS 2000. Lack of calbindin-D28k does not affect hearing level or survival of hair cells in acoustic trauma. ORL J Otorhinolaryngol Relat Spec 62: 9–12 [DOI] [PubMed] [Google Scholar]
- Airaksinen MS, Eilers J, Garaschuk O, Thoenen H, Konnerth A, Meyer M 1997. Ataxia and altered dendritic calcium signaling in mice carrying a targeted null mutation of the calbindin D28k gene. Proc Natl Acad Sci U S A 94: 1488–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akke M, Forsen S, Chazin WJ 1991. Molecular basis for co-operativity in Ca2+ binding to calbindin D9k. 1H nuclear magnetic resonance studies of (Cd2+)1-bovine calbindin D9k. J Mol Biol 220: 173–189 [DOI] [PubMed] [Google Scholar]
- Allbritton NL, Meyer T, Stryer L 1992. Range of messenger action of calcium ion and inositol 1,4,5,-trisphophate. Science 258: 1812–1815 [DOI] [PubMed] [Google Scholar]
- Babini E, Bertini I, Capozzi F, Del Bianco C, Hollender D, Kiss T, Luchinat C, Quattrone A 2004. Solution structure of human beta-parvalbumin and structural comparison with its paralog alpha-parvalbumin and with their rat orthologs. Biochemistry 43: 16076–16085 [DOI] [PubMed] [Google Scholar]
- Baldellon C, Alattia JR, Strub MP, Pauls T, Berchtold MW, Cave A, Padilla A 1998. N-15 NMR relaxation studies of calcium-loaded parvalbumin show tight dynamics compared to those of other EF-hand proteins. Biochemistry 37: 9964–9975 [DOI] [PubMed] [Google Scholar]
- Barski JJ, Hartmann J, Rose CR, Hoebeek F, Morl K, Noll-Hussong M, De Zeeuw CI, Konnerth A, Meyer M 2003. Calbindin in cerebellar Purkinje cells is a critical determinant of the precision of motor coordination. J Neurosci 23: 3469–3477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearzatto B, Servais L, Roussel C, Gall D, Baba-Aissa F, Schurmans S, de Kerchove d’Exaerde A, Cheron G, Schiffmann SN 2006. Targeted calretinin expression in granule cells of calretinin-null mice restores normal cerebellar functions. FASEB J 20: 380–382 [DOI] [PubMed] [Google Scholar]
- Bellido T, Huening M, Raval-Pandya M, Manolagas SC, Christakos S 2000. Calbindin-D28k is expressed in osteoblastic cells and suppresses their apoptosis by inhibiting caspase-3 activity. J Biol Chem 275: 26328–26332 [DOI] [PubMed] [Google Scholar]
- Berggard T, Miron S, Onnerfjord P, Thulin E, Akerfeldt KS, Enghild JJ, Akke M, Linse S 2002a. Calbindin D28k exhibits properties characteristic of a Ca2+ sensor. J Biol Chem 277: 16662–16672 [DOI] [PubMed] [Google Scholar]
- Berggard T, Szczepankiewicz O, Thulin E, Linse S 2002b. Myo-inositol monophosphatase is an activated target of calbindin D28k. J Biol Chem 277: 41954–41959 [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Bootman MD, Roderick HL 2003. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol 4: 517–529 [DOI] [PubMed] [Google Scholar]
- Billing-Marczak K, Kuznicki J 1999. Calretinin–sensor or buffer–function still unclear. Pol J Pharmacol 51: 173–178 [PubMed] [Google Scholar]
- Billups B, Forsythe ID 2002. Presynaptic mitochondrial calcium sequestration influences transmission at mammalian central synapses. J Neurosci 22: 5840–5847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boitier E, Rea R, Duchen MR 1999. Mitochondria exert a negative feedback on the propagation of intracellular Ca2+ waves in rat cortical astrocytes. J Cell Biol 145: 795–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindels RJ, Hartog A, Timmermans JA, van Os CH 1991a. Immunocytochemical localization of calbindin-D28k, calbindin-D9k and parvalbumin in rat kidney. Contrib Nephrol 91: 7–13 [DOI] [PubMed] [Google Scholar]
- Bindels RJ, Timmermans JA, Hartog A, Coers W, van Os CH 1991b. Calbindin-D9k and parvalbumin are exclusively located along basolateral membranes in rat distal nephron. J Am Soc Nephrol 2: 1122–1129 [DOI] [PubMed] [Google Scholar]
- Bindreither D, Lackner P 2009. Structural diversity of calcium binding sites. Gen Physiol Biophys 28: F82–F88 [PubMed] [Google Scholar]
