Abstract
The mammary gland is composed of a diverse array of cell types that form intricate interaction networks essential for its normal development and physiologic function. Abnormalities in these interactions play an important role throughout different stages of tumorigenesis. Branching ducts and alveoli are lined by an inner layer of secretory luminal epithelial cells that produce milk during lactation and are surrounded by contractile myoepithelial cells and basement membrane. The surrounding stroma comprised of extracellular matrix and various cell types including fibroblasts, endothelial cells, and infiltrating leukocytes not only provides a scaffold for the organ, but also regulates mammary epithelial cell function via paracrine, physical, and hormonal interactions. With rare exceptions breast tumors initiate in the epithelial compartment and in their initial phases are confined to the ducts but this barrier brakes down with invasive progression because of a combination of signals emitted by tumor epithelial and various stromal cells. In this article, we overview the importance of cellular interactions and microenvironmental signals in mammary gland development and cancer.
Fibroblasts, infiltrating leukocytes, and the extracellular matrix regulate the behavior of milk-producing mammary epithelial cells. Changes in this microenvironment influence, and may even drive, tumorigenesis.
The mammary gland is composed of a combination of multiple cell types that together form complex interaction networks required for the proper development and functioning of the organ. The branching milk ducts are formed by an outer myoepithelial cell layer producing the basement membrane (BM) and an inner luminal epithelial cell layer producing milk during lactation. The ducts are surrounded by the microenvironment composed of extracellular matrix (ECM) and various stromal cell types (e.g., endothelial cells, fibroblasts, myofibroblasts, and leukocytes). Large amount of data suggest that cell-cell and cell-microenvironment interactions modify the proliferation, survival, polarity, differentiation, and invasive capacity of mammary epithelial cells. However, the molecular mechanisms underlying these effects are poorly understood. The purification and comprehensive characterization of each cell type comprising normal and neoplastic human breast tissue combined with hypothesis testing in cell culture and animal models are likely to improve our understanding of the role these cells play in the normal functioning of the mammary gland and in breast tumorigenesis. In this article, we overview cellular and microenvironmental interactions that play important roles in the normal functioning of the mammary gland and their abnormalities in breast cancer.
THE ROLE OF THE MICROENVIRONMENT IN MAMMARY GLAND DEVELOPMENT AND FUNCTION
Contrary to that of most organs, the development of the mammary gland mostly occurs postnatally and it is only completed in adulthood and some aspects of mammary epithelial cell differentiation even require the completion of a full-term pregnancy, lactation, and involution cycle. The mammary gland is also unique as it is continuously remodeled following puberty because of the cyclical influence of reproductive hormones. Most of our knowledge of mammary gland development has been derived from observations made in mice and interpolated for humans despite the well-known differences between human and mouse mammary gland development and function. Studies addressing human mammary gland development have been limited to the structural and immunohistochemical analyses of a limited number of samples collected at different stages of fetal, infantile, childhood, and pubertal development (Anbazhagan et al. 1998; Osin et al. 1998; Naccarato et al. 2000; Jolicoeur et al. 2003).
The mammary gland is derived from the ectoderm and in the human embryo the breast bud arises as a result of proliferation of basal cells of the epidermis because of factors secreted by mesenchymal cells present in the breast bud (Anbazhagan et al. 1998). Mammary epithelial cells remain responsive to signals emitted by embryonic mesenchyme even to adulthood, but only in nulliparous mice. In fact, signals emitted by embryonic mesenchyme dictate the differentiation of epithelial cells, and mammary epithelial cells form salivary gland–like structures when placed on top of salivary gland mesenchyme (Sakakura et al. 1979). This differentiation-inducing effect of embryonic mesenchyme is so pronounced that it is able to alter the phenotype of mammary carcinoma cells to a more benign differentiated state (DeCosse et al. 1973, 1975). This could potentially be explained by the up-regulation of embryonic programs in the tumor cells and then their normalization in response to mesenchymal-derived differentiation inducing signals. Indeed, more recent studies have shown that the embryonic morphogen Nodal is overexpressed in highly metastatic breast cancer cells and in melanomas. Nodal expression, and consequently the invasive phenotype of the cancer cells, can be down-regulated by placing the cells into human embryonic stem cell (hESC) microenvironment (Postovit et al. 2008).
The importance of proper cellular context for normal mammary epithelial cell differentiation was also shown by the observation that cells plated on plastic tissue culture dishes displayed very different phenotype and were not able to differentiate into milk producing mammary epithelium in contrast to cells plated in three-dimensional (3D) reconstituted basement membrane (Matrigel) cultures (Petersen et al. 1992; Howlett and Bissell 1993). More recent follow up studies have shown that transition from monolayer to 3D cultures dramatically influences the epigenetic programs of the cells, particularly histone modification patterns (Le Beyec et al. 2007). Specifically, plating mammary epithelial cells in laminin-rich extracellular matrix (Matrigel) induces rapid deacetylation of histones H3 and H4 at the global level accompanied with decreased overall transcription.
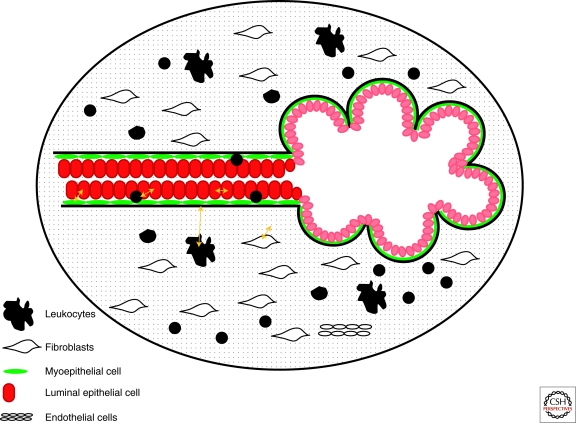
However, in the normal mammary gland the majority of luminal epithelial cells are not in direct contact with the basement membrane, but rather lay on top of myoepithelial cells (Fig. 1). Myoepithelial cells influence the differentiation, polarity, proliferation, and invasion/migration of adjacent luminal epithelial cells. They also produce the basement membrane (BM) that forms a physical barrier separating the epithelial and stromal compartments. Luminal epithelial cells display apical-basal polarity as shown by the localization of sialomucin (MUC1), epithelial specific antigen (ESA), and occludin on the apical membrane and integrin β4 on the basolateral membrane. This polarity is observed in cell culture when luminal epithelial cells are grown in Matrigel but not in collagen I (Gudjonsson et al. 2002). However, coculture with normal myoepithelial cells restores luminal epithelial cell polarity even in collagen cultures and this is at least in part mediated by laminin-1 secreted by the myoepithelial cells. Other studies have suggested that 3D culture of luminal epithelial cells in Matrigel can induce the production of non-Matrigel basement membrane molecules such as laminin 5 (Zahir et al. 2003). These results suggest that cells that are embedded in 3D environment can produce unique BM components that sometimes resemble the in vivo conditions.
