Abstract
The nuclear lamins are type V intermediate filament proteins that are critically important for the structural properties of the nucleus. In addition, they are involved in the regulation of numerous nuclear processes, including DNA replication, transcription and chromatin organization. The developmentally regulated expression of lamins suggests that they are involved in cellular differentiation. Their assembly dynamic properties throughout the cell cycle, particularly in mitosis, are influenced by posttranslational modifications. Lamins may regulate nuclear functions by direct interactions with chromatin and determining the spatial organization of chromosomes within the nuclear space. They may also regulate chromatin functions by interacting with factors that epigenetically modify the chromatin or directly regulate replication or transcription.
Intermediate filaments form a polymeric mesh beneath the nuclear membrane that controls the shape and stability of the nucleus but also regulates chromatin positioning and gene expression.
A filamentous layer located between the inner nuclear membrane (INM) and peripheral heterochromatin was evident even in early electron microscopic studies of vertebrate cell nuclei (Fawcett 1966). This layer, later termed the nuclear lamina, is also found to be closely associated with nuclear pore complexes (NPCs) and contains three major structurally related polypeptides (Aaronson and Blobel 1975). These proteins are named nuclear lamins A, B, and C according to their molecular weights (Gerace and Blobel 1980). Further biochemical characterization and cDNA cloning of the nuclear lamins classifies them as type V intermediate filament proteins (Goldman et al. 1986; McKeon et al. 1986).
STRUCTURE AND BIOCHEMICAL PROPERTIES OF NUCLEAR LAMINS
Lamin Isoforms and Expression Patterns
Lamins are present in all metazoans examined to date ranging from hydra to human, but are not found in unicellular organisms and plants (Cohen et al. 2001; Melcer et al. 2007). Extensive characterization in several model organisms including humans, mice, frogs, fruit flies and nematodes shows that their properties are shared across species (Melcer et al. 2007; Dechat et al. 2008b). Based on their sequence homologies, expression patterns, structural features, and biochemical and dynamic properties, lamins are subdivided into A- and B-types. All metazoans express at least one B-type lamin. Typically, invertebrates have only a single lamin gene of the B-type, with some exceptions such as Drosophila, which expresses one B-type (lamin Dm0) and one A-type lamin (lamin C) encoded by two distinct genes. Most vertebrates have one A-type lamin and two B-type lamin genes except for Xenopus, which has three B-type genes (Melcer et al. 2007).
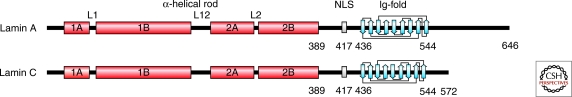
The two major isoforms of vertebrate A-type lamins, lamins A and C, are derived from a single gene (LMNA) by alternative splicing (see Fig. 1) (Lin and Worman 1993). In some vertebrates, alternative splicing can also produce two less abundant isoforms, lamins AΔ10 and C2, from LMNA (Nakajima and Abe 1995; Machiels et al. 1996). Lamins B1 and B2 are the two major B-type lamins in most vertebrates. They are encoded by the LMNB1 and LMNB2 genes, respectively (Peter et al. 1989; Vorburger et al. 1989). The latter also encodes the minor isoform lamin B3 (Furukawa and Hotta 1993). Although at least one B-type lamin is expressed in all cells throughout development, the expression of A-type lamins is developmentally regulated (Benavente et al. 1985; Schatten et al. 1985; Lehner et al. 1987). During mouse development, lamins A and C are not expressed until days 10–12 of mouse embryogenesis and then primarily in primordial muscle cells (Stewart and Burke 1987; Rober et al. 1989). Lamin A/C expression in other organs does not occur until after birth (Rober et al. 1989). Cells of hematopoietic lineage express only B-type lamins (Guilly et al. 1990; Rober et al. 1990). Similar patterns of expression of A and B-type lamins take place during the developmental progression of other vertebrates (Benavente et al. 1985; Lehner et al. 1987; Prather et al. 1989) and Drosophila (Frasch et al. 1988; Riemer et al. 1995). The regulated expression of A- and B-type lamins is also evident during differentiation of stem cells in culture. For example, undifferentiated human and mouse embryonic stem (ES) cells lack lamins A and C, but express lamins B1 and B2 (Constantinescu et al. 2006). The minor mammalian isoforms, lamins C2 and B3, are expressed exclusively in germ cells (Furukawa and Hotta 1993; Machiels et al. 1995; Nakajima and Abe 1995), whereas small amounts of lamin AΔ10 appear to be present in a variety of cell types (Machiels et al. 1995).
Figure 1.
Structure of nuclear lamins. Schematic drawing of mature lamin A and lamin C polypeptide chains. The lamin structure consists of a short amino terminal head domain, a central α-helical rod domain (red), and the carboxy-terminal domain containing the NLS and the Ig-fold (blue; the nine β-strands of the Ig-fold motif are depicted). Modified, with permission, from (Dechat et al. 2008b).
The importance of the developmental regulation of lamin expression is evident from studies in LMNA knockout (LMNA−/−) and LMNB1 mutant (LMNBΔ/Δ) mice. In mice null for the A-type lamins, no obvious embryonic defects can be detected, but these animals show severe postnatal growth retardation and muscular dystrophy (Sullivan et al. 1999). On the other hand, mice with an insertional mutation in LMNB1 develop defects in their lungs and bones during embryogenesis and die at birth, even though they continue to express lamin B2 (Vergnes et al. 2004).
Structure and Assembly of the Nuclear Lamins
The nuclear lamins have the typical tripartite structure of intermediate filament (IF) proteins, consisting of a highly α-helical central rod domain flanked by a short globular amino-terminal “head” domain and a longer carboxy-terminal “tail” domain (Parry et al. 1986). The central rod domain is composed of four subhelical regions comprised of heptad repeats and designated as coil 1A, 1B, 2A, and 2B. These individual “coils” are separated from each other by three short linker segments, L1, L12, and L2, of which L12 is the most flexible (Parry et al. 1986). Although the head domain appears to be unstructured, the tail domain contains a highly conserved structural motif similar to a type s immunoglobulin fold (Ig-fold) (Dhe-Paganon et al. 2002; Krimm et al. 2002). A nuclear localization signal (NLS), required for transport into the nucleus, is present in all lamins between the carboxy-terminal end of the central rod domain and the Ig-fold (Loewinger and McKeon 1988) (Fig. 1).
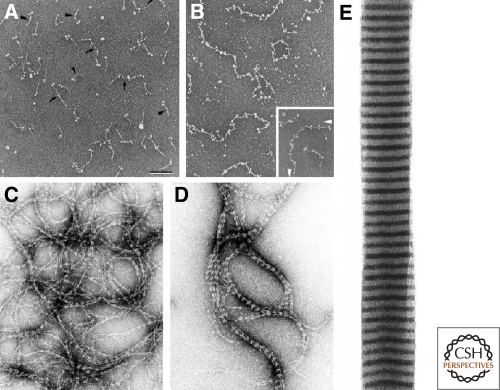
In vitro, lamins self-assemble into higher order structures through a series of steps. The first step involves the formation of a coiled-coil dimer by the in register and in parallel association of two α-helical rod domains into a left handed superhelix (Heitlinger et al. 1991) (Fig. 2A). Next, the lamin dimers associate in a head-to-tail fashion (Heitlinger et al. 1991; Stuurman et al. 1996) and these polarized arrays interact in an antiparallel fashion to form apolar tetrameric protofilaments (Heitlinger et al. 1991) (Fig. 2B). The interaction of four lamin protofilaments leads to the formation of ∼10-nm filaments (Heitlinger et al. 1991; Stuurman et al. 1996; Ben-Harush et al. 2009) (Fig. 2C,D). Although the assembly of some invertebrate lamins appears to terminate at this stage of the assembly process, vertebrate lamins continue to assemble into well ordered paracrystalline arrays in vitro (Zackroff and Goldman 1979; Heitlinger et al. 1991; Stuurman et al. 1996; Ben-Harush et al. 2009) (Fig. 2E). The assembly of lamin proteins differs from their cytoplasmic counterparts in several ways. For example, lamin dimers associate by polar “head-to-tail” interactions to form protofilaments, which is very different from the half-staggered, antiparallel side-by-side association of cytoplasmic IF dimers, which assemble into 10 nm filaments (Strelkov et al. 2004). This difference is at least in part explained by the crystal structure of the coiled-coil dimer of the lamin A coil 2B region (Strelkov et al. 2004). Although this structure appears to be similar to the overall structure of the homologous segment of the cytoplasmic IF protein vimentin, the distribution of charged residues varies causing significant changes in the patterns of intra- and interhelical salt bridges. Additionally, vertebrate lamins have six extra heptads in the central rod domain (McKeon et al. 1986), which may also help to explain assembly differences between cytoplasmic IF and lamins. The central rod domains of some invertebrates lamins contain fewer extra heptads and these lamins stop assembling into higher order structures at the 10-nm (IF) stage of assembly, similar to cytoplasmic IF proteins (Ben-Harush et al. 2009).
Figure 2.
Assembly of the nuclear lamins in vitro. Lamins self assemble to form dimers (A) which then join to form linear head-to-tail polymers (protofilaments) (B). Bar = 100 nm; electron micrograph of rotary shadowed chicken lamin B2. These protofilaments further assemble into “beaded” filaments or fibers (C) which in turn associate laterally into thicker fibers (D), and eventually into paracrystalline arrays (E); C,D are negatively stained electron microscope preparations. Reprinted from Stuurman et al. (1998) with permission from Elsevier.
Posttranslational Processing and Modifications of the Nuclear Lamins
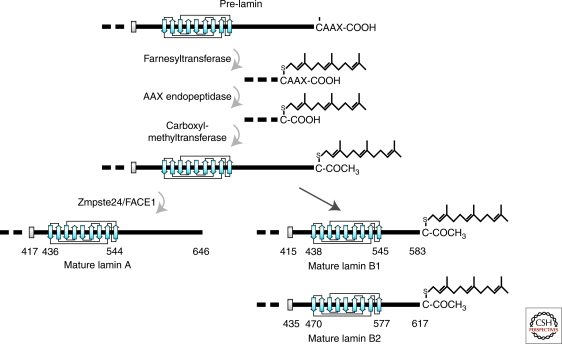
Lamins A, B1, and B2 are expressed as prelamins that require extensive posttranslational modifications of their carboxy-terminal –CAAX box to become mature lamins (Rusinol and Sinensky 2006; Davies et al. 2009). Modification of the –CAAX box takes place in a highly regulated temporal sequence starting with the farnesylation of the cysteine residue (Farnsworth et al. 1990; Lutz et al. 1992) by a farnesyltransferase (Zhang and Casey 1996). This modification initiates a sequence of processing steps (Beck et al. 1990) beginning with the removal of the –AAX by a CAAX prenyl protease. Two members of this zinc metalloproteinase family have been identified in humans: Rce1 (Ras-converting enzyme 1) and Zmpste24 (Zinc metalloprotease related to the STE24 homolog in yeast), also known as FACE1 (farnesylated-proteins converting enzyme)(Boyartchuk et al. 1997; Leung et al. 2001; Corrigan et al. 2005); followed by carboxymethylation of the carboxy-terminal cysteine by isoprenylcysteine carboxyl methyltransferase (Icmt) (Winter-Vann and Casey 2005). In contrast to B-type lamins, which remain permanently farnesylated and carboxymethylated, an additional 15 amino acids are removed from the carboxyl terminus of farnesylated/carboxymethylated prelamin A by Zmpste24/FACE1 (Corrigan et al. 2005). This final processing step results in the production of mature lamin A lacking the carboxy-terminal farnesyl- and carboxymethyl-modifications (Fig. 3). This sequence of lamin processing steps is highly regulated and each step depends on the preceding modification (Kilic et al. 1997). Lamin C, which is 74 residues shorter than mature lamin A, does not possess a –CAAX box and therefore is not farnesylated or otherwise modified (see Fig. 1). The precise location of the intracellular post-translational processing sites for the lamins remains largely unknown. Although the enzymes required for processing the carboxyl terminus of the lamins are present in both the inner nuclear membrane and endoplasmic reticulum membranes, the complete processing of prelamin A can occur when the protein is confined either to the cytoplasm or to the nucleus (Barrowman et al. 2008). Even though the prelamins can be processed in the cytoplasm, it is more likely that all lamin processing occurs in the nucleus (Lutz et al. 1992), because lamins are rapidly transported into the nucleus after translation in the cytoplasm (Lehner et al. 1986).
Figure 3.