- Blatow M, Caputi A, Burnashev N, Monyer H, Rozov A 2003. Ca2+ buffer saturation underlies paired pulse facilitation in calbindin-D28k-containing terminals. Neuron 38: 79–88 [DOI] [PubMed] [Google Scholar]
- Boros S, Bindels RJ, Hoenderop JG 2009. Active Ca2+ reabsorption in the connecting tubule. Pflugers Arch 458: 99–109 [DOI] [PubMed] [Google Scholar]
- Bucurenciu I, Kulik A, Schwaller B, Frotscher M, Jonas P 2008. Nanodomain Coupling between Ca2+ Channels and Ca2+ Sensors Promotes Fast and Efficient Transmitter Release at a Cortical GABAergic Synapse. Neuron 57: 536–545 [DOI] [PubMed] [Google Scholar]
- Caillard O, Moreno H, Schwaller B, Llano I, Celio MR, Marty A 2000. Role of the calcium-binding protein parvalbumin in short-term synaptic plasticity. Proc Natl Acad Sci U S A 97: 13372–13377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho P, Lechleiter JD 1995. Calreticulin inhibits repetitive intracellular Ca2+ waves. Cell 82: 765–771 [DOI] [PubMed] [Google Scholar]
- Cao LP, Bolt MJ, Wei M, Sitrin MD, Chun Li Y 2002. Regulation of calbindin-D9k expression by 1,25-dihydroxyvitamin D(3) and parathyroid hormone in mouse primary renal tubular cells. Arch Biochem Biophys 400: 118–124 [DOI] [PubMed] [Google Scholar]
- Carafoli E, Genazzani A, Guerini D 1999. Calcium controls the transcription of its own transporters and channels in developing neurons. Biochem Biophys Res Commun 266: 624–632 [DOI] [PubMed] [Google Scholar]
- Celio M, Pauls T, Schwaller B (ed.) 1996. Guidebook to the Calcium-Binding Proteins. Oxford University Press, Oxford [Google Scholar]
- Chen G, Carroll S, Racay P, Dick J, Pette D, Traub I, Vrbova G, Eggli P, Celio M, Schwaller B 2001. Deficiency in parvalbumin increases fatigue resistance in fast-twitch muscle and upregulates mitochondria. Am J Physiol (Cell Physiol) 281: C114–C122 [DOI] [PubMed] [Google Scholar]
- Chen G, Racay P, Bichet S, Celio MR, Eggli P, Schwaller B 2006. Deficiency in parvalbumin, but not in calbindin D-28k upregulates mitochondrial volume and decreases smooth endoplasmic reticulum surface selectively in a peripheral, subplasmalemmal region in the soma of Purkinje cells. Neuroscience 142: 97–105 [DOI] [PubMed] [Google Scholar]
- Cheron G, Gall D, Servais L, Dan B, Maex R, Schiffmann SN 2004. Inactivation of calcium-binding protein genes induces 160 Hz oscillations in the cerebellar cortex of alert mice. J Neurosci 24: 434–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung WT, Richards DE, Rogers JH 1993. Calcium binding by chick calretinin and rat calbindin D28k synthesised in bacteria. Eur J Biochem 215: 401–410 [DOI] [PubMed] [Google Scholar]
- Chin D, Means AR 2000. Calmodulin: a prototypical calcium sensor. Trends Cell Biol 10: 322–328 [DOI] [PubMed] [Google Scholar]
- Choi KC, Jeung EB 2008. Molecular mechanism of regulation of the calcium-binding protein calbindin-D(9k), and its physiological role(s) in mammals: a review of current research. J Cell Mol Med 12: 409–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christakos S, Dhawan P, Peng X, Obukhov AG, Nowycky MC, Benn BS, Zhong Y, Liu Y, Shen Q 2007. New insights into the function and regulation of vitamin D target proteins. J Steroid Biochem Mol Biol 103: 405–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin T, Chat M, Lucas MG, Moreno H, Racay P, Schwaller B, Marty A, Llano I 2005. Developmental changes in parvalbumin regulate presynaptic Ca2+ signaling. J Neurosci 25: 96–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox JA, Milos M, MacManus JP 1990. Calcium- and magnesium-binding properties of oncomodulin. Direct binding studies and microcalorimetry. J Biol Chem 265: 6633–6637 [PubMed] [Google Scholar]
- Cox JA, Durussel I, Scott DJ, Berchtold MW 1999. Remodeling of the AB site of rat parvalbumin and oncomodulin into a canonical EF-hand. Eur J Biochem 264: 790–799 [DOI] [PubMed] [Google Scholar]
- Csillik B, Schwaller B, Mihaly A, Henzi T, Losonczic E, Knyihar-Csillik E 2010. Upregulated expression of oncomodulin, the beta isoform of parvalbumin, in perikarya and axons in the diencephalon of parvalbumin knockout mice. Neuroscience 165: 749–757 [DOI] [PubMed] [Google Scholar]