Figure 1.
Schematic view of a normal mammary duct and lobule. Black line indicates the basement membrane. In the ducts myoepithelial cells form a nearly complete layer around the luminal epithelial cells, whereas in the alveoli the myoepithelial layer is more fenestrated and some luminal epithelial cells can be in direct contact with the basement membrane. Yellow arrows indicate potential cell–cell and cell–matrix interactions.
Besides myoepithelial cells, the effects of numerous other stromal cell types have also been tested in cell culture assays. For example, co-culture of endothelial cells with MCF-7 breast cancer cells leads to apoptosis of endothelial cells and such apoptosis requires cell–cell contacts. Although the functional impact of such studies remains to be elucidated, these experiments suggest that cancer cells can impact the integrity of the tumor endothelium (Kebers et al. 1998). Coculture studies using different mesenchymal cells and MCF10A and preneoplastic MCF10AT1-EIII8 mammary epithelial cells showed that fibroblasts derived from normal reduction mammoplasty inhibit or retard the morphological conversion and growth of MCF10A and EIII8 cells, whereas tumor derived fibroblasts evoke ductal-alveolar morphogenesis of both cell types (Shekhar et al. 2001). Recently, Caveolin-1 deficient (Cav1-/-) mammary stromal fibroblasts were shown to mimic the effects of human breast cancer associated fibroblasts as they show similar profile of RB/E2F-regulated genes that are up-regulated and confer a poor prognosis with enhanced epithelial-mesenchymal transition (EMT) (Sotgia et al. 2009).
ABNORMALITIES IN THE MICROENVIRONMENT DURING TUMOR PROGRESSION
Early studies showed that the normal mammary microenvironment is capable of “reverting” the malignant phenotype of breast cancer cells by inducing a more differentiated state (DeCosse et al. 1973, 1975), suggesting that cancer cells can only thrive in an abnormal environment in which they evolved. Pathologists have also long noted the prognostic value of certain histopathological features of breast tumors, including lymphocytic infiltration, fibrosis, and angio- and lymphangiogenesis (Fig. 2). The contribution of host factors as shown by differences in tumor initiation, progression, and angiogenesis depending on variability in germline genotypes also support a role for nonepithelial cells in carcinogenesis (Rohan et al. 2000; Hunter 2004). In fact, cancer is a systemic disease involving the whole body, because tumor cells perturb the homeostasis of multiple organs not only that of those where they reside. For example, the hematopoietic system and blood clotting cascade appear to be different between normal individuals and cancer patients (Bluff et al. 2008; Psaila and Lyden 2009). Furthermore, primary tumors can influence (promote or inhibit depending on the specific tumor) the growth of cancer cells at distant metastatic sites via hormonal factors (Fig. 3). This was shown in a xenograft model whereas MDA-MB-231 cells injected into one side of the mouse were able to “instigate” the growth of a less tumorigenic cell line injected to the other side (McAllister et al. 2008).
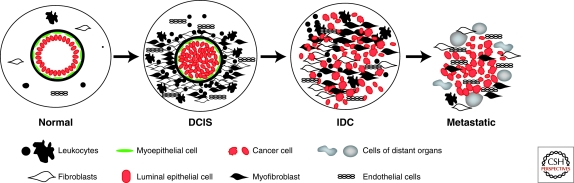
Figure 2.
Microenvironmental alterations during tumor progression. Schematic view of normal breast, DCIS (ductal carcinoma in situ), IDC (invasive ductal carcinoma), and metastatic breast carcinoma. Normal breast ducts are composed of the basement membrane and an inner layer of luminal epithelial sitting on an outer layer of myoepithelial cells. Cells composing the stroma include various leukocytes, fibroblasts, myofibroblasts, and endothelial cells. In DCIS, the myoepithelial cells are epigenetically and phenotypically altered and their number decreases potentially because of signals coming from the tumor epithelial and various stromal cells. The number of stromal fibroblasts, myofibroblasts, lymphocytes, and endothelial cells increases as tumors progress, although the degree of this is tumor subtype-specific.
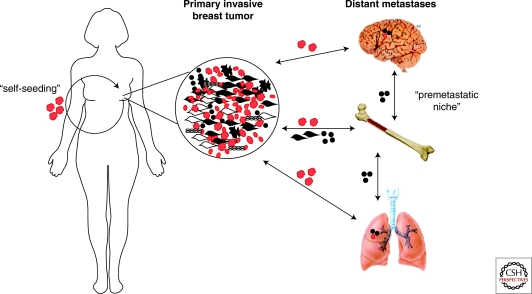
Figure 3.
Systemic effects of breast tumors. Tumor cells shed into the circulation and migrate to various organs. At the same time signals emitted by cells within the primary tumor mobilize bone marrow-derived mesenchymal stem cells (BMD-MSCs). These BMD-MSCs are the recruited to the primary tumor and also to other organs such as lungs and brain creating a premetastatic niche prior to the arrival of the disseminated cancer cells. The interaction between cancer cells and BMD-MSCs within the primary tumor enhances the growth, survival, motility, invasive and metastatic capacity of tumor cells via paracrine interactions. In addition, BMD-MSCs can differentiate into myofibroblasts and other stromal cell types that further support the growth and progression of the tumor. Disseminated cancer cells preferentially grow at sites where BMD-MSCs are localized forming distant metastases. Disseminated tumor cells can also return to the original primary tumor and promote its growth and further metastatic spread in a process called “self-seeding.”
Because metastases are responsible for most cancer-related deaths, understanding the systemic effects of tumors and factors that regulate the ability of disseminated cancer cells to grow at distant sites are of primary importance. In line with the “seed and soil” model of metastasis formation (Fidler 2003), the concept of “premetastatic niche” has recently been formulated by David Lyden and colleagues, proposing that bone marrow-derived hematopoietic progenitors may preferentially localize to future sites of metastases and “prepare” them for the arrival and outgrowth of disseminated cancer cells (Kaplan et al. 2005; Kerbel et al. 2008; Psaila and Lyden 2009). Although the identity of factors that regulate the distribution of bone marrow-derived hematopoietic progenitors is still unclear, it appears to be serum-derived and tumor-specific as transfer of serum from melanoma bearing mice to healthy mouse and subsequent injection of lung cancer cells resulted in preferential metastasis formation at sites favored by melanoma and not by lung tumors (Kaplan et al. 2005).