Posttranslational processing of the carboxyl terminus of prelamins A, B1, and B2. Processing takes place in a series of steps: (1) addition of a farnesyl group to the cysteine residue of the –CAAX box of pre-lamin A, prelamin B1 and prelamin B2 by a farnesyltransferase; (2) removal of the last three residues (−AAX) by an AAX endopeptidase; (3) methylation of the terminal carboxylic acid group (−COOH) by a carboxyl methyltransferase; (4) removal of the carboxyl terminal 15 amino acids of lamin A with the farnesyl attached by the metalloprotease Zmpste24/FACE1. This last proteolysis step does not occur on B-type lamins and therefore they remain farnesylated. Modified, with permission, from Dechat et al. (2008b).
In addition to farnesylation and carboxymethylation, lamins are also posttranslationally modified by phosphorylation (Ottaviano and Gerace 1985), sumoylation (Zhang and Sarge 2008), ADP-ribosylation (Adolph 1987), and possibly by glycosylation (Ferraro et al. 1989). Phosphorylation of serine and threonine residues proximal to the NLS by protein kinase C is known to play a role in the regulated import of lamins into the nucleus (Hennekes et al. 1993; Leukel and Jost 1995). At the onset of mitosis, the phosphorylation of lamins at specific sites by cyclin-dependent kinase (Cdk) 1 and protein kinase C (PKC) is required to drive disassembly of the lamina (Gerace and Blobel 1980; Dessev and Goldman 1988; Dessev et al. 1988; Dessev et al. 1989; Heald and McKeon 1990; Peter et al. 1990; Ward and Kirschner 1990; Dessev et al. 1991; Molloy and Little 1992; Goss et al. 1994; Collas 1999). Subsequently, dephosphorylation of the mitotic sites by protein phosphatase 1a is required for lamin/lamina assembly during the telophase/early G1 transition (Thompson et al. 1997).
Lamin Structure and Dynamic Properties within the Lamina and Nucleoplasm
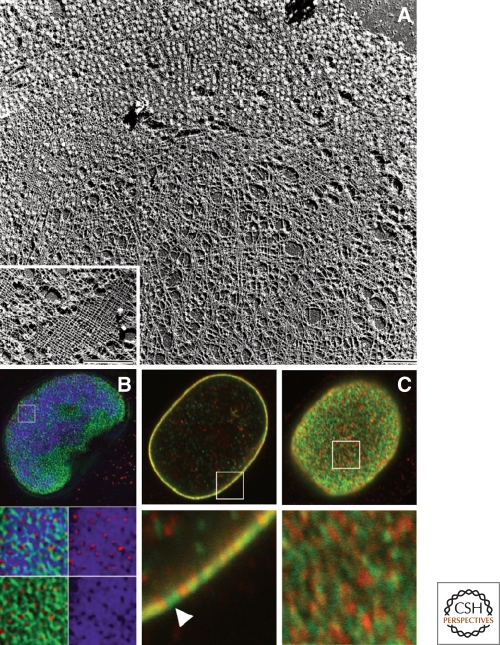
Very little is known about the formation, composition, and structure of lamin polymers in vivo. The structures of the lamins within the lamina that have been described to date range from a regular meshwork of ∼10–15 nm filaments observed in Xenopus oocyte germinal vesicles and in sperm pronuclei assembled in vitro (Aebi et al. 1986; Zhang and Casey 1996; Goldberg et al. 2008a) (Fig. 4A), to a more irregular filamentous meshwork seen in mammalian cells (Belmont et al. 1993; Schermelleh et al. 2008; Shimi et al. 2008) (Fig. 4B,C). These meshworks have long been assumed to be stable structures based on the resistance of the nuclear lamins within the lamina to extraction in detergent containing high salt solutions, and the propensity of purified lamins to polymerize into higher order insoluble structures in vitro at relatively low critical concentrations (Aebi et al. 1986; Lourim and Lin 1989; Glass and Gerace 1990). In further support of this assumption, measurements of lamin mobility by fluorescence recovery after photobleaching (FRAP) reveal that GFP-tagged A- and B-type lamins do not appreciably exchange subunits within the lamina for up to several hours throughout most of interphase (Broers et al. 1999; Moir et al. 2000; Dahl et al. 2006).
Figure 4.
The nuclear lamins form a meshwork of filaments within the lamina. (A) Spread nuclear envelope from Xenopus oocytes after detergent extraction and preparation for transmission electron microscopy by freeze-drying/unidirectional metal shadowing. The micrograph shows the nuclear lamina meshwork partially studded with nuclear pore complexes. (Inset) Higher-magnification view of a particularly well-preserved area clearly shows the near-tetragonal lamina meshwork. Bars, 1 µm. Reprinted from Stuurman et al. (1998), with permission from Elsevier. (B) Structured illumination microsopy (SIM) reveals that there is an irregular meshwork of nuclear lamin B as revealed by immunofluorescence (green). This preparation is also stained with antibodies directed against nuclear pores and is stained with DAPI for DNA/chromatin. Pores (red), DAPI (blue). From Schermelleh et al. (2008). Reprinted with permission from AAAS. (C) Confocal immunofluorescence localization of lamin A/C (green) and lamin B1 (red) in HeLa cells. Lamins A/C and B1 in a single nucleus are seen in an equatorial section (left panels) and the nuclear surface (right panels). The areas indicated by white squares in the top panels are enlarged fivefold in the lower panels. These images demonstrate that lamins form mainly separate networks with some overlapping regions. Bar, 5 µm. Adapted, with permission, from Shimi et al. (2008).
Recently it has become apparent that the A- and B-type lamins form separate filamentous networks in the lamina. These structures can be identified by both high resolution light microscopy (Schermelleh et al. 2008; Shimi et al. 2008) and whole mount electron microscopy in lamin B containing Xenopus oocyte nuclei ectopically expressing A-type lamins (Goldberg et al. 2008b). Even though the A-type and B-type lamin networks appear to be mainly separate structures (Shimi et al. 2008), there is evidence that the individual networks overlap and interact to varying degrees. Evidence for interaction between these two lamin meshworks is based on studies using fluorescence resonance energy transfer (FRET) in combination with time domain fluorescence lifetime imaging and high resolution confocal immunofluorescence (Moir et al. 2000; Delbarre et al. 2006; Shimi et al. 2008).
Other evidence supporting the interactions between the A and B type lamins is derived from studies of nuclei in which either A or B type lamins are perturbed. Fibroblasts derived from LMNA−/− mouse embryos (MEFs) or a limb girdle muscular dystrophy patient with a homozygous nonsense mutation in LMNA contain abnormally shaped nuclei containing blebs or lobules (Sullivan et al. 1999; Muchir et al. 2003). A significant number of these blebbed regions lack B-type lamins and NPCs. In addition, nuclei in LMNBΔ/Δ MEFs are also highly lobulated, showing a dramatic increase in the mesh size of the lamin A/C network (Vergnes et al. 2004). HeLa cells depleted of lamin B1 by shRNA silencing frequently develop extensively enlarged lamin A/C meshworks and nuclear envelope blebs which lack lamin B2 and NPCs (Shimi et al. 2008). Many of these nuclear phenotypes are also found in cells endogenously or ectopically expressing either point mutations or truncations of lamins A/C (Ostlund et al. 2001; Vaughan et al. 2001; Vigouroux et al. 2001; Bechert et al. 2003; Favreau et al. 2003; Muchir et al. 2003; Goldman et al. 2004). It is also noteworthy that lamin A is more mobile in the nuclear lamina in nuclei with decreased amounts of lamin B1 (Tang et al. 2008).
The A- and B-type lamins have different disassembly and assembly properties during mitosis as revealed by biochemical fractionation, immunofluorescence, and live cell imaging analysis, further supporting the idea that the two types of lamins form separate networks in the lamina. As described earlier, depolymerization of the lamins is regulated by mitotic kinases and reassembly requires dephosphorylation by protein phosphatase 1a (Thompson et al. 1997; Ito et al. 2007). When the nuclear envelope is disassembled during late prophase, A-type lamins become dispersed throughout the cytoplasm in an apparently freely diffusible state whereas the majority of B-type lamins remain associated with the nuclear membranes, which appear to be mainly dispersed into the endoplasmic reticulum (Gerace and Blobel 1980; Stick et al. 1988). This association of B-type lamins with membranes during mitosis appears to be mainly attributable to their permanently farnesylated state (Rusinol and Sinensky 2006).
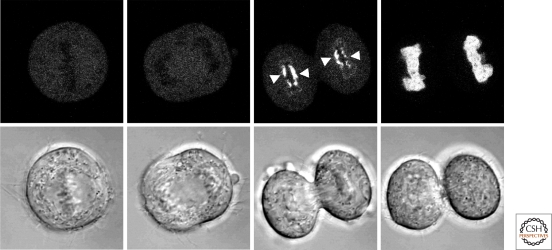
Further support for differences in the properties of the A- and B-type lamins is derived from studies revealing their spatial and temporal order of assembly into the nuclear lamina at anaphase/telophase and in the early stages of G1. For example, in mouse keratinocytes and hamster cells, lamin B1 accumulates along the entire periphery of the decondensing daughter chromosomes and assembles into a relatively stable polymer by mid- to late- telophase (Broers et al. 1999; Moir et al. 2000). In these cells, lamin A begins to accumulate within the nucleus mainly after the major components of the nuclear envelope including NPCs are assembled in daughter cells in late telophase. In HeLa cells, however, a small fraction of A-type lamins associates with chromosomes much earlier than in mouse cells, first assembling in the central or “core” region of chromosomes in close association with kinetochores (Dechat et al. 2004; Dechat et al. 2007; Haraguchi et al. 2008) (Fig. 5). The remainder of the A-type lamins is then transported into the nucleus after formation of an intact nuclear envelope. Later, other elements of the nuclear envelope begin to assemble in daughter cells (Dechat et al. 2004; Dechat et al. 2007; Haraguchi et al. 2008).
Figure 5.
Association of lamin A with chromosomes during mitosis. HeLa cells expressing GFP-lamin A were followed by time-lapse microscopy from the metaphase/anaphase transition (far left panels) into early G1 (far right panels). GFP-lamin A first associates with the core regions of chromosomes during telophase and spreads to cover the entire chromatin surface by early G1. DIC images of the same series are shown in the bottom row (Dechat et al. 2007).
Although the major fraction of the various lamin isoforms is associated with the nuclear lamina, these proteins are also present throughout the nucleoplasm, especially in interphase (Goldman et al. 1992; Lutz et al. 1992). These nucleoplasmic lamins are likely to have different functions, for example in DNA replication and transcription (Dechat et al. 2008b), and a small fraction may represent assembly intermediates that are subsequently incorporated into the nuclear lamina (Goldman et al. 1992). Interestingly, B-type lamins in the nucleoplasm appear to be relatively static, similar to those in the lamina, whereas the nucleoplasmic A-type lamins are much more dynamic. These differences in the properties of nucleoplasmic A- and B-type lamins are supported experimentally by their biochemical extractability and their dynamic properties as determined in vivo by FRAP analyses and fluorescence correlation spectroscopy (FCS) (Broers et al. 1999; Moir et al. 2000; Shimi et al. 2008). For example, FCS measurements of GFP-tagged lamins A/C and lamins B1/B2 showed that nucleoplasmic lamins A/C are highly mobile, whereas lamin B1 and lamin B2 are mainly immobile (Shimi et al. 2008). This latter observation suggests that nucleoplasmic B-type lamins are either assembled into some type of structure or are tightly associated with other unknown immobile structural components. Interestingly, decreasing the amount of nucleoplasmic lamin B1 by shRNA silencing increases the rate of mobility for a large fraction of nucleoplasmic lamin A (Shimi et al. 2008). Taken together, these FCS results suggest that, as in the case of the lamina, the A- and B-type lamins form separate yet interacting structures within the nucleoplasm. Obviously, it will be of great interest in the future to determine the specific functions of these nucleoplasmic lamins.
FUNCTIONS OF THE LAMINS
Regulation of Nuclear Shape and Mechanical Stability
It is becoming increasingly obvious that some of the functions of nuclear lamins are analogous to those of cytoskeletal intermediate filament proteins, which are known to be involved in the determination and maintenance of cell shape and mechanical properties (Goldman et al. 2008). In support of this, the nuclei of LMNA−/− MEFs display increased deformability and impaired viability under mechanical strain compared to the nuclei in control MEFs (Houben et al. 2007). Increased nuclear deformability is also observed in human ES cells lacking A-type lamins as compared to nuclei in differentiated cells expressing the A-type lamins (Pajerowski et al. 2007). In addition, cells either deficient in lamins or expressing mutant lamin proteins often contain misshapen nuclei (Ostlund et al. 2001; Vaughan et al. 2001; Vigouroux et al. 2001; Bechert et al. 2003; Favreau et al. 2003; Muchir et al. 2003; Goldman et al. 2004). Quantitative rheological measurements, particle tracking methods and differential interference contrast microscopy reveal that in vitro reconstituted lamin B1 networks are extremely porous, with elastic stiffness providing resistance to shear deformations (Panorchan et al. 2004a; Panorchan et al. 2004b). Interestingly, measurements made by atomic force microscopy show that germinal vesicles isolated from Xenopus oocytes, which normally contain only B-type lamins, display a significant increase in stiffness on the ectopic expression of lamin A (Schape et al. 2009).