- D’Orlando C, Fellay B, Schwaller B, Salicio V, Bloc A, Gotzos V, Celio MR 2001. Calretinin and calbindin D-28k delay the onset of cell death after excitotoxic stimulation in transfected P19 cells. Brain Res 909: 145–158 [DOI] [PubMed] [Google Scholar]
- Dargan SL, Schwaller B, Parker I 2004. Spatiotemporal patterning of IP3-mediated Ca2+ signals in Xenopus oocytes by Ca2+-binding proteins. J Physiol 556: 447–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwish H, Krisinger J, Furlow JD, Smith C, Murdoch FE, DeLuca HF 1991. An estrogen-responsive element mediates the transcriptional regulation of calbindin D-9K gene in rat uterus. J Biol Chem 266: 551–558 [PubMed] [Google Scholar]
- de Solages C, Szapiro G, Brunel N, Hakim V, Isope P, Buisseret P, Rousseau C, Barbour B, Lena C 2008. High-frequency organization and synchrony of activity in the purkinje cell layer of the cerebellum. Neuron 58: 775–788 [DOI] [PubMed] [Google Scholar]
- Dove LS, Nahm SS, Murchison D, Abbott LC, Griffith WH 2000. Altered calcium homeostasis in cerebellar Purkinje cells of leaner mutant mice. J Neurophysiol 84: 513–524 [DOI] [PubMed] [Google Scholar]
- Eberhard M, Erne P 1994. Calcium and magnesium binding to rat parvalbumin. Eur J Biochem 222: 21–26 [DOI] [PubMed] [Google Scholar]
- Edmonds B, Reyes R, Schwaller B, Roberts WM 2000. Calretinin modifies presynaptic calcium signaling in frog saccular hair cells. Nat Neurosci 3: 786–790 [DOI] [PubMed] [Google Scholar]
- Empson RM, Garside ML, Knopfel T 2007. Plasma membrane Ca2+ ATPase 2 contributes to short-term synapse plasticity at the parallel fiber to Purkinje neuron synapse. J Neurosci 27: 3753–3758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faas GC, Schwaller B, Vergara JL, Mody I 2007. Resolving the fast kinetics of cooperative binding: Ca2+ buffering by calretinin. PLoS Biol 5: e311 doi: 310.1371/journal.pbio.0050311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farre-Castany MA, Schwaller B, Gregory P, Barski J, Mariethoz C, Eriksson JL, Tetko IV, Wolfer D, Celio MR, Schmutz I, Albrecht U, Villa AE 2007. Differences in locomotor behavior revealed in mice deficient for the calcium-binding proteins parvalbumin, calbindin D-28k or both. Behav Brain Res 178: 250–261 [DOI] [PubMed] [Google Scholar]
- Feher JJ 1984. Measurement of facilitated calcium diffusion by a soluble calcium-binding protein. Biochim Biophys Acta 773: 91–98 [DOI] [PubMed] [Google Scholar]
- Fierro L, Llano I 1996. High endogenous calcium buffering in Purkinje cells from rat cerebellar slices. J Physiol 496: 617–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn BE, Kordel J, Thulin E, Sellers P, Forsen S 1992. Dissection of calbindin D9k into two Ca2+-binding subdomains by a combination of mutagenesis and chemical cleavage. FEBS Lett 298: 211–214 [DOI] [PubMed] [Google Scholar]
- Freund TF 2003. Interneuron Diversity series: Rhythm and mood in perisomatic inhibition. Trends Neurosci 26: 489–495 [DOI] [PubMed] [Google Scholar]
- Gabso M, Neher E, Spira ME 1997. Low mobility of the Ca2+ buffers in axons of cultured Aplysia neurons. Neuron 18: 473–481 [DOI] [PubMed] [Google Scholar]
- Gall D, Roussel C, Susa I, D’Angelo E, Rossi P, Bearzatto B, Galas MC, Blum D, Schurmans S, Schiffmann SN 2003. Altered neuronal excitability in cerebellar granule cells of mice lacking calretinin. J Neurosci 23: 9320–9327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford JL, Walsh MP, Vogel HJ 2007. Structures and metal-ion-binding properties of the Ca2+-binding helix-loop-helix EF-hand motifs. Biochem J 405: 199–221 [DOI] [PubMed] [Google Scholar]
- Gurden H, Schiffmann SN, Lemaire M, Bohme GA, Parmentier M, Schurmans S 1998. Calretinin expression as a critical component in the control of dentate gyrus long-term potentiation induction in mice. Eur J Neurosci 10: 3029–3033 [DOI] [PubMed] [Google Scholar]