A related observation was described by Charlotte Kuperwasser's group whereas bone-marrow derived progenitors promoted the outgrowth of estrogen receptor negative (ER-) breast tumors in an estrogen dependent manner (Gupta et al. 2007). Specifically, the authors found that xenografts injected into postpartum mice grew faster than those injected into nulliparous mice and this was because of the proangiogenic effects of estrogen and parturition. Subsequent experiments showed that estrogen promoted the immobilization of bone marrow-derived stromal progenitors, which then colonized to sites of tumor injection and enhanced angiogenesis.
The role of bone marrow-derived mesenchymal stem cells (BM-MSCs) in promoting distant metastasis formation has also been described in a recent study by Bob Weinberg's laboratory, whereas coinjection of poorly metastatic breast cancer cells with BM-MSCs promoted lung metastases (Karnoub et al. 2007). Follow up experiments showed that this metastasis promoting-effect is mediated by CCL5 induced in MSCs following their interaction with breast cancer cells. Thus, inhibitors of CCR5, the signaling receptor for CCL5, might be useful for the prevention of distant metastases.
Despite these convincing observations implicating a role for microenvironmental and systemic alterations in breast tumorigenesis, our understanding of the genes and pathways mediating cellular interactions and paracrine regulatory networks among various cell types in normal and neoplastic breast tissue is still limited. Numerous studies have analyzed the expression of selected candidate genes in normal and neoplastic primary human breast tissue samples by immunohistochemistry or mRNA in situ hybridization and found up-regulation of invasion and angiogenesis related genes (e.g., MMPs and TIMPs) and growth factors (e.g., HGF and CTGF) in tumor-associated fibroblasts, endothelial cells, and myofibroblasts (Vrana et al. 1996; Guidi et al. 1997; Brown et al. 1999; Nielsen et al. 2001; Bisson et al. 2003). Some of these proinvasive and proangiogenic genes showed increased expression in ductal carcinomas in situ (DCIS)-associated myoepithelial cells as well, implying the potential role for these changes in the gradual brake down of the BM separating epithelial and stromal cells.
To analyze molecular alterations in cells composing the microenvironment in a comprehensive and unbiased way, Allinen et al. purified and characterized the genome-wide gene expression and genetic profiles of all major cell types (luminal epithelial, myoepithelial, and endothelial cells, infiltrating leukocytes, fibroblasts, and myofibroblasts) from normal breast tissue and in situ and invasive breast carcinomas. Each cell type was purified using cell type-specific cell surface markers and their comprehensive gene expression profiles were analyzed by SAGE (Serial Analysis of Gene Expression), whereas genetic changes were detected by cDNA array CGH (Comprehensive Genomic Hybridization) and SNP (Single Nucleotide Polymorphism) arrays. Using this approach the authors concluded that gene expression changes occur in all cell types during breast tumor progression, but clonally selected genetic alterations are restricted to tumor epithelial cells (Allinen et al. 2004). Interestingly, the comparison of myoepithelial cells from normal breast tissue and DCIS yielded the highest number of consistently differentially expressed genes and a significant fraction of these encoded for secreted proteins and cell surface receptors suggesting intensive autocrine/paracrine regulatory loops. Many of the genes reflecting normal myoepithelial cell differentiation (SMA, OXTR) were absent or dramatically downregulated in DCIS-associated myoepithelial cells implying perturbed myoepithelial cell differentiation.
Independent studies by Dennis Sgroi's group using a different approach (laser capture microdissection and array-based profiling) reached similar conclusions as they detected gene expression changes associated with tumor progression both in the stromal and epithelial compartment when comparing normal breast, in situ and invasive breast carcinomas (Ma et al. 2009). The gene expression signature of epithelial cells correlated with tumor grade, but not with histologic stage. Genes up-regulated in tumor-associated stroma included many ECM-related molecules and the expression of MMP1, MMP2, and MMP14 was significantly higher in invasive compared with in situ tumors suggesting a potential role for these matrix metalloproteases in promoting invasive progression. One of the key basement membrane-degrading MMPs, MMP14, was also shown to be up-regulated in DCIS-associated myoepithelial cells and in tumor-associated myofibroblasts (Hu et al. 2008).
The dramatic changes in gene expression patterns but lack of clonally selected somatic genetic alterations in the tumor-associated myoepithelial and stromal cells suggested potential epigenetic alterations, because stromal cells isolated from normal and tumor tissue are known to maintain their differences even after prolonged cell culture and in xenograft studies (Bissell and Radisky 2001; Tlsty and Hein 2001; Bissell et al. 2002; Orimo et al. 2005). Indeed, a follow up study by Hu et al. analyzing global DNA methylation profiles using MSDK (methylation-specific digital karyotyping) identified altered DNA methylation patterns not only in tumor epithelial but also in DCIS-associated myoepithelial cells and stromal fibroblasts (Hu et al. 2005). Independent studies testing the methylation of selected candidate genes in breast carcinomas also described DNA methylation differences between normal and cancer-associated stroma and the frequency of these alterations was dependent on tumor subtype (Fiegl et al. 2006).
The molecular mechanisms responsible for the altered epigenetic profiles of cancer-associated stromal cells and the stage of tumor progression when these first can be detected have not been clarified yet. Prolonged paracrine stimulation by cancer cells may initiate epigenetic alterations in the surrounding stromal cells or the differentiation of local or recruited circulating stem cells is perturbed by the tumor. Alternatively, epigenetic alterations in stromal cells might be initiated by chronic inflammation thereby resulting in abnormal microenvironment and increased risk of tumor initiation. Most likely each one of these potential mechanisms can occur and their relative contribution varies depending on tumor type and progression stage.
THE FUNCTIONAL RELEVANCE OF MICROENVIRONMENTAL ALTERATIONS IN BREAST TUMORIGENESIS
The historically prevailing view of breast tumor progression is focused on tumor epithelial cells, whereby gradual progression of a tumor through defined steps is entirely because of the accumulation of genetic and epigenetic alterations that confer progressively malignant phenotypes. However, this model has been challenged by multiple recent studies, which highlight the importance of microenvironment in shaping tumor evolution and progression. Numerous studies using diverse experimental systems have shown that the proliferation, survival, polarity, differentiation state, and invasive capacity of breast cancer cells can be modulated by myoepithelial and various stromal cells (Sternlicht and Barsky 1997; Sternlicht et al. 1997; Elenbaas and Weinberg 2001; Radisky et al. 2001; Tlsty 2001; Tlsty and Hein 2001; Bissell et al. 2002; Coussens and Werb 2002; Deugnier et al. 2002; Gudjonsson et al. 2002; Wiseman and Werb 2002; Kenny and Bissell 2003; Shekhar et al. 2003). The diagnostic criteria that pathologists use to distinguish in situ from invasive carcinomas is the presence or absence of an intact myoepithelial cell layer confirmed by immunohistochemical analyses of myoepithelial cell-specific markers including smooth muscle actin, calponin, and CD10 (Damiani et al. 1999). However, it is unknown what leads to the progressive disappearance of the myoepithelial cells in DCIS and how this contributes to invasive progression.
