Abstract
Second messenger molecules relay, amplify, and diversify cell surface receptor signals. Two important examples are phosphorylated D-myo-inositol derivatives, such as phosphoinositide lipids within cellular membranes, and soluble inositol phosphates. Here, we review how phosphoinositide metabolism generates multiple second messengers with important roles in T-cell development and function. They include soluble inositol(1,4,5)trisphosphate, long known for its Ca2+-mobilizing function, and phosphatidylinositol(3,4,5)trisphosphate, whose generation by phosphoinositide 3-kinase and turnover by the phosphatases PTEN and SHIP control a key “hub” of TCR signaling. More recent studies unveiled important second messenger functions for diacylglycerol, phosphatidic acid, and soluble inositol(1,3,4,5)tetrakisphosphate (IP4) in immune cells. Inositol(1,3,4,5)tetrakisphosphate acts as a soluble phosphatidylinositol(3,4,5)trisphosphate analog to control protein membrane recruitment. We propose that phosphoinositide lipids and soluble inositol phosphates (IPs) can act as complementary partners whose interplay could have broadly important roles in cellular signaling.
IP3 and PIP3 are important second messengers in T-cell signaling, as are the PIP2 derivatives diacylglycerol and phosphatidic acid. IP4 may act as a soluble PIP3 analog that regulates recruitment of proteins to the membrane.
Second messenger molecules relay, amplify, and diversify signals perceived by cell surface receptors. One important second messenger family is comprised of the phosphorylated derivatives of the small cyclic poly-alcohol D-myo-inositol. They include phosphoinositide lipids within cellular membranes, and soluble inositol phosphates, here termed IPs. In most stimulatory cells, the plasma membrane phosphoinositide, phosphatidylinositol(4,5)bisphosphate (here termed PIP2 for improved readability, although several different PIP2 isomers exist) is a key precursor for both other phosphoinositides and soluble IPs. Many of these regulate distinct and overlapping downstream effectors (Irvine and Schell 2001; Alcazar-Roman and Wente 2008; Resnick and Saiardi 2008; Sauer et al. 2009; Shears 2009; Sauer and Cooke 2010). In particular, class I phosphoinositide 3-kinases (PI3K) phosphorylate PIP2 at the 3-position of its inositol-ring into phosphatidylinositol(3,4,5)trisphosphate (here termed PIP3) (Fig. 1) after receptor stimulation (Vanhaesebroeck et al. 2005; Juntilla and Koretzky 2008; Buitenhuis and Coffer 2009; Fruman and Bismuth 2009). Receptor-induced PIP2-hydrolysis by phospholipases such as PLCγ1/2 in lymphocytes generates the lipid diacylglycerol (DAG) and the soluble IP inositol(1,4,5)trisphosphate (IP3). PIP3, DAG, and IP3 have essential second messenger functions in many cells, including lymphocytes. Here, we review the importance of phosphoinositide signaling in T cells and highlight the importance of a recently identified, intriguing molecular interplay between second messenger lipids and their soluble IP counterparts.
Figure 1.
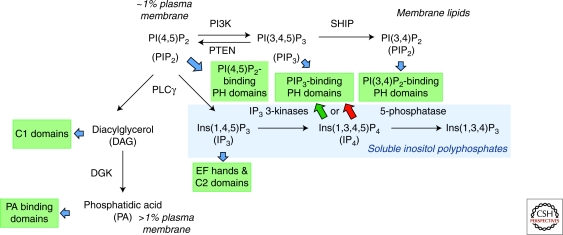
The phosphoinositide PI(4,5)P2 is a key node of second messenger metabolism in lymphocytes. PI(4,5)P2-phosphorylation by PI3-kinases and PI(4,5)P2-hydrolysis by PLCγ initiate several second messenger cascades within cellular membranes and soluble compartments. These second messengers can have various functions, including the recruitment and/or activation of effector proteins by binding to specific domains within them (blue arrows and green boxes). In particular, the phosphatases SHIP1/2 or PTEN limit or down-regulate PIP3 levels and the functions of PIP3 and its effectors by hydrolyzing PIP3 into PI(3,4)P2 or PI(4,5)P2, respectively. For group 1D, PH domains such as that of Akt, PI(3,4)P2 binding sustains effector activity (Cozier et al. 2004; DiNitto and Lambright 2006; Lemmon 2008). PI(4,5)P2 recruits proteins such as RASA3/GAP1IP4BP by binding to their PH domains (Lockyer et al. 1997; Cozier et al. 2000a; Cozier et al. 2000b). Hence, both PIP2s and PIP3 can have partially overlapping functions depending on the lipid-binding protein domain involved. PLCγ hydrolyzes PI(4,5)P2 into protein C1 domain binding DAG and into IP3, which binds EF and C2 domain containing proteins and mobilizes Ca2+. IP3 3-kinases convert IP3 into IP4, which acts as a soluble PIP3 analog and controls the abilities of certain PH domains to bind to PIP3 either positively (green arrow) or negatively (red arrow). An unknown 5-phosphatase metabolizes IP4 into I(1,3,4)P3, a precursor for higher-order IPs. In vitro, SHIP1 and PTEN can dephosphorylate IP4 at the 5- or 3-positions, respectively (Erneux et al. 1998; Maehama and Dixon 1998; Pesesse et al. 1998; Caffrey et al. 2001). Whether this occurs physiologically is unknown. Finally, DAG kinases (DGKs) down-regulate DAG function by phosphorylating it into PA, itself a ligand for certain proteins. Further metabolism of all these second messengers results in the generation of many lipid and soluble metabolites. Several of these have important signaling functions (Irvine 2001; Irvine and Schell 2001; York and Hunter 2004; York 2006; Alcazar-Roman and Wente 2008; Huang et al. 2008; Jia et al. 2008b; Miller et al. 2008; Burton et al. 2009; Shears 2009; Sauer and Cooke 2010; Schell 2010). Their functions in immunocytes are unknown.
PHOSPHOINOSITIDES CONTROL SIGNALING BY BINDING TO SPECIFIC PROTEIN DOMAINS
All phosphoinositides contain a hydrophobic membrane-embedded diacylglyceride and a hydrophilic solvent-exposed IP moiety. The inositol ring hydroxyl groups can be stereo-specifically phosphorylated by phosphoinositide-kinases. Most phosphatidylinositol-bisphosphate in the plasma membrane of unstimulated cells is phosphorylated at the inositol 4- and 5-positions. This PIP2 comprises <1% of the inner leaflet of the plasma membrane, contrasting with much more abundant phospholipids such as phosphatidylserine and phosphatidic acid (PA) (Lemmon 2008). Despite its low abundance, PIP2 is an important second messenger. It recruits and regulates multiple signaling proteins by binding to their pleckstrin homology (PH), epsin N-terminal homology (ENTH), 4.1, ezrin, radixin, moesin (FERM), Tubby, or Phox-homology (PX) domains with varying affinities and specificities (McLaughlin et al. 2002). Due to the constitutive PIP2 availability in resting cells, these PIP2-associated proteins likely maintain signaling pathways in a preactivation state. Evidence supporting this notion in T cells comes from the inhibitory effects of PIP2 binding to the guanine nucleotide exchange factor, Vav (Han et al. 1998), and the guanine nucleotide activating factor, ArhGAP9, which maintains Rho-family GTPases in an inactive state (Ang et al. 2007; Ceccarelli et al. 2007).
PHOSPHOINOSITIDE 3-KINASES CONVERT PIP2 INTO PHOSPHATIDYLINOSITOL(3,4,5) TRISPHOSPHATE
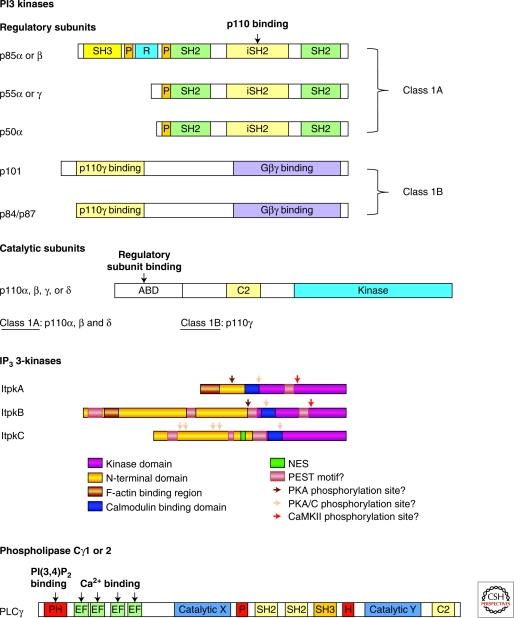
Despite the importance of PIP2, much greater attention has been given to the products of PIP2 phosphorylation or PIP2 hydrolysis that are induced following receptor activation. PIP2 phosphorylation is mediated by PI3Ks. PI3Ks fall into four classes based upon their substrate specificity. Each class comprises one or more regulatory and catalytic subunits with partially distinct and overlapping functions (Fig. 2). Class II and III PI3Ks phosphorylate phosphatidylinositol (PI) into PI(3)P and PI(3,4)P2. Their functions in lymphocytes are largely unexplored. Class I PI3Ks phosphorylate PIP2 into PIP3, an inducible ligand that mediates recruitment and activation of various important signaling proteins. Class IA PI3Ks are activated by most stimulatory receptors on lymphocytes including T- and B-cell antigen receptors (TCR, BCR), and co-stimulatory, Toll-like, and cytokine receptors (Vanhaesebroeck et al. 2005; Buitenhuis and Coffer 2009; Fruman and Bismuth 2009). Five regulatory (p50α, p55α, p55γ, p85α, p85β) and three catalytic (p110α, β, and δ) subunits participate in Class IA PI3K signaling. Recruitment of Class IA regulatory subunits via binding of their SH2 domains to receptor-induced tyrosine-phosphorylated ITAM motifs leads to colocalization of PI3K catalytic subunits with their substrate, membrane PIP2. In contrast, Class IB PI3Ks are activated downstream of G-protein-coupled receptors, including chemokine receptors. Class IB comprises two regulatory subunits (p101, p84/87) and one catalytic (p110γ) subunit (Stephens et al. 1994; Stoyanov et al. 1995; Stephens et al. 1997; Suire et al. 2005). Following GPCR engagement, class IB PI3K regulatory subunits bind through their C-terminal domains to Gβγ subunits. Both class IA (Okkenhaug et al. 2002; Okkenhaug et al. 2006; Patton et al. 2006; Matheu et al. 2007; Liu et al. 2009a; Liu and Uzonna 2010; Soond et al. 2010) and class IB (Sasaki et al. 2000; Swat et al. 2006; Alcazar et al. 2007) PI3Ks have important functions in T-cell development and function. The T-cell phenotypes of mice with individual or combined PI3K subunit deficiencies are summarized in Table 1.
Figure 2.
Schematic structures of key PIP2 or IP3 kinases and PIP2 lipases in lymphocytes. For details, see text.
Table 1.
Phenotypic consequences of deficiencies in phosphoinositol lipid metabolizing enzymes in lymphocytes.