- Hackney CM, Mahendrasingam S, Penn A, Fettiplace R 2005. The concentrations of calcium buffering proteins in mammalian cochlear hair cells. J Neurosci 25: 7867–7875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauk TG, Muller A, Lee J, Schwendener R, Fischer D 2008. Neuroprotective and axon growth promoting effects of intraocular inflammation do not depend on oncomodulin or the presence of large numbers of activated macrophages. Exp Neurol 209: 469–482 [DOI] [PubMed] [Google Scholar]
- Heizmann CW, Berchtold MW, Rowlerson AM 1982. Correlation of parvalbumin concentration with relaxation speed in mammalian muscles. Proc Natl Acad Sci U S A 79: 7243–7247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henzl MT, Tanner JJ 2007. Solution structure of Ca2+-free rat beta-parvalbumin (oncomodulin). Protein Sci 16: 1914–1926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henzl MT, Tanner JJ 2008. Solution structure of Ca2+-free rat alpha-parvalbumin. Protein Sci 17: 431–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Qian J, Borisov O, Pan S, Li Y, Liu T, Deng L, Wannemacher K, Kurnellas M, Patterson C, Elkabes S, Li H 2006. Optimized proteomic analysis of a mouse model of cerebellar dysfunction using amine-specific isobaric tags. Proteomics 6: 4321–4334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huybers S, Naber TH, Bindels RJ, Hoenderop JG 2007. Prednisolone-induced Ca2+ malabsorption is caused by diminished expression of the epithelial Ca2+ channel TRPV6. Am J Physiol Gastrointest Liver Physiol 292: G92–G97 [DOI] [PubMed] [Google Scholar]
- Jouaville LS, Ichas F, Holmuhamedov EL, Camacho P, Lechleiter JD 1995. Synchronization of calcium waves by mitochondrial substrates in Xenopus laevis oocytes. Nature 377: 438–441 [DOI] [PubMed] [Google Scholar]
- Kallfelz FA, Taylor AN, Wasserman RH 1967. Vitamin D-induced calcium binding factor in rat intestinal mucosa. Proc Soc Exp Biol Med 125: 54–58 [DOI] [PubMed] [Google Scholar]
- Kang JS, Tian JH, Pan PY, Zald P, Li C, Deng C, Sheng ZH 2008. Docking of axonal mitochondria by syntaphilin controls their mobility and affects short-term facilitation. Cell 132: 137–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MH, Korogod N, Schneggenburger R, Ho WK, Lee SH 2005. Interplay between Na+/Ca2+ exchangers and mitochondria in Ca2+ clearance at the calyx of Held. J Neurosci 25: 6057–6065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapstein GJ, Vietla S, Lieberman DN, Gray PA, Airaksinen MS, Thoenen H, Meyer M, Mody I 1998. Calbindin-D28k fails to protect hippocampal neurons against ischemia in spite of its cytoplasmic calcium buffering properties: evidence from calbindin-D28k knockout mice. Neuroscience 85: 361–373 [DOI] [PubMed] [Google Scholar]
- Kojetin DJ, Venters RA, Kordys DR, Thompson RJ, Kumar R, Cavanagh J 2006. Structure, binding interface and hydrophobic transitions of Ca2+-loaded calbindin-D(28K). Nat Struct Mol Biol 13: 641–647 [DOI] [PubMed] [Google Scholar]
- Kordel J, Skelton NJ, Akke M, Chazin WJ 1993. High-resolution structure of calcium-loaded calbindin D9k. J Mol Biol 231: 711–734 [DOI] [PubMed] [Google Scholar]
- Kreiner L, Lee A 2005. Endogenous and exogenous Ca2+ buffers differentially modulate Ca2+-dependent inactivation of CaV2.1 Ca2+ channels. J Biol Chem 281: 4691–4698 [DOI] [PubMed] [Google Scholar]
- Kreiner L, Christel CJ, Benveniste M, Schwaller B, Lee A 2010. Compensatory regulation of Cav2.1 Ca2+ channels in cerebellar Purkinje neurons lacking parvalbumin and calbindin D-28k. J Neurophysiol 103: 371–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretsinger RH, Nockolds CE 1973. Carp muscle calcium-binding protein. II. Structure determination and general description. J Biol Chem 248: 3313–3326 [PubMed] [Google Scholar]