Building on their prior results demonstrating dramatic alterations in myoepithelial cells and various stromal cell types in DCIS, Hu and colleagues tested the role of these cell types in the progression of in situ to invasive breast carcinomas using the MCF10DCIS.com (Miller et al. 2000) xenograft model of human DCIS (Hu et al. 2008). This cell line has the unique property that it is able to differentiate into luminal epithelial and myoepithelial cells when injected into immunodeficient mice and forms tumors with high histologic and molecular resemblance to human comedo DCIS that with time progresses to invasive tumors. Using this xenograft model and coinjection experiments with normal myoepithelial cells and various fibroblasts (e.g., isolated from normal breast, breast tumors, and rheumatoid arthritis), the authors found that normal myoepithelial cells efficiently suppressed tumor growth and transition to IDC whereas all fibroblasts but particularly cancer and chronic inflammation-associated cells had progression-promoting effects (Hu et al. 2008). Experiments using primary human breast tissue samples corroborated the findings obtained in the xenograft model and suggest that the key event of in situ to invasive breast carcinoma progression is the disappearance of myoepithelial cells resulting from the aberrant differentiation of myoepithelial progenitors because of a combination of signals emitted by tumor epithelial and various stromal cells (Fig. 4). Because myoepithelial cell differentiation is critically dependent on contact with the basement membrane, which is produced by the myoepithelial cells, slight perturbation of this differentiation process initiates a positive feed-back loop that leads to the progressive disappearance of differentiated myoepithelial cells. In a follow up study, Hu et al. showed that anti-inflammatory drugs such as cycloxygenase (COX) inhibitors are able to decrease the tumor progression-promoting effects of fibroblasts by delaying the loss of myoepithelial cells (Hu et al. 2009) highlighting the potential of cancer therapeutic and preventative approaches targeting epithelial-stromal cell interactions.
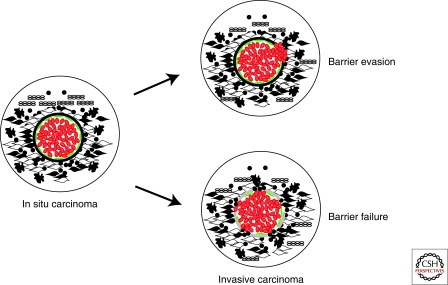
Figure 4.
Hypothetical model depicting two different views of in situ to invasive breast carcinoma transition. Cells are depicted as in Figure 2. In the “barrier evasion” model the tumor epithelial cells disrupt the myoepithelial cell layer, degrade the basement membrane, and migrate into the stroma. In the “barrier failure” model the myoepithelial cells become progressively lost and the basement membrane is disrupted at sites coinciding with areas of leukocytic infiltration and accumulation of myofibroblasts. The loss of the myoepithelial cell layer and basement membrane results in invasive carcinomas where tumor cells can invade surrounding tissues and can migrate to distant organs.
ANIMAL MODELS
The importance of microenvironmental alterations in tumorigenesis was dramatically illustrated by the demonstration that systemic inactivation of TGFβ type II receptor in stromal fibroblasts led to prostate and gastric epithelial neoplasia (Bhowmick et al. 2004), whereas its inactivation in mammary stromal fibroblasts led to abnormal ductal development and promoted the growth of transplanted tumors (Cheng et al. 2005). A major function of TGFβ signaling is the suppression of expression of growth factors, cytokines and chemokines (Bierie and Moses 2006). Conditional knockout of Tgfbr2 resulting in loss of expression of the receptor and thus abrogation of all TGFβ signaling, when combined with oncogene activation or tumor suppressor gene attenuation, accelerates the development of carcinomas and enhances tumor progression, including metastasis. Mechanistic studies using the mammary gland models have shown that a major mechanism whereby loss or attenuation of TGFβ signaling accelerates tumor progression is increased secretion of chemokines, particularly Cxcl1 and Cxcl5, by the knockout carcinoma cells (Bierie et al. 2009). Importantly, global gene expression signatures associated with loss of TGFβ signaling within carcinoma cells were shown to correlate with poor prognosis, particularly in estrogen receptor positive human breast cancer (Bierie et al. 2009). In the mouse models, increased chemokine expression resulted in recruitment of myeloid-derived suppressor cells (MDSCs), also called immature myeloid cells (iMCs) to the invasive edge of the primary carcinoma with resultant enhancement of invasion and metastasis. Significantly, inhibition of the cognate receptor for Cxcl1 and Cxcl5, Cxcr2 with a small molecule inhibitor, resulted in decreased MDSC infiltration and decreased pulmonary metastases (Yang et al. 2008).
In the recent years, heterogeneity of the stromal fibroblasts is being appreciated. FSP1 (also know in the literature as S100A4 protein/mts1 protein) was originally identified as a fibroblast-specific transcript in a differential display screen between renal epithelial cells and interstitial fibroblasts (Strutz et al. 1995). FSP1 shows specific expression in various fibroblast cultures and is not expressed in epithelial cells, at least in the mouse (Strutz et al. 1995; El-Rifai et al. 2002). Specificity of FSP1 expression was confirmed in vivo by the observation that dividing fibroblasts exposed to nucleoside analogues are also selectively eliminated in transgenic mice expressing thymidine kinase under control of the FSP1 promoter (Iwano et al. 2001). Expression of FSP1 is observed in some fibroblasts of the body and it is first observed at embryonic day 9 (Strutz et al. 1995). Therefore, FSP1 serves as an excellent marker for one class of fibroblasts, both in normal and activated state (Kalluri and Neilson 2003) and because of this, FSP1 targeted Cre (cre recombinase) has been used for the selective elimination of specific genes in fibroblasts.
A recent study showed that genetic inactivation of PTEN in stromal fibroblasts of mouse mammary glands accelerates the initiation, progression and malignant transformation of mammary epithelial tumors in association with massive remodeling of the ECM, innate immune cell infiltration and increased angiogenesis (Trimboli et al. 2009). Loss of PTEN in stromal fibroblasts leads to increased phosphorylation and recruitment of Ets2 to target promoters that enhance malignant transformation. These findings identify the Pten-Ets2 axis as a critical stroma-specific signaling pathway that suppresses mammary epithelial tumors.