| Deficient- or functionally-impaired protein(s) | T-cell phenotype | References |
|---|---|---|
| PI3 Kinase Regulatory Subunits | ||
| p85α- (pik3r1) deficient mice | Reduced T-cell development, reduced migrational velocity, normal mature T-cell function. | (Matheu et al. 2007; Shiroki et al. 2007) |
| p85β- (pik3r2) deficient mice | Normal T-cell development, reduced cell velocity. Elevated anti-CD3/IL-2-induced proliferation in one study. Defective peripheral T cell recall responses and CD28 signaling in another study. | (Deane et al. 2004; Matheu et al. 2007; Alcazar et al. 2009) |
| p85α, p55α, p50α (pik3r1) multi-deficient mice | None reported. Perinatal lethality. | (Fruman et al. 2000) |
| p85α, p55α, p50α (pik3r1) & p85β (pik3r2) multi-deficient T cells | Largely normal T-cell development. Impaired T cell Ca2+ signaling, proliferation and cytokines, defective Treg function and Th2 responses but normal Th1 responses, reduced cell velocity and polarization. Sjoegren's like autoimmune disease. | (Oak et al. 2006; Deane et al. 2007; Matheu et al. 2007; Fruman and Bismuth 2009) |
| PI3 Kinase Catalytic Subunits | ||
| p110α−/− mice or p110β−/− mice | Not determined. Embryonic lethal. | (Buitenhuis and Coffer 2009) |
| p110δ−/− mice | No major abnormalities. | (Clayton et al. 2002) |
| p110δD910A catalytically inactive mutant expressing mice | Impaired TCR-induced Ca2+ signaling, proliferation, migration and cytokine production, reduced Th1, Th2 and Treg function. Protection from type 2 cytokine responses like airway hyperresponsiveness. Colitis, widespread autoimmunity. | (Okkenhaug et al. 2002; Okkenhaug et al. 2006; Patton et al. 2006; Nashed et al. 2007; Jarmin et al. 2008) |
| p110γ−/− mice | Some controversies between groups. Impaired T-cell development and activation, proliferation, actin polymerization. Normal activation but impaired migration in another study. Similar cell velocity but with more turning. Reduced CD4+ memory cell survival, autoantibody production, glomerulonephritis, and systemic lupus. | (Sasaki et al. 2000; Rodriguez-Borlado et al. 2003; Barber et al. 2006; Alcazar et al. 2007; Martin et al. 2008; Thomas et al. 2008; Fruman and Bismuth 2009) |
| p110γ−/−p110δ−/− or p110γ−/−p110δD910A mice | Impaired T-cell development, proliferation and cell survival, exaggerated Th2 over Th1 responses, high serum IgE levels. | (Webb et al. 2005; Swat et al. 2006; Ji et al. 2007) |
| IP33-Kinases | ||
| ItpkA−/− mice | None reported. | (Jun et al. 1998; Kim et al. 2009) |
| ItpkB−/− mice | Blocked T-cell development and thymocyte positive selection. Normal IP3-accumulation and Ca2+ signaling, but defective DAG production, Itk recruitment, and activation in thymocytes. | (Pouillon et al. 2003; Wen et al. 2004; Chamberlain et al. 2005; Huang et al. 2006; Huang et al. 2007; Jia et al. 2007; Marechal et al. 2007; Miller et al. 2007b; Huang et al. 2008; Jia et al. 2008a; Jia et al. 2008b; Miller et al. 2008; Miller et al. 2009) |
| ItpkC−/− mice | No thymic phenotype. Normal IP3 3-kinase activity in thymocytes. | (Pouillon et al. 2003) |
| ItpkB−/−ItpkC−/− mice | Same T-cell developmental block as ItpkB−/− mice. | (Pouillon et al. 2003) |
| ITPKC loss-of-function polymorphism, humans | Associated with Kawasaki disease susceptibility. | (Onouchi et al. 2008) |
| ITPKC knockdown, Jurkat cells | Hyperactivation. | (Onouchi et al. 2008) |
| ITPKC overexpression, Jurkat cells | Reduced activation. | (Onouchi et al. 2008) |
| IPMK−/− mice | None reported. Embryonic lethal. | (Frederick et al. 2005) |
| PIP3/IP4Phosphatases | ||
| SHIP−/− mice | Altered Th1/Th2 ratio, reduced CD8 cytotoxicity. Shortened lifespan, splenomegaly, autoimmunity. | (Helgason et al. 1998; Tarasenko et al. 2007) |
| PTEN+/− mice | Hyperproliferative T cells. Increased tumor incidence, autoimmunity. | (Di Cristofano et al. 1999; Di Cristofano and Pandolfi 2000) |
| Conditional PTEN knockout in T cells in mice | Impaired T-cell development, hyperresponsive to suboptimal TCR signals, increased cytokine production, Th2 bias, autoimmune pathology. | (Suzuki et al. 2001; Hagenbeek et al. 2004; Buckler et al. 2008) |
| PLCγ1−/− mice | Conditional knockout: Impaired thymocyte positive and negative selection, regulatory Treg cell development and function, TCR-induced peripheral T-cell proliferation and cytokine production. Autoimmune disease. Conventional knockout is embryonic lethal. Chimeric mice: Severe defects in early hematopoiesis. | (Ji et al. 1997; Shirane et al. 2001; Fu et al. 2010) |
| PLCγ2−/− mice | No T-cell defect reported. Defects in mast cells, dendritic cells, osteoclasts, and neutrophils. | (Wang et al. 2000; Graham et al. 2007; Cremasco et al. 2008; Epple et al. 2008; Cremasco et al. 2010) |
| DGKα−/− mice | Hyper-responsive T cells. | (Outram et al. 2002; Olenchock et al. 2006) |
| DGKξ−/− mice | Hyper-responsive T cells. | (Zhong et al. 2003; Olenchock et al. 2006) |
| DGKα−/−DGKξ−/− mice | Lymphopenia due to a partial block in T-cell development. Reduced Tregs, peripheral T cell resistant to anergy-induction. | (Olenchock et al. 2006; Zha et al. 2006; Guo et al. 2008) |
THE SECOND MESSENGER PIP3 RECRUITS PROTEINS TO MEMBRANES BY BINDING TO THEIR PH DOMAINS
Taken together, immunoreceptor-induced PIP3 generation is important for lymphocyte proliferation and differentiation (for reviews, see Juntilla and Koretzky 2008; Buitenhuis and Coffer 2009; Fruman and Bismuth 2009). PIP3 mediates the cellular effects of PI3K activation by recruiting effector proteins that stereospecifically bind to PIP3 through a subclass of pleckstrin homology or PH domains—protein modules of approximately 120 amino acids that were originally identified as a repeated domain in Pleckstrin (Haslam et al. 1993; Mayer et al. 1993). The mammalian genome contains approximately 250 PH domain proteins. Although there is little amino acid sequence conservation among PH domains, they share a common β-barrel fold comprised of two β sheets and a C-terminal α-helical cap (Downing et al. 1994; Ferguson et al. 1994; Macias et al. 1994; Timm et al. 1994; Yoon et al. 1994). In approximately 60 PH domains, the N-terminal portion contains a pocket that binds phosphoinositols (Cozier et al. 2004; Hirata et al. 1998). These PH domains are divided into two classes. Class I PH domains bind with high affinity and specificity to PI(4,5)P2, PI(3,4)P2, PIP3, and/or IP4. Class II PH domains bind PIs more promiscuously and with lower affinity.
Different positively charged amino acid side chains in the PI-binding pocket of a PH domain determine its ligand specificity by interacting with specific negatively charged phosphate groups within the inositol ring. The Akt (Protein kinase B) and Tec protein kinase families are part of a small but immunologically important subset of PH-domain-containing proteins whose activities are regulated by PIP3 binding, which colocalizes the kinases with their upstream activators (August et al. 1997; Heyeck et al. 1997; Stokoe et al. 1997). In addition, the Akt PH domain can also bind to PI(3,4)P2 (Cozier et al. 2004; DiNitto and Lambright 2006; James et al. 1996; Lemmon 2008).
PIP3 CONTROLS AKT FUNCTION IN LYMPHOCYTES
Akt and its upstream activator PDK1 (Phosphoinositide-dependent kinase 1) are nonreceptor serine/threonine kinases of the AGC family. They regulate survival and proliferation of many cell types (Manning and Cantley 2007). Both kinase families are structurally relatively simple—containing only a kinase domain, a PH domain, and in the case of Akt1, a short hydrophobic motif. Before activation, the Akt and PDK1 PH domains seem to function in an inhibitory manner that is released upon membrane PIP3 binding (Stokoe et al. 1997; Gao and Harris 2006). Full PDK1 activation requires PIP3 binding to the PDK1 PH domain, which allows trans-phosphorylation of a C-terminal residue (Gao and Harris 2006). In the absence of lipid ligands, the Akt1 PH domain occludes access to the activation site, prohibiting its phosphorylation by PDK1 (Stokoe et al. 1997; Calleja et al. 2007). PH-domain-mediated PDK1 and Akt recruitment to membrane PIP3 leads to phosphorylation of the catalytic T-loop of Akt by PDK1, activating Akt (Stephens et al. 1998). A second kinase of uncertain identity phosphorylates the Akt C-terminal hydrophobic motif, leading to optimal Akt kinase activity.
There are three Akt paralogs in mammals (Akt1/2/3). All are expressed in lymphocytes and have partially redundant functions. Akt promotes cell survival primarily through inhibition of apoptosis. Akt can directly or indirectly inhibit the function of proapoptotic Bcl-2 family members by phosphorylation (del Peso et al. 1997; Gardai et al. 2004; Qi et al. 2006; Hubner et al. 2008). Akt also activates the transcription factor NF-kB (Madrid et al. 2000) and induces expression of antiapoptotic Bcl-2, FLIP, and IAP proteins (Wang et al. 1998; Panka et al. 2001). Akt, moreover, promotes cell proliferation by increasing cell metabolism (Frauwirth et al. 2002; Rathmell et al. 2003; Jacobs et al. 2008), facilitating protein translation (Dufner et al. 1999), and cell cycle progression (Liang et al. 2002; Shin et al. 2002; Viglietto et al. 2002). Consequently, genetic alterations that enhance Akt activity either directly or indirectly via amplification of PI3K activity often lead to cellular transformation (Liu et al. 2009b).
Akt1 and Akt2 are particularly important during early T-cell development (Juntilla et al. 2007; Juntilla and Koretzky 2008). CD4 and CD8 T cells develop from a common thymic progenitor through a series of developmental stages that can be partially characterized by differential cell-surface expression of the CD4 and CD8 coreceptors. At the early, CD4−CD8− double-negative (DN) stage, productive TCRβ gene rearrangement results in a pre-TCR signal that suppresses rearrangement of the second TCRβ allele and triggers proliferative expansion and progression to the CD4+CD8+ double-positive (DP) stage. During this process, the cells rearrange their TCRα chain genes. Successful TCRα protein expression results in pre-TCR replacement by a mature TCR composed of α and β chains. Akt1 or Akt2 deficiency results in a two-fold reduction in thymic cellularity due to a partial block between DN and DP cells. In contrast, Akt1 and Akt2 double-deficiency results in a 45-fold decrease in thymic cellularity (Juntilla et al. 2007). This highlights the importance of Akt kinases during the highly proliferative stage of thymic development.
TEC FAMILY PROTEIN TYROSINE KINASES ARE KEY PIP3 EFFECTORS IN LYMPHOCYTES
The expression of certain Tec family nonreceptor protein–tyrosine–kinases (TFKs) is restricted to specific immune cell types (Readinger et al. 2009). Itk and Rlk are largely restricted to T cells and NK cells. Btk is not expressed in these, but enriched in B cells, mast cells, and myeloid cells. Tec is broadly expressed in hematopoietic cells. Despite some functional redundancies, deficiency in individual TFKs causes profound functional defects (for a review, see Readinger et al. 2009). Itk deficiency causes defects in T-cell development and in certain peripheral T-cell functions (Liao and Littman 1995; Fowell et al. 1999; Schaeffer et al. 1999; Schaeffer et al. 2000; Schaeffer et al. 2001; Tomlinson et al. 2004; Finkelstein et al. 2005; Atherly et al. 2006; Broussard et al. 2006; Horai et al. 2007; Lucas et al. 2007).
At the DP stage, thymocytes are evaluated for the ability of their TCR to interact with self MHC/peptide complexes on the surfaces of thymic antigen-presenting cells. DP cells whose TCRs do not interact with sufficient strength or duration die by “neglect.” DP cells whose TCRs interact moderately with self-MHC class I or II are positively selected to mature into CD8 or CD4 single positive T cells, respectively. DP cells carrying potentially autoreactive TCRs that interact too strongly or for an inappropriate duration with self-MHC are induced to die by “negative selection.” A major unresolved question in immunological research is how TCR engagement can have such different outcomes.
Positively and negatively selecting TCR signals appear to activate the same proximal signaling molecules, including Itk. In Itk-deficient mice, the efficiencies of positive and negative selection are dramatically decreased (Liao and Littman 1995; Schaeffer et al. 2000). In contrast, Itk deficiency favors the accumulation of a nonconventional thymic T cell subset with an effector/memory or “innate” phenotype, particularly in the CD8 lineage (Atherly et al. 2006; Broussard et al. 2006; Horai et al. 2007). In addition, TCR signals that normally induce negative selection can, under some circumstances, promote positive selection in Itk-deficient mice (Schaeffer et al. 2000). In mature T cells, Itk appears to be required to sustain TH2 responses (Fowell et al. 1999; Schaeffer et al. 2001).
TFKs have a relatively complex domain structure with an N-terminal PH domain, followed by a proline-rich Tec homology domain, Src homology 3 (SH3) and SH2 domains, and a C-terminal kinase domain. In Rlk, a missing N-terminal PH domain is functionally substituted by palmitoylation, resulting in constitutive membrane association (Debnath et al. 1999). Membrane recruitment of the other TFKs predominantly depends on interactions between their PH domains and membrane PIP3 (Ching et al. 1999; Shan et al. 2000), together with interactions between their SH2 domains and adaptor proteins such as SLP-76 in T cells (Ching et al. 1999; Su et al. 1999). However, recent data may suggest that Btk membrane recruitment can also occur in a PH-domain-independent manner (Fruman and Bismuth 2009). Activation-induced association with cytosolic SLP-76 and the membrane adaptor LAT colocalizes TFKs with their upstream activators, Src kinases, and their downstream target, PLCγ1 (Wange 2000; Qi and August 2007). Mechanistically, the consequences of TFK deficiency have primarily been attributed to defective PLCγ phosphorylation and activation (Liu et al. 1998). In addition, TFKs appear required for Vav-dependent cytoskeletal organization and cell adhesion (Gomez-Rodriguez et al. 2007). However, TFK promotion of Vav function appears to involve TFK adaptor functions, rather than their kinase activities (Grasis et al. 2003; Dombroski et al. 2005).