- Kurnellas MP, Lee AK, Li H, Deng L, Ehrlich DJ, Elkabes S 2007. Molecular alterations in the cerebellum of the plasma membrane calcium ATPase 2 (PMCA2)-null mouse indicate abnormalities in Purkinje neurons. Mol Cell Neurosci 34: 178–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambers TT, Mahieu F, Oancea E, Hoofd L, de Lange F, Mensenkamp AR, Voets T, Nilius B, Clapham DE, Hoenderop JG, Bindels RJ 2006. Calbindin-D28K dynamically controls TRPV5-mediated Ca2+ transport. Embo J 25: 2978–2988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, Scheuer T, Catterall WA 2000a. Ca2+/calmodulin-dependent facilitation and inactivation of P/Q-type Ca2+ channels. J Neurosci 20: 6830–6838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GS, Choi KC, Jeung EB 2006. Glucocorticoids differentially regulate expression of duodenal and renal calbindin-D9k through glucocorticoid receptor-mediated pathway in mouse model. Am J Physiol Endocrinol Metab 290: E299–E307 [DOI] [PubMed] [Google Scholar]
- Lee SH, Rosenmund C, Schwaller B, Neher E 2000b. Differences in Ca2+ buffering properties between excitatory and inhibitory hippocampal neurons from the rat. J Physiol 525: 405–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Schwaller B, Neher E 2000c. Kinetics of Ca2+ binding to parvalbumin in bovine chromaffin cells: implications for [Ca2+] transients of neuronal dendrites. J Physiol 525: 419–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linse S, Johansson C, Brodin P, Grundstrom T, Drakenberg T, Forsen S 1991. Electrostatic contributions to the binding of Ca2+ in calbindin D9k. Biochemistry 30: 154–162 [DOI] [PubMed] [Google Scholar]
- Lips MB, Keller BU 1998. Endogenous calcium buffering in motoneurones of the nucleus hypoglossus from mouse. J Physiol (Lond) 511: 105–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas W, Jones KA 1994. Cortical neurons containing calretinin are selectively resistant to calcium overload and excitotoxicity. Neuroscience 61: 307–316 [DOI] [PubMed] [Google Scholar]
- Lutz W, Frank EM, Craig TA, Thompson R, Venters RA, Kojetin D, Cavanagh J, Kumar R 2003. Calbindin D28K interacts with Ran-binding protein M: identification of interacting domains by NMR spectroscopy. Biochem Biophys Res Commun 303: 1186–1192 [DOI] [PubMed] [Google Scholar]
- Maeda H, Ellis-Davies GC, Ito K, Miyashita Y, Kasai H 1999. Supralinear Ca2+ signaling by cooperative and mobile Ca2+ buffering in Purkinje neurons. Neuron 24: 989–1002 [DOI] [PubMed] [Google Scholar]
- Maetzler W, Nitsch C, Bendfeldt K, Racay P, Vollenweider F, Schwaller B 2004. Ectopic parvalbumin expression in mouse forebrain neurons increases excitotoxic injury provoked by ibotenic acid injection into the striatum. Exp Neurol 186: 78–88 [DOI] [PubMed] [Google Scholar]
- Maki M, Kitaura Y, Satoh H, Ohkouchi S, Shibata H 2002. Structures, functions and molecular evolution of the penta-EF-hand Ca2+-binding proteins. Biochim Biophys Acta 1600: 51–60 [DOI] [PubMed] [Google Scholar]
- Marenholz I, Heizmann CW, Fritz G 2004. S100 proteins in mouse and man: from evolution to function and pathology (including an update of the nomenclature). Biochem Biophys Res Commun 322: 1111–1122 [DOI] [PubMed] [Google Scholar]
- Marsden BJ, Shaw GS, Sykes BD 1990. Calcium binding proteins. Elucidating the contributions to calcium affinity from an analysis of species variants and peptide fragments. Biochem Cell Biol 68: 587–601 [DOI] [PubMed] [Google Scholar]
- Martin SR, Linse S, Johansson C, Bayley PM, Forsen S 1990. Protein surface charges and Ca2+ binding to individual sites in calbindin D9k: stopped-flow studies. Biochemistry 29: 4188–4193 [DOI] [PubMed] [Google Scholar]
- Maughan DW, Godt RE 1999. Parvalbumin concentration and diffusion coefficient in frog myoplasm. J Muscle Res Cell Motil 20: 199–209 [DOI] [PubMed] [Google Scholar]
- McLaughlin S, Mulrine N, Gresalfi T, Vaio G, McLaughlin A 1981. Adsorption of divalent cations to bilayer membranes containing phosphatidylserine. J Gen Physiol 77: 445–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihaly A, Szente M, Dubravcsik Z, Boda B, Kiraly E, Nagy T, Domonkos A 1997. Parvalbumin- and calbindin-containing neurons express c-fos protein in primary and secondary (mirror) epileptic foci of the rat neocortex. Brain Res 761: 135–145 [DOI] [PubMed] [Google Scholar]