Tumor-associated macrophages are one of the most prominent and well-characterized subset of leukocytes that can promote or inhibit tumorigenesis depending on tumor type and progression stage. Multiple animal models have tested their roles in mammary tumorigenesis. Using animal models of breast cancer metastasis, Jeff Pollard and colleagues showed that a population of host macrophages displaying a distinct phenotype is recruited to extravasating pulmonary metastatic breast cancer cells regardless of their species of origin. Ablation of this macrophage population through several independent means (genetic and chemical) revealed that these macrophages are required for efficient metastatic seeding and growth of breast cancer cells (Qian et al. 2009). In another recent paper, using the MMTV-PyMT model of mammary carcinogenesis, Lisa Coussens and colleagues showed that IL-4-expressing CD4(+) T lymphocytes indirectly promote invasion and subsequent metastasis of mammary adenocarcinomas by directly regulating the phenotype and effector function of tumor-associated CD11b(+)Gr1(−)F4/80(+) macrophages that in turn enhance metastasis through activation of epidermal growth factor receptor signaling in malignant mammary epithelial cells (DeNardo et al. 2009).
CLINICAL RELEVANCE OF MICROENVIRONMENTAL ALTERATIONS IN BREAST TUMORIGENESIS
Harol Dvorak's well-known statement “tumors are wounds that never heal” was substantiated by results of comprehensive molecular profiling studies as many of the genes up-regulated in tumor-associated stroma were known to play a role in wound-healing (Allinen et al. 2004). Further highlighting the similarities between tumor-associated stroma and wound healing response was the demonstration that gene expression signatures initiated in fibroblasts in response to serum stimulation were present in breast and other solid (but not hematopoietic) tumors and predicted relapse-free survival (Chang et al. 2004). Many genes present in this “wound-response signature” include ECM-modifying enzymes such as LOXL2 (lysyl oxidase-like 2), PLOD2 (procollagen lysine, 2-oxoglutarate 5-dioxygenase 2), and PLAUR (plasminogen activator urokinase receptor). Subsequent follow up study by the same group showed that the wound-response signature outperformed other known prognostic markers in breast cancer for the identification of patients with shorter relapse and distant metastasis-free survival (Chang et al. 2005).
A similar study analyzed tumor stromal gene signatures by laser-capture microdissecting 53 primary breast tumors and comprehensive profiling of the isolated cells (Finak et al. 2008). Using this approach the authors defined a 26-gene stroma-derived prognostic predictor (SDPP) that classified breast cancer patients according to their overall and relapse-free survival independent of known clinical prognostic markers and previously published expression signatures. However, stromal expression profiles somewhat correlated with breast tumor subtypes that may have influenced the prognostic value of this SDPP as basal-like tumors more frequently expressed an activated stroma signature.
Microenvironmental alterations in breast cancer not only influence tumor progression and predict prognosis, but they also have major effect on the efficacy of cancer therapy especially targeted therapy aimed at growth factor receptors and secreted proteins. An elegant study showed the importance of tissue organization and interactions with the ECM in defining apoptotic responses to chemotherapy (Weaver et al. 2002). Specifically, polarized epithelial cells (normal or malignant) are resistant to several different apoptosis-inducing stimuli and this resistance is at least in part mediated by the activation of NF-κB mediated by ligation of β4 integrin to the ECM. Thus, disrupting integrin-ECM interactions may improve chemotherapeutic responses.
Abnormal tissue organization and homeostasis can increase the chance of tumor initiation as illustrated by the increased cancer risk associated with chronic inflammation and tissue injury (Dvorak 1986; Schafer and Werner 2008). This link is most clearly recognizable in colon, gastric, and hepatocellular carcinomas, but human epidemiological data demonstrating decreased risk in users of NSAIDs (nonsteroidal anti-inflammatory drugs) also supports the relevance of inflammation in breast cancer (Moran 2002). One possible mechanism by which chronic inflammation and tissue damage may contribute to tumor initiation is alterations in the epithelial stem cell niche and expansion of the stem or progenitor cell pool because of factors secreted by inflammatory cells. Because tumors thought to initiate from cells with stem or progenitor-like properties, increasing the numbers of these cells might increase the probability of tumor initiation by increasing target size for oncogenic mutations. Furthermore, alterations in the microenvironment alter the fitness landscape providing possible growth advantage for cells with tumor-initiating genetic or epigenetic changes (Blagosklonny 2002; Marusyk and DeGregori 2008; Marusyk and Polyak 2010). ROS (reactive oxygen species) generated by infiltrating leukocytes (macrophages and neutrophils) and resulting from intermittent hypoxia induced by tissue damage, might also lead to increased mutation rates and alter the epigenetic profiles of epithelial and stromal cells. Indeed, a recent report described alterations in DNA methylation in hypoxic conditions (Pal et al. 2009).
Inflammation was also shown to be responsible for the induction of wound-induced tumors in chickens infected with the Rous sarcoma virus (Martins-Green et al. 1994). This classic study by Mina Bissell's lab provided a good example for the importance of tissue injury in promoting tumor initiation. Despite the fact that the oncogenic virus was present in the circulation of the chick embryos, tumors only developed at the site of injection or at experimentally induced wounds. In subsequent experiments the authors showed that a combination of growth factors including TGFβ and acidic and basic FGF are responsible for this wounding-induced tumor initiation.
Inflammation and infiltrating leukocytes are also involved in later stages of tumor progression by promoting progression to invasion and metastasis. One mechanism by which this can occur is the induction of EMT in breast cancer cells by infiltrating T cells (Santisteban et al. 2009). Specifically, CD8+ T cells can induce the CD44+/CD24- stem cell-like phenotype of breast cancer cells in co-cultures, which correlates with increased invasive and metastatic capacity and resistance to therapeutic interventions.
A recent report also showed a direct link between inflammation and breast tumorigenesis involving an epigenetic switch induced by Src, NF-κB, microRNAs, and IL6 in tumor epithelial cells leading to increasingly malignant phenotype (Iliopoulos et al. 2009). The authors showed the link between these molecules using MCF-10A cells as model, but subsequently showed that the activation signature is also present in primary human breast tumors implicating IL6-STAT3 signaling in breast cancer.
In summary, cancer progression is not an entirely cell-autonomous process. Instead, Darwinian evolution of tumors and resulting clinical progression are influenced, and perhaps even driven, by changes that occur in the tumor microenvironment. Therefore, cancer risk-stratification, diagnostic, and preventative approaches have to consider the potential contribution of microenvironmental alterations in tumor initiation and progression, and use this knowledge for the design of more efficient approaches toward cancer prevention and therapy.