Nuclear Lamins and the Regulation of Chromatin Positioning and Gene Expression
Lamins may regulate transcription by organizing chromatin into active and inactive domains. For example, electron microscopic and light microscopic observations show that there is a close association between peripherally localized heterochromatin and the nuclear lamina (Fawcett 1966; Paddy et al. 1990) (Fig. 6). This suggests that lamins may be involved in anchoring or organizing interphase chromosomes (Sullivan et al. 1999; Goldman et al. 2004; Nikolova et al. 2004; Galiova et al. 2008). Additional support for this idea comes from biochemical experiments showing that lamins A/C bind to mitotic chromosomes and to polynucleosomal particles in vitro (Burke 1990; Glass and Gerace 1990; Yuan et al. 1991). The interaction of lamins with chromatin could be mediated either by their direct binding to histones (Taniura et al. 1995; Goldberg et al. 1999; Mattout et al. 2007), or to specific DNA sequences in the matrix attachment/scaffold-associated regions (MARs/SARs) (Luderus et al. 1992; Luderus et al. 1994; Baricheva et al. 1996; Zhao et al. 1996).
Figure 6.
The nuclear lamina is a fibrous network (fibrous lamina) located between peripheral heterochromatin and the inner nuclear membrane. A transmission electron micrograph of a thin section showing a portion of a smooth muscle nucleus in guinea pig epedidymis. Adapted from Fawcett (1966), copyright 1995, Wiley-Liss, Inc. Reprinted with permission of John Wiley & Sons, Inc. American Journal of Anatomy, Vol. 119, No. 1, pg. 140.
The Role of Lamins in Mitosis
In addition to nuclear disassembly, several lines of evidence suggest a direct role for lamins in nuclear assembly following mitosis. The microinjection of lamin antibodies into mitotic cells causes arrest in a telophase-like state with the chromosomes remaining condensed (Benavente and Krohne 1986). In addition, decreased expression of lamin Dm0 in Drosophila inhibits nuclear membrane assembly and causes an enrichment of NPCs in cytoplasmic annulate lamellae (Lenz-Bohme et al. 1997). In Caenorhabditis elegans, the down-regulation of the single lamin leads to a loss of chromosomes, defects in chromosome separation into daughter cell nuclei, and abnormal condensation of chromatin (Liu et al. 2000). In vitro nuclear assembly in frog egg extracts can be inhibited by addition of the C-terminal tail fragment of lamin B3, in part because of the Ig-fold motif in the lamin tail, which can inhibit lamin polymerization in vitro (Shumaker et al. 2005). Lastly, the expression of the mutant lamin A which causes Hutchinson-Gilford Progeria Syndrome (HGPS) leads to defects in cell division including a delay in cytokinesis, delayed completion of nuclear reassembly at the end of mitosis and an increase in mitotic cells (Cao et al. 2007; Dechat et al. 2007).
B-type lamins also play a role in the formation of the mitotic spindle. In cultured cells, a diffuse localization of B-type lamins can be observed associated with the mitotic spindle (Georgatos et al. 1997; Maison et al. 1997; Moir et al. 2000; Beaudouin et al. 2002; Tsai et al. 2006). In frog egg extracts, a mitotic spindle can be induced to form around added DNA or on beads coated with a variety of factors important for mitotic spindle formation (Tsai and Zheng 2005). In these lysates, lamin B3, the major lamin in frog eggs, is required to organize a “spindle matrix” structure. This matrix contains membrane vesicles and proteins known to regulate the assembly and dynamic properties of the microtubules comprising the mitotic spindle (Tsai et al. 2006). Depletion of lamin B3 from the egg extracts with antibodies or the addition of dominant negative fragments of lamin B3 known to disrupt lamin assembly, also disrupt the formation of the mitotic spindle. Furthermore, lamin B3 is associated with several spindle assembly factors within the spindle matrix such as NuMA and Nudel and it appears to play an important role in regulating the activity of these proteins during mitosis (Tsai et al. 2006; Ma et al. 2009).
The Role of Lamins in DNA Replication and Repair
Several lines of evidence suggest that lamins play a role in DNA replication. In cultured cells, sites of DNA replication can be visualized as discrete early or late replication foci in the nucleoplasm. These foci can be detected by incorporation of bromodeoxyuridine into sites of replication (Moir et al. 1994) or by immunolocalization of replication-associated proteins such as proliferating cell nuclear antigen (PCNA) (Shumaker et al. 2008). It has been shown that lamin B1 colocalizes with these replication foci during late S phase in mouse 3T3 cells (Moir et al. 1994) and that lamins A/C are present at sites of early replication in normal human fibroblasts (Kennedy et al. 2000). Furthermore, DNA replication in Xenopus nuclei assembled in vitro is inhibited by depletion of lamin B3 or by the addition of a dominant-negative fragment of lamin B3, which drives the disassembly of the endogenous lamin network (Lopez-Soler et al. 2001; Shumaker et al. 2005; Shumaker et al. 2008). There is evidence that the lamins play a direct role in regulating replication by their binding to DNA replication factors such as PCNA. The lamin binding site for PCNA resides within the highly conserved Ig-fold motif located in the carboxyl terminus of both the A- and B-type lamins (Shumaker et al. 2008). In addition, there is a close association between lamin B3 and PCNA at the surface of sperm head chromatin during the earliest stages of nuclear assembly in Xenopus interphase egg extracts (Shumaker et al. 2008).
Nuclear lamins have also been implicated in DNA repair, although the mechanistic basis of this remains unclear. For example, the expression of disease-causing mutant lamins impairs the formation of DNA repair foci (Liu et al. 2005; Manju et al. 2006). Furthermore, genetic instability because of defects in telomere function and DNA repair has been implicated in progeria, the premature aging syndrome (Gonzalez-Suarez et al. 2009a; Gonzalez-Suarez et al. 2009b).
The Role of Lamins in Transcription
Several lines of experimental evidence support the possibility that lamins play a role in transcription. An amino-terminally deleted dominant negative lamin A, which disassembles lamin networks, specifically inhibits RNA pol II activity in hamster cells or in nuclei isolated from Xenopus embryos (Spann et al. 2002). In addition, over-expression of lamins A/C or silencing of lamin B1 leads to a significant inhibition of pol II transcription in HeLa cells (Kumaran and Spector 2008; Shimi et al. 2008; Tang et al. 2008).
Lamins also associate with several transcription factors leading to the suggestion that they are involved in specific regulatory pathways (Heessen and Fornerod 2007; Andres and Gonzalez 2009). For example, the association of Oct-1 with lamin B1 at the nuclear envelope appears to be important for the oxidative stress response in MEFs, as lamin B1 deficiency leads to a dysregulation of Oct-1-dependent genes and to an increase in reactive oxygen species (Malhas et al. 2009). Lamins A/C also interact with the transcription factors c-Fos, MOK2, and sterol response element binding protein 1 (SREBP1) (Dreuillet et al. 2002; Lloyd et al. 2002; Ivorra et al. 2006; Dreuillet et al. 2008; Harper et al. 2009). In the case of c-Fos, its interaction with lamins A/C at the nuclear envelope appear to suppress AP-1 (activating protein 1) binding to DNA and transcriptional activity in an extracellular signal-regulated kinase (ERK) 1/2 activity-dependent fashion (Ivorra et al. 2006; Gonzalez et al. 2008). In addition to regulating transcription factor function by direct interaction, lamins are also associated with transcription factors indirectly via several lamin-binding proteins including emerin, LAP2β and pRb. Although the evidence supporting a role for the lamins in regulating transcription is convincing, it remains unclear whether this regulation involves direct or indirect binding to transcription factors.
Lamins as Regulators of the Positioning and Organization of Chromatin
The positioning of genes near or in contact with the nuclear lamina may be a mechanism for modulating gene expression. Inactive genes and gene-poor chromatin are frequently found in association with the lamina region, which suggests that the nuclear lamina may be a transcriptionally silent microdomain (Boyle et al. 2001; Cremer et al. 2001). Furthermore, lamins may play a role in establishing this microdomain by directly interacting with chromatin. The interactions of lamins with chromatin/DNA at the nuclear lamina can be identified in more detail by DamID labeling (Pickersgill et al. 2006). In this technique, an expression vector encoding a potential chromatin/DNA binding protein fused to the Escherichia coli enzyme DNA adenine methyl transferase (Dam) is expressed in cells. DNA methylated by the lamin Dam fusion protein is then analyzed by sequencing. In Drosophila Kc cells, lamin Dm0 seems to mainly associate with transcriptionally inactive, mid-to-late replicating genome regions, lacking active histone marks and enriched in large intergenic regions (Pickersgill et al. 2006). In human lung fibroblasts lamin B1-associated domains (LADs) are present along chromosomes in a distinct pattern interspersed with “lamin B1 poor” regions (Guelen et al. 2008). These LADs are ≥1 Mb in size, are mostly heterochromatic. Genes present within LADs are 5–10-fold less active than genes outside LADs.
The lamina appears to have gene or chromatin-specific effects on activation or repression of transcription. Evidence for the lamina constituting a repressive environment for transcription comes from studies localizing inactive gene loci to the nuclear periphery (Kosak et al. 2002; Zink et al. 2004; Williams et al. 2006). For example, the inactive Ig-heavy and Ig-κ loci are preferentially positioned at the nuclear periphery in non-B-cell lineages, but are centrally located in B-cell nuclei where they are actively engaged in transcription (Kosak et al. 2002). In addition, transcriptionally inactive testis-specific gene clusters are frequently associated with lamin Dm0 in the lamina of Drosophila S2 cells. Upon down-regulation of lamin Dm0, these gene clusters relocate from the lamina to the nuclear interior and become transcriptionally active (Shevelyov et al. 2009). Artificial tethering of a reporter gene to the nuclear envelope using the membrane spanning domain of the inner nuclear membrane protein emerin results in the transcriptional inactivation of the gene in mouse cells (Reddy et al. 2008). However, the relocalization of chromosomes from the nuclear interior to the nuclear periphery using the inner membrane protein Lap2β inactivates some genes, but not others (Finlan et al. 2008). The idea that the nuclear lamina is not repressive to all transcription is further supported by the finding that targeting a genetic locus to the nuclear lamina by artificial tethering to lamin B1 does not interfere with its transcriptional activation (Kumaran and Spector 2008). In addition, lamin A can act as a transcriptional repressor in mammalian cells and in yeast when artificially targeted to specific promoters (Lee et al. 2009). This latter finding is intriguing because yeast do not contain lamin genes. These somewhat conflicting results demonstrate that more research is required to determine the role of lamins in transcription both in the region of the lamina and throughout the nucleoplasm.
Lamins are Involved in the Epigenetic Regulation of Chromatin
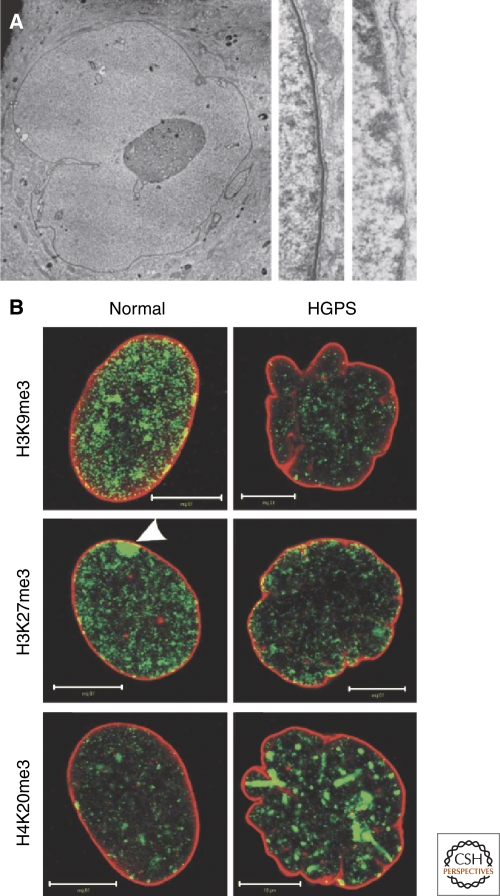
The dramatic loss of peripheral heterochromatin in the nuclei of cells expressing one of the mutant forms of lamin A, progerin, that causes the premature aging disorder HGPS emphasizes the important role of lamins in the modification and/or the organization of chromatin (Shumaker et al. 2006) (Fig. 7). A partial loss of peripheral heterochromatin is also seen in LMNA−/− MEFs (Sullivan et al. 1999; Nikolova et al. 2004). Furthermore, down-regulation of lamin B1 in HeLa cells results in lamin A/C-rich and lamin B2 deficient nuclear blebs or microdomains characterized by the absence of heterochromatin (Shimi et al. 2008). These changes in chromatin organization related to lamin expression are reflected in alterations in histone modifications including reductions in trimethylated H3K9, trimethylated H3K27 and an increase in trimethylation of H4K20 in HGPS cells (Scaffidi and Misteli 2006; Shumaker et al. 2006; Shimi et al. 2008) (Fig. 7). These studies demonstrate that the interaction of chromatin with lamins, whether direct or indirect, has strong effects on the epigenetic modification of histones, and that lamin-associated microdomains in the nucleus might regulate modification of chromatin (Pegoraro et al. 2009). One potential link between lamins and chromatin modification may involve the tumor suppressor ING1. This protein, which interacts with histone acetyltransferases and histone deacetylases, appears to be stabilized and targeted to the nucleus by its interaction with A-type lamins (Han et al. 2008). HGPS cells have reduced ING1 levels and the expression of ING1 protein lacking its lamin interacting domain causes a loss of peripheral heterochromatin in normal cells.
Figure 7.
The association of the nuclear lamina with chromatin in normal and progeria patients' cells. (A) Thin section electron micrograph of a late passage blebbed nucleus in an HGPS patient's skin fibroblast (left and center panels) and normal human foreskin fibroblasts (right panel). A high-magnification view of the nuclear envelope in a normal human foreskin fibroblast shows a normal array of heterochromatin adjacent to the nuclear envelope, making any lamina structure difficult to detect (right panel). A higher-magnification view of a HGPS cell showing a loss of peripheral heterochromatin and a prominent electron-dense lamina region associated with the inner nuclear envelope membrane (the nucleus is to the left in center and right panels) (Goldman et al. 2004). (B) Alterations of histone methylation patterns in HGPS fibroblasts. Normal and HGPS fibroblasts from female donors were double-labeled with antibodies against lamins A/C (red) and trimethylated Lys 9 in histone H3 (H3K9me3), Lys 27 in histone H3 (H3K27me3), or Lys 20 in histone H4 (H4K20me3) (all green). Note the decrease of H3K9me3 and H3K27me3 and the increase of H4K20me3 in the lobulated HGPS nuclei compared with normal nuclei. The decrease in H3K27me3 is best observed at the inactive X chromosome, which is normally enriched in this histone modification (see arrowhead in center left panel). Bars, 10 µm. Reprinted, with permission, from Dechat et al. (2008b).
Lamins and Interphase Chromosome Organization
The organization of chromosome territories and domains is influenced by the expression of lamins. For example, in LMNBΔ/Δ MEFs and in cells derived from patients suffering from some types of laminopathies, gene-poor chromosome 18 is positioned away from its normal location at the nuclear periphery toward the nuclear interior (Malhas et al. 2007; Meaburn et al. 2007). Furthermore, in HeLa cells silenced for the expression of lamin B1, the lamin A/C rich and lamin B2-deficient nuclear envelope blebs that form are associated predominantly with gene-rich chromosome regions (Shimi et al. 2008) (Fig. 8). Together, these findings suggest that A-type lamins are preferentially associated with gene-rich chromatin regions and B-type lamins are preferentially associated with gene-poor regions of chromosomes. It must be emphasized that these studies are of a preliminary nature and that much more work is required to establish the precise relationships between the different types of lamins and gene rich/gene poor chromosome domains within the interphase nucleus.
Figure 8.
Localization of specific gene rich chromosomal regions in nuclear blebs in LB1-silenced HeLa cells. Chromosomes are detected by fluorescence in situ hybridization. DNA is counterstained with Hoechst 33258 (blue). Chromosome 6p (or 19), and chromosome 6q (or 18) are shown in green and red, respectively. Bars, 5 µm. Adapted, with permission, from Shimi et al. (2008).
Lamins may also play a role in the localization and function of centromeres and telomeres in the nucleus. Centromeres are typically arranged near the nuclear periphery in an interphase nucleus (Solovei et al. 2004). However, the lamin A/C-rich, lamin B2-deficient nuclear envelope blebs that form in lamin B1-silenced cells are devoid of centromeres, suggesting that B-type lamins are involved in anchoring these heterochromatic structures at the nuclear lamina (Shimi et al. 2008). Telomeres are also influenced by the composition and structure of the lamina, because in LMNA−/− MEFs, the distribution, length and structure of telomeres is altered leading to increased genomic instability compared to control MEFs (Gonzalez-Suarez et al. 2009a). Human fibroblasts ectopically expressing HGPS lamin A mutant proteins show rapid telomere shortening and an accelerated replicative senescence phenotype (McClintock et al. 2006; Huang et al. 2008). Furthermore, the proliferative defects seen in normal dermal fibroblasts expressing progerin can be overcome by expression of the catalytic subunit of telomerase or the inactivation of p53 (Kudlow et al. 2008). Other support for the involvement of lamins in telomere function comes from the finding that the D4Z4 human subtelomeric repeat can localize an adjacent telomere to the nuclear periphery probably by interacting with A-type lamins and CTCF (CCCTC-binding factor) (Ottaviani et al. 2009a; Ottaviani et al. 2009b). The D4Z4 repeat acts as a CTCF and A-type lamin-dependent transcriptional insulator, suggesting that nuclear lamin architecture may organize specific regions of chromatin and influence gene expression (Ottaviani et al. 2009a).
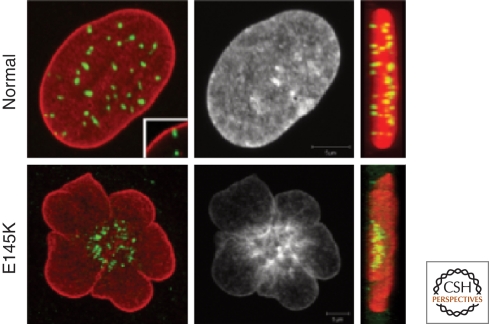
Strong support for the role of the lamins in interphase chromosome organization comes from a study of cells obtained from patients with a form of atypical HGPS (Taimen et al. 2009b). Unlike the most common form of HGPS, which results in only defective lamin A, these patients have a mutation resulting in the expression of a point mutation, E145K, in the 1B segment of the central rod domain of both lamins A and C (see Fig. 1). The cells from these patients have a flower-shaped nucleus with centrally clustered centromeres and abnormal peripherally displaced telomeres (Fig. 9). This abnormal configuration of chromosomes requires a round of cell division and persists throughout interphase. The multilobulated nuclei are therefore unlikely to correctly establish normal interphase chromosome territory arrangements. Both the abnormal nuclear shapes and chromosome configurations in E145K cells appear to be due to the aberrant assembly of the nuclear lamina by the mutant lamin A. In vitro analysis of E145K lamin A polymerization shows that this mutant lamin forms disorganized higher-order structures, a defect not seen in the more common form of progeria resulting from a truncation near the carboxyl terminus (Taimen et al. 2009b).
Figure 9.
Mislocalization of heterochromatin and centromeres in HGPS E145K cells. Control and E145K patient cells were stained with anti-lamin A (red), CREST antiserum (green), and Hoechst (white). Maximum projections of series of z-sections spanning the entire nucleus and side projections are shown. Centromeres are clustered in the central region of the nucleus in E145K cells, while they are either closely associated with the peripheral lamina region or elsewhere in the nucleoplasm in control cells. Inset shows an example of the close association of one centromere with the lamina in a single confocal section. In the right hand panels, side projections from merged images are shown. Centromeres are distributed throughout the nuclei in normal cells. In E145K cells centromeres are clustered in the middle of one region of the nucleus (Taimen et al. 2009a).
Lamins in Cell Proliferation and Differentiation
Deficiencies in A-type lamins or the expression of mutant A-type lamins both lead to an array of proliferative defects because of cell cycle acceleration, cell cycle arrest and/or premature senescence (Verstraeten et al. 2007; Dechat et al. 2008a). There is evidence that lamins A/C are involved in the regulation of the pRb/E2F pathway responsible for the G1/S cell cycle transition. One current model suggests that pRb in its suppressive hypophosphorylated state is in a complex with lamins A/C and LAP2α, and that the loss of lamins A/C leads to the degradation of pRb (Ozaki et al. 1994; Markiewicz et al. 2002; Johnson et al. 2004). In addition to pRb, cyclin D3, another critical regulator of G1 progression, interacts directly with lamins A/C (Mariappan et al. 2007).
The findings that lamins are involved in the regulation of cell cycle progression and that they are differentially expressed during development, suggest that they also function in the regulation of cell differentiation. In support of this, during myoblast differentiation there is a decrease in the soluble nucleoplasmic lamin A/C pool, most likely in a pRb and cyclin D3-dependent manner (Muralikrishna et al. 2001; Mariappan and Parnaik 2005). Furthermore, the down-regulation of lamins A/C or the expression of lamin A/C mutants associated with Autosomal Dominant Emery Dreifuss Muscular Dystrophy (AD-EDMD) lead to impaired myoblast differentiation possibly by disrupting the Rb-MyoD pathway (Bakay et al. 2006; Frock et al. 2006). As further evidence for the importance of the lamin A/pRb interaction, the expression of either an AD-EDMD lamin A mutant protein or the HGPS lamin A mutant protein, progerin, results in the inhibition of the phosphorylation of pRb (Markiewicz et al. 2005; Dechat et al. 2007). In the case of the AD-EDMD mutation, a further decrease in pRb levels along with the failure to hyperphosphorylate the available pRB inhibits myoblast differentiation (Favreau et al. 2004; Kandert et al. 2009). In adipocyte differentiation, the overexpression of either wild-type lamin A or a lamin A mutant associated with familial partial lipodystrophy impairs the potential of mouse fibroblasts to differentiate into mature adipocytes; and LMNA−/− mouse embryonic fibroblasts differentiate more readily into fat-containing cells compared to control cells (Boguslavsky et al. 2006). There is also evidence that lamins A/C regulate adipocyte differentiation in a complex with the inner nuclear membrane protein emerin, by influencing the nucleocytoplasmic distribution of β-catenin (Tilgner et al. 2009). In addition, lamins A/C appear to be involved in osteoblast differentiation, as silencing their expression causes impaired osteoblastogenesis and accelerated osteoclastogenesis in human bone marrow stromal cells (Akter et al. 2009; Rauner et al. 2009). Lamin A may also be involved in adult stem cell differentiation as the expression of progerin causes defects mouse stem cell populations (Espada et al. 2008) in the differentiation potential of human mesenchymal stem cells, probably by affecting the Notch-signaling pathway (Espada et al. 2008; Scaffidi and Misteli 2008).
Another aspect of the involvement of the lamins in differentiation is related to aging. In this regard, progerin can be detected in cells from healthy individuals and the protein appears to accumulate with increasing age (McClintock et al. 2006; Scaffidi and Misteli 2006; Cao et al. 2007; Rodriguez et al. 2009). Furthermore, dermal fibroblasts from older individuals (Scaffidi and Misteli 2006), and cells in aged C. elegans (Haithcock et al. 2005) display changes in nuclear shape similar to those found in premature aging.
The Lamins are Connected to the Cytoskeleton
There is evidence that the nuclear lamins are connected to the cytoskeleton through a complex of proteins called the LINC complex (linker of nucleoskeleton and cytoskeleton) (Crisp et al. 2006; Ketema et al. 2007; Stewart-Hutchinson et al. 2008; Burke and Roux 2009). In this complex the integral proteins of the inner nuclear membrane, Sun1 and Sun2, interact with the outer nuclear membrane proteins, nesprin-1, nesprin-2, and nesprin-3α,in the luminal space between the inner and outer nuclear membranes. Although the Sun proteins bind to the lamins (Crisp et al. 2006), the nesprins are associated with both the microfilament and intermediate filament cytoskeletons via direct actin binding and plectin (Tzur et al. 2006). Another lamin binding protein of the inner nuclear membrane, emerin, binds directly to nesprin isoforms, providing additional linkages between the lamins and the cytoskeleton (Wheeler et al. 2007; Zhang et al. 2007). These interactions between the nuclear lamina and the cytoskeleton may have important functional significance. For example, nuclei in fibroblasts deficient in lamin B1 rotate at a higher frequency than nuclei in control cells (Ji et al. 2007). LMNA−/− MEFs, on the other hand, are reported to show a decrease in mechanical stiffness, defects in mechanotransduction resulting in impaired strain-induced signaling, defects in cell polarization and cell migration, and a disturbed organization of microfilaments, vimentin IF networks and microtubules (Houben et al. 2007; Lee et al. 2007; Dahl et al. 2008; Houben et al. 2009).
CONCLUSIONS AND FUTURE PERSPECTIVES
Over the past decade it has become clear that the nuclear lamins are one of the key players in determining nuclear architecture and function. There is growing evidence that these Type V IF proteins provide a filamentous scaffold built from interconnecting A- and B-type lamin networks that pervade the entire nucleus. This scaffold not only determines the shape and mechanical properties of the nucleus, but also serves as a docking site for chromatin and for numerous proteins involved in chromatin organization and various nuclear functions. Besides the extensive lamin structures located within the lamina, smaller and more dynamic lamin polymers appear to be components of large protein complexes known to be involved in a wide range of nuclear housekeeping functions such as DNA replication, DNA repair and RNA PolII transcription.
We have learned much about the nuclear lamins from the analysis of the changes in nuclear form and function that take place in cells from patients suffering from the remarkably large number of diseases attributable to hundreds of mutations in the LMNA gene. However, the precise roles that normal lamins play in nuclear functions remain largely unknown. To gain more insights into their specific functions we need a better understanding of their normal structure at the highest levels of resolution. To date only a few subdomains of the lamins have been shown to be amenable to analysis by X-ray diffraction, because of the well known difficulties inherent in crystallizing IF proteins. Other challenges for future studies include determining the steps in lamin polymerization and depolymerization in vivo and determining how complex networks of the A- and B-type lamins are assembled and interact with each other both at the nuclear periphery and within the nucleoplasm throughout the cell cycle. Finally, it will be of great interest to determine the different roles of the A- and B-type lamins in determining the overall architecture of the nucleus and the mechanisms involved in their linkages to other nuclear structures, such as the nuclear membrane and pore complexes.
ACKNOWLEDGMENTS
Work in the Goldman lab on nuclear lamins is funded by the National Institute on Aging and the National Cancer Institute. PT received a fellowship from Sigrid Juselius Foundation, Orion-Farmos Research Foundation, Cancer Society of Southwestern Finland, and Finnish Cultural Foundation.
Footnotes
Editors: Tom Misteli and David L. Spector
Additional Perspectives on The Nucleus available at www.cshperspectives.org
REFERENCES
- Aaronson RP, Blobel G 1975. Isolation of nuclear pore complexes in association with a lamina. Proc Natl Acad Sci 72: 1007–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolph KW 1987. ADPribosylation of nuclear proteins labeled with [3H]adenosine: changes during the HeLa cycle. Biochim Biophys Acta 909: 222–230 [DOI] [PubMed] [Google Scholar]
- Aebi U, Cohn J, Buhle L, Gerace L 1986. The nuclear lamina is a meshwork of intermediate-type filaments. Nature 323: 560–564 [DOI] [PubMed] [Google Scholar]
- Akter R, Rivas D, Geneau G, Drissi H, Duque G 2009. Effect of lamin A/C knockdown on osteoblast differentiation and function. J Bone Miner Res 24: 283–293 [DOI] [PubMed] [Google Scholar]
- Andres V, Gonzalez JM 2009. Role of A-type lamins in signaling, transcription, and chromatin organization. J Cell Biol 187: 945–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakay M, Wang Z, Melcon G, Schiltz L, Xuan J, Zhao P, Sartorelli V, Seo J, Pegoraro E, Angelini C, et al. 2006. Nuclear envelope dystrophies show a transcriptional fingerprint suggesting disruption of Rb-MyoD pathways in muscle regeneration. Brain 129: 996–1013 [DOI] [PubMed] [Google Scholar]
- Baricheva EA, Berrios M, Bogachev SS, Borisevich IV, Lapik ER, Sharakhov IV, Stuurman N, Fisher PA 1996. DNA from Drosophila melanogaster β-heterochromatin binds specifically to nuclear lamins in vitro and the nuclear envelope in situ. Gene 171: 171–176 [DOI] [PubMed] [Google Scholar]
- Barrowman J, Hamblet C, George CM, Michaelis S 2008. Analysis of prelamin A biogenesis reveals the nucleus to be a CaaX processing compartment. Mol Biol Cell 19: 5398–5408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudouin J, Gerlich D, Daigle N, Eils R, Ellenberg J 2002. Nuclear envelope breakdown proceeds by microtubule-induced tearing of the lamina. Cell 108: 83–96 [DOI] [PubMed] [Google Scholar]
- Bechert K, Lagos-Quintana M, Harborth J, Weber K, Osborn M 2003. Effects of expressing lamin A mutant protein causing Emery-Dreifuss muscular dystrophy and familial partial lipodystrophy in HeLa cells. Exp Cell Res 286: 75–86 [DOI] [PubMed] [Google Scholar]
- Beck LA, Hosick TJ, Sinensky M 1990. Isoprenylation is required for the processing of the lamin A precursor. J Cell Biol 110: 1489–1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmont AS, Zhai Y, Thilenius A 1993. Lamin B distribution and association with peripheral chromatin revealed by optical sectioning and electron microscopy tomography. J Cell Biol 123: 1671–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Harush K, Wiesel N, Frenkiel-Krispin D, Moeller D, Soreq E, Aebi U, Herrmann H, Gruenbaum Y, Medalia O 2009. The supramolecular organization of the C elegans nuclear lamin filament. J Mol Biol 386: 1392–1402 [DOI] [PubMed] [Google Scholar]
- Benavente R, Krohne G 1986. Involvement of nuclear lamins in postmitotic reorganization of chromatin as demonstrated by microinjection of lamin antibodies. J Cell Biol 103: 1847–1854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benavente R, Krohne G, Franke WW 1985. Cell type-specific expression of nuclear lamina proteins during development of Xenopus laevis. Cell 41: 177–190 [DOI] [PubMed] [Google Scholar]
- Boguslavsky RL, Stewart CL, Worman HJ 2006. Nuclear lamin A inhibits adipocyte differentiation: implications for Dunnigan-type familial partial lipodystrophy. Hum Mol Genet 15: 653–663 [DOI] [PubMed] [Google Scholar]
- Boyartchuk VL, Ashby MN, Rine J 1997. Modulation of Ras and a-factor function by carboxyl-terminal proteolysis. Science 275: 1796–1800 [DOI] [PubMed] [Google Scholar]
- Boyle S, Gilchrist S, Bridger JM, Mahy NL, Ellis JA, Bickmore WA 2001. The spatial organization of human chromosomes within the nuclei of normal and emerin-mutant cells. Hum Mol Genet 10: 211–219 [DOI] [PubMed] [Google Scholar]
- Broers JL, Machiels BM, van Eys GJ, Kuijpers HJ, Manders EM, van Driel R, Ramaekers FC 1999. Dynamics of the nuclear lamina as monitored by GFP-tagged A-type lamins. J Cell Sci 112: 3463–3475 [DOI] [PubMed] [Google Scholar]
- Burke B 1990. On the cell-free association of lamins A and C with metaphase chromosomes. Exp Cell Res 186: 169–176 [DOI] [PubMed] [Google Scholar]
- Burke B, Roux KJ 2009. Nuclei take a position: Managing nuclear location. Dev Cell 17: 587–597 [DOI] [PubMed] [Google Scholar]
- Cao K, Capell BC, Erdos MR, Djabali K, Collins FS 2007. A lamin A protein isoform overexpressed in Hutchinson-Gilford progeria syndrome interferes with mitosis in progeria and normal cells. Proc Natl Acad Sci 104: 4949–4954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M, Lee KK, Wilson KL, Gruenbaum Y 2001. Transcriptional repression, apoptosis, human disease and the functional evolution of the nuclear lamina. Trends Biochem Sci 26: 41–47 [DOI] [PubMed] [Google Scholar]
- Collas P 1999. Sequential PKC- and Cdc2-mediated phosphorylation events elicit zebrafish nuclear envelope disassembly. J Cell Sci 112: 977–987 [DOI] [PubMed] [Google Scholar]
- Constantinescu D, Gray HL, Sammak PJ, Schatten GP, Csoka AB 2006. Lamin A/C expression is a marker of mouse and human embryonic stem cell differentiation. Stem Cells 24: 177–185 [DOI] [PubMed] [Google Scholar]
- Corrigan DP, Kuszczak D, Rusinol AE, Thewke DP, Hrycyna CA, Michaelis S, Sinensky MS 2005. Prelamin A endoproteolytic processing in vitro by recombinant Zmpste24. Biochem J 387: 129–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer M, von Hase J, Volm T, Brero A, Kreth G, Walter J, Fischer C, Solovei I, Cremer C, Cremer T 2001. Non-random radial higher-order chromatin arrangements in nuclei of diploid human cells. Chromosome Res 9: 541–567 [DOI] [PubMed] [Google Scholar]
- Crisp M, Liu Q, Roux K, Rattner JB, Shanahan C, Burke B, Stahl PD, Hodzic D 2006. Coupling of the nucleus and cytoplasm: Role of the LINC complex. J Cell Biol 172: 41–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl KN, Ribeiro AJ, Lammerding J 2008. Nuclear shape, mechanics, and mechanotransduction. Circ Res 102: 1307–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl KN, Scaffidi P, Islam MF, Yodh AG, Wilson KL, Misteli T 2006. Distinct structural and mechanical properties of the nuclear lamina in Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci 103: 10271–10276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies BS, Fong LG, Yang SH, Coffinier C, Young SG 2009. The posttranslational processing of prelamin A and disease. Annu Rev Genomics Hum Genet 10: 153–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechat T, Adam SA, Goldman RD 2008a. Nuclear lamins and chromatin: When structure meets function. Adv Enzyme Regul 49: 157–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechat T, Gajewski A, Korbei B, Gerlich D, Daigle N, Haraguchi T, Furukawa K, Ellenberg J, Foisner R 2004. LAP2α and BAF transiently localize to telomeres and specific regions on chromatin during nuclear assembly. J Cell Sci 117: 6117–6128 [DOI] [PubMed] [Google Scholar]
- Dechat T, Pfleghaar K, Sengupta K, Shimi T, Shumaker DK, Solimando L, Goldman RD 2008b. Nuclear lamins: Major factors in the structural organization and function of the nucleus and chromatin. Genes Dev 22: 832–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechat T, Shimi T, Adam SA, Rusinol AE, Andres DA, Spielmann HP, Sinensky MS, Goldman RD 2007. Alterations in mitosis and cell cycle progression caused by a mutant lamin A known to accelerate human aging. Proc Natl Acad Sci 104: 4955–4960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbarre E, Tramier M, Coppey-Moisan M, Gaillard C, Courvalin JC, Buendia B 2006. The truncated prelamin A in Hutchinson-Gilford progeria syndrome alters segregation of A-type and B-type lamin homopolymers. Hum Mol Genet 15: 1113–1122 [DOI] [PubMed] [Google Scholar]
- Dessev G, Goldman R 1988. Meiotic breakdown of nuclear envelope in oocytes of Spisula solidissima involves phosphorylation and release of nuclear lamin. Dev Biol 130: 543–550 [DOI] [PubMed] [Google Scholar]
- Dessev G, Iovcheva-Dessev C, Bischoff JR, Beach D, Goldman R 1991. A complex containing p34cdc2 and cyclin B phosphorylates the nuclear lamin and disassembles nuclei of clam oocytes in vitro. J Cell Biol 112: 523–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessev G, Iovcheva C, Tasheva B, Goldman R 1988. Protein kinase activity associated with the nuclear lamina. Proc Natl Acad Sci 85: 2994–2998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessev G, Palazzo R, Rebhun L, Goldman R 1989. Disassembly of the nuclear envelope of spisula oocytes in a cell-free system. Dev Biol 131: 496–504 [DOI] [PubMed] [Google Scholar]
- Dhe-Paganon S, Werner ED, Chi YI, Shoelson SE 2002. Structure of the globular tail of nuclear lamin. J Biol Chem 277: 17381–17384 [DOI] [PubMed] [Google Scholar]
- Dreuillet C, Harper M, Tillit J, Kress M, Ernoult-Lange M 2008. Mislocalization of human transcription factor MOK2 in the presence of pathogenic mutations of lamin A/C. Biol Cell 100: 51–61 [DOI] [PubMed] [Google Scholar]
- Dreuillet C, Tillit J, Kress M, Ernoult-Lange M 2002. In vivo and in vitro interaction between human transcription factor MOK2 and nuclear lamin A/C. Nucleic Acids Res 30: 4634–4642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espada J, Varela I, Flores I, Ugalde AP, Cadinanos J, Pendas AM, Stewart CL, Tryggvason K, Blasco MA, Freije JM, et al. 2008. Nuclear envelope defects cause stem cell dysfunction in premature-aging mice. J Cell Biol 181: 27–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnsworth CC, Gelb MH, Glomset JA 1990. Identification of geranylgeranyl-modified proteins in HeLa cells. Science 247: 320–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favreau C, Dubosclard E, Ostlund C, Vigouroux C, Capeau J, Wehnert M, Higuet D, Worman HJ, Courvalin JC, Buendia B 2003. Expression of lamin A mutated in the carboxyl-terminal tail generates an aberrant nuclear phenotype similar to that observed in cells from patients with Dunnigan-type partial lipodystrophy and Emery-Dreifuss muscular dystrophy. Exp Cell Res 282: 14–23 [DOI] [PubMed] [Google Scholar]
- Favreau C, Higuet D, Courvalin JC, Buendia B 2004. Expression of a mutant lamin A that causes Emery-Dreifuss muscular dystrophy inhibits in vitro differentiation of C2C12 myoblasts. Mol Cell Biol 24: 1481–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett DW 1966. On the occurrence of a fibrous lamina on the inner aspect of the nuclear envelope in certain cells of vertebrates. Am J Anat 119: 129–145 [DOI] [PubMed] [Google Scholar]
- Ferraro A, Eufemi M, Cervoni L, Marinetti R, Turano C 1989. Glycosylated forms of nuclear lamins. FEBS Lett 257: 241–246 [DOI] [PubMed] [Google Scholar]
- Finlan LE, Sproul D, Thomson I, Boyle S, Kerr E, Perry P, Ylstra B, Chubb JR, Bickmore WA 2008. Recruitment to the nuclear periphery can alter expression of genes in human cells. PLoS Genet 4: e1000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasch M, Paddy M, Saumweber H 1988. Developmental and mitotic behaviour of two novel groups of nuclear envelope antigens of Drosophila melanogaster. J Cell Sci 90: 247–263 [DOI] [PubMed] [Google Scholar]
- Frock RL, Kudlow BA, Evans AM, Jameson SA, Hauschka SD, Kennedy BK 2006. Lamin A/C and emerin are critical for skeletal muscle satellite cell differentiation. Genes Dev 20: 486–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K, Hotta Y 1993. cDNA cloning of a germ cell specific lamin B3 from mouse spermatocytes and analysis of its function by ectopic expression in somatic cells. Embo J 12: 97–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galiova G, Bartova E, Raska I, Krejci J, Kozubek S 2008. Chromatin changes induced by lamin A/C deficiency and the histone deacetylase inhibitor trichostatin A. Eur J Cell Biol 87: 291–303 [DOI] [PubMed] [Google Scholar]
- Georgatos SD, Pyrpasopoulou A, Theodoropoulos PA 1997. Nuclear envelope breakdown in mammalian cells involves stepwise lamina disassembly and microtubule-drive deformation of the nuclear membrane. J Cell Sci 110: 2129–2140 [DOI] [PubMed] [Google Scholar]
- Gerace L, Blobel G 1980. The nuclear envelope lamina is reversibly depolymerized during mitosis. Cell 19: 277–287 [DOI] [PubMed] [Google Scholar]
- Glass JR, Gerace L 1990. Lamins A and C bind and assemble at the surface of mitotic chromosomes. J Cell Biol 111: 1047–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg M, Harel A, Brandeis M, Rechsteiner T, Richmond TJ, Weiss AM, Gruenbaum Y 1999. The tail domain of lamin Dm0 binds histones H2A and H2B. Proc Natl Acad Sci 96: 2852–2857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg MW, Fiserova J, Huttenlauch I, Stick R 2008a. A new model for nuclear lamina organization. Biochem Soc Trans 36: 1339–1343 [DOI] [PubMed] [Google Scholar]
- Goldberg MW, Huttenlauch I, Hutchison CJ, Stick R 2008b. Filaments made from A- and B-type lamins differ in structure and organization. J Cell Sci 121: 215–225 [DOI] [PubMed] [Google Scholar]
- Goldman RD, Grin B, Mendez MG, Kuczmarski ER 2008. Intermediate filaments: versatile building blocks of cell structure. Curr Opin Cell Biol 20: 28–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman AE, Maul G, Steinert PM, Yang HY, Goldman RD 1986. Keratin-like proteins that coisolate with intermediate filaments of BHK-21 cells are nuclear lamins. Proc Natl Acad Sci 83: 3839–3843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman AE, Moir RD, Montag-Lowy M, Stewart M, Goldman RD 1992. Pathway of incorporation of microinjected lamin A into the nuclear envelope. J Cell Biol 119: 725–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman RD, Shumaker DK, Erdos MR, Eriksson M, Goldman AE, Gordon LB, Gruenbaum Y, Khuon S, Mendez M, Varga R, et al. 2004. Accumulation of mutant lamin A causes progressive changes in nuclear architecture in Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci 101: 8963–8968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez JM, Navarro-Puche A, Casar B, Crespo P, Andres V 2008. Fast regulation of AP-1 activity through interaction of lamin A/C, ERK1/2, and c-Fos at the nuclear envelope. J Cell Biol 183: 653–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Suarez I, Redwood AB, Gonzalo S 2009a. Loss of A-type lamins and genomic instability. Cell Cycle 8: 3860–3865 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Suarez I, Redwood AB, Perkins SM, Vermolen B, Lichtensztejin D, Grotsky DA, Morgado-Palacin L, Gapud EJ, Sleckman BP, Sullivan T, et al. 2009b. Novel roles for A-type lamins in telomere biology and the DNA damage response pathway. Embo J 28: 2414–2427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss VL, Hocevar BA, Thompson LJ, Stratton CA, Burns DJ, Fields AP 1994. Identification of nuclear β II protein kinase C as a mitotic lamin kinase. J Biol Chem 269: 19074–19080 [PubMed] [Google Scholar]
- Guelen L, Pagie L, Brasset E, Meuleman W, Faza MB, Talhout W, Eussen BH, de Klein A, Wessels L, de Laat W, et al. 2008. Domain organization of human chromosomes revealed by mapping of nuclear lamina interactions. Nature 453: 948–951 [DOI] [PubMed] [Google Scholar]
- Guilly MN, Kolb JP, Gosti F, Godeau F, Courvalin JC 1990. Lamins A and C are not expressed at early stages of human lymphocyte differentiation. Exp Cell Res 189: 145–147 [DOI] [PubMed] [Google Scholar]
- Haithcock E, Dayani Y, Neufeld E, Zahand AJ, Feinstein N, Mattout A, Gruenbaum Y, Liu J 2005. Age-related changes of nuclear architecture in Caenorhabditis elegans. Proc Natl Acad Sci USA 102: 16690–16695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Feng X, Rattner JB, Smith H, Bose P, Suzuki K, Soliman MA, Scott MS, Burke BE, Riabowol K 2008. Tethering by lamin A stabilizes and targets the ING1 tumour suppressor. Nat Cell Biol 10: 1333–1340 [DOI] [PubMed] [Google Scholar]
- Haraguchi T, Kojidani T, Koujin T, Shimi T, Osakada H, Mori C, Yamamoto A, Hiraoka Y 2008. Live cell imaging and electron microscopy reveal dynamic processes of BAF-directed nuclear envelope assembly. J Cell Sci 121: 2540–2554 [DOI] [PubMed] [Google Scholar]
- Harper M, Tillit J, Kress M, Ernoult-Lange M 2009. Phosphorylation-dependent binding of human transcription factor MOK2 to lamin A/C. Febs J 276: 3137–3147 [DOI] [PubMed] [Google Scholar]
- Heald R, McKeon F 1990. Mutations of phosphorylation sites in lamin A that prevent nuclear lamina disassembly in mitosis. Cell 61: 579–589 [DOI] [PubMed] [Google Scholar]
- Heessen S, Fornerod M 2007. The inner nuclear envelope as a transcription factor resting place. EMBO Rep 8: 914–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitlinger E, Peter M, Haner M, Lustig A, Aebi U, Nigg EA 1991. Expression of chicken lamin B2 in Escherichia coli: Characterization of its structure, assembly, and molecular interactions. J Cell Biol 113: 485–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennekes H, Peter M, Weber K, Nigg EA 1993. Phosphorylation on protein kinase C sites inhibits nuclear import of lamin B2. J Cell Biol 120: 1293–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houben F, Ramaekers FC, Snoeckx LH, Broers JL 2007. Role of nuclear lamina-cytoskeleton interactions in the maintenance of cellular strength. Biochim Biophys Acta 1773: 675–686 [DOI] [PubMed] [Google Scholar]
- Houben F, Willems CH, Declercq IL, Hochstenbach K, Kamps MA, Snoeckx LH, Ramaekers FC, Broers JL 2009. Disturbed nuclear orientation and cellular migration in A-type lamin deficient cells. Biochim Biophys Acta 1793: 312–324 [DOI] [PubMed] [Google Scholar]
- Huang S, Risques RA, Martin GM, Rabinovitch PS, Oshima J 2008. Accelerated telomere shortening and replicative senescence in human fibroblasts overexpressing mutant and wild-type lamin A. Exp Cell Res 314: 82–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H, Koyama Y, Takano M, Ishii K, Maeno M, Furukawa K, Horigome T 2007. Nuclear envelope precursor vesicle targeting to chromatin is stimulated by protein phosphatase 1 in Xenopus egg extracts. Exp Cell Res 313: 1897–1910 [DOI] [PubMed] [Google Scholar]
- Ivorra C, Kubicek M, Gonzalez JM, Sanz-Gonzalez SM, Alvarez-Barrientos A, O'Connor JE, Burke B, Andres V 2006. A mechanism of AP-1 suppression through interaction of c-Fos with lamin A/C. Genes Dev 20: 307–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji JY, Lee RT, Vergnes L, Fong LG, Stewart CL, Reue K, Young SG, Zhang Q, Shanahan CM, Lammerding J 2007. Cell nuclei spin in the absence of lamin b1. J Biol Chem 282: 20015–20026 [DOI] [PubMed] [Google Scholar]
- Johnson BR, Nitta RT, Frock RL, Mounkes L, Barbie DA, Stewart CL, Harlow E, Kennedy BK 2004. A-type lamins regulate retinoblastoma protein function by promoting subnuclear localization and preventing proteasomal degradation. Proc Natl Acad Sci 101: 9677–9682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandert S, Wehnert M, Muller CR, Buendia B, Dabauvalle MC 2009. Impaired nuclear functions lead to increased senescence and inefficient differentiation in human myoblasts with a dominant p.R545C mutation in the LMNA gene. Eur J Cell Biol 88: 593–608 [DOI] [PubMed] [Google Scholar]
- Kennedy BK, Barbie DA, Classon M, Dyson N, Harlow E 2000. Nuclear organization of DNA replication in primary mammalian cells. Genes Dev 14: 2855–2868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketema M, Wilhelmsen K, Kuikman I, Janssen H, Hodzic D, Sonnenberg A 2007. Requirements for the localization of nesprin-3 at the nuclear envelope and its interaction with plectin. J Cell Sci 120: 3384–3394 [DOI] [PubMed] [Google Scholar]
- Kilic F, Dalton MB, Burrell SK, Mayer JP, Patterson SD, Sinensky M 1997. In vitro assay and characterization of the farnesylation-dependent prelamin A endoprotease. J Biol Chem 272: 5298–5304 [DOI] [PubMed] [Google Scholar]
- Kosak ST, Skok JA, Medina KL, Riblet R, Le Beau MM, Fisher AG, Singh H 2002. Subnuclear compartmentalization of immunoglobulin loci during lymphocyte development. Science 296: 158–162 [DOI] [PubMed] [Google Scholar]
- Krimm I, Ostlund C, Gilquin B, Couprie J, Hossenlopp P, Mornon JP, Bonne G, Courvalin JC, Worman HJ, Zinn-Justin S 2002. The Ig-like structure of the C-terminal domain of lamin A/C, mutated in muscular dystrophies, cardiomyopathy, and partial lipodystrophy. Structure 10: 811–823 [DOI] [PubMed] [Google Scholar]
- Kudlow BA, Stanfel MN, Burtner CR, Johnston ED, Kennedy BK 2008. Suppression of proliferative defects associated with processing-defective lamin A mutants by hTERT or inactivation of p53. Mol Biol Cell 19: 5238–5248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran RI, Spector DL 2008. A genetic locus targeted to the nuclear periphery in living cells maintains its transcriptional competence. J Cell Biol 180: 51–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Hale CM, Panorchan P, Khatau SB, George JP, Tseng Y, Stewart CL, Hodzic D, Wirtz D 2007. Nuclear lamin A/C deficiency induces defects in cell mechanics, polarization, and migration. Biophys J 93: 2542–2552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DC, Welton KL, Smith ED, Kennedy BK 2009. A-type nuclear lamins act as transcriptional repressors when targeted to promoters. Exp Cell Res 315: 996–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner CF, Furstenberger G, Eppenberger HM, Nigg EA 1986. Biogenesis of the nuclear lamina: in vivo synthesis and processing of nuclear protein precursors. Proc Natl Acad Sci 83: 2096–2099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner CF, Stick R, Eppenberger HM, Nigg EA 1987. Differential expression of nuclear lamin proteins during chicken development. J Cell Biol 105: 577–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz-Bohme B, Wismar J, Fuchs S, Reifegerste R, Buchner E, Betz H, Schmitt B 1997. Insertional mutation of the Drosophila nuclear lamin Dm0 gene results in defective nuclear envelopes, clustering of nuclear pore complexes, and accumulation of annulate lamellae. J Cell Biol 137: 1001–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leukel M, Jost E 1995. Two conserved serines in the nuclear localization signal flanking region are involved in the nuclear targeting of human lamin A. Eur J Cell Biol 68: 133–142 [PubMed] [Google Scholar]
- Leung GK, Schmidt WK, Bergo MO, Gavino B, Wong DH, Tam A, Ashby MN, Michaelis S, Young SG 2001. Biochemical studies of Zmpste24-deficient mice. J Biol Chem 276: 29051–29058 [DOI] [PubMed] [Google Scholar]
- Lin F, Worman HJ 1993. Structural organization of the human gene encoding nuclear lamin A and nuclear lamin C. J Biol Chem 268: 16321–16326 [PubMed] [Google Scholar]
- Liu J, Rolef Ben-Shahar T, Riemer D, Treinin M, Spann P, Weber K, Fire A, Gruenbaum Y 2000. Essential roles for Caenorhabditis elegans lamin gene in nuclear organization, cell cycle progression, and spatial organization of nuclear pore complexes. Mol Biol Cell 11: 3937–3947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Wang J, Chan KM, Tjia WM, Deng W, Guan X, Huang JD, Li KM, Chau PY, Chen DJ, et al. 2005. Genomic instability in laminopathy-based premature aging. Nat Med 11: 780–785 [DOI] [PubMed] [Google Scholar]
- Lloyd DJ, Trembath RC, Shackleton S 2002. A novel interaction between lamin A and SREBP1: Implications for partial lipodystrophy and other laminopathies. Hum Mol Genet 11: 769–777 [DOI] [PubMed] [Google Scholar]
- Loewinger L, McKeon F 1988. Mutations in the nuclear lamin proteins resulting in their aberrant assembly in the cytoplasm. Embo J 7: 2301–2309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Soler RI, Moir RD, Spann TP, Stick R, Goldman RD 2001. A role for nuclear lamins in nuclear envelope assembly. J Cell Biol 154: 61–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourim D, Lin JJ 1989. Expression of nuclear lamin A and muscle-specific proteins in differentiating muscle cells in ovo and in vitro. J Cell Biol 109: 495–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luderus ME, de Graaf A, Mattia E, den Blaauwen JL, Grande MA, de Jong L, van Driel R 1992. Binding of matrix attachment regions to lamin B1. Cell 70: 949–959 [DOI] [PubMed] [Google Scholar]
- Luderus ME, den Blaauwen JL, de Smit OJ, Compton DA, van Driel R 1994. Binding of matrix attachment regions to lamin polymers involves single-stranded regions and the minor groove. Mol Cell Biol 14: 6297–6305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz RJ, Trujillo MA, Denham KS, Wenger L, Sinensky M 1992. Nucleoplasmic localization of prelamin A: Implications for prenylation-dependent lamin A assembly into the nuclear lamina. Proc Natl Acad Sci 89: 3000–3004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Tsai MY, Wang S, Lu B, Chen R, Iii JR, Zhu X, Zheng Y 2009. Requirement for Nudel and dynein for assembly of the lamin B spindle matrix. Nat Cell Biol 11: 247–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machiels BM, Broers JL, Raymond Y, de Ley L, Kuijpers HJ, Caberg NE, Ramaekers FC 1995. Abnormal A-type lamin organization in a human lung carcinoma cell line. Eur J Cell Biol 67: 328–335 [PubMed] [Google Scholar]
- Machiels BM, Henfling ME, Schutte B, van Engeland M, Broers JL, Ramaekers FC 1996. Subcellular localization of proteasomes in apoptotic lung tumor cells and persistence as compared to intermediate filaments. Eur J Cell Biol 70: 250–259 [PubMed] [Google Scholar]
- Maison C, Pyrpasopoulou A, Theodoropoulos PA, Georgatos SD 1997. The inner nuclear membrane protein LAP1 forms a native complex with B-type lamins and partitions with spindle-associated mitotic vesicles. Embo J 16: 4839–4850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhas AN, Lee CF, Vaux DJ 2009. Lamin B1 controls oxidative stress responses via Oct-1. J Cell Biol 184: 45–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhas A, Lee CF, Sanders R, Saunders NJ, Vaux DJ 2007. Defects in lamin B1 expression or processing affect interphase chromosome position and gene expression. J Cell Biol 176: 593–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manju K, Muralikrishna B, Parnaik VK 2006. Expression of disease-causing lamin A mutants impairs the formation of DNA repair foci. J Cell Sci 119: 2704–2714 [DOI] [PubMed] [Google Scholar]
- Mariappan I, Parnaik VK 2005. Sequestration of pRb by cyclin D3 causes intranuclear reorganization of lamin A/C during muscle cell differentiation. Mol Biol Cell 16: 1948–1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariappan I, Gurung R, Thanumalayan S, Parnaik VK 2007. Identification of cyclin D3 as a new interaction partner of lamin A/C. Biochem Biophys Res Commun 355: 981–985 [DOI] [PubMed] [Google Scholar]
- Markiewicz E, Ledran M, Hutchison CJ 2005. Remodelling of the nuclear lamina and nucleoskeleton is required for skeletal muscle differentiation in vitro. J Cell Sci 118: 409–420 [DOI] [PubMed] [Google Scholar]
- Markiewicz E, Dechat T, Foisner R, Quinlan RA, Hutchison CJ 2002. Lamin A/C binding protein LAP2alpha is required for nuclear anchorage of retinoblastoma protein. Mol Biol Cell 13: 4401–4413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattout A, Goldberg M, Tzur Y, Margalit A, Gruenbaum Y 2007. Specific and conserved sequences in D melanogaster and C elegans lamins and histone H2A mediate the attachment of lamins to chromosomes. J Cell Sci 120: 77–85 [DOI] [PubMed] [Google Scholar]
- McClintock D, Gordon LB, Djabali K 2006. Hutchinson-Gilford progeria mutant lamin A primarily targets human vascular cells as detected by an anti-Lamin A G608G antibody. Proc Natl Acad Sci 103: 2154–2159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeon FD, Kirschner MW, Caput D 1986. Homologies in both primary and secondary structure between nuclear envelope and intermediate filament proteins. Nature 319: 463–468 [DOI] [PubMed] [Google Scholar]
- Meaburn KJ, Cabuy E, Bonne G, Levy N, Morris GE, Novelli G, Kill IR, Bridger JM 2007. Primary laminopathy fibroblasts display altered genome organization and apoptosis. Aging Cell 6: 139–153 [DOI] [PubMed] [Google Scholar]
- Melcer S, Gruenbaum Y, Krohne G 2007. Invertebrate lamins. Exp Cell Res 313: 2157–2166 [DOI] [PubMed] [Google Scholar]
- Moir RD, Montag-Lowy M, Goldman RD 1994. Dynamic properties of nuclear lamins: lamin B is associated with sites of DNA replication. J Cell Biol 125: 1201–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moir RD, Yoon M, Khuon S, Goldman RD 2000. Nuclear lamins A and B1: Different pathways of assembly during nuclear envelope formation in living cells. J Cell Biol 151: 1155–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy S, Little M 1992. p34cdc2 kinase-mediated release of lamins from nuclear ghosts is inhibited by cAMP-dependent protein kinase. Exp Cell Res 201: 494–499 [DOI] [PubMed] [Google Scholar]
- Muchir A, van Engelen BG, Lammens M, Mislow JM, McNally E, Schwartz K, Bonne G 2003. Nuclear envelope alterations in fibroblasts from LGMD1B patients carrying nonsense Y259X heterozygous or homozygous mutation in lamin A/C gene. Exp Cell Res 291: 352–362 [DOI] [PubMed] [Google Scholar]
- Muralikrishna B, Dhawan J, Rangaraj N, Parnaik VK 2001. Distinct changes in intranuclear lamin A/C organization during myoblast differentiation. J Cell Sci 114: 4001–4011 [DOI] [PubMed] [Google Scholar]
- Nakajima N, Abe K 1995. Genomic structure of the mouse A-type lamin gene locus encoding somatic and germ cell-specific lamins. FEBS Lett 365: 108–114 [DOI] [PubMed] [Google Scholar]
- Nikolova V, Leimena C, McMahon AC, Tan JC, Chandar S, Jogia D, Kesteven SH, Michalicek J, Otway R, Verheyen F, et al. 2004. Defects in nuclear structure and function promote dilated cardiomyopathy in lamin A/C-deficient mice. J Clin Invest 113: 357–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund C, Bonne G, Schwartz K, Worman HJ 2001. Properties of lamin A mutants found in Emery-Dreifuss muscular dystrophy, cardiomyopathy and Dunnigan-type partial lipodystrophy. J Cell Sci 114: 4435–4445 [DOI] [PubMed] [Google Scholar]
- Ottaviani A, Rival-Gervier S, Boussouar A, Foerster AM, Rondier D, Sacconi S, Desnuelle C, Gilson E, Magdinier F 2009a. The D4Z4 macrosatellite repeat acts as a CTCF and A-type lamins-dependent insulator in facio-scapulo-humeral dystrophy. PLoS Genet 5: e1000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottaviani A, Schluth-Bolard C, Rival-Gervier S, Boussouar A, Rondier D, Foerster AM, Morere J, Bauwens S, Gazzo S, Callet-Bauchu E, et al. 2009b. Identification of a perinuclear positioning element in human subtelomeres that requires A-type lamins and CTCF. Embo J 28: 2428–2436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottaviano Y, Gerace L 1985. Phosphorylation of the nuclear lamins during interphase and mitosis. J Biol Chem 260: 624–632 [PubMed] [Google Scholar]
- Ozaki T, Saijo M, Murakami K, Enomoto H, Taya Y, Sakiyama S 1994. Complex formation between lamin A and the retinoblastoma gene product: Identification of the domain on lamin A required for its interaction. Oncogene 9: 2649–2653 [PubMed] [Google Scholar]
- Paddy MR, Belmont AS, Saumweber H, Agard DA, Sedat JW 1990. Interphase nuclear envelope lamins form a discontinuous network that interacts with only a fraction of the chromatin in the nuclear periphery. Cell 62: 89–106 [DOI] [PubMed] [Google Scholar]
- Pajerowski JD, Dahl KN, Zhong FL, Sammak PJ, Discher DE 2007. Physical plasticity of the nucleus in stem cell differentiation. Proc Natl Acad Sci 104: 15619–15624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panorchan P, Wirtz D, Tseng Y 2004b. Structure-function relationship of biological gels revealed by multiple-particle tracking and differential interference contrast microscopy: The case of human lamin networks. Phys Rev E Stat Nonlin Soft Matter Phys 70: 041906. [DOI] [PubMed] [Google Scholar]
- Panorchan P, Schafer BW, Wirtz D, Tseng Y 2004a. Nuclear envelope breakdown requires overcoming the mechanical integrity of the nuclear lamina. J Biol Chem 279: 43462–43467 [DOI] [PubMed] [Google Scholar]
- Parry DA, Conway JF, Steinert PM 1986. Structural studies on lamin. Similarities and differences between lamin and intermediate-filament proteins. Biochem J 238: 305–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegoraro G, Kubben N, Wickert U, Gohler H, Hoffmann K, Misteli T 2009. Ageing-related chromatin defects through loss of the NURD complex. Nat Cell Biol 11: 1261–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter M, Kitten GT, Lehner CF, Vorburger K, Bailer SM, Maridor G, Nigg EA 1989. Cloning and sequencing of cDNA clones encoding chicken lamins A and B1 and comparison of the primary structures of vertebrate A- and B-type lamins. J Mol Biol 208: 393–404 [DOI] [PubMed] [Google Scholar]
- Peter M, Nakagawa J, Doree M, Labbe JC, Nigg EA 1990. In vitro disassembly of the nuclear lamina and M phase-specific phosphorylation of lamins by cdc2 kinase. Cell 61: 591–602 [DOI] [PubMed] [Google Scholar]
- Pickersgill H, Kalverda B, de Wit E, Talhout W, Fornerod M, van Steensel B 2006. Characterization of the Drosophila melanogaster genome at the nuclear lamina. Nat Genet 38: 1005–1014 [DOI] [PubMed] [Google Scholar]
- Prather RS, Sims MM, Maul GG, First NL, Schatten G 1989. Nuclear lamin antigens are developmentally regulated during porcine and bovine embryogenesis. Biol Reprod 41: 123–132 [DOI] [PubMed] [Google Scholar]
- Rauner M, Sipos W, Goettsch C, Wutzl A, Foisner R, Pietschmann P, Hofbauer LC 2009. Inhibition of lamin A/C attenuates osteoblast differentiation and enhances RANKL-dependent osteoclastogenesis. J Bone Miner Res 24: 78–86 [DOI] [PubMed] [Google Scholar]
- Reddy KL, Zullo JM, Bertolino E, Singh H 2008. Transcriptional repression mediated by repositioning of genes to the nuclear lamina. Nature 452: 243–247 [DOI] [PubMed] [Google Scholar]
- Riemer D, Stuurman N, Berrios M, Hunter C, Fisher PA, Weber K 1995. Expression of Drosophila lamin C is developmentally regulated: Analogies with vertebrate A-type lamins. J Cell Sci 108: 3189–3198 [DOI] [PubMed] [Google Scholar]
- Rober RA, Weber K, Osborn M 1989. Differential timing of nuclear lamin A/C expression in the various organs of the mouse embryo and the young animal: A developmental study. Development 105: 365–378 [DOI] [PubMed] [Google Scholar]
- Rober RA, Sauter H, Weber K, Osborn M 1990. Cells of the cellular immune and hemopoietic system of the mouse lack lamins A/C: distinction versus other somatic cells. J Cell Sci 95: 587–598 [DOI] [PubMed] [Google Scholar]
- Rodriguez S, Coppede F, Sagelius H, Eriksson M 2009. Increased expression of the Hutchinson-Gilford progeria syndrome truncated lamin A transcript during cell aging. Eur J Hum Genet 17: 928–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusinol AE, Sinensky MS 2006. Farnesylated lamins, progeroid syndromes and farnesyl transferase inhibitors. J Cell Sci 119: 3265–3272 [DOI] [PubMed] [Google Scholar]
- Scaffidi P, Misteli T 2006. Lamin A-dependent nuclear defects in human aging. Science 312: 1059–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi P, Misteli T 2008. Lamin A-dependent misregulation of adult stem cells associated with accelerated ageing. Nat Cell Biol 10: 452–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schape J, Prausse S, Radmacher M, Stick R 2009. Influence of lamin A on the mechanical properties of amphibian oocyte nuclei measured by atomic force microscopy. Biophys J 96: 4319–4325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatten G, Maul GG, Schatten H, Chaly N, Simerly C, Balczon R, Brown DL 1985. Nuclear lamins and peripheral nuclear antigens during fertilization and embryogenesis in mice and sea urchins. Proc Natl Acad Sci 82: 4727–4731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schermelleh L, Carlton PM, Haase S, Shao L, Winoto L, Kner P, Burke B, Cardoso MC, Agard DA, Gustafsson MG, et al. 2008. Subdiffraction multicolor imaging of the nuclear periphery with 3D structured illumination microscopy. Science 320: 1332–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevelyov YY, Lavrov SA, Mikhaylova LM, Nurminsky ID, Kulathinal RJ, Egorova KS, Rozovsky YM, Nurminsky DI 2009. The B-type lamin is required for somatic repression of testis-specific gene clusters. Proc Natl Acad Sci 106: 3282–3287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimi T, Pfleghaar K, Kojima S, Pack CG, Solovei I, Goldman AE, Adam SA, Shumaker DK, Kinjo M, Cremer T, et al. 2008. The A- and B-type nuclear lamin networks: microdomains involved in chromatin organization and transcription. Genes Dev 22: 3409–3421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumaker DK, Dechat T, Kohlmaier A, Adam SA, Bozovsky MR, Erdos MR, Eriksson M, Goldman AE, Khuon S, Collins FS, et al. 2006. Mutant nuclear lamin A leads to progressive alterations of epigenetic control in premature aging. Proc Natl Acad Sci 103: 8703–8708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumaker DK, Lopez-Soler RI, Adam SA, Herrmann H, Moir RD, Spann TP, Goldman RD 2005. Functions and dysfunctions of the nuclear lamin Ig-fold domain in nuclear assembly, growth, and Emery-Dreifuss muscular dystrophy. Proc Natl Acad Sci USA 102: 15494–15499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumaker DK, Solimando L, Sengupta K, Shimi T, Adam SA, Grunwald A, Strelkov SV, Aebi U, Cardoso MC, Goldman RD 2008. The highly conserved nuclear lamin Ig-fold binds to PCNA: Its role in DNA replication. J Cell Biol 181: 269–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solovei I, Schermelleh L, During K, Engelhardt A, Stein S, Cremer C, Cremer T 2004. Differences in centromere positioning of cycling and postmitotic human cell types. Chromosoma 112: 410–423 [DOI] [PubMed] [Google Scholar]
- Spann TP, Goldman AE, Wang C, Huang S, Goldman RD 2002. Alteration of nuclear lamin organization inhibits RNA polymerase II-dependent transcription. J Cell Biol 156: 603–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart-Hutchinson PJ, Hale CM, Wirtz D, Hodzic D 2008. Structural requirements for the assembly of LINC complexes and their function in cellular mechanical stiffness. Exp Cell Res 314: 1892–1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C, Burke B 1987. Teratocarcinoma stem cells and early mouse embryos contain only a single major lamin polypeptide closely resembling lamin B. Cell 51: 383–392 [DOI] [PubMed] [Google Scholar]
- Stick R, Angres B, Lehner CF, Nigg EA 1988. The fates of chicken nuclear lamin proteins during mitosis: Evidence for a reversible redistribution of lamin B2 between inner nuclear membrane and elements of the endoplasmic reticulum. J Cell Biol 107: 397–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strelkov SV, Schumacher J, Burkhard P, Aebi U, Herrmann H 2004. Crystal structure of the human lamin A coil 2B dimer: Implications for the head-to-tail association of nuclear lamins. J Mol Biol 343: 1067–1080 [DOI] [PubMed] [Google Scholar]
- Stuurman N, Heins S, Aebi U 1998. Nuclear lamins: Their structure, assembly, and interactions. J Struct Biol 122: 42–66 [DOI] [PubMed] [Google Scholar]
- Stuurman N, Sasse B, Fisher PA 1996. Intermediate filament protein polymerization: Molecular analysis of Drosophila nuclear lamin head-to-tail binding. J Struct Biol 117: 1–15 [DOI] [PubMed] [Google Scholar]
- Sullivan T, Escalante-Alcalde D, Bhatt H, Anver M, Bhat N, Nagashima K, Stewart CL, Burke B 1999. Loss of A-type lamin expression compromises nuclear envelope integrity leading to muscular dystrophy. J Cell Biol 147: 913–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taimen P, Pfleghaar K, Shimi T, Moller D, Ben-Harush K, Erdos MR, Adam SA, Herrmann H, Medalia O, Collins FS, et al. 2009a. A progeria mutation reveals functions for lamin A in nuclear assembly, architecture, and chromosome organization. Proc Natl Acad Sci 106: 20788–20793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taimen P, Pfleghaar K, Shimi T, Moller D, Ben-Harush K, Erdos MR, Adam SA, Herrmann H, Medalia O, Collins FS, et al. 2009b. A progeria mutation reveals functions for lamin A in nuclear assembly, architecture, and chromosome organization. Proc Natl Acad Sci 106: 20788–20793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang CW, Maya-Mendoza A, Martin C, Zeng K, Chen S, Feret D, Wilson SA, Jackson DA 2008. The integrity of a lamin-B1-dependent nucleoskeleton is a fundamental determinant of RNA synthesis in human cells. J Cell Sci 121: 1014–1024 [DOI] [PubMed] [Google Scholar]
- Taniura H, Glass C, Gerace L 1995. A chromatin binding site in the tail domain of nuclear lamins that interacts with core histones. J Cell Biol 131: 33–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson LJ, Bollen M, Fields AP 1997. Identification of protein phosphatase 1 as a mitotic lamin phosphatase. J Biol Chem 272: 29693–29697 [DOI] [PubMed] [Google Scholar]
- Tilgner K, Wojciechowicz K, Jahoda C, Hutchison C, Markiewicz E 2009. Dynamic complexes of A-type lamins and emerin influence adipogenic capacity of the cell via nucleocytoplasmic distribution of beta-catenin. J Cell Sci 122: 401–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai MY, Zheng Y 2005. Aurora A kinase-coated beads function as microtubule-organizing centers and enhance RanGTP-induced spindle assembly. Curr Biol 15: 2156–2163 [DOI] [PubMed] [Google Scholar]
- Tsai MY, Wang S, Heidinger JM, Shumaker DK, Adam SA, Goldman RD, Zheng Y 2006. A mitotic lamin B matrix induced by RanGTP required for spindle assembly. Science 311: 1887–1893 [DOI] [PubMed] [Google Scholar]
- Tzur YB, Wilson KL, Gruenbaum Y 2006. SUN-domain proteins: ‘Velcro’ that links the nucleoskeleton to the cytoskeleton. Nat Rev Mol Cell Biol 7: 782–788 [DOI] [PubMed] [Google Scholar]
- Vaughan A, Alvarez-Reyes M, Bridger JM, Broers JL, Ramaekers FC, Wehnert M, Morris GE, Whitfield WGF, Hutchison CJ 2001. Both emerin and lamin C depend on lamin A for localization at the nuclear envelope. J Cell Sci 114: 2577–2590 [DOI] [PubMed] [Google Scholar]
- Vergnes L, Peterfy M, Bergo MO, Young SG, Reue K 2004. Lamin B1 is required for mouse development and nuclear integrity. Proc Natl Acad Sci 101: 10428–10433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstraeten VL, Broers JL, Ramaekers FC, van Steensel MA 2007. The nuclear envelope, a key structure in cellular integrity and gene expression. Curr Med Chem 14: 1231–1248 [DOI] [PubMed] [Google Scholar]
- Vigouroux C, Auclair M, Dubosclard E, Pouchelet M, Capeau J, Courvalin JC, Buendia B 2001. Nuclear envelope disorganization in fibroblasts from lipodystrophic patients with heterozygous R482Q/W mutations in the lamin A/C gene. J Cell Sci 114: 4459–4468 [DOI] [PubMed] [Google Scholar]
- Vorburger K, Kitten GT, Nigg EA 1989. Modification of nuclear lamin proteins by a mevalonic acid derivative occurs in reticulocyte lysates and requires the cysteine residue of the C-terminal CXXM motif. Embo J 8: 4007–4013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward GE, Kirschner MW 1990. Identification of cell cycle-regulated phosphorylation sites on nuclear lamin C Cell 61: 561–577 [DOI] [PubMed] [Google Scholar]
- Wheeler MA, Davies JD, Zhang Q, Emerson LJ, Hunt J, Shanahan CM, Ellis JA 2007. Distinct functional domains in nesprin-1α and nesprin-2β bind directly to emerin and both interactions are disrupted in X-linked Emery-Dreifuss muscular dystrophy. Exp Cell Res 313: 2845–2857 [DOI] [PubMed] [Google Scholar]
- Williams RR, Azuara V, Perry P, Sauer S, Dvorkina M, Jorgensen H, Roix J, McQueen P, Misteli T, Merkenschlager M, et al. 2006. Neural induction promotes large-scale chromatin reorganisation of the Mash1 locus. J Cell Sci 119: 132–140 [DOI] [PubMed] [Google Scholar]
- Winter-Vann AM, Casey PJ 2005. Post-prenylation-processing enzymes as new targets in oncogenesis. Nat Rev Cancer 5: 405–412 [DOI] [PubMed] [Google Scholar]
- Yuan J, Simos G, Blobel G, Georgatos SD 1991. Binding of lamin A to polynucleosomes. J Biol Chem 266: 9211–9215 [PubMed] [Google Scholar]
- Zackroff RV, Goldman RD 1979. In vitro assembly of intermediate filaments from baby hamster kidney (BHK-21) cells. Proc Natl Acad Sci 76: 6226–6230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang FL, Casey PJ 1996. Protein prenylation: Molecular mechanisms and functional consequences. Annu Rev Biochem 65: 241–269 [DOI] [PubMed] [Google Scholar]
- Zhang YQ, Sarge KD 2008. Sumoylation regulates lamin A function and is lost in lamin A mutants associated with familial cardiomyopathies. J Cell Biol 182: 35–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Bethmann C, Worth NF, Davies JD, Wasner C, Feuer A, Ragnauth CD, Yi Q, Mellad JA, Warren DT, et al. 2007. Nesprin-1 and -2 are involved in the pathogenesis of Emery Dreifuss muscular dystrophy and are critical for nuclear envelope integrity. Hum Mol Genet 16: 2816–2833 [DOI] [PubMed] [Google Scholar]
- Zhao K, Harel A, Stuurman N, Guedalia D, Gruenbaum Y 1996. Binding of matrix attachment regions to nuclear lamin is mediated by the rod domain and depends on the lamin polymerization state. FEBS Lett 380: 161–164 [DOI] [PubMed] [Google Scholar]
- Zink D, Amaral MD, Englmann A, Lang S, Clarke LA, Rudolph C, Alt F, Luther K, Braz C, Sadoni N, et al. 2004. Transcription-dependent spatial arrangements of CFTR and adjacent genes in human cell nuclei. J Cell Biol 166: 815–825 [DOI] [PMC free article] [PubMed] [Google Scholar]