- Moreland JL, Gramada A, Buzko OV, Zhang Q, Bourne PE 2005. The Molecular Biology Toolkit (MBT): A Modular Platform for Developing Molecular Visualization Applications. BMC Bioinformatics 6:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan DW, Welton AF, Heick AE, Christakos S 1986. Specific in vitro activation of Ca,Mg-ATPase by vitamin D-dependent rat renal calcium binding protein (calbindin D28K). Biochem Biophys Res Commun 138: 547–553 [DOI] [PubMed] [Google Scholar]
- Muller A, Kukley M, Stausberg P, Beck H, Muller W, Dietrich D 2005. Endogenous Ca2+ buffer concentration and Ca2+ microdomains in hippocampal neurons. J Neurosci 25: 558–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M, Felmy F, Schwaller B, Schneggenburger R 2007. Parvalbumin is a mobile presynaptic Ca2+ buffer in the calyx of held that accelerates the decay of Ca2+ and short-term facilitation. J Neurosci 27: 2261–2271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muntener M, Kaser L, Weber J, Berchtold MW 1995. Increase of skeletal muscle relaxation speed by direct injection of parvalbumin cDNA. Proc Natl Acad Sci U S A 92: 6504–6508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchison D, Griffith WH 2000. Mitochondria buffer non-toxic calcium loads and release calcium through the mitochondrial permeability transition pore and sodium/calcium exchanger in rat basal forebrain neurons. Brain Res 854: 139–151 [DOI] [PubMed] [Google Scholar]
- Murchison D, Dove LS, Abbott LC, Griffith WH 2002. Homeostatic compensation maintains Ca2+ signaling functions in Purkinje neurons in the leaner mutant mouse. Cerebellum 1: 119–127 [DOI] [PubMed] [Google Scholar]
- Nagerl UV, Mody I 1998. Calcium-dependent inactivation of high-threshold calcium currents in human dentate gyrus granule cells. J Physiol 509: 39–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagerl UV, Novo D, Mody I, Vergara JL 2000. Binding kinetics of calbindin-D(28k) determined by flash photolysis of caged Ca2+. Biophys J 79: 3009–3018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E 1998. Usefulness and limitations of linear approximations to the understanding of Ca2+ signals. Cell Calcium 24: 345–357 [DOI] [PubMed] [Google Scholar]
- Nelson MR, Thulin E, Fagan PA, Forsen S, Chazin WJ 2002. The EF-hand domain: a globally cooperative structural unit. Protein Sci 11: 198–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orduz D, Llano I 2007. Recurrent axon collaterals underlie facilitating synapses between cerebellar Purkinje cells. Proc Natl Acad Sci U S A 104: 17831–17836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palczewska M, Groves P, Ambrus A, Kaleta A, Kover KE, Batta G, Kuznicki J 2001. Structural and biochemical characterization of neuronal calretinin domain I-II (residues 1–100). Comparison to homologous calbindin D28k domain I-II (residues 1–93). Eur J Biochem 268: 6229–6237 [DOI] [PubMed] [Google Scholar]
- Pike CJ, Cotman CW 1995. Calretinin-immunoreactivity neurons are resistant to B-amyloid toxicity in vitro. Brain Res 671: 293–298 [DOI] [PubMed] [Google Scholar]
- Raymackers JM, Gailly P, Schoor MC, Pette D, Schwaller B, Hunziker W, Celio MR, Gillis JM 2000. Tetanus relaxation of fast skeletal muscles of the mouse made parvalbumin deficient by gene inactivation. J Physiol 527: 355–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy D, Pollock AS, Clark SA, Sooy K, Vasavada RC, Stewart AF, Honeyman T, Christakos S 1997. Transfection and overexpression of the calcium binding protein calbindin-D28k results in a stimulatory effect on insulin synthesis in a rat beta cell line (RIN 1046–38). Proc Natl Acad Sci U S A 94: 1961–1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisner PD, Christakos S, Vanaman TC 1992. In vitro enzyme activation with calbindin-D28k, the vitamin D-dependent 28 kDa calcium binding protein. FEBS Lett 297: 127–131 [DOI] [PubMed] [Google Scholar]
- Rimessi A, Giorgi C, Pinton P, Rizzuto R 2008. The versatility of mitochondrial calcium signals: from stimulation of cell metabolism to induction of cell death. Biochim Biophys Acta 1777: 808–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodenbaugh DW, Wang W, Davis J, Edwards T, Potter JD, Metzger JM 2007. Parvalbumin isoforms differentially accelerate cardiac myocyte relaxation kinetics in an animal model of diastolic dysfunction. Am J Physiol Heart Circ Physiol 293: H1705–H1713 [DOI] [PubMed] [Google Scholar]