CONCLUDING REMARKS
Even after deciphering the molecular mechanisms underlying cellular and microenvironmental interactions in the normal mammary gland during different stages of its development and the abnormalities of these in breast tumors, translating of these findings into clinical practice will remain a challenge. The redundancy of cell types and paracrine factors eliciting similar effects predicts that blocking any one of these is likely to be therapeutically ineffective. The similarities between tumor-associated microenvironmental alterations and those induced by physiologic or other pathologic processes such as wound healing and inflammation imply that any therapy targeting these may have serious and unpredictable side effects. Lack of suitable models that faithfully reproduce the normal mammary gland and breast tumor microenvironments poses a challenge for functional studies aimed at testing hypotheses built based on observations in human tissues, thereby hindering the development of novel preventative and therapeutic interventions targeting microenvironmental alterations. Deficiencies of currently used cell culture and animal models might also explain their poor predictive value of the in vivo efficacy of therapeutic agents. To overcome these deficiencies improved culture models that better reflect the physiologic environment and, thus, sensitivity to therapeutic agents such as 3D epithelial-stromal cell co-cultures are being developed by multiple labs (Weaver et al. 2002; Krause et al. 2008). However, despite all these challenges, anti-angiogenic and anti-inflammatory therapies have shown promise for cancer treatment and prevention (Ellis and Hicklin 2008), giving hope that improved knowledge will facilitate the development of a wider array of similar and more successful approaches.
ACKNOWLEDGMENTS
The authors thank members of their laboratories for their critical reading of the manuscript and constructive discussions. Work in the authors' laboratories is supported by the National Cancer Institute (CA89393, CA116235, CA143233, and CA080111), Department of Defense (W81XWH-07-1-0294 and W81XWH-09-1-0131), Avon Foundation, Susan G. Komen Breast Cancer Research Foundation, Breast Cancer Research Foundation, and Novartis Oncology grants awarded to KP, and CA125550, DK55001, and Champalimaud Metastasis Program grants awarded to RK.
Footnotes
Editors: Mina J. Bissell, Kornelia Polyak, and Jeffrey Rosen
Additional Perspectives on Mammary Gland Biology available at www.cshperspectives.org
REFERENCES
- Allinen M, Beroukhim R, Cai L, Brennan C, Lahti-Domenici J, Huang H, Porter D, Hu M, Chin L, Richardson A, et al. 2004. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell 6: 17–32 [DOI] [PubMed] [Google Scholar]
- Anbazhagan R, Osin PP, Bartkova J, Nathan B, Lane EB, Gusterson BA 1998. The development of epithelial phenotypes in the human fetal and infant breast. J Pathol 184: 197–206 [DOI] [PubMed] [Google Scholar]
- Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, Washington MK, Neilson EG, Moses HL 2004. TGF-β signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science 303: 848–851 [DOI] [PubMed] [Google Scholar]
- Bierie B, Moses HL 2006. Tumour microenvironment: TGF:β The molecular Jekyll and Hyde of cancer. Nat Rev Cancer 6: 506–520 [DOI] [PubMed] [Google Scholar]
- Bierie B, Chung CH, Parker JS, Stover DG, Cheng N, Chytil A, Aakre M, Shyr Y, Moses HL 2009. Abrogation of TGF-β signaling enhances chemokine production and correlates with prognosis in human breast cancer. J Clin Invest 119: 1571–1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell MJ, Radisky D 2001. Putting tumours in context. Nat Rev Cancer 1: 46–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell MJ, Radisky DC, Rizki A, Weaver VM, Petersen OW 2002. The organizing principle: Microenvironmental influences in the normal and malignant breast. Differentiation 70: 537–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisson C, Blacher S, Polette M, Blanc JF, Kebers F, Desreux J, Tetu B, Rosenbaum J, Foidart JM, Birembaut P, et al. 2003. Restricted expression of membrane type 1-matrix metalloproteinase by myofibroblasts adjacent to human breast cancer cells. Int J Cancer 105: 7–13 [DOI] [PubMed] [Google Scholar]
- Blagosklonny MV 2002. Oncogenic resistance to growth-limiting conditions. Nat Rev Cancer 2: 221–225 [DOI] [PubMed] [Google Scholar]
- Bluff JE, Brown NJ, Reed MW, Staton CA 2008. Tissue factor, angiogenesis and tumour progression. Breast Cancer Res 10: 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LF, Guidi AJ, Schnitt SJ, Van De Water L, Iruela-Arispe ML, Yeo TK, Tognazzi K, Dvorak HF 1999. Vascular stroma formation in carcinoma in situ, invasive carcinoma, and metastatic carcinoma of the breast. Clin Cancer Res 5: 1041–1056 [PubMed] [Google Scholar]
- Chang HY, Nuyten DS, Sneddon JB, Hastie T, Tibshirani R, Sorlie T, Dai H, He YD, van't Veer LJ, Bartelink H, et al. 2005. Robustness, scalability, and integration of a wound-response gene expression signature in predicting breast cancer survival. Proc Natl Acad Sci 102: 3738–3743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HY, Sneddon JB, Alizadeh AA, Sood R, West RB, Montgomery K, Chi JT, van de Rijn M, Botstein D, Brown PO 2004. Gene expression signature of fibroblast serum response predicts human cancer progression: Similarities between tumors and wounds. PLoS Biol 2: E7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng N, Bhowmick NA, Chytil A, Gorksa AE, Brown KA, Muraoka R, Arteaga CL, Neilson EG, Hayward SW, Moses HL 2005. Loss of TGF-β type II receptor in fibroblasts promotes mammary carcinoma growth and invasion through upregulation of TGF-α-, MSP- and HGF-mediated signaling networks. Oncogene 24: 5053–5068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Werb Z 2002. Inflammation and cancer. Nature 420: 860–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiani S, Ludvikova M, Tomasic G, Bianchi S, Gown AM, Eusebi V 1999. Myoepithelial cells and basal lamina in poorly differentiated in situ duct carcinoma of the breast. An immunocytochemical study. Virchows Arch 434: 227–234 [DOI] [PubMed] [Google Scholar]
- DeCosse JJ, Gossens CL, Kuzma JF, Unsworth BR 1973. Breast cancer: Induction of differentiation by embryonic tissue. Science 181: 1057–1058 [DOI] [PubMed] [Google Scholar]
- DeCosse JJ, Gossens C, Kuzma JF, Unsworth BR 1975. Embryonic inductive tissues that cause histologic differentiation of murine mammary carcinoma in vitro. J Natl Cancer Inst 54: 913–922 [PubMed] [Google Scholar]
- DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, Coussens LM 2009. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell 16: 91–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deugnier MA, Teuliere J, Faraldo MM, Thiery JP, Glukhova MA 2002. The importance of being a myoepithelial cell. Breast Cancer Res 4: 224–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak HF 1986. Tumors: Wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 315: 1650–1659 [DOI] [PubMed] [Google Scholar]
- El-Rifai W, Moskaluk CA, Abdrabbo MK, Harper J, Yoshida C, Riggins GJ, Frierson HF Jr, Powell SM 2002. Gastric cancers overexpress S100A calcium-binding proteins. Cancer Res 62: 6823–6826 [PubMed] [Google Scholar]
- Elenbaas B, Weinberg RA 2001. Heterotypic signaling between epithelial tumor cells and fibroblasts in carcinoma formation. Exp Cell Res 264: 169–184 [DOI] [PubMed] [Google Scholar]
- Ellis LM, Hicklin DJ 2008. VEGF-targeted therapy: Mechanisms of anti-tumour activity. Nat Rev Cancer 8: 579–591 [DOI] [PubMed] [Google Scholar]
- Fidler IJ 2003. The pathogenesis of cancer metastasis: The ‘seed and soil’ hypothesis revisited. Nat Rev Cancer 3: 453–458 [DOI] [PubMed] [Google Scholar]
- Fiegl H, Millinger S, Goebel G, Muller-Holzner E, Marth C, Laird PW, Widschwendter M 2006. Breast cancer DNA methylation profiles in cancer cells and tumor stroma: Association with HER-2/neu status in primary breast cancer. Cancer Res 66: 29–33 [DOI] [PubMed] [Google Scholar]
- Finak G, Bertos N, Pepin F, Sadekova S, Souleimanova M, Zhao H, Chen H, Omeroglu G, Meterissian S, Omeroglu A, et al. 2008. Stromal gene expression predicts clinical outcome in breast cancer. Nat Med 14: 518–527 [DOI] [PubMed] [Google Scholar]
- Gudjonsson T, Ronnov-Jessen L, Villadsen R, Rank F, Bissell MJ, Petersen OW 2002. Normal and tumor-derived myoepithelial cells differ in their ability to interact with luminal breast epithelial cells for polarity and basement membrane deposition. J Cell Sci 115: 39–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidi AJ, Schnitt SJ, Fischer L, Tognazzi K, Harris JR, Dvorak HF, Brown LF 1997. Vascular permeability factor (vascular endothelial growth factor) expression and angiogenesis in patients with ductal carcinoma in situ of the breast. Cancer 80: 1945–1953 [DOI] [PubMed] [Google Scholar]
- Gupta PB, Proia D, Cingoz O, Weremowicz J, Naber SP, Weinberg RA, Kuperwasser C 2007. Systemic stromal effects of estrogen promote the growth of estrogen receptor-negative cancers. Cancer Res 67: 2062–2071 [DOI] [PubMed] [Google Scholar]
- Howlett AR, Bissell MJ 1993. The influence of tissue microenvironment (stroma and extracellular matrix) on the development and function of mammary epithelium. Epithelial Cell Biol 2: 79–89 [PubMed] [Google Scholar]
- Hu M, Peluffo G, Chen H, Gelman R, Schnitt S, Polyak K 2009. Role of COX-2 in epithelial-stromal cell interactions and progression of ductal carcinoma in situ of the breast. Proc Natl Acad Sci 106: 3372–3377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Yao J, Cai L, Bachman KE, van den Brule F, Velculescu V, Polyak K 2005. Distinct epigenetic changes in the stromal cells of breast cancers. Nat Genet 37: 899–905 [DOI] [PubMed] [Google Scholar]
- Hu M, Yao J, Carroll DK, Weremowicz S, Chen H, Carrasco D, Richardson A, Violette S, Nikolskaya T, Nikolsky Y, et al. 2008. Regulation of in situ to invasive breast carcinoma transition. Cancer Cell 13: 394–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter KW. 2004. Host genetics and tumour metastasis. Br J Cancer 90: 752–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliopoulos D, Hirsch HA, Struhl K 2009. An epigenetic switch involving NF-κB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell 139: 693–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwano M, Fischer A, Okada H, Plieth D, Xue C, Danoff TM, Neilson EG 2001. Conditional abatement of tissue fibrosis using nucleoside analogs to selectively corrupt DNA replication in transgenic fibroblasts. Mol Ther 3: 149–159 [DOI] [PubMed] [Google Scholar]
- Jolicoeur F, Gaboury LA, Oligny LL 2003. Basal cells of second trimester fetal breasts: Immunohistochemical study of myoepithelial precursors. Pediatr Dev Pathol 6: 398–413 [DOI] [PubMed] [Google Scholar]
- Kalluri R, Neilson EG 2003. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest 112: 1776–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, et al. 2005. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438: 820–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA 2007. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 449: 557–563 [DOI] [PubMed] [Google Scholar]
- Kebers F, Lewalle JM, Desreux J, Munaut C, Devy L, Foidart JM, Noel A 1998. Induction of endothelial cell apoptosis by solid tumor cells. Exp Cell Res 240: 197–205 [DOI] [PubMed] [Google Scholar]
- Kenny PA, Bissell MJ 2003. Tumor reversion: Correction of malignant behavior by microenvironmental cues. Int J Cancer 107: 688–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerbel RS, Benezra R, Lyden DC, Hattori K, Heissig B, Nolan DJ, Mittal V, Shaked Y, Dias S, Bertolini F, et al. 2008. Endothelial progenitor cells are cellular hubs essential for neoangiogenesis of certain aggressive adenocarcinomas and metastatic transition but not adenomas. Proc Natl Acad Sci 105: E54; author reply E55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause S, Maffini MV, Soto AM, Sonnenschein C 2008. A novel 3D in vitro culture model to study stromal-epithelial interactions in the mammary gland. Tissue Eng Part C Methods 14: 261–271 [DOI] [PubMed] [Google Scholar]
- Le Beyec J, Xu R, Lee SY, Nelson CM, Rizki A, Alcaraz J, Bissell MJ 2007. Cell shape regulates global histone acetylation in human mammary epithelial cells. Exp Cell Res 313: 3066–3075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XJ, Dahiya S, Richardson E, Erlander M, Sgroi DC 2009. Gene expression profiling of the tumor microenvironment during breast cancer progression. Breast Cancer Res 11: R7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins-Green M, Boudreau N, Bissell MJ 1994. Inflammation is responsible for the development of wound-induced tumors in chickens infected with Rous sarcoma virus. Cancer Res 54: 4334–4341 [PubMed] [Google Scholar]
- Marusyk A, DeGregori J 2008. Declining cellular fitness with age promotes cancer initiation by selecting for adaptive oncogenic mutations. Biochim Biophys Acta 1785: 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusyk A, Polyak K 2010. Tumor heterogeneity: Causes and consequences. BBA Reviews on Cancer 1805: 105–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister SS, Gifford AM, Greiner AL, Kelleher SP, Saelzler MP, Ince TA, Reinhardt F, Harris LN, Hylander BL, Repasky EA, et al. 2008. Systemic endocrine instigation of indolent tumor growth requires osteopontin. Cell 133: 994–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller FR, Santner SJ, Tait L, Dawson PJ 2000. MCF10DCIS.com xenograft model of human comedo ductal carcinoma in situ [letter]. J Natl Cancer Inst 92: 1185–1186 [DOI] [PubMed] [Google Scholar]