PHOSPHOLIPASE-Cγ HYDROLYZES PIP2 INTO THE SECOND MESSENGERS DIACYLGLYCEROL AND INOSITOL(1,4,5)TRISPHOSPHATE
PI(4,5)P2 is a substrate for another immunologically important enzyme, phosphatidylinositol-specific phospholipase-Cγ (PLCγ). PLCγ hydrolyzes PIP2 into its hydrophobic and hydrophilic components, the membrane-lipid diacylglycerol (DAG) and soluble inositol(1,4,5)trisphosphate (IP3). Both are second messengers that regulate proteins through specific binding domains.
The two mammalian PLCγ isoforms, PLCγ1 and 2, have partially overlapping expression patterns and functions (Wilde and Watson 2001). T cells exclusively express PLCγ1. T-cell-specific PLCγ1-deletion impaired thymocyte positive and negative selection, regulatory Treg cell development and function, TCR-induced peripheral T-cell proliferation, and cytokine production (Fu et al. 2010). Defective TCR activation of the MAP kinases Erk and Jnk, and of the transcription factors NFAT, AP-1, and NF-κB indicates the broad importance of PLCγ1 in TCR signaling through several pathways. Autoimmune disease symptoms show the physiological importance of PLCγ1 in T cells (Fu et al. 2010). Severe blocks in T-cell development and late onset autoimmunity in mice expressing a PLCγ1-binding-deficient LAT allele indicate the importance of LAT interactions for PLCγ1 function (Sommers et al. 2002; Sommers et al. 2005). Finally, severe defects in early hematopoiesis in chimeric mice generated with PLCγ1-deficient embryonic stem cells suggest important PLCγ1 functions in hematopoietic stem or progenitor cells (Shirane et al. 2001). In contrast, PLCγ2-deficient mice are viable with specific defects in B cells, mast cells, dendritic cells, osteoclasts, and neutrophils (Wang et al. 2000; Graham et al. 2007; Cremasco et al. 2008; Epple et al. 2008; Cremasco et al. 2010).
PLCγ1/2 have complex domain structures with an N-terminal PH domain, followed by a number of EF hands, a catalytic domain that is split by an internal regulatory domain, and a C-terminal Ca2+-binding-C2 domain (Fig. 2). The regulatory domain contains a core of two tandem SH2 domains and an SH3 domain that is flanked by a split PH domain. This split PH domain can self-associate to form a functional PH domain, or associate in trans with a split PH domain in TRPC-family Ca2+ channels (van Rossum et al. 2005). Either one or both PH domains contribute to PLCγ1 membrane association by binding to PIP3 (Bae et al. 1998; Falasca et al. 1998). Additional interactions are provided by the other modular domains. Once PLCγ1 is at the membrane, phosphorylation of tyrosine residues within its regulatory domain by TFKs induces PLCγ1 activation. Recent studies suggest that this requires phosphotyrosine-independent PLCγ1 SH2 domain binding to a noncanonical ligand motif in Itk (Joseph et al. 2007; Min et al. 2009).
DIACYLGLYCEROL CONTROLS Ras AND PKC ACTIVATION IN LYMPHOCYTES
The membrane second messenger, DAG, propagates signals via membrane recruitment of cytosolic signaling proteins by binding to their C1 domains, cysteine-rich domains of approximately 50 amino acids. Two β-sheets harbor the DAG-binding cavity.
Several well-characterized DAG-effector families include Ras guanine-nucleotide-exchange-factors/releasing proteins (RasGRPs), protein kinase C-related kinases (PKCs, PKD), chimaerin Rho/Rac-GTPase-activating proteins (Yang and Kazanietz 2007), Munc13 proteins (Betz et al. 1998), and diacylglycerol kinases (DGKs). There is some effector selectivity for different DAG species that differ in their subcellular localization. For example, RasGRPs preferentially bind to DAG in Golgi membranes (Carrasco and Merida 2004). PKCs preferentially bind to DAG in the plasma membrane (Spitaler et al. 2006).
RasGRP membrane recruitment by DAG colocalizes these Ras activators with their substrate, inducing release of Ras-bound GDP, GTP binding, and Ras activation. Ras then activates the kinase Raf, which activates the downstream Erk cascade. The four mammalian RasGRPs1–4 have partially overlapping expression patterns and partially redundant functions. T cells predominantly express RasGRP1, the main mediator of TCR-induced Ras/Erk activation (Dower et al. 2000; Priatel et al. 2002). RasGRP1 deficiency causes a significant block of T-cell development with strong defects in positive, and some defects in negative selection (Dower et al. 2000; Priatel et al. 2002). However, this developmental block is incomplete and can be partially rescued by strong TCR activation (Priatel et al. 2006). Nevertheless, a moderate lymphopenia occurs due to T-cell exhaustion (Priatel et al. 2007). Interestingly, Treg cells accumulate in the periphery of RasGRP1-deficient mice, despite perturbed Treg development (Chen et al. 2008).
In contrast to RasGRPs, DAG-mediated membrane recruitment allosterically induces PKC activitation by abrogating an autoinhibitory association between the PKC pseudo-substrate and substrate-binding domains (Rosse et al. 2010). DAG promotes activation of classic (PKCα, PKCβI, PKCβII, PKCγ), novel (PKCδ, PKCε, PKCη, PKCθ), and atypical PKCs (PKCξ, λ/ι). However, classic and novel PKCs also require Ca2+ binding to their C2 domains (Rosse et al. 2010). Multiple studies have shown essential PKC roles in lymphocyte development and function (for reviews, see Isakov and Altman 2002; Barouch-Bentov and Altman 2006; Manicassamy et al. 2006). In particular, PKCθ is important for TCR signaling and required for thymocyte-positive selection (Morley et al. 2008). Incomplete developmental defects likely reflect redundancy among thymocyte-expressed PKCs.
Several recent publications suggest important functions for chimaerins in TCR signaling, T-cell adhesion, and chemotaxis that involve their ability to inactivate Rac (Siliceo et al. 2006; Caloca et al. 2008; Siliceo and Merida 2009). No munc13 protein roles in the immune system have been reported.
DIACYLGLYCEROL KINASES CONVERT DAG INTO PHOSPHATIDIC ACID
Aside from their production by PLCγ in lymphocytes, DAG levels are also regulated through their phosphorylation into phosphatidic acid (PA) by DAG kinases (DGKs). In T cells, this down-regulates PKC and RasGRP functions and TCR-induced-Erk activation (reviewed in Zhong et al. 2008). However, in many cell types, receptor-induced PA generation activates a series of PA-effector proteins with various functions, including vesicular trafficking, cell survival, and proliferation (Wang et al. 2006).
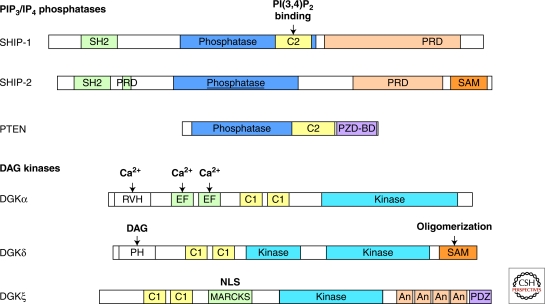
The ten mammalian DGKs form five groups based on their domain structure (Fig. 3). All DGKs have two to three C1 domains and a kinase domain. However, these C1 domains do not necessarily participate in DAG binding. Instead, the domains that distinguish the DGK types direct differential localization or activation requirements. T cells express at least three DAG kinases: DGKα (type I), δ (type II), and ξ (type IV). DGKα has an N-terminal RVH domain and two EF hands. Ca2+ binding to these three domains induces DGKα activation. DGKδ has an N-terminal PH domain, two EF hands, and a C-terminal SAM domain. The PH domain facilitates DAG binding. The SAM domain mediates DGKδ oligomerization and ER targeting. DGKξ has a central MARCKS domain, multiple ankyrin repeats, and a C-terminal PDZ-BM domain. The MARCKs domain contains a nuclear localization sequence. The PDZ-BM domain of DGKξ regulates Rac activity and membrane ruffling.
Figure 3.
Schematic structures of key PIP3 or IP4 phosphatases and DAG kinases in lymphocytes. For details, see text.
DGKα and DGKξ cooperatively down-regulate T-cell activation and facilitate T-cell tolerance (Guo et al. 2008). Different T-cell developmental and activation stages express different DGKα and DGKξ splice variants (Macian et al. 2002). DGKα- (Outram et al. 2002; Olenchock et al. 2006) or DGKξ- (Zhong et al. 2003; Olenchock et al. 2006) deficient mice have increased DAG-dependent signaling and produce hyper-responsive T cells. DGKα/ξ double deficiency causes lymphopenia due to a partial block in T-cell development (Guo et al. 2008). The peripheral T cells contain reduced Treg proportions and are resistant to anergy induction (Olenchock et al. 2006; Zha et al. 2006). Therefore, limiting DAG function in T cells is of great physiological importance, although defective PA production may contribute to the phenotype (Zhong et al. 2008).
IP3 MEDIATES ANTIGEN-RECEPTOR-INDUCED Ca2+ MOBILIZATION AND CAN ACT AS A PRECURSOR FOR HIGHER-ORDER INOSITOL PHOSPHATES IN LYMPHOCYTES
Ca2+ is a rapid and robust, soluble second messenger with multiple downstream effectors (Feske 2007; Oh-hora and Rao 2008; Vig and Kinet 2009). mM extracellular Ca2+ concentrations contrast with nM cytosolic Ca2+ concentrations that are tightly maintained by pumps, which sequester Ca2+ into the extracellular space or intracellular storage compartments (Feske 2007; Oh-hora and Rao 2008). Following TCR engagement, Ca2+ signaling is initiated by the soluble product of PIP2 hydrolysis, Ins(1,4,5)P3 (here IP3). IP3 binding to receptors in the ER membrane releases the stored Ca2+ into the cytosol (Taylor et al. 2009). Subsequent sensing of the store depletion by STIM proteins induces STIM translocation to the plasma membrane, where STIM-induced opening of ORAI-family Ca2+ channels triggers a store-operated Ca2+ entry (SOCE). This causes a sustained increase of the cytoplasmic Ca2+ concentration that is essential for T-cell activation (Feske 2007; Oh-hora and Rao 2008; Vig and Kinet 2009).
Cytosolic Ca2+ binds to proteins with C2 domains and EF hands, inducing conformational changes that alter activity (Niki et al. 1996). Important examples with EF hands are the cytosolic adapter Calmodulin and STIM proteins (Feske 2007; Oh-hora and Rao 2008; Vig and Kinet 2009).
IP3 CONVERSION INTO INOSITOL(1,3,4,5)TETRAKISPHOSPHATE BY IP3 3-KINASES CONTROLS LYMPHOCYTE DEVELOPMENT AND FUNCTION
Aside from mediating receptor-induced Ca2+ mobilization, IP3 can also act as a precursor for other IPs. A key step in their generation is IP3 phosphorylation at its 3-position into IP4 by IP3 3-kinases (IP3Ks). Mammals have four IP3Ks: the closely related ITPK-family members ItpkA/B/C and the unrelated IP multikinase (IPMK).
Genetic studies in yeast and biochemical data have suggested many potential IP4 functions (Irvine 2001; Irvine and Schell 2001; Nalaskowski and Mayr 2004; Pattni and Banting 2004; Irvine 2005; Irvine et al. 2006; York 2006; Otto et al. 2007; Huang et al. 2008; Jia et al. 2008b; Miller et al. 2008; Sauer and Cooke 2010; Schell 2010). Much research has explored potential IP3K roles in regulating Ca2+ mobilization either by controlling IP3 turnover, or through distinct functions of IP4 or IP4-derived other IPs. The results caused a long-standing controversy that could be attributed to differences in cell type, stimulation regimen, or experimental conditions. Studies with human Jurkat T cells suggest that IP4 can sustain TCR-induced IP3 accumulation and Ca2+ mobilization through competitive inhibition of an inositol 5-phosphatase (Hermosura et al. 2000). Moreover, a recent study suggested that ITPKC knockdown in Jurkat cells promotes TCR-induced activation of the Ca2+-effector NFAT and IL-2 production (Onouchi et al. 2008). These data are consistent with one IP3K role in controlling IP3 levels or function. The precise mechanism, identity of the 5-phosphatase, and physiological relevance remain unclear. Normal anti-TCR antibody-induced IP3 accumulation and Ca2+ mobilization in ItpkB-deficient thymocytes suggest that ItpkB may not regulate Ca2+ mobilization in murine thymocytes (Pouillon et al. 2003; Wen et al. 2004). However, IP4 levels and total IP3K activity were only approximately 50% reduced. Thus, other IP3Ks likely contribute to IP4 production in thymocytes, and the consequences of complete IP3K/IP4 deficiency on Ca2+ mobilization still needs to be determined.
IP4 chemically resembles the PH-domain-binding headgroup of PIP3, and less well those of PI(4,5)P2 and PI(3,4)P2. Not surprisingly, IP4 can bind to some of the protein domains that bind to these phosphoinositides. This property is often used to facilitate PH domain crystallization for X-ray structure determination (Lemmon 2008). Biochemical data suggest that IP4 binding to the PH domains of Ras GTPase-activating proteins (RASA2/GAP1m, RASA3/GAP1IP4BP) (Cullen et al. 1995; Cozier et al. 2000b), BTK, AKT, or certain regulators of vesicular trafficking (synaptotagmins, centaurin-α1/p42IP4), might inhibit their membrane recruitment, activation, or protein interactions. However, until recently, the physiological relevance of these findings was unknown.
Lymphocytes predominantly express two Itpks, ItpkB and ItpkC (Vanweyenberg et al. 1995; Wen et al. 2004). ItpkC is broadly expressed by many tissues. ItpkB expression appears restricted to hematopoietic cells and the brain (Wen et al. 2004; Jia et al. 2008a). ItpkB-deficient mice recently unveiled the physiological importance of ItpkB in lymphocyte development and activation (Table 1) (Pouillon et al. 2003; Wen et al. 2004). These mice are profoundly immunocompromised due to severe peripheral T-cell deficiency (Pouillon et al. 2003; Wen et al. 2004), reduced numbers and defective activation of B cells (Marechal et al. 2007; Miller et al. 2007a; Miller et al. 2009), and increased numbers of functionally perturbed neutrophils (Jia et al. 2007). Several recent reviews describe current models for ItpkB function in B cells and neutrophils (Huang et al. 2008; Jia et al. 2008b; Miller et al. 2008; Sauer and Cooke 2010). Here, we highlight the function of IP4 in T cells.
ItpkB-deficient mice have a block of T-cell development due to defective positive selection (Pouillon et al. 2003; Wen et al. 2004). Whether negative selection is affected is still unclear (Sauer and Cooke 2010). Selected effectors of both IP3 (Ca2+) and DAG are specifically required for positive selection. Deficiency in Calcineurin B, a Ca2+-dependent phosphatase that activates NFAT (Neilson et al. 2004; Gallo et al. 2007), or in RasGRP1, partially blocks positive selection (Dower et al. 2000; Priatel et al. 2002). Intriguingly, ItpkB deficiency blocks positive selection much more profoundly in a manner that depends on the ability of ItpkB to generate IP4 (Huang et al. 2007). Normal IP3 levels and Ca2+ mobilization in ItpkB-deficient thymocytes argue against significant contributions by these messengers. Instead, the block results from an inability of ItpkB-deficient thymocytes to produce sufficient DAG amounts in response to mild, positively selecting TCR stimuli (Huang et al. 2007). Consequently, mild TCR engagement induced Ras/Erk activation is impaired in ItpkB-deficient DP cells. This likely contributes to defective positive selection (Starr et al. 2003; McGargill et al. 2009). Addition of the DAG analog, PMA, restored Erk activation and allowed ItpkB-deficient CD4 and CD8 T-cell maturation (Huang et al. 2007). Thus, IP4 is required for DAG-induced Erk activation in response to positively selecting TCR stimuli. Yet, how does IP4 promote DAG accumulation?
SOLUBLE IP4 CONTROLS ITK-PH-DOMAIN BINDING TO PIP3 IN THYMOCYTES
Mechanistic analyses revealed that IP4 is required for membrane recruitment and subsequent activation of the TFK Itk following mild TCR stimulation (Huang et al. 2007). This was surprising since generation of PIP3, the established membrane ligand for the Itk PH domain, was thought to be the limiting factor for Itk membrane localization. In vitro analyses then showed that low μM IP4 concentrations, as previously found in TCR-stimulated T cells (Imboden and Pattison 1987), promoted Itk or Itk-PH-domain binding to PIP3 (Huang et al. 2007). Very high, likely super-physiological IP4 concentrations, competed with PIP3 binding. The ability of the Itk-PH domain to oligomerize (Huang et al. 2007) then resulted in a model where IP4 binding to one Itk-PH domain in a multimeric Itk complex induced conformational changes in all PH domains within the complex that allosterically increase their affinities for PIP3 and IP4. This promotes PIP3 binding, as long as IP4 is not in excess over PIP3. Here, IP4 catalyzes PIP3 binding at low, but competes with it at very high concentrations (Sauer and Cooke 2010). These findings identified Itk as the first physiological IP4 “receptor” and unveiled the first physiological IP4 function as a second messenger that controls Itk membrane recruitment and activation downstream of the TCR (Irvine 2007). They suggest that IP4 and PIP3 act as partners where soluble IP4 controls the ability of its membrane lipid counterpart to interact with the Itk-PH domain. It is exciting to hypothesize that this new principle of regulating PH-domain function through an interplay of a soluble IP and its phosphoinositide-lipid partner has broader relevance in biological signaling. Indeed, IP4 could have similar bimodal effects on PIP3 binding to the Tec- and RASA3-PH domains, but whether this is physiologically relevant still needs to be determined (Huang et al. 2007). In neutrophils, IP4 has recently been suggested to inhibit Akt-PH domain interactions with membrane PIP3 or PI(3,4)P2 (Jia et al. 2007). Loss of this function may contribute to an accumulation of granulocyte-macrophage progenitors and functionally-impaired neutrophils in ItpkB−/− mice (Jia et al. 2007; Jia et al. 2008a; Jia et al. 2008b). On the other hand, IP4 did not affect PLCγ1 binding to PIP3, which is mediated through its classic- and split-PH domains (Fig. 2) (Huang et al. 2007). Thus, not all PIP3-binding PH domains may be subject to IP4 control. One possible explanation is that the affinities of a given PH domain for PIP3 and IP4 can differ, with some PH domains preferring IP4 or PIP3, possibly due to differential contributions of the 1-phosphate or the lipid environment to the binding free energy (DiNitto and Lambright 2006).
Finally, it is important to keep in mind that IP4 can be a precursor for multiple soluble IPs in mammalian cells. Some of these have been found in lymphocytes (for reviews, see Sauer et al. 2009; Sauer and Cooke 2010). Hence, some aspects of the ItpkB−/− phenotype might reflect deficiencies in other IPs.
Due to the blocked T-cell development in ItpkB-deficient mice, analyses of specific ItpkB functions in peripheral T cells will require conditionally ItpkB-deficient mice. However, studies linking ItpkC loss-of-function to augmented Jurkat cell activation and human Kawasaki disease support an IP4 role in regulating peripheral T-cell activation (Onouchi et al. 2008).
PHOSPHATASES CONTROL PIP3 AND IP4 TURNOVER
PIP3 levels are down-regulated by lipid phosphatases, including PTEN and SHIP1/2 in lymphocytes (Figs. 1 and 3; Table 1) (Harris et al. 2008). PTEN reverses the PI3K reaction and is generally thought to limit PI3K function. SHIP1/2 remove the PIP3 5-phosphate, generating PI(3,4)P2 and also down-modulating PIP3 levels (Damen et al. 1996; Kavanaugh et al. 1996; Lioubin et al. 1996). However, effectors such as Akt that predominantly bind to the inositol 3/4-phosphates may continue to be recruited and activated by PI(3,4)P2. Thus, differential use of PTEN and SHIP1/2 can selectively alter the activities of a subset of PIP3 effectors, qualitatively changing PI3K responses.
Complete PTEN deficiency in mice results in embryonic lethality (Di Cristofano et al. 1998). PTEN hemizygosity, or conditional deficiency caused T-cell hyperproliferation, autoimmunity, and increased tumor incidence (Di Cristofano et al. 1999; Di Cristofano and Pandolfi 2000). PTEN deletion in T cells revealed the importance of precise PIP3 regulation for T-cell development through defective positive and negative selection (Suzuki et al. 2001). PTEN-deficient peripheral T cells were hyper-responsive to suboptimal TCR stimulation and less dependent on co-stimulation, resulting in autoimmune pathology (Suzuki et al. 2001). Activated T cells secreted increased cytokine amounts (Suzuki et al. 2001) and showed a bias toward the TH2 lineage (Buckler et al. 2008). Thus, PIP3 down-regulation by PTEN is required for limiting T-cell responses and maintaining self-tolerance.
PIP3 dephosphorylation at the 5-position by SHIP1/2 is also important for T-cell tolerance. However, while the effects of SHIP deficiency partially overlap with those of PTEN deficiency, the additional importance of generating PI(3,4)P2 is evident from the additional shortened lifespan of SHIP-deficient mice (Helgason et al. 1998). SHIP-deficient CD4 T cells have an altered Th1/Th2 cell ratio. SHIP-deficient CD8 T cells are less cytotoxic (Tarasenko et al. 2007). Thus far, these phenotypes have been attributed to defective PIP3 conversion into PI(3,4)P2.
However, in vitro, both SHIPs and PTEN can dephosphorylate IP4 into I(1,3,4)P3 or Ins(1,4,5)P3, respectively (Fig. 1) (Erneux et al. 1998; Maehama and Dixon 1998; Pesesse et al. 1998; Caffrey et al. 2001). Whether this is relevant in vivo is unknown. If it were, then the phenotypes of PTEN−/− or SHIP−/− mice might need reinterpretation to consider potential contributions of perturbed IP4 down-regulation. On the other hand, the presence of an IP4-inhibited 5-phosphatase in Jurkat cells that lack SHIP and PTEN (and also show hyperactivation) suggests that other phosphatases might also regulate IP4 levels in T cells (Hermosura et al. 2000).
CONCLUDING REMARKS
We have highlighted the importance of phosphoinositol signaling in lymphocytes, focusing on T cells. The importance of phosphoinositide lipids and of the Ca2+-mobilizing soluble I(1,4,5)P3 in lymphocyte signaling have long been established, although many open questions remain. For example, what influence do the specific fatty acids in a phosphoinositide have? There is evidence that many different fatty acids can be found in a given phosphoinositide isomer. They might control where it is located or other aspects of its function. Is PIP3 really a universe of diverse molecules that differ in their fatty acid moieties and, possibly, functions?
More recent data unveiled important functions for the lipids DAG and PA, and for soluble IP4 in lymphocyte development and function. Some insight into the molecular mechanisms through which these novel messengers act has been gained, but our understanding of their molecular functions is far from complete. In particular, the roles of additional DAG effectors other than RasGRPs and PKCs in T cells need assessment, and the molecular mechanisms through which PA contributes to DGK function in lymphocytes are only beginning to be understood (Zhong et al. 2008).
One of the most intriguing, possibly broadly relevant, recent findings is the ability of IP4 to control PIP3 interactions with its effectors. Because PIP3 is the key mediator of PI3K, PTEN, and SHIP function, IP4 might ultimately act to control the functions of these important enzymes. Biochemically, PI3K and IP3Ks catalyze the very same reaction: phosphorylation of the inositol 3-position. Physiologically, they create the Tessa Virtue and Scott Moir, respectively, of a couple of second messengers whose dance in the cellular ice ring is reminiscent of the intricacy and elegance of its 2010 Winter Olympics counterpart. In DP thymocytes, the dance establishes a feedback loop of Itk-membrane recruitment and PLCγ1 activation that is essential to allow mild TCR stimuli to cause sufficient DAG production, such that Ras/Erk can be activated and trigger positive selection (Huang et al. 2007; Sauer and Cooke 2010). It will be important to explore how broadly relevant PIP3 control through soluble IP4 is in other cell types and downstream of other receptors. Moreover, clarifying what determines whether a PIP3-binding protein domain is subject to IP4 control remains a challenging and important question. Finally, it will be exciting to determine the functions of the other higher-order IPs found in lymphocytes (Sauer et al. 2009; Sauer and Cooke 2010), and to determine which ones are IP4-derived and might, thus, mediate aspects of IP4 function. Clearly, exploring phosphoinositide-lipid signaling and the functions of the soluble IP code in lymphocytes will remain an exciting and important research area for years to come.
ACKNOWLEDGMENTS
We thank Claire Conche and Sabine Siegemund for critical reading of the manuscript and valuable comments. K.S. is supported by NIH grants AI070845 and GM088647, and by Scholar Award 1440-11 from The Leukemia & Lymphoma Society. The authors declare no competing financial interests.
Footnotes
Editors: Lawrence E. Samelson and Andrey Shaw
Additional Perspectives on Immunoreceptor Signaling available at www.cshperspectives.org
REFERENCES
- Alcazar I, Cortes I, Zaballos A, Hernandez C, Fruman DA, Barber DF, Carrera AC 2009. p85beta phosphoinositide 3-kinase regulates CD28 coreceptor function. Blood 113: 3198–3208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcazar I, Marques M, Kumar A, Hirsch E, Wymann M, Carrera AC, Barber DF 2007. Phosphoinositide 3-kinase gamma participates in T cell receptor-induced T cell activation. J Exp Med 204: 2977–2987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcazar-Roman AR, Wente SR 2008. Inositol polyphosphates: a new frontier for regulating gene expression. Chromosoma 117: 1–13 [DOI] [PubMed] [Google Scholar]
- Ang BK, Lim CY, Koh SS, Sivakumar N, Taib S, Lim KB, Ahmed S, Rajagopal G, Ong SH 2007. ArhGAP9, a novel MAP kinase docking protein, inhibits Erk and p38 activation through WW domain binding. J Mol Signal 2: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atherly LO, Lucas JA, Felices M, Yin CC, Reiner SL, Berg LJ 2006. The Tec family tyrosine kinases Itk and Rlk regulate the development of conventional CD8+ T cells. Immunity 25: 79–91 [DOI] [PubMed] [Google Scholar]
- August A, Sadra A, Dupont B, Hanafusa H 1997. Src-induced activation of inducible T cell kinase (ITK) requires phosphatidylinositol 3-kinase activity and the Pleckstrin homology domain of inducible T cell kinase. Proc Natl Acad Sci U S A 94: 11227–11232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae YS, Cantley LG, Chen CS, Kim SR, Kwon KS, Rhee SG 1998. Activation of phospholipase C-gamma by phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem 273: 4465–4469 [DOI] [PubMed] [Google Scholar]
- Barber DF, Bartolome A, Hernandez C, Flores JM, Fernandez-Arias C, Rodriguez-Borlado L, Hirsch E, Wymann M, Balomenos D, Carrera AC 2006. Class IB-phosphatidylinositol 3-kinase (PI3K) deficiency ameliorates IA-PI3K-induced systemic lupus but not T cell invasion. J Immunol 176: 589–593 [DOI] [PubMed] [Google Scholar]
- Barouch-Bentov R, Altman A 2006. Protein Kinase C-Theta (PKCtheta): New Perspectives on Its Functions in T-Cell Biology. In Advances in Experimental Medicine and Biology (ed. Tsoukas CD), pp. 1–14 Springer, New York, NY: [DOI] [PubMed] [Google Scholar]
- Betz A, Ashery U, Rickmann M, Augustin I, Neher E, Sudhof TC, Rettig J, Brose N 1998. Munc13–1 is a presynaptic phorbol ester receptor that enhances neurotransmitter release. Neuron 21: 123–136 [DOI] [PubMed] [Google Scholar]
- Broussard C, Fleischacker C, Horai R, Chetana M, Venegas AM, Sharp LL, Hedrick SM, Fowlkes BJ, Schwartzberg PL 2006. Altered development of CD8+ T cell lineages in mice deficient for the Tec kinases Itk and Rlk. Immunity 25: 93–104 [DOI] [PubMed] [Google Scholar]
- Buckler JL, Liu X, Turka LA 2008. Regulation of T-cell responses by PTEN. Immunol Rev 224: 239–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buitenhuis M, Coffer PJ 2009. The role of the PI3K-PKB signaling module in regulation of hematopoiesis. Cell Cycle 8: 560–566 [DOI] [PubMed] [Google Scholar]
- Burton A, Hu X, Saiardi A 2009. Are inositol pyrophosphates signalling molecules? J Cell Physiol 220: 8–15 [DOI] [PubMed] [Google Scholar]
- Caffrey JJ, Darden T, Wenk MR, Shears SB 2001. Expanding coincident signaling by PTEN through its inositol 1,3,4,5,6-pentakisphosphate 3-phosphatase activity. FEBS Lett 499: 6–10 [DOI] [PubMed] [Google Scholar]
- Calleja V, Alcor D, Laguerre M, Park J, Vojnovic B, Hemmings BA, Downward J, Parker PJ, Larijani B 2007. Intramolecular and intermolecular interactions of protein kinase B define its activation in vivo. PLoS Biol 5: e95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caloca MJ, Delgado P, Alarcon B, Bustelo XR 2008. Role of chimaerins, a group of Rac-specific GTPase activating proteins, in T-cell receptor signaling. Cell Signal 20: 758–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco S, Merida I 2004. Diacylglycerol-dependent binding recruits PKCtheta and RasGRP1 C1 domains to specific subcellular localizations in living T lymphocytes. Mol Biol Cell 15: 2932–2942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli DF, Blasutig IM, Goudreault M, Li Z, Ruston J, Pawson T, Sicheri F 2007. Non-canonical interaction of phosphoinositides with pleckstrin homology domains of Tiam1 and ArhGAP9. J Biol Chem 282: 13864–13874 [DOI] [PubMed] [Google Scholar]
- Chamberlain PP, Sandberg ML, Sauer K, Cooke MP, Lesley SA, Spraggon G 2005. Structural insights into enzyme regulation for inositol 1,4,5-trisphosphate 3-kinase B. Biochem 44: 14486–14493 [DOI] [PubMed] [Google Scholar]
- Chen X, Priatel JJ, Chow MT, Teh HS 2008. Preferential development of CD4 and CD8 T regulatory cells in RasGRP1-deficient mice. J Immunol 180: 5973–5982 [DOI] [PubMed] [Google Scholar]
- Ching KA, Kawakami Y, Kawakami T, Tsoukas CD 1999. Emt/Itk associates with activated TCR complexes: role of the pleckstrin homology domain. J Immunol 163: 6006–6013 [PubMed] [Google Scholar]
- Clayton E, Bardi G, Bell SE, Chantry D, Downes CP, Gray A, Humphries LA, Rawlings D, Reynolds H, Vigorito E, et al. 2002. A crucial role for the p110delta subunit of phosphatidylinositol 3-kinase in B cell development and activation. J Exp Med 196: 753–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozier G, Sessions R, Bottomley JR, Reynolds JS, Cullen PJ 2000a. Molecular modelling and site-directed mutagenesis of the inositol 1,3,4,5-tetrakisphosphate-binding pleckstrin homology domain from the Ras GTPase-activating protein GAP1IP4BP. Biochem J 349: 333–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozier GE, Carlton J, Bouyoucef D, Cullen PJ 2004. Membrane targeting by pleckstrin homology domains. Curr Top Microbiol Immunol 282: 49–88 [DOI] [PubMed] [Google Scholar]
- Cozier GE, Lockyer PJ, Reynolds JS, Kupzig S, Bottomley JR, Millard TH, Banting G, Cullen PJ 2000b. GAP1IP4BP contains a novel group I pleckstrin homology domain that directs constitutive plasma membrane association. J Biol Chem 275: 28261–28268 [DOI] [PubMed] [Google Scholar]
- Cremasco V, Benasciutti E, Cella M, Kisseleva M, Croke M, Faccio R 2010. Phospholipase C gamma 2 is critical for development of a murine model of inflammatory arthritis by affecting actin dynamics in dendritic cells. PLoS One 5: e8909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremasco V, Graham DB, Novack DV, Swat W, Faccio R 2008. Vav/Phospholipase Cgamma2-mediated control of a neutrophil-dependent murine model of rheumatoid arthritis. Arthritis Rheum 58: 2712–2722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen PJ, Hsuan JJ, Truong O, Letcher AJ, Jackson TR, Dawson AP, Irvine RF 1995. Identification of a specific Ins(1,3,4,5)P4-binding protein as a member of the GAP1 family. Nature 376: 527–530 [DOI] [PubMed] [Google Scholar]
- Damen JE, Liu L, Rosten P, Humphries RK, Jefferson AB, Majerus PW, Krystal G 1996. The 145-kDa protein induced to associate with Shc by multiple cytokines is an inositol tetraphosphate and phosphatidylinositol 3,4,5-triphosphate 5-phosphatase. Proc Natl Acad Sci U S A 93: 1689–1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane JA, Kharas MG, Oak JS, Stiles LN, Luo J, Moore TI, Ji H, Rommel C, Cantley LC, Lane TE, et al. 2007. T-cell function is partially maintained in the absence of class IA phosphoinositide 3-kinase signaling. Blood 109: 2894–2902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane JA, Trifilo MJ, Yballe CM, Choi S, Lane TE, Fruman DA 2004. Enhanced T cell proliferation in mice lacking the p85beta subunit of phosphoinositide 3-kinase. J Immunol 172: 6615–6625 [DOI] [PubMed] [Google Scholar]
- Debnath J, Chamorro M, Czar MJ, Schaeffer EM, Lenardo MJ, Varmus HE, Schwartzberg PL 1999. rlk/TXK encodes two forms of a novel cysteine string tyrosine kinase activated by Src family kinases. Mol Cell Biol 19: 1498–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G 1997. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science 278: 687–689 [DOI] [PubMed] [Google Scholar]
- Di Cristofano A, Kotsi P, Peng YF, Cordon-Cardo C, Elkon KB, Pandolfi PP 1999. Impaired Fas response and autoimmunity in Pten+/- mice. Science 285: 2122–2125 [DOI] [PubMed] [Google Scholar]
- Di Cristofano A, Pandolfi PP 2000. The multiple roles of PTEN in tumor suppression. Cell 100: 387–390 [DOI] [PubMed] [Google Scholar]
- Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi PP 1998. Pten is essential for embryonic development and tumour suppression. Nat Genet 19: 348–355 [DOI] [PubMed] [Google Scholar]
- DiNitto JP, Lambright DG 2006. Membrane and juxtamembrane targeting by PH and PTB domains. Biochim Biophys Acta 1761: 850–867 [DOI] [PubMed] [Google Scholar]
- Dombroski D, Houghtling RA, Labno CM, Precht P, Takesono A, Caplen NJ, Billadeau DD, Wange RL, Burkhardt JK, Schwartzberg PL 2005. Kinase-independent functions for Itk in TCR-induced regulation of Vav and the actin cytoskeleton. J Immunol 174: 1385–1392 [DOI] [PubMed] [Google Scholar]
- Dower NA, Stang SL, Bottorff DA, Ebinu JO, Dickie P, Ostergaard HL, Stone JC 2000. RasGRP is essential for mouse thymocyte differentiation and TCR signaling. Nat Immunol 1: 317–321 [DOI] [PubMed] [Google Scholar]
- Downing AK, Driscoll PC, Gout I, Salim K, Zvelebil MJ, Waterfield MD 1994. Three-dimensional solution structure of the pleckstrin homology domain from dynamin. Curr Biol 4: 884–891 [DOI] [PubMed] [Google Scholar]
- Dufner A, Andjelkovic M, Burgering BM, Hemmings BA, Thomas G 1999. Protein kinase B localization and activation differentially affect S6 kinase 1 activity and eukaryotic translation initiation factor 4E-binding protein 1 phosphorylation. Mol Cell Biol 19: 4525–4534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epple H, Cremasco V, Zhang K, Mao D, Longmore GD, Faccio R 2008. Phospholipase Cgamma2 modulates integrin signaling in the osteoclast by affecting the localization and activation of Src kinase. Mol Cell Biol 28: 3610–3622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erneux C, Govaerts C, Communi D, Pesesse X 1998. The diversity and possible functions of the inositol polyphosphate 5-phosphatases. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids 1436: 185–199 [DOI] [PubMed] [Google Scholar]
- Falasca M, Logan SK, Lehto VP, Baccante G, Lemmon MA, Schlessinger J 1998. Activation of phospholipase C gamma by PI 3-kinase-induced PH domain-mediated membrane targeting. Embo J 17: 414–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KM, Lemmon MA, Schlessinger J, Sigler PB 1994. Crystal structure at 2.2 A resolution of the pleckstrin homology domain from human dynamin. Cell 79: 199–209 [DOI] [PubMed] [Google Scholar]
- Feske S 2007. Calcium signalling in lymphocyte activation and disease. Nat Rev Immunol 7: 690–702 [DOI] [PubMed] [Google Scholar]
- Finkelstein LD, Shimizu Y, Schwartzberg PL 2005. Tec kinases regulate TCR-mediated recruitment of signaling molecules and integrin-dependent cell adhesion. J Immunol 175: 5923–5930 [DOI] [PubMed] [Google Scholar]
- Fowell DJ, Shinkai K, Liao XC, Beebe AM, Coffman RL, Littman DR, Locksley RM 1999. Impaired NFATc translocation and failure of Th2 development in Itk-deficient CD4+ T cells. Immunity 11: 399–409 [DOI] [PubMed] [Google Scholar]
- Frauwirth KA, Riley JL, Harris MH, Parry RV, Rathmell JC, Plas DR, Elstrom RL, June CH, Thompson CB 2002. The CD28 signaling pathway regulates glucose metabolism. Immunity 16: 769–777 [DOI] [PubMed] [Google Scholar]
- Frederick JP, Mattiske D, Wofford JA, Megosh LC, Drake LY, Chiou ST, Hogan BL, York JD 2005. An essential role for an inositol polyphosphate multikinase, Ipk2, in mouse embryogenesis and second messenger production. Proc Natl Acad Sci U S A 102: 8454–8459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruman DA, Bismuth G 2009. Fine tuning the immune response with PI3K. Immunol Rev 228: 253–272 [DOI] [PubMed] [Google Scholar]
- Fruman DA, Mauvais-Jarvis F, Pollard DA, Yballe CM, Brazil D, Bronson RT, Kahn CR, Cantley LC 2000. Hypoglycaemia, liver necrosis and perinatal death in mice lacking all isoforms of phosphoinositide 3-kinase p85 alpha. Nat Genet 26: 379–382 [DOI] [PubMed] [Google Scholar]
- Fu G, Chen Y, Yu M, Podd A, Schuman J, He Y, Di L, Yassai M, Haribhai D, North PE, et al. 2010. Phospholipase C{gamma}1 is essential for T cell development, activation, and tolerance. J Exp Med 207: 309–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo EM, Winslow MM, Cante-Barrett K, Radermacher AN, Ho L, McGinnis L, Iritani B, Neilson JR, Crabtree GR 2007. Calcineurin sets the bandwidth for discrimination of signals during thymocyte development. Nature 450: 731–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Harris TK 2006. Role of the PH domain in regulating in vitro autophosphorylation events required for reconstitution of PDK1 catalytic activity. Bioorg Chem 34: 200–223 [DOI] [PubMed] [Google Scholar]
- Gardai SJ, Hildeman DA, Frankel SK, Whitlock BB, Frasch SC, Borregaard N, Marrack P, Bratton DL, Henson PM 2004. Phosphorylation of Bax Ser184 by Akt regulates its activity and apoptosis in neutrophils. J Biol Chem 279: 21085–21095 [DOI] [PubMed] [Google Scholar]
- Gomez-Rodriguez J, Readinger JA, Viorritto IC, Mueller KL, Houghtling RA, Schwartzberg PL 2007. Tec kinases, actin, and cell adhesion. Immunol Rev 218: 45–64 [DOI] [PubMed] [Google Scholar]
- Graham DB, Robertson CM, Bautista J, Mascarenhas F, Diacovo MJ, Montgrain V, Lam SK, Cremasco V, Dunne WM, Faccio R, et al. 2007. Neutrophil-mediated oxidative burst and host defense are controlled by a Vav-PLCgamma2 signaling axis in mice. J Clin Invest 117: 3445–3452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasis JA, Browne CD, Tsoukas CD 2003. Inducible T cell tyrosine kinase regulates actin-dependent cytoskeletal events induced by the T cell antigen receptor. J Immunol 170: 3971–3976 [DOI] [PubMed] [Google Scholar]
- Guo R, Wan CK, Carpenter JH, Mousallem T, Boustany RM, Kuan CT, Burks AW, Zhong XP 2008. Synergistic control of T cell development and tumor suppression by diacylglycerol kinase alpha and zeta. Proc Natl Acad Sci U S A 105: 11909–11914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenbeek TJ, Naspetti M, Malergue F, Garcon F, Nunes JA, Cleutjens KBJM, Trapman J, Krimpenfort P, Spits H 2004. The Loss of PTEN Allows TCR {alpha}{beta} Lineage Thymocytes to Bypass IL-7 and Pre-TCR-mediated Signaling. J Exp Med 200: 883–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Luby-Phelps K, Das B, Shu X, Xia Y, Mosteller RD, Krishna UM, Falck JR, White MA, Broek D 1998. Role of substrates and products of PI 3-kinase in regulating activation of Rac-related guanosine triphosphatases by Vav. Science 279: 558–560 [DOI] [PubMed] [Google Scholar]
- Harris SJ, Parry RV, Westwick J, Ward SG 2008. Phosphoinositide lipid phosphatases: natural regulators of phosphoinositide 3-kinase signaling in T lymphocytes. J Biol Chem 283: 2465–2469 [DOI] [PubMed] [Google Scholar]
- Haslam RJ, Koide HB, Hemmings BA 1993. Pleckstrin domain homology. Nature 363: 309–310 [DOI] [PubMed] [Google Scholar]
- Helgason CD, Damen JE, Rosten P, Grewal R, Sorensen P, Chappel SM, Borowski A, Jirik F, Krystal G, Humphries RK 1998. Targeted disruption of SHIP leads to hemopoietic perturbations, lung pathology, and a shortened life span. Genes Dev 12: 1610–1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermosura MC, Takeuchi H, Fleig A, Riley AM, Potter BV, Hirata M, Penner R 2000. InsP4 facilitates store-operated calcium influx by inhibition of InsP3 5-phosphatase. Nature 408: 735–740 [DOI] [PubMed] [Google Scholar]
- Heyeck SD, Wilcox HM, Bunnell SC, Berg LJ 1997. Lck phosphorylates the activation loop tyrosine of the Itk kinase domain and activates Itk kinase activity. J Biol Chem 272: 25401–25408 [DOI] [PubMed] [Google Scholar]
- Hirata M, Kanematsu T, Takeuchi H, Yagisawa H 1998. Pleckstrin homology domain as an inositol compound binding module. Jpn J Pharmacol 76: 255–263 [DOI] [PubMed] [Google Scholar]
- Horai R, Mueller KL, Handon RA, Cannons JL, Anderson SM, Kirby MR, Schwartzberg PL 2007. Requirements for selection of conventional and innate T lymphocyte lineages. Immunity 27: 775–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YH, Barouch-Bentov R, Herman A, Walker J, Sauer K 2006. Integrating traditional and postgenomic approaches to investigate lymphocyte development and function. Adv Exp Med Biol 584: 245–276 [DOI] [PubMed] [Google Scholar]
- Huang YH, Grasis JA, Miller AT, Xu R, Soonthornvacharin S, Andreotti AH, Tsoukas CD, Cooke MP, Sauer K 2007. Positive regulation of Itk PH domain function by soluble IP4. Science 316: 886–889 [DOI] [PubMed] [Google Scholar]
- Huang YH, Hoebe K, Sauer K 2008. New therapeutic targets in immune disorders: ItpkB, Orai1 and UNC93B. Expert Opin Ther Targets 12: 391–413 [DOI] [PubMed] [Google Scholar]
- Hubner A, Barrett T, Flavell RA, Davis RJ 2008. Multisite phosphorylation regulates Bim stability and apoptotic activity. Mol Cell 30: 415–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imboden JB, Pattison G 1987. Regulation of inositol 1,4,5-trisphosphate kinase activity after stimulation of human T cell antigen receptor. J Clin Invest 79: 1538–1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R 2001. Inositol phosphates: Does IP(4) run a protection racket? Curr Biol 11: R172–174 [DOI] [PubMed] [Google Scholar]
- Irvine R 2007. Cell signaling. The art of the soluble. Science 316: 845–846 [DOI] [PubMed] [Google Scholar]
- Irvine RF 2005. Inositide evolution - towards turtle domination? J Physiol 566: 295–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine RF, Lloyd-Burton SM, Yu JC, Letcher AJ, Schell MJ 2006. The regulation and function of inositol 1,4,5-trisphosphate 3-kinases. Adv Enzyme Regul 46: 314–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine RF, Schell MJ 2001. Back in the water: the return of the inositol phosphates. Nat Rev Mol Cell Biol 2: 327–338 [DOI] [PubMed] [Google Scholar]
- Isakov N, Altman A 2002. Protein kinase C(theta) in T cell activation. Annu Rev Immunol 20: 761–794 [DOI] [PubMed] [Google Scholar]
- Jacobs SR, Herman CE, Maciver NJ, Wofford JA, Wieman HL, Hammen JJ, Rathmell JC 2008. Glucose uptake is limiting in T cell activation and requires CD28-mediated Akt-dependent and independent pathways. J Immunol 180: 4476–4486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James SR, Downes CP, Gigg R, Grove SJ, Holmes AB, Alessi DR 1996. Specific binding of the Akt-1 protein kinase to phosphatidylinositol 3,4,5-trisphosphate without subsequent activation. Biochem J 315: 709–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarmin SJ, David R, Ma L, Chai JG, Dewchand H, Takesono A, Ridley AJ, Okkenhaug K, Marelli-Berg FM 2008. T cell receptor-induced phosphoinositide-3-kinase p110delta activity is required for T cell localization to antigenic tissue in mice. J Clin Invest 118: 1154–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, Rintelen F, Waltzinger C, Bertschy Meier D, Bilancio A, Pearce W, Hirsch E, Wymann MP, Ruckle T, Camps M, et al. 2007. Inactivation of PI3Kgamma and PI3Kdelta distorts T-cell development and causes multiple organ inflammation. Blood 110: 2940–2947 [DOI] [PubMed] [Google Scholar]
- Ji QS, Winnier GE, Niswender KD, Horstman D, Wisdom R, Magnuson MA, Carpenter G 1997. Essential role of the tyrosine kinase substrate phospholipase C-gamma1 in mammalian growth and development. Proc Natl Acad Sci U S A 94: 2999–3003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Loison F, Hattori H, Li Y, Erneux C, Park SY, Gao C, Chai L, Silberstein LE, Schurmans S, et al. 2008a. Inositol trisphosphate 3-kinase B (InsP3KB) as a physiological modulator of myelopoiesis. Proc Natl Acad Sci U S A 105: 4739–4744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Schurmans S, Luo HR 2008b. Regulation of innate immunity by inositol 1,3,4,5-tetrakisphosphate. Cell Cycle 7: 2803–2808 [DOI] [PubMed] [Google Scholar]
- Jia Y, Subramanian KK, Erneux C, Pouillon V, Hattori H, Jo H, You J, Zhu D, Schurmans S, Luo HR 2007. Inositol 1,3,4,5-tetrakisphosphate negatively regulates phosphatidylinositol-3,4,5-trisphosphate signaling in neutrophils. Immunity 27: 453–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph RE, Min L, Xu R, Musselman ED, Andreotti AH 2007. A remote substrate docking mechanism for the tec family tyrosine kinases. Biochem 46: 5595–5603 [DOI] [PubMed] [Google Scholar]
- Jun K, Choi G, Yang SG, Choi KY, Kim H, Chan GC, Storm DR, Albert C, Mayr GW, Lee CJ, et al. 1998. Enhanced hippocampal CA1 LTP but normal spatial learning in inositol 1,4,5-trisphosphate 3-kinase(A)-deficient mice. Learn Mem 5: 317–330 [PMC free article] [PubMed] [Google Scholar]
- Juntilla MM, Koretzky GA 2008. Critical roles of the PI3K/Akt signaling pathway in T cell development. Immunol Lett 116: 104–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juntilla MM, Wofford JA, Birnbaum MJ, Rathmell JC, Koretzky GA 2007. Akt1 and Akt2 are required for alphabeta thymocyte survival and differentiation. Proc Natl Acad Sci U S A 104: 12105–12110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanaugh WM, Pot DA, Chin SM, Deuter-Reinhard M, Jefferson AB, Norris FA, Masiarz FR, Cousens LS, Majerus PW, Williams LT 1996. Multiple forms of an inositol polyphosphate 5-phosphatase form signaling complexes with Shc and Grb2. Curr Biol 6: 438–445 [DOI] [PubMed] [Google Scholar]
- Kim IH, Park SK, Hong ST, Jo YS, Kim EJ, Park EH, Han SB, Shin HS, Sun W, Kim HT, et al. 2009. Inositol 1,4,5-trisphosphate 3-kinase a functions as a scaffold for synaptic Rac signaling. J Neurosci 29: 14039–14049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon MA 2008. Membrane recognition by phospholipid-binding domains. Nat Rev Mol Cell Biol 9: 99–111 [DOI] [PubMed] [Google Scholar]
- Liang J, Zubovitz J, Petrocelli T, Kotchetkov R, Connor MK, Han K, Lee JH, Ciarallo S, Catzavelos C, Beniston R, et al. 2002. PKB/Akt phosphorylates p27, impairs nuclear import of p27 and opposes p27-mediated G1 arrest. Nat Med 8: 1153–1160 [DOI] [PubMed] [Google Scholar]
- Liao XC, Littman DR 1995. Altered T cell receptor signaling and disrupted T cell development in mice lacking Itk. Immunity 3: 757–769 [DOI] [PubMed] [Google Scholar]
- Lioubin MN, Algate PA, Tsai S, Carlberg K, Aebersold A, Rohrschneider LR 1996. p150Ship, a signal transduction molecule with inositol polyphosphate-5-phosphatase activity. Genes Dev 10: 1084–1095 [DOI] [PubMed] [Google Scholar]
- Liu D, Uzonna JE 2010. The p110{delta} Isoform of Phosphatidylinositol 3-Kinase Controls the Quality of Secondary Anti-Leishmania Immunity by Regulating Expansion and Effector Function of Memory T Cell Subsets. J Immunol 184: 3098–3105 [DOI] [PubMed] [Google Scholar]
- Liu D, Zhang T, Marshall AJ, Okkenhaug K, Vanhaesebroeck B, Uzonna JE 2009a. The p110delta isoform of phosphatidylinositol 3-kinase controls susceptibility to Leishmania major by regulating expansion and tissue homing of regulatory T cells. J Immunol 183: 1921–1933 [DOI] [PubMed] [Google Scholar]
- Liu KQ, Bunnell SC, Gurniak CB, Berg LJ 1998. T cell receptor-initiated calcium release is uncoupled from capacitative calcium entry in Itk-deficient T cells. J Exp Med 187: 1721–1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Cheng H, Roberts TM, Zhao JJ 2009b. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov 8: 627–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockyer PJ, Bottomley JR, Reynolds JS, McNulty TJ, Venkateswarlu K, Potter BV, Dempsey CE, Cullen PJ 1997. Distinct subcellular localisations of the putative inositol 1,3,4,5-tetrakisphosphate receptors GAP1IP4BP and GAP1m result from the GAP1IP4BP PH domain directing plasma membrane targeting. Curr Biol 7: 1007–1010 [DOI] [PubMed] [Google Scholar]
- Lucas JA, Felices M, Evans JW, Berg LJ 2007. Subtle defects in pre-TCR signaling in the absence of the Tec kinase Itk. J Immunol 179: 7561–7567 [DOI] [PubMed] [Google Scholar]
- Macian F, Garcia-Cozar F, Im SH, Horton HF, Byrne MC, Rao A 2002. Transcriptional mechanisms underlying lymphocyte tolerance. Cell 109: 719–731 [DOI] [PubMed] [Google Scholar]
- Macias MJ, Musacchio A, Ponstingl H, Nilges M, Saraste M, Oschkinat H 1994. Structure of the pleckstrin homology domain from beta-spectrin. Nature 369: 675–677 [DOI] [PubMed] [Google Scholar]
- Madrid LV, Wang CY, Guttridge DC, Schottelius AJ, Baldwin AS Jr, Mayo MW 2000. Akt suppresses apoptosis by stimulating the transactivation potential of the RelA/p65 subunit of NF-kappaB. Mol Cell Biol 20: 1626–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehama T, Dixon JE 1998. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem 273: 13375–13378 [DOI] [PubMed] [Google Scholar]
- Manicassamy S, Gupta S, Sun Z 2006. Selective function of PKC-theta in T cells. Cell Mol Immunol 3: 263–270 [PubMed] [Google Scholar]
- Manning BD, Cantley LC 2007. AKT/PKB signaling: navigating downstream. Cell 129: 1261–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marechal Y, Pesesse X, Jia Y, Pouillon V, Perez-Morga D, Daniel J, Izui S, Cullen PJ, Leo O, Luo HR, et al. 2007. Inositol 1,3,4,5-tetrakisphosphate controls proapoptotic Bim gene expression and survival in B cells. Proc Natl Acad Sci U S A 104: 13978–13983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AL, Schwartz MD, Jameson SC, Shimizu Y 2008. Selective regulation of CD8 effector T cell migration by the p110 gamma isoform of phosphatidylinositol 3-kinase. J Immunol 180: 2081–2088 [DOI] [PubMed] [Google Scholar]
- Matheu MP, Deane JA, Parker I, Fruman DA, Cahalan MD 2007. Class IA phosphoinositide 3-kinase modulates basal lymphocyte motility in the lymph node. J Immunol 179: 2261–2269 [DOI] [PubMed] [Google Scholar]
- Mayer BJ, Ren R, Clark KL, Baltimore D 1993. A putative modular domain present in diverse signaling proteins. Cell 73: 629–630 [DOI] [PubMed] [Google Scholar]
- McGargill MA, Ch'en IL, Katayama CD, Pages G, Pouyssegur J, Hedrick SM 2009. Cutting Edge: Extracellular Signal-Related Kinase Is Not Required for Negative Selection of Developing T Cells. J Immunol 183: 4838–4842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S, Wang J, Gambhir A, Murray D 2002. PIP(2) and proteins: interactions, organization, and information flow. Annu Rev Biophys Biomol Struct 31: 151–175 [DOI] [PubMed] [Google Scholar]
- Michell RH, Heath VL, Lemmon MA, Dove SK 2006. Phosphatidylinositol 3,5-bisphosphate: metabolism and cellular functions. Trends Biochem Sci 31: 52–63 [DOI] [PubMed] [Google Scholar]
- Miller AT, Beisner DR, Liu D, Cooke MP 2009. Inositol 1,4,5-trisphosphate 3-kinase B is a negative regulator of BCR signaling that controls B cell selection and tolerance induction. J Immunol 182: 4696–4704 [DOI] [PubMed] [Google Scholar]
- Miller AT, Chamberlain PP, Cooke MP 2008. Beyond IP3: roles for higher order inositol phosphates in immune cell signaling. Cell Cycle 7: 463–467 [DOI] [PubMed] [Google Scholar]
- Miller AT, Sandberg M, Huang YH, Young M, Sutton S, Sauer K, Cooke MP 2007a. Production of Ins(1,3,4,5)P4 mediated by the kinase Itpkb inhibits store-operated calcium channels and regulates B cell selection and activation. Nat Immunol 8: 514–521 [DOI] [PubMed] [Google Scholar]
- Miller AT, Sandberg M, Huang YH, Young M, Sutton S, Sauer K, Cooke MP 2007b. Production of Ins(1,3,4,5)P(4) mediated by the kinase Itpkb inhibits store-operated calcium channels and regulates B cell selection and activation. Nat Immunol 8: 514–521 [DOI] [PubMed] [Google Scholar]
- Min L, Joseph RE, Fulton DB, Andreotti AH 2009. Itk tyrosine kinase substrate docking is mediated by a nonclassical SH2 domain surface of PLCgamma1. Proc Natl Acad Sci U S A 106: 21143–21148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley SC, Weber KS, Kao H, Allen PM 2008. Protein kinase C-theta is required for efficient positive selection. J Immunol 181: 4696–4708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalaskowski MM, Mayr GW 2004. The families of kinases removing the Ca2+ releasing second messenger Ins(1,4,5)P3. Curr Mol Med 4: 277–290 [DOI] [PubMed] [Google Scholar]
- Nashed BF, Zhang T, Al-Alwan M, Srinivasan G, Halayko AJ, Okkenhaug K, Vanhaesebroeck B, Hayglass KT, Marshall AJ 2007. Role of the phosphoinositide 3-kinase p110delta in generation of type 2 cytokine responses and allergic airway inflammation. Eur J Immunol 37: 416–424 [DOI] [PubMed] [Google Scholar]
- Neilson JR, Winslow MM, Hur EM, Crabtree GR 2004. Calcineurin B1 is essential for positive but not negative selection during thymocyte development. Immunity 20: 255–266 [DOI] [PubMed] [Google Scholar]
- Niki I, Yokokura H, Sudo T, Kato M, Hidaka H 1996. Ca2+ signaling and intracellular Ca2+ binding proteins. J Biochem 120: 685–698 [DOI] [PubMed] [Google Scholar]
- Oak JS, Deane JA, Kharas MG, Luo J, Lane TE, Cantley LC, Fruman DA 2006. Sjogren's syndrome-like disease in mice with T cells lacking class 1A phosphoinositide-3-kinase. Proc Natl Acad Sci U S A 103: 16882–16887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh-hora M, Rao A 2008. Calcium signaling in lymphocytes. Curr Opin Immunol 20: 250–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okkenhaug K, Bilancio A, Farjot G, Priddle H, Sancho S, Peskett E, Pearce W, Meek SE, Salpekar A, Waterfield MD, et al. 2002. Impaired B and T cell antigen receptor signaling in p110delta PI 3-kinase mutant mice. Science 297: 1031–1034 [DOI] [PubMed] [Google Scholar]
- Okkenhaug K, Patton DT, Bilancio A, Garcon F, Rowan WC, Vanhaesebroeck B 2006. The p110delta isoform of phosphoinositide 3-kinase controls clonal expansion and differentiation of Th cells. J Immunol 177: 5122–5128 [DOI] [PubMed] [Google Scholar]
- Olenchock BA, Guo R, Carpenter JH, Jordan M, Topham MK, Koretzky GA, Zhong XP 2006. Disruption of diacylglycerol metabolism impairs the induction of T cell anergy. Nat Immunol 7: 1174–1181 [DOI] [PubMed] [Google Scholar]
- Onouchi Y, Gunji T, Burns JC, Shimizu C, Newburger JW, Yashiro M, Nakamura Y, Yanagawa H, Wakui K, Fukushima Y, et al. 2008. ITPKC functional polymorphism associated with Kawasaki disease susceptibility and formation of coronary artery aneurysms. Nat Genet 40: 35–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto JC, Mulugu S, Fridy PC, Chiou ST, Armbruster BN, Ribeiro AA, York JD 2007. Biochemical analysis of inositol phosphate kinases. Methods Enzymol 434: 171–185 [DOI] [PubMed] [Google Scholar]
- Outram SV, Crompton T, Merida I, Varas A, Martinez AC 2002. Diacylglycerol kinase alpha activity promotes survival of CD4+ 8+ double positive cells during thymocyte development. Immunology 105: 391–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panka DJ, Mano T, Suhara T, Walsh K, Mier JW 2001. Phosphatidylinositol 3-kinase/Akt activity regulates c-FLIP expression in tumor cells. J Biol Chem 276: 6893–6896 [DOI] [PubMed] [Google Scholar]
- Pattni K, Banting G 2004. Ins(1,4,5)P3 metabolism and the family of IP3–3Kinases. Cell Signal 16: 643–654 [DOI] [PubMed] [Google Scholar]
- Patton DT, Garden OA, Pearce WP, Clough LE, Monk CR, Leung E, Rowan WC, Sancho S, Walker LS, Vanhaesebroeck B, et al. 2006. Cutting edge: the phosphoinositide 3-kinase p110 delta is critical for the function of CD4+ CD25+ Foxp3+ regulatory T cells. J Immunol 177: 6598–6602 [DOI] [PubMed] [Google Scholar]
- Pesesse X, Moreau C, Drayer AL, Woscholski R, Parker P, Erneux C 1998. The SH2 domain containing inositol 5-phosphatase SHIP2 displays phosphatidylinositol 3,4,5-trisphosphate and inositol 1,3,4,5-tetrakisphosphate 5-phosphatase activity. FEBS Lett 437: 301–303 [DOI] [PubMed] [Google Scholar]
- Pouillon V, Hascakova-Bartova R, Pajak B, Adam E, Bex F, Dewaste V, Van Lint C, Leo O, Erneux C, Schurmans S 2003. Inositol 1,3,4,5-tetrakisphosphate is essential for T lymphocyte development. Nat Immunol 4: 1136–1143 [DOI] [PubMed] [Google Scholar]
- Priatel JJ, Chen X, Dhanji S, Abraham N, Teh HS 2006. RasGRP1 transmits prodifferentiation TCR signaling that is crucial for CD4 T cell development. J Immunol 177: 1470–1480 [DOI] [PubMed] [Google Scholar]
- Priatel JJ, Chen X, Zenewicz LA, Shen H, Harder KW, Horwitz MS, Teh HS 2007. Chronic immunodeficiency in mice lacking RasGRP1 results in CD4 T cell immune activation and exhaustion. J Immunol 179: 2143–2152 [DOI] [PubMed] [Google Scholar]
- Priatel JJ, Teh SJ, Dower NA, Stone JC, Teh HS 2002. RasGRP1 transduces low-grade TCR signals which are critical for T cell development, homeostasis, and differentiation. Immunity 17: 617–627 [DOI] [PubMed] [Google Scholar]
- Qi Q, August A 2007. Keeping the (kinase) party going: SLP-76 and ITK dance to the beat. Sci STKE 2007: e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi XJ, Wildey GM, Howe PH 2006. Evidence that Ser87 of BimEL is phosphorylated by Akt and regulates BimEL apoptotic function. J Biol Chem 281: 813–823 [DOI] [PubMed] [Google Scholar]
- Rathmell JC, Elstrom RL, Cinalli RM, Thompson CB 2003. Activated Akt promotes increased resting T cell size, CD28-independent T cell growth, and development of autoimmunity and lymphoma. Eur J Immunol 33: 2223–2232 [DOI] [PubMed] [Google Scholar]
- Readinger JA, Mueller KL, Venegas AM, Horai R, Schwartzberg PL 2009. Tec kinases regulate T-lymphocyte development and function: new insights into the roles of Itk and Rlk/Txk. Immunol Rev 228: 93–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick AC, Saiardi A 2008. Inositol polyphosphate multikinase: metabolic architect of nuclear inositides. Front Biosci 13: 856–866 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Borlado L, Barber DF, Hernandez C, Rodriguez-Marcos MA, Sanchez A, Hirsch E, Wymann M, Martinez AC, Carrera AC 2003. Phosphatidylinositol 3-kinase regulates the CD4/CD8 T cell differentiation ratio. J Immunol 170: 4475–4482 [DOI] [PubMed] [Google Scholar]
- Rosse C, Linch M, Kermorgant S, Cameron AJ, Boeckeler K, Parker PJ 2010. PKC and the control of localized signal dynamics. Nat Rev Mol Cell Biol 11: 103–112 [DOI] [PubMed] [Google Scholar]
- Sasaki T, Irie-Sasaki J, Jones RG, Oliveira-dos-Santos AJ, Stanford WL, Bolon B, Wakeham A, Itie A, Bouchard D, Kozieradzki I, et al. 2000. Function of PI3Kgamma in thymocyte development, T cell activation, and neutrophil migration. Science 287: 1040–1046 [DOI] [PubMed] [Google Scholar]
- Sauer K, Cooke MP 2010. Regulation of immune cell development through soluble inositol(1,3,4,5)tetrakisphosphate. Nat Rev Immunol 10, 257–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer K, Huang YH, Ying H, Sandberg M, Mayr GW 2009. Phosphoinositide Analysis in Lymphocyte Activation. Curr Protoc Immunol 87: 11.11.11–11.11.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer EM, Broussard C, Debnath J, Anderson S, McVicar DW, Schwartzberg PL 2000. Tec family kinases modulate thresholds for thymocyte development and selection. J Exp Med 192: 987–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer EM, Debnath J, Yap G, McVicar D, Liao XC, Littman DR, Sher A, Varmus HE, Lenardo MJ, Schwartzberg PL 1999. Requirement for Tec kinases Rlk and Itk in T cell receptor signaling and immunity. Science 284: 638–641 [DOI] [PubMed] [Google Scholar]
- Schaeffer EM, Yap GS, Lewis CM, Czar MJ, McVicar DW, Cheever AW, Sher A, Schwartzberg PL 2001. Mutation of Tec family kinases alters T helper cell differentiation. Nat Immunol 2: 1183–1188 [DOI] [PubMed] [Google Scholar]
- Schell MJ 2010. Inositol trisphosphate 3-kinases: focus on immune and neuronal signaling. Cell Mol Life Sci 67: 1755–1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan X, Czar MJ, Bunnell SC, Liu P, Liu Y, Schwartzberg PL, Wange RL 2000. Deficiency of PTEN in Jurkat T cells causes constitutive localization of Itk to the plasma membrane and hyperresponsiveness to CD3 stimulation. Mol Cell Biol 20: 6945–6957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shears SB 2009. Molecular basis for the integration of inositol phosphate signaling pathways via human ITPK1. Adv Enzyme Regul 49: 87–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin I, Yakes FM, Rojo F, Shin NY, Bakin AV, Baselga J, Arteaga CL 2002. PKB/Akt mediates cell-cycle progression by phosphorylation of p27(Kip1) at threonine 157 and modulation of its cellular localization. Nat Med 8: 1145–1152 [DOI] [PubMed] [Google Scholar]
- Shirane M, Sawa H, Kobayashi Y, Nakano T, Kitajima K, Shinkai Y, Nagashima K, Negishi I 2001. Deficiency of phospholipase C-gamma1 impairs renal development and hematopoiesis. Development 128: 5173–5180 [DOI] [PubMed] [Google Scholar]
- Shiroki F, Matsuda S, Doi T, Fujiwara M, Mochizuki Y, Kadowaki T, Suzuki H, Koyasu S 2007. The p85alpha regulatory subunit of class IA phosphoinositide 3-kinase regulates beta-selection in thymocyte development. J Immunol 178: 1349–1356 [DOI] [PubMed] [Google Scholar]
- Siliceo M, Garcia-Bernal D, Carrasco S, Diaz-Flores E, Coluccio Leskow F, Teixido J, Kazanietz MG, Merida I 2006. Beta2-chimaerin provides a diacylglycerol-dependent mechanism for regulation of adhesion and chemotaxis of T cells. J Cell Sci 119: 141–152 [DOI] [PubMed] [Google Scholar]
- Siliceo M, Merida I 2009. T cell receptor-dependent tyrosine phosphorylation of beta2-chimaerin modulates its Rac-GAP function in T cells. J Biol Chem 284: 11354–11363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommers CL, Lee J, Steiner KL, Gurson JM, Depersis CL, El-Khoury D, Fuller CL, Shores EW, Love PE, Samelson LE 2005. Mutation of the phospholipase C-gamma1-binding site of LAT affects both positive and negative thymocyte selection. J Exp Med 201: 1125–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommers CL, Park CS, Lee J, Feng C, Fuller CL, Grinberg A, Hildebrand JA, Lacana E, Menon RK, Shores EW, et al. 2002. A LAT mutation that inhibits T cell development yet induces lymphoproliferation. Science 296: 2040–2043 [DOI] [PubMed] [Google Scholar]
- Soond DR, Bjorgo E, Moltu K, Dale VQ, Patton DT, Torgersen KM, Galleway F, Twomey B, Clark J, Gaston JH, et al. 2010. PI3K p110{delta} regulates T cell cytokine production during primary and secondary immune responses in mice and humans. Blood 115: 2203–2213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitaler M, Emslie E, Wood CD, Cantrell D 2006. Diacylglycerol and protein kinase D localization during T lymphocyte activation. Immunity 24: 535–546 [DOI] [PubMed] [Google Scholar]
- Starr TK, Jameson SC, Hogquist KA 2003. Positive and negative selection of T cells. Annu Rev Immunol 21: 139–176 [DOI] [PubMed] [Google Scholar]
- Stephens L, Anderson K, Stokoe D, Erdjument-Bromage H, Painter GF, Holmes AB, Gaffney PR, Reese CB, McCormick F, Tempst P, et al. 1998. Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase B. Science 279: 710–714 [DOI] [PubMed] [Google Scholar]
- Stephens L, Smrcka A, Cooke FT, Jackson TR, Sternweis PC, Hawkins PT 1994. A novel phosphoinositide 3 kinase activity in myeloid-derived cells is activated by G protein beta gamma subunits. Cell 77: 83–93 [DOI] [PubMed] [Google Scholar]
- Stephens LR, Eguinoa A, Erdjument-Bromage H, Lui M, Cooke F, Coadwell J, Smrcka AS, Thelen M, Cadwallader K, Tempst P, et al. 1997. The G beta gamma sensitivity of a PI3K is dependent upon a tightly associated adaptor, p101. Cell 89: 105–114 [DOI] [PubMed] [Google Scholar]
- Stokoe D, Stephens LR, Copeland T, Gaffney PR, Reese CB, Painter GF, Holmes AB, McCormick F, Hawkins PT 1997. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science 277: 567–570 [DOI] [PubMed] [Google Scholar]
- Stoyanov B, Volinia S, Hanck T, Rubio I, Loubtchenkov M, Malek D, Stoyanova S, Vanhaesebroeck B, Dhand R, Nurnberg B, et al. 1995. Cloning and characterization of a G protein-activated human phosphoinositide-3 kinase. Science 269: 690–693 [DOI] [PubMed] [Google Scholar]
- Su YW, Zhang Y, Schweikert J, Koretzky GA, Reth M, Wienands J 1999. Interaction of SLP adaptors with the SH2 domain of Tec family kinases. Eur J Immunol 29: 3702–3711 [DOI] [PubMed] [Google Scholar]
- Suire S, Coadwell J, Ferguson GJ, Davidson K, Hawkins P, Stephens L 2005. p84, a new Gbetagamma-activated regulatory subunit of the type IB phosphoinositide 3-kinase p110gamma. Curr Biol 15: 566–570 [DOI] [PubMed] [Google Scholar]
- Suzuki A, Yamaguchi MT, Ohteki T, Sasaki T, Kaisho T, Kimura Y, Yoshida R, Wakeham A, Higuchi T, Fukumoto M, et al. 2001. T cell-specific loss of Pten leads to defects in central and peripheral tolerance. Immunity 14: 523–534 [DOI] [PubMed] [Google Scholar]
- Swat W, Montgrain V, Doggett TA, Douangpanya J, Puri K, Vermi W, Diacovo TG 2006. Essential role of PI3Kdelta and PI3Kgamma in thymocyte survival. Blood 107: 2415–2422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarasenko T, Kole HK, Chi AW, Mentink-Kane MM, Wynn TA, Bolland S 2007. T cell-specific deletion of the inositol phosphatase SHIP reveals its role in regulating Th1/Th2 and cytotoxic responses. Proc Natl Acad Sci U S A 104: 11382–11387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CW, Rahman T, Tovey SC, Dedos SG, Taylor EJ, Velamakanni S 2009. IP3 receptors: some lessons from DT40 cells. Immunol Rev 231: 23–44 [DOI] [PubMed] [Google Scholar]
- Thomas MS, Mitchell JS, DeNucci CC, Martin AL, Shimizu Y 2008. The p110gamma isoform of phosphatidylinositol 3-kinase regulates migration of effector CD4 T lymphocytes into peripheral inflammatory sites. J Leukoc Biol 84: 814–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timm D, Salim K, Gout I, Guruprasad L, Waterfield M, Blundell T 1994. Crystal structure of the pleckstrin homology domain from dynamin. Nat Struct Biol 1: 782–788 [DOI] [PubMed] [Google Scholar]
- Tomlinson MG, Kane LP, Su J, Kadlecek TA, Mollenauer MN, Weiss A 2004. Expression and function of Tec, Itk, and Btk in lymphocytes: evidence for a unique role for Tec. Mol Cell Biol 24: 2455–2466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rossum DB, Patterson RL, Sharma S, Barrow RK, Kornberg M, Gill DL, Snyder SH 2005. Phospholipase Cgamma1 controls surface expression of TRPC3 through an intermolecular PH domain. Nature 434: 99–104 [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Ali K, Bilancio A, Geering B, Foukas LC 2005. Signalling by PI3K isoforms: insights from gene-targeted mice. Trends Biochem Sci 30: 194–204 [DOI] [PubMed] [Google Scholar]
- Vanweyenberg V, Communi D, D'Santos CS, Erneux C 1995. Tissue- and cell-specific expression of Ins(1,4,5)P3 3-kinase isoenzymes. Biochem J 306: 429–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vig M, Kinet JP 2009. Calcium signaling in immune cells. Nat Immunol 10: 21–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viglietto G, Motti ML, Bruni P, Melillo RM, D'Alessio A, Califano D, Vinci F, Chiappetta G, Tsichlis P, Bellacosa A, et al. 2002. Cytoplasmic relocalization and inhibition of the cyclin-dependent kinase inhibitor p27(Kip1) by PKB/Akt-mediated phosphorylation in breast cancer. Nat Med 8: 1136–1144 [DOI] [PubMed] [Google Scholar]
- Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS Jr 1998. NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science 281: 1680–1683 [DOI] [PubMed] [Google Scholar]
- Wang D, Feng J, Wen R, Marine JC, Sangster MY, Parganas E, Hoffmeyer A, Jackson CW, Cleveland JL, Murray PJ, et al. 2000. Phospholipase Cgamma2 is essential in the functions of B cell and several Fc receptors. Immunity 13: 25–35 [DOI] [PubMed] [Google Scholar]
- Wang X, Devaiah SP, Zhang W, Welti R 2006. Signaling functions of phosphatidic acid. Prog Lipid Res 45: 250–278 [DOI] [PubMed] [Google Scholar]
- Wange RL 2000. LAT, the linker for activation of T cells: a bridge between T cell-specific and general signaling pathways. Sci STKE 2000: re1. [DOI] [PubMed] [Google Scholar]
- Webb LM, Vigorito E, Wymann MP, Hirsch E, Turner M 2005. Cutting edge: T cell development requires the combined activities of the p110gamma and p110delta catalytic isoforms of phosphatidylinositol 3-kinase. J Immunol 175: 2783–2787 [DOI] [PubMed] [Google Scholar]
- Wen BG, Pletcher MT, Warashina M, Choe SH, Ziaee N, Wiltshire T, Sauer K, Cooke MP 2004. Inositol (1,4,5) trisphosphate 3 kinase B controls positive selection of T cells and modulates Erk activity. Proc Natl Acad Sci U S A 101: 5604–5609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde JI, Watson SP 2001. Regulation of phospholipase C gamma isoforms in haematopoietic cells: why one, not the other? Cell Signal 13: 691–701 [DOI] [PubMed] [Google Scholar]
- Yang C, Kazanietz MG 2007. Chimaerins: GAPs that bridge diacylglycerol signalling and the small G-protein Rac. Biochem J 403: 1–12 [DOI] [PubMed] [Google Scholar]
- Yoon HS, Hajduk PJ, Petros AM, Olejniczak ET, Meadows RP, Fesik SW 1994. Solution structure of a pleckstrin-homology domain. Nature 369: 672–675 [DOI] [PubMed] [Google Scholar]
- York JD 2006. Regulation of nuclear processes by inositol polyphosphates. Biochim Biophys Acta 1761: 552–559 [DOI] [PubMed] [Google Scholar]
- York JD, Hunter T 2004. Signal transduction. Unexpected mediators of protein phosphorylation. Science 306: 2053–2055 [DOI] [PubMed] [Google Scholar]
- Zha Y, Marks R, Ho AW, Peterson AC, Janardhan S, Brown I, Praveen K, Stang S, Stone JC, Gajewski TF 2006. T cell anergy is reversed by active Ras and is regulated by diacylglycerol kinase-alpha. Nat Immunol 7: 1166–1173 [DOI] [PubMed] [Google Scholar]
- Zhong XP, Guo R, Zhou H, Liu C, Wan CK 2008. Diacylglycerol kinases in immune cell function and self-tolerance. Immunol Rev 224: 249–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong XP, Hainey EA, Olenchock BA, Jordan MS, Maltzman JS, Nichols KE, Shen H, Koretzky GA 2003. Enhanced T cell responses due to diacylglycerol kinase zeta deficiency. Nat Immunol 4: 882–890 [DOI] [PubMed] [Google Scholar]