- Sabatini BL, Oertner TG, Svoboda K 2002. The life cycle of Ca2+ ions in dendritic spines. Neuron 33: 439–452 [DOI] [PubMed] [Google Scholar]
- Sakaguchi N, Henzl MT, Thalmann I, Thalmann R, Schulte BA 1998. Oncomodulin is expressed exclusively by outer hair cells in the organ of Corti. J Histochem Cytochem 46: 29–40 [DOI] [PubMed] [Google Scholar]
- Schiffmann SN, Cheron G, Lohof A, d’Alcantara P, Meyer M, Parmentier M, Schurmans S 1999. Impaired motor coordination and Purkinje cell excitability in mice lacking calretinin. Proc Natl Acad Sci U S A 96: 5257–5262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H, Brown EB, Schwaller B, Eilers J 2003a. Diffusional mobility of parvalbumin in spiny dendrites of cerebellar purkinje neurons quantified by fluorescence recovery after photobleaching. Biophys J 84: 2599–2608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H, Stiefel KM, Racay P, Schwaller B, Eilers J 2003b. Mutational analysis of dendritic Ca2+ kinetics in rodent Purkinje cells: role of parvalbumin and calbindin D28k. J Physiol 551: 13–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H, Schwaller B, Eilers J 2005. Calbindin D28k targets myo-inositol monophosphatase in spines and dendrites of cerebellar Purkinje neurons. Proc Natl Acad Sci U S A 102: 5850–5855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H, Arendt O, Brown EB, Schwaller B, Eilers J 2007a. Parvalbumin is freely mobile in axons, somata and nuclei of cerebellar Purkinje neurones. J Neurochem 100: 727–735 [DOI] [PubMed] [Google Scholar]
- Schmidt H, Kunerth S, Wilms C, Strotmann R, Eilers J 2007b. Spino-dendritic cross-talk in rodent Purkinje neurons mediated by endogenous Ca2+-binding proteins. J Physiol 581: 619–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H, Eilers J 2009. Spine neck geometry determines spino-dendritic cross-talk in the presence of mobile endogenous calcium binding proteins. J Comput Neurosci 27: 229–243 [DOI] [PubMed] [Google Scholar]
- Schurmans S, Schiffmann SN, Gurden H, Lemaire M, Lipp H-P, Schwam V, Pochet R, Imperato A, Böhme GA, Parmentier M 1997. Impaired LTP induction in the dentate gyrus of calretinin-deficient mice. Proc. Natl. Acad. Sci. 94: 10415–10420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaller B, Durussel I, Jermann D, Herrmann B, Cox JA 1997. Comparison of the Ca2+-binding properties of human recombinant calretinin-22k and calretinin. J Biol Chem 272: 29663–29671 [DOI] [PubMed] [Google Scholar]
- Schwaller B, Dick J, Dhoot G, Carroll S, Vrbova G, Nicotera P, Pette D, Wyss A, Bluethmann H, Hunziker W, Celio MR 1999. Prolonged contraction-relaxation cycle of fast-twitch muscles in parvalbumin knockout mice. Am J Physiol (Cell Physiol) 276: C395–403 [DOI] [PubMed] [Google Scholar]
- Schwaller B, Meyer M, Schiffmann S 2002. ‘New’ functions for ‘old’ proteins: the role of the calcium-binding proteins calbindin D-28k, calretinin and parvalbumin, in cerebellar physiology. Studies with knockout mice. Cerebellum 1: 241–258 [DOI] [PubMed] [Google Scholar]
- Schwaller B, Tetko IV, Tandon P, Silveira DC, Vreugdenhil M, Henzi T, Potier MC, Celio MR, Villa AE 2004. Parvalbumin deficiency affects network properties resulting in increased susceptibility to epileptic seizures. Mol Cell Neurosci 25: 650–663 [DOI] [PubMed] [Google Scholar]
- Schwaller B 2007. Emerging Functions of the “Ca2+ Buffers” Parvalbumin, Calbindin D-28k and Calretinin in the Brain. In Handbook of Neurochemistry and Molecular Neurobiology Neural Protein Metabolism and function (ed. Lajtha A, Banik N), pp. 197–222 Springer, New York [Google Scholar]
- Schwaller B 2009. The continuing disappearance of "pure" Ca2+ buffers. Cell Mol Life Sci 66: 275–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servais L, Bearzatto B, Schwaller B, Dumont M, De Saedeleer C, Dan B, Barski JJ, Schiffmann SN, Cheron G 2005. Mono- and dual-frequency fast cerebellar oscillation in mice lacking parvalbumin and/or calbindin D-28k. Eur J Neurosci 22: 861–870 [DOI] [PubMed] [Google Scholar]
- Skelton NJ, Kördel J, Akke M, Forsén S, Chazin WJ 1994. Signal transduction versus buffering activity in Ca++-binding proteins. Nat Struct Biol 1: 239–245 [DOI] [PubMed] [Google Scholar]
- Sooy K, Schermerhorn T, Noda M, Surana M, Rhoten WB, Meyer M, Fleischer N, Sharp GW, Christakos S 1999. Calbindin-D(28k) controls [Ca2+]i and insulin release. Evidence obtained from calbindin-d(28k) knockout mice and beta cell lines. J Biol Chem 274: 34343–34349 [DOI] [PubMed] [Google Scholar]
- Stevens J, Rogers JH 1997. Chick calretinin: purification, composition, and metal binding activity of native and recombinant forms. Protein Expr Purif 9: 171–181 [DOI] [PubMed] [Google Scholar]
- Swain AL, Kretsinger RH, Amma EL 1989. Restrained least squares refinement of native (calcium) and cadmium-substituted carp parvalbumin using X-ray crystallographic data at 1.6-A resolution. J Biol Chem 264: 16620–16628 [PubMed] [Google Scholar]
- Szabadkai G, Duchen MR 2008. Mitochondria: the hub of cellular Ca2+ signaling. Physiology 23: 84–94 [DOI] [PubMed] [Google Scholar]
- Szatkowski ML, Westfall MV, Gomez CA, Wahr PA, Michele DE, DelloRusso C, Turner II, Hong KE, Albayya FP, Metzger JM 2001. In vivo acceleration of heart relaxation performance by parvalbumin gene delivery. J Clin Invest 107: 191–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Zucker RS 1997. Mitochondrial involvement in post-tetanic potentiation of synaptic transmission. Neuron 18: 483–491 [DOI] [PubMed] [Google Scholar]
- Thalmann I, Shibasaki O, Comegys TH, Henzl MT, Senarita M, Thalmann R 1995. Detection of a beta-parvalbumin isoform in the mammalian inner ear. Biochem Biophys Res Commun 215: 142–147 [DOI] [PubMed] [Google Scholar]
- Tikunov BA, Rome LC 2009. Is high concentration of parvalbumin a requirement for superfast relaxation? J Muscle Res Cell Motil 30: 57–65 [DOI] [PubMed] [Google Scholar]
- Ueno T, Kameyama K, Hirata M, Ogawa M, Hatsuse H, Takagaki Y, Ohmura M, Osawa N, Kudo Y 2002. A mouse with a point mutation in plasma membrane Ca2+-ATPase isoform 2 gene showed the reduced Ca2+ influx in cerebellar neurons. Neurosci Res 42: 287–297 [DOI] [PubMed] [Google Scholar]
- Van Den Bosch L, Schwaller B, Vleminckx V, Meijers B, Stork S, Ruehlicke T, Van Houtte E, Klaassen H, Celio MR, Missiaen L, Robberecht W, Berchtold MW 2002. Protective effect of parvalbumin on excitotoxic motor neuron death. Exp Neurol 174: 150–161 [DOI] [PubMed] [Google Scholar]
- Vecellio M, Schwaller B, Meyer M, Hunziker W, Celio MR 2000. Alterations in Purkinje cell spines of calbindin D-28 k and parvalbumin knock-out mice. Eur J Neurosci 12: 945–954 [DOI] [PubMed] [Google Scholar]
- Vreugdenhil M, Jefferys JG, Celio MR, Schwaller B 2003. Parvalbumin-deficiency facilitates repetitive IPSCs and gamma oscillations in the hippocampus. J Neurophysiol 89: 1414–1422 [DOI] [PubMed] [Google Scholar]
- Wang W, Metzger JM 2008. Parvalbumin isoforms for enhancing cardiac diastolic function. Cell Biochem Biophys 51: 1–8 [DOI] [PubMed] [Google Scholar]
- Wassle H, Peichl L, Airaksinen MS, Meyer M 1998. Calcium-binding proteins in the retina of a calbindin-null mutant mouse. Cell Tissue Res 292: 211–218 [DOI] [PubMed] [Google Scholar]
- Yin Y, Henzl MT, Lorber B, Nakazawa T, Thomas TT, Jiang F, Langer R, Benowitz LI 2006. Oncomodulin is a macrophage-derived signal for axon regeneration in retinal ganglion cells. Nat Neurosci 9: 843–852 [DOI] [PubMed] [Google Scholar]
- Zheng W, Xie Y, Li G, Kong J, Feng JQ, Li YC 2004. Critical role of calbindin-D28k in calcium homeostasis revealed by mice lacking both vitamin D receptor and calbindin-D28k. J Biol Chem 279: 52406–52413 [DOI] [PubMed] [Google Scholar]
- Zhou Z, Neher E 1993. Mobile and immobile calcium buffers in bovine adrenal chromaffin cells. J Physiol 469: 245–273 [DOI] [PMC free article] [PubMed] [Google Scholar]