- Moran EM 2002. Epidemiological and clinical aspects of nonsteroidal anti-inflammatory drugs and cancer risks. J Environ Pathol Toxicol Oncol 21: 193–201 [PubMed] [Google Scholar]
- Naccarato AG, Viacava P, Vignati S, Fanelli G, Bonadio AG, Montruccoli G, Bevilacqua G 2000. Bio-morphological events in the development of the human female mammary gland from fetal age to puberty. Virchows Arch 436: 431–438 [DOI] [PubMed] [Google Scholar]
- Nielsen BS, Rank F, Lopez JM, Balbin M, Vizoso F, Lund LR, Dano K, Lopez-Otin C 2001. Collagenase-3 expression in breast myofibroblasts as a molecular marker of transition of ductal carcinoma in situ lesions to invasive ductal carcinomas. Cancer Res 61: 7091–7100 [PubMed] [Google Scholar]
- Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA 2005. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 121: 335–348 [DOI] [PubMed] [Google Scholar]
- Osin PP, Anbazhagan R, Bartkova J, Nathan B, Gusterson BA 1998. Breast development gives insights into breast disease. Histopathology 33: 275–283 [DOI] [PubMed] [Google Scholar]
- Pal A, Srivastava T, Sharma MK, Mehndiratta M, Das P, Sinha S, Chattopadhyay P 2009. Aberrant methylation and associated transcriptional mobilization of Alu elements contributes to genomic instability in hypoxia. J Cell Mol Med (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen OW, Ronnov-Jessen L, Howlett AR, Bissell MJ 1992. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells [published erratum appears in Proc Natl Acad Sci 1993 Mar 15;90(6): 2556]. Proc Natl Acad Sci 89: 9064–9068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postovit LM, Margaryan NV, Seftor EA, Kirschmann DA, Lipavsky A, Wheaton WW, Abbott DE, Seftor RE, Hendrix MJ 2008. Human embryonic stem cell microenvironment suppresses the tumorigenic phenotype of aggressive cancer cells. Proc Natl Acad Sci 105: 4329–4334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psaila B, Lyden D 2009. The metastatic niche: Adapting the foreign soil. Nat Rev Cancer 9: 285–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian B, Deng Y, Im JH, Muschel RJ, Zou Y, Li J, Lang RA, Pollard JW 2009. A distinct macrophage population mediates metastatic breast cancer cell extravasation, establishment and growth. PLoS One 4: e6562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radisky D, Hagios C, Bissell MJ 2001. Tumors are unique organs defined by abnormal signaling and context. Semin Cancer Biol 11: 87–95 [DOI] [PubMed] [Google Scholar]
- Rohan RM, Fernandez A, Udagawa T, Yuan J, D'Amato RJ 2000. Genetic heterogeneity of angiogenesis in mice. Faseb J 14: 871–876 [DOI] [PubMed] [Google Scholar]
- Sakakura T, Sakagami Y, Nishizuka Y 1979. Persistence of responsiveness of adult mouse mammary gland to induction by embryonic mesenchyme. Dev Biol 72: 201–210 [DOI] [PubMed] [Google Scholar]
- Santisteban M, Reiman JM, Asiedu MK, Behrens MD, Nassar A, Kalli KR, Haluska P, Ingle JN, Hartmann LC, Manjili MH, et al. 2009. Immune-induced epithelial to mesenchymal transition in vivo generates breast cancer stem cells. Cancer Res 69: 2887–2895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer M, Werner S 2008. Cancer as an overhealing wound: An old hypothesis revisited. Nat Rev Mol Cell Biol 9: 628–638 [DOI] [PubMed] [Google Scholar]
- Shekhar MP, Pauley R, Heppner G 2003. Host microenvironment in breast cancer development: Extracellular matrix-stromal cell contribution to neoplastic phenotype of epithelial cells in the breast. Breast Cancer Res 5: 130–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar MP, Werdell J, Santner SJ, Pauley RJ, Tait L 2001. Breast stroma plays a dominant regulatory role in breast epithelial growth and differentiation: Implications for tumor development and progression. Cancer Res 61: 1320–1326 [PubMed] [Google Scholar]
- Sotgia F, Del Galdo F, Casimiro MC, Bonuccelli G, Mercier I, Whitaker-Menezes D, Daumer KM, Zhou J, Wang C, Katiyar S, et al. 2009. Caveolin-1-/- null mammary stromal fibroblasts share characteristics with human breast cancer-associated fibroblasts. Am J Pathol 174: 746–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternlicht MD, Barsky SH 1997. The myoepithelial defense: A host defense against cancer. Med Hypotheses 48: 37–46 [DOI] [PubMed] [Google Scholar]
- Sternlicht MD, Kedeshian P, Shao ZM, Safarians S, Barsky SH 1997. The human myoepithelial cell is a natural tumor suppressor. Clin Cancer Res 3: 1949–1958 [PubMed] [Google Scholar]
- Strutz F, Okada H, Lo CW, Danoff T, Carone RL, Tomaszewski JE, Neilson EG 1995. Identification and characterization of a fibroblast marker: FSP1. J Cell Biol 130: 393–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tlsty TD 2001. Stromal cells can contribute oncogenic signals. Semin Cancer Biol 11: 97–104 [DOI] [PubMed] [Google Scholar]
- Tlsty TD, Hein PW 2001. Know thy neighbor: Stromal cells can contribute oncogenic signals. Curr Opin Genet Dev 11: 54–59 [DOI] [PubMed] [Google Scholar]
- Trimboli AJ, Cantemir-Stone CZ, Li F, Wallace JA, Merchant A, Creasap N, Thompson JC, Caserta E, Wang H, Chong JL, et al. 2009. Pten in stromal fibroblasts suppresses mammary epithelial tumours. Nature 461: 1084–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrana JA, Stang MT, Grande JP, Getz MJ 1996. Expression of tissue factor in tumor stroma correlates with progression to invasive human breast cancer: Paracrine regulation by carcinoma cell-derived members of the transforming growth factor β family. Cancer Res 56: 5063–5070 [PubMed] [Google Scholar]
- Weaver VM, Lelievre S, Lakins JN, Chrenek MA, Jones JC, Giancotti F, Werb Z, Bissell MJ 2002. β4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell 2: 205–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman BS, Werb Z 2002. Stromal effects on mammary gland development and breast cancer. Science 296: 1046–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Huang J, Ren X, Gorska AE, Chytil A, Aakre M, Carbone DP, Matrisian LM, Richmond A, Lin PC, et al. 2008. Abrogation of TGF β signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell 13: 23–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahir N, Lakins JN, Russell A, Ming W, Chatterjee C, Rozenberg GI, Marinkovich MP, Weaver VM 2003. Autocrine laminin-5 ligates α6β4 integrin and activates RAC and NFκB to mediate anchorage-independent survival of mammary tumors. J Cell Biol 163: 1397